Аннотация
The fractional composition of proteins of the cod-fish family - polar cod ( Boreogadus saida ) and blue whiting ( Micromesistius poutassou ) which are currently underutilized in the food industry has been studied. A method of multiple extraction of homogenized fish raw material with solutions of increasing ionic strength and pH was used for protein fractionation. Water-soluble (WSP), salt-soluble (SSP) and alkaline-soluble (ASP) fractions of fish proteins have been separated. The content of protein in a whole fish and its aqueous extracts was determined by the Kjeldahl method as well as photometric method. The amino acid composition of the proteins was studied by high-performance liquid chromatography. Protein of blue whiting comprises 92% of WSP and SSP fractions; while polar cod comprisesonly 60%. All proteins under study contain the full set of the amino acids including essential ones. The content of lysine both in non-fractionated proteins contained in a whole fish and their fractions is considerable (from 59.5 to 121 g kg-1). When evaluating the biological value of proteins, their usefulness is established (there are no limiting amino acids). The data of the rationality coefficient Rc which presents the balance of the amino acid composition of the proteins indicate the high biological value of proteins of the whole polar cod and their ASP fraction. The fish species being studied can be used for production high-grade protein products - fish hydrolysates and isolates. The possibility of using unrefined fish is shown that is peculiarity of novel food technologies for obtaining protein products of high biological valueКлючевые слова
Amino acid balance, blue whiting, fractional and amino acid composition, polar cod, protein biological valueВВЕДЕНИЕ
The analysis of a range of foods consumed in most countries has proved the growing phenomenon of a protein deficiency in products of animal origin [1, 2]. Currently, industrially processed hydrobionts are a source of high biological value protein that closely resembles that of animal origin.
Fish products play a major role in the rational food intake of millions of people worldwide. Therefore, the creation of new generation food products, based on marine hydrobionts having improved nutritional and biological value, is of great significance to accommodate the increasing demand for high-quality protein fish products. These types of foods, rich in essential components, are intended to replenish the amino acid and bioenergetic deficiencies of the organism.
The World Health Organization and Institute of Nutrition of the Russian Academy of Science have developed the main requirements for a balanced diet and its components [3–5]. When using marine bioresources, the principle task is to develop new technologies for separating organic components, which is linked with the production of new types of fish products that can meet specific biological, medical and technological standards [4–6].
Currently, the physicochemical characteristics and biochemical composition of the majority of traditional, commercially exploited marine species, including the Arctic bioresources, have been studied quite comprehensively. Yet, the decreased fishing volume of the most nutritionally valuable species in the Arctic region, and the changed structure of harvested fish have led to the real need to start using non-traditional and small-sized fish, such as the polar cod (Boreogadus saida) and the blue whiting (Micromesistius poutassou), for instance. Thus, an abundance of data shows significant cumulative average stocks of blue whiting and polar cod [6, 7]. This suggests the prospect of increasing the production volume of these species and a need to develop new fish processing technologies.
The existing processing methods can be broadly classified into two universal, technological schemes for obtaining non-specific protein. This approach is widely used in processing protein-containing raw materials [8, 9] and manufacturing fish products, including hydrolysates, isolates and concentrated protein [9]. However, development of innovative technologies to separate protein from the whole sum of chemical components of fish raw materials is of vital importance.
At the research stage, it is important to fractionate the proteins. This protein isolation is based on the solubility principle of various types of proteins in water, salt and alkaline solutions. The necessity of such research is linked with the need to obtain data on the value and technological characteristics of the raw materials used for processing [10, 11, 12]. The qualitative and quantitative protein composition and the protein distribution patterns in the tissue of hydrobionts are important when choosing processing methods for raw materials with the aim of maximizing the output volume of the final products.
In this context, the use of the so-called low-value fishes as raw materials for processing is highly valuable. Recent research devoted to characterizing the chemical and biochemical composition of the abovementioned species exists. However, there has been done no in-depth research into the nutritional value of the blue whiting and polar cod, which are representatives of the cod family available in large quantities.
Therefore, this study aims to examine the protein composition of the raw material obtained from low-value fish species of the cod family, i.e. blue whiting and polar cod, for further utilization and to develop an innovative hydrobiont processing technology.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Food Samples Analyzed. Both blue whiting (M. poutassou) (whole fish) and polar cod (B. saida) (whole fish) were analyzed. The blue whiting was caught by the Murmansk Trawl Fleet JSC in the central and eastern part of the Atlantic in spring (March) and in autumn (October) of 2015. The polar cod was caught by the same company in the Barents sea at the same time. Atlantic cod (Gadus morhua) fillets (boneless without skin) were used as a reference sample for comparison. The Atlantic cod was caught by the Murmansk Trawl Fleet JSC in the fishing parts of the Atlantic in April 2015. All the fish were frozen and delivered to the port of Murmansk (Russia). Fish was frozen in vertical quick-freezing plate devices VPF-10 produced by "Factory of cold" LTD (Russia), and then it was stored during 1 month at the temperature not higher than minus 18ºC.
Chemicals and Standards. All chemical reagents used were either reagent or analytical grade. All chemical reagents were purchased from “Petersburg’s Red Chemist” LTD (Russia). Standard amino acids for chromatography and crystalline bovine serum albumin for spectrometry were purchased from Sigma-Aldrich (Germany).
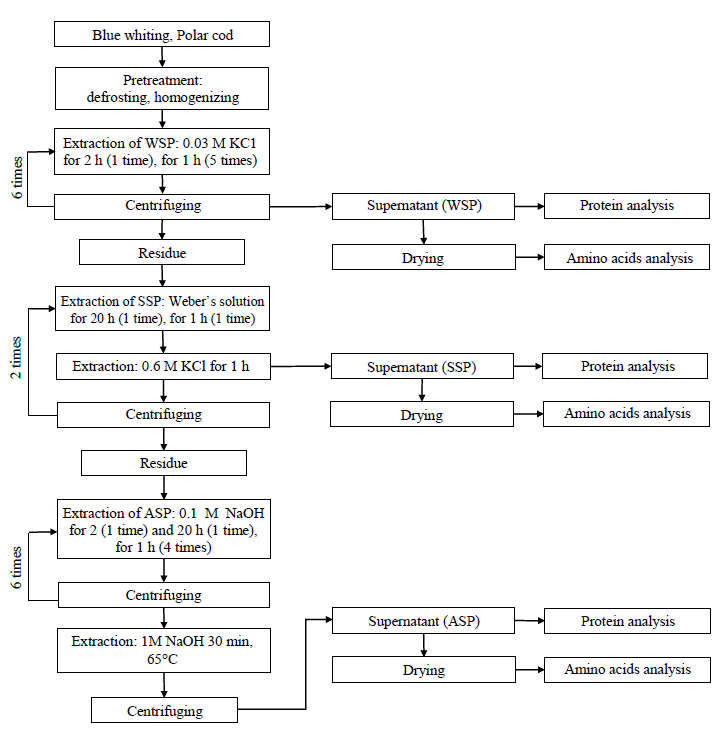
Protein Extraction. Fish protein was fractionated by multiple extractions of homogenous fish raw material with solutions of increasing ionic strength and pH [18]. This method is based on the solubility of the various proteins in salt and alkaline solutions.
The frozen samples (see section Food Samples Analyzed) were defrosted with air at 18ºC and cut into small pieces. The pieces were homogenized (Ase homogenizer, Nihonseiki Kaisha Ltd, Japan) at 5000 rpm for 5 min. Three protein fractions, i.e. water-soluble proteins (WSP), salt-soluble proteins (SSP) and alkali-soluble proteins (ASP) were extracted. The extraction process is shown in Fig. 1.
The WSP were extracted by soaking 1 g of homogeneous sample in 10 mL of 0.03 М KCl solution. The prepared solution was kept at 1–5ºС for 2 h, then centrifuged at 4000 rpm for 20 min, and the solution was separated from the residue by decantation. The residue was 5 times extracted with 0.03 М KCl solutions (1 : 5 v/v) for 1 h, respectively. The supernatant (WSP) and residue were kept for further processing. So, 6 subfractions of WSP were extracted.
The SSP was obtained by soaking the residue left from extracting the WSP with Weber’s solution (a mixture of 0.60 M KCl, 0.0010 M Na2CO3 and 0.040 M NaHCO3) at a 1 : 10 v/v ratio, for 20 h. Subsequently, the liquid part was decanted, and the residue was extracted with 0.6 М KCl solution, then with Weber’s solution and finally with 0.6 М KCl solution for 1 h at 1 : 5 v/v, and 1 h infusion time, respectively. The supernatant (SSP) and residue were kept for further processing. This method made it possible to extract 5 subfractions of SSP.
The ASP were obtained by extracting the residue remaining from the isolated SSP with 0.1 М NaOH solution (1 : 10 v/v) twice, for 2 and 20 h, respectively. Then, the whole procedure was repeated 4 times at 1 : 5 v/v and 1 h infusion time, respectively. The protein was extracted by centrifugation at 6000 rpm for 30 min in 0.1 М NaOH solution. This procedure helped to extraction of 6 subfractions of proteins soluble in 0.1 M NaOH. The remaining residue was soaked in 1 М NaOH solution (1 : 10 v/v) at 65ºС for 30 min. The supernatant (ASP) and residue were kept for further processing.
Protein Determination. The protein content of the original blue whiting and polar cod (see section Food Samples Analyzed) samples was defined according to the total nitrogen content (TN, g kg-1) determined using Kjeldahl apparatus (JP Selecta, Spain) which contains of two modules: the BLOCK–DIGEST–12 for protein mineralization, and the PRO–NITRO A for ammonia distillation. The amount of protein (P, g kg-1) was calculated by multiplying the TN by a conversion factor (usually of 6.25 for fish protein).
The protein content of the polar cod and blue whiting extracts from fractioning (see section Protein Extraction) was determined by Lowry’s method based on the reaction of the proteins with the copper (II) salts in the alkaline solution followed by reduction using phosphomolybdenum-tungsten reagent (Folin’s reagent). These reactions results in production of colored substances which can be determined by measurement at 750 nm using a T70 UV/visible spectrometer (PG Instruments, UK). Bovine serum albumin (0.05–0.80 mg/mL) was used to establish a calibration curve [13, 14].
Amino Acid Determination. Samples (10 g) of homogenized whole fish (polar cod, blue whiting) and cod fillet (see section Food Samples Analyzed) were combined with 96% (v/v) ethyl alcohol (1 : 5 w/w). The protein precipitate was degreased with diethyl ether and air-dried. Extracts of protein fractions were freeze and vacuum-dried using a freeze-dryer LSM-07 (Russia) at 45ºС. The samples (20 mg) were then hydrolyzed with 4 mL 6 M HCl in glass tubes at 120ºC for 24 h under vacuum. The hydrolysates were dried by a vacuum concentrator PE-8920 (Ecros, Russia) at 60ºC and then dissolved in citrate buffer (pH 2.2). The amino content was measured [15] by an automatic amino acid analyzer (AAA-88, Ingos Laboratory Instruments, Czech Republic).
The tryptophan content was determined by hydrolyzing the samples in 2% (w/v) KOH at 80ºC for 30 min, followed by photometric assay with p-dimethylamine benzaldehyde and measurement at 650 nm using a photoelectric colorimeter. Tryptophan (0.02–1.00 mg/mL) was used to establish a calibration curve [16].
Protein Biological Value Determination. The biological value of the proteins and protein fractions was defined by their amino acid contents. The obtained data was compared with the reference protein, etalon, which fully complies with a wellbalanced amino acid protein (the reference protein).
Etalon (ideal) protein according to World Health Organization is a hypothetical protein which can completely satisfy the human requirement in all the essential amino acids.
The amino acid score of the i-amino acid (AASi, %) was calculated as a ratio of the essential amino acids in the examined protein (Ai, g kg-1) to its content in the reference protein (Aie, g kg-1), according to the following equation (1):
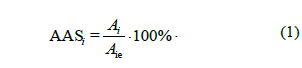

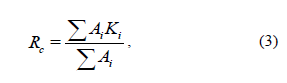
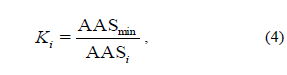
where AASmin (%) is a minimum amino acid score, and Ki is the utility factor of i-amino acid.
Statistics. Experiments were done in triplicate. The data were analyzed by one-way analysis of variance (ANOVA) using Origin Pro 8.0. Differences among means were considered significant at p ˂ 0.05.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
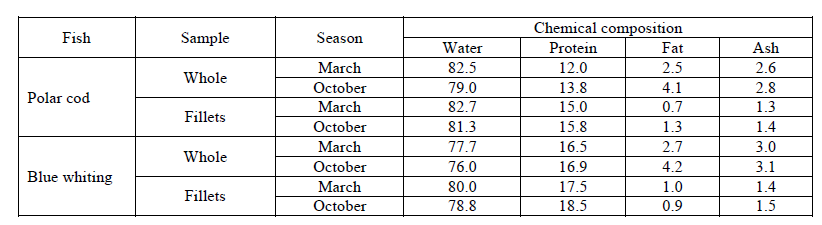
Basic chemical composition of the whole and gutted fish. Chemical composition of the fish is not constant; it depends on its feeding, and it is vary along the year. The feeding intensity of the polar cod and the blue whiting is usually minimal in spring (during spawning period), but it is maximal in autumn (during interspawning period). Table 1 shows the results of researches of the basic chemical compounds in the spring and autumn polar cod and blue whiting (both whole fish and fillets).
The fish caught in spring was used for the further researches.
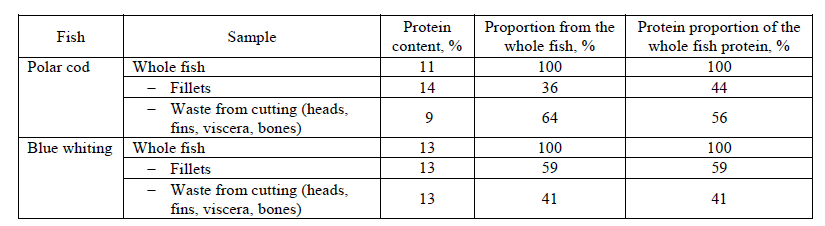
Protein content of whole, cutted fish and waste. Table 2 presents data on the protein content in the whole fish, fish fillets, and fish waste (heads, fins, viscera, bones). The protein content in the whole fish is from 12 to 14% for the polar cod, and from 16 to 19% for blue whiting. The protein content in the blue whiting and polar cod fillets is somewhat higher (15–17%) than in the whole fish, but even the wastes from fish gutting is significant (from 10 to 16%), which is comparable to the whole fish. Thus, the fish waste produced during cutting, as well as the fillets, is a good source of proteins, and they can be used for extracting water- and salt-soluble proteins.
The analysis of the data presented in the Table 2 shows that a significant part of proteinous raw material can be lost during fish cutting. In case of polar cod cutting the losses are about 64% (of mass), in case of blue whiting they about 41% (of mass). These numbers theoretically correspond the losses of 55 and 37% of proteins contained in the whole fish. The obtained results showed the rationality of processing these fish without cutting which can use the maximal amount of proteins contained in fish for the finished product (hydrolysate or isolate).
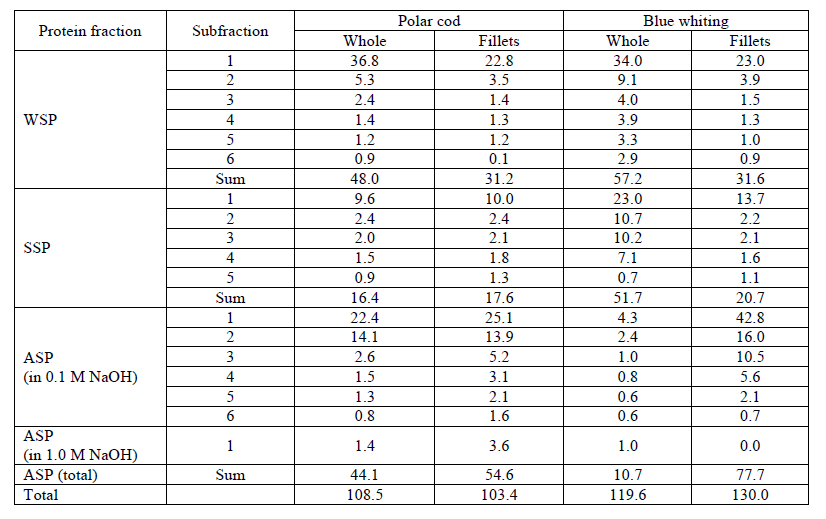
Fractional composition of proteins and amino acids content. Table 3 presents the protein fractionation results obtained from multiple extractions (Fig. 1) of polar cod and blue whiting.
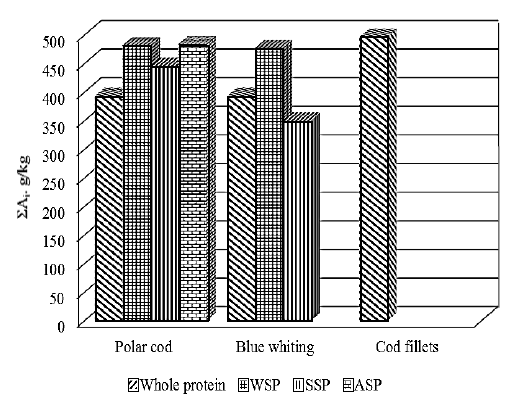
The content of the WSP, SSP and ASP in the whole polar cod and its muscle tissue (fillet) is almost identical (48 and 31 g kg-1 of WSP, 16 and 18 g kg-1 of SSP, 44-55 g rg-1 of ASP), while blue whiting proteins are somewhat different: WSP and SSP in the muscles are almost twice less (57 and 32 g kg-1 of WSP, 52 and 21 g kg-1 of SSP), but ASP is almost 7 times higher. Analysis of Table 3 shows that the amount of WSP, SSP and ASP yields from the blue whiting were 57, 52 and 11 g kg-1, while the corresponding values from the polar cod were 48, 16 and 44 g kg-1, respectively. Therefore, WSP and SSP comprise 92 and 60% of the blue whiting and polar cod protein, respectively. The blue whiting fillets (muscle tissue) contain more ASP (60% of total), while the polar cod fillets contain almost equal of ASP and sum of WSP and WSP (52 and 47% correspondently).
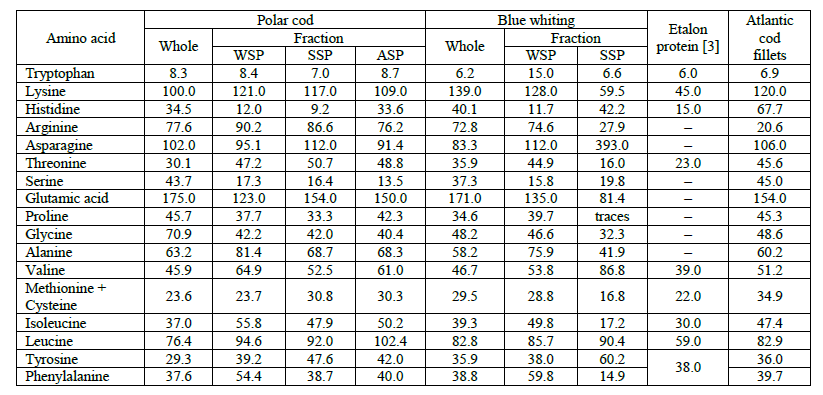
The amino acid content of the blue whiting and polar cod (whole fishes) protein is shown in Table 4, which also provides the WSP, SSP and ASP amino acid compositions. The research of amino acid composition of ASP of the blue whiting has not been carried because of the low content of the ASP. Data on the sample of Atlantic cod fillet are also included in Table 4 for comparison. Atlantic cod muscle tissue contains about 20% of protein and less than 0.5% of fat, so Atlantic cod is considered a reference fish raw material of high protein content.
All the examined proteins in Table 4 are characterized according to the full range of amino acids, including essential acids. Almost all the samples contained glutamic acid, whose content ranged from 123 to 174 g kg-1. The blue whiting SSP, with the highest content of asparaginic acid at 393 g kg-1 but almost complete absence of pyrrolidine carboxylic acid, was an exception. Tryptophan had the lowest content in all the samples, and it ranged from 3.6 to 15.0 g kg-1. A notable content of lysine, which is an essential amino acid, was found in WSP, SSP and ASP of blue whiting and polar cod (from 59.5 to 121 g kg-1). Thus, proteins extracted from polar cod and blue whiting can be recommended as a food supplement to enrich protein foods low in lysine, for example, vegetable food.
The data analysis confirmed that the amino acid content of the proteins from polar cod and blue whiting compare favorably with the amino acids in Atlantic cod fillet proteins, particularly the blue whiting protein samples.
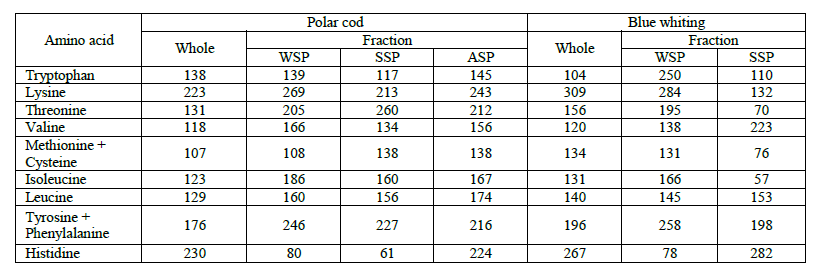
Biological value of underutilized fish species. Table 5 lists the amino acid scores (AASi), which characterizes the protein quality of polar cod and blue whiting (whole fishes, as well as their WSP, SSP and ASP fractions). The data analysis revealed that polar cod and blue whiting proteins do not contain limiting amino acids. Therefore, they are complete. However, the polar cod and blue whiting WSP, and blue whiting SSP contained the limiting amino acid, histidine, while blue whiting SSP contained three limiting amino acids, i.e. isoleucine, threonine and methionine.
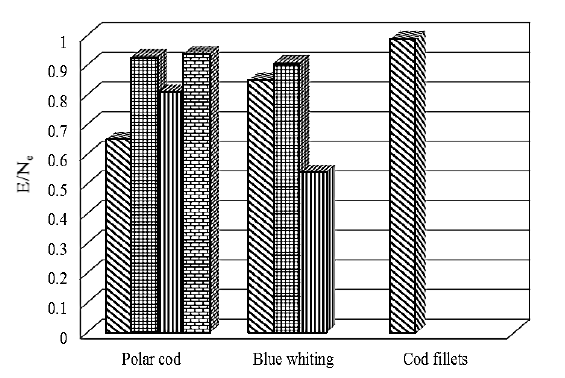
Fig. 2 reveals the blue whiting had the highest biological value (E/Ne). Among the fractions, the WSP of polar cod and blue whiting had the highest biological value.
It is possible to evaluate biological value of the protein by the sum of essential amino acids; Fig. 3 contains these results.
The rationality coefficient (Rс) considers both deficit and excess essential amino acids in the protein. Therefore, it can be assumed that this parameter provides the most objective characteristic of the protein completeness based on the amino acid composition of the protein and thus, its biological value. The Rс for the whole blue whiting proteins in their entirety and their ASP was the highest, confirming its high biological value (Fig. 4). The Rс value for blue whiting was 18 % superior to that of Atlantic cod fillet. Blue whiting (whole fish) proteins and cod fillet proteins were equally balanced.
The rationality coefficient (Rс) considers both deficit and excess essential amino acids in the protein. Therefore, it can be assumed that this parameter provides the most objective characteristic of the protein completeness based on the amino acid composition of the protein and thus, its biological value. The Rс for the whole blue whiting proteins in their entirety and their ASP was the highest, confirming its high biological value (Fig. 4). The Rс value for blue whiting was 18% superior to that of Atlantic cod fillet. Blue whiting (whole fish) proteins and cod fillet proteins were equally balanced.
When the proteins of all the examined samples were graded according to their biological value i.e. increasing value of Rс, the following order was observed: polar cod SSP < blue whiting SSP < blue whiting WSP < polar cod WSP < whole blue whiting protein < Atlantic cod fillet < whole polar cod proteins < polar cod ASP.
Conclusions of using the raw material for protein hydrolysate and isolate in food production. The fraction composition of the proteins of whole and gutted (for fillets) polar cod and blue whiting has been researched for the first time. The obtained data shows that gutting the polar cod and the blue whiting results in losses of almost a half of the proteins from the whole fish, so it is reasonable to use whole fish while producing hydrolysates and isolates for maximal usage of the protein component. The protein content of the whole fish and its muscle tissue is almost independent from the fishing season, but the fat content of the whole fish is almost twice higher in autumn than in spring. It is found that the whole fish - blue whiting and polar cod – contains the full-grade proteins with the high biological value. So, the explored raw material can (and must) be possible to be used for obtaining such valuable products as hydrolysates and isolates. Fish protein hydrolysates contain from peptides and amino acids. Fish protein isolates contain more than 75% of protein in its native form.
The obtained research data makes it possible to recommend all basic technological methods of processing of small fishes during producing fish hydrolysates and isolates. It is recommended to use the whole fish from spring fishing season for producing proteinous products (hydrolysates and isolates).
It is also recommended to hydrolyze minced whole fish during producing hydrolysates. As for isolates (especially actual for the polar cod because of the high content of ASP), it is better to chop the whole fish, and then to carry out the alkaline treatment of the mince. Thus the alkaline extraction makes it possible to get the whole complex of proteins without substantial losses.
It should be said in the conclusion that it is most rational to use blue whiting and polar cod without gutting to reduce energy and labor costs during such operation as “gutting”. Moreover, it will increase the yield of fish hydrolysates and isolates.
Official statistical data of the catch of the blue whiting and the polar cod shows that these fishes have a significant proportion in the total catches. They are not inferior to traditional fishing objects in their protein content. But small sizes and some other disadvantages (for example, high infection with nematodes of the blue whiting) require preliminary gutting using traditional technologies. The possibility to use undressed fish is a feature of the new technologies of producing proteinous products with enhanced biological value.
БЛАГОДАРНОСТИ
The work was funded by the Russian Science Foundation, project 16-16-00076.
СПИСОК ЛИТЕРАТУРЫ
- Hambræus L. Protein and Amino Acids in Human Nutrition: Reference Module in Biomedical Sciences; 2014. DOI: 10.1016/B978-0-12-801238-3.00028-3.
- Shahidi F. (ed.) Maximizing the value of marine by-products. CRC Press: Boca Raton, Boston, New York, Washington DC, 2007. 375 p.
- WHO/FAO/UNU Expert Consultation. Proteins and amino acids requirements in human nutrition (WHO Technical Report Series 935). Geneva: World Health Organization, 2007. 265 p.
- Normy fiziologicheskikh potrebnostey v energii i pishchevykh veshchestvakh dlya razlichnykh grupp naseleniya Rossiyskoy Federatsii [Norms of physiological requirements in energy and nutrients for different population groups in the Russian Federation]. Moscow, 2008. 41 p.
- Pokrovskiy V.I., Romanenko G.A., Knyazhev V.A., et al. Politika zdorovogo pitaniya [Policy of healthy nutrition]. Novosibirsk: Sib. Univ. Publ., 2002. 339 p.
- Zhong C., Sun Z., Zhou Z., et al. Chemical characterization and nutritional analysis of protein isolates from Caragana korshinskii Kom. Journal of Agricultural and Food Chemistry, 2014, vol. 62, pp. 3217-3222
- Sostoyanie syr'evykh biologicheskikh resursov Barentseva morya i Severnoy Atlantiki v 2016 g [The Status of Reserves of Biological Resources of the Barents Sea and Northern. Atlantic in 2016]. Murmansk: PINRO Publ., 2016. 107 p.
- Kharakteristika sostoyania zapasov promyslovykh obyektov v moryakh Severo-Yevropyeyskogo basseina, v Severnoy Atlantike i Zapadnom sektore Rossiyskoy Arktiki v 2014 godu i prognoz vozmozhnogo vylova na 2016 god [Stock state characteristics of fishing objects in the seas of Northern European Basin, in the Northern Atlantic, and in the Western Sector of Russian Arctic in 2014 and the possible catch forecast for 2016]. Murmansk: PINRO Publ., 2015.
- Abdollahi M., Rezaei M., Jafarpour A., and Undeland I. Dynamic rheological, microstructural and physicochemical properties of blend fish protein recovered from kilka (Clupeonella cultriventris) and silver carp (Hypophthalmichthys molitrix) by the pH-shift process or washing-based technology). Food Chemistry, 2017, vol. 229, pp. 695-709. DOI: 10.1016/j.foodchem.2017.02.133.
- Vareltzis P.K. and Undeland I. Protein isolation from blue mussels (Mytilus edulis) using an acid and alkaline solubilisation technique - process characteristics and functionality of the isolates. Journal of the Science of Food and Agriculture, 2012, vol. 92, pp. 3055-3064. DOI: 10.1002/jsfa.5723.
- Tahergorabi R., Sivanandan L., Beamer S.K., Matak K.E., and Jaczynski J. A three-prong strategy to develop functional food using protein isolates recovered from chicken processing by-products with isoelectric solubilization/precipitation. Journal of the Science of Food and Agriculture, 2012, vol. 92, pp. 2534-2542. DOI: 10.1002/jsfa.5668.
- Marmon S.K., Liljelind P., and Undeland I. Removal of lipids, dioxins, and polychlorinated biphenyls during production of protein isolates from baltic herring (Clupea harengus) using pH-shift processes. Journal of Agricultural and Food Chemistry, 2009, vol. 57, pp. 7819-7825.
- Theodore A.E., Raghavan S., and Kristinsson H.G. Antioxidative activity of protein hydrolysates prepared from alkaline-aided channel catfish protein isolates. Journal of Agricultural and Food Chemistry, 2008, vol. 56, pp. 7459-7466. DOI: 10.1021/jf901266v.
- Leemput J., Masson C., Bigot K., et al. ATM localization and gene expression in the adult mouse eye. Molecular Vision, 2009, vol. 15, pp. 393-416.
- Gehring C.K., Gigliotti J.C., Moritz J.S., Tou J.C., and Jaczynski J. Functional and nutritional characteristics of proteins and lipids recovered by isoelectric processing of fish by-products and low-value fish: a review. Food Chemistry, 2011, vol. 124, pp. 422-431. DOI: 10.1016/j.foodchem.2010.06.078.
- Nikitenko E.A., Zaytsev V. G., and Ostrovskiy O. V. Influence of tryptophan content in proteins on the possibility of their determination by a photochemical method with p-dimethylaminobenzaldehyde. Biomedical chemistry, 2007, vol. 53, no. 2, pp. 216-220. (In Russian).