Аннотация
Reproduction is key to the survival and development of a species. Anthropogenic activities release significant amounts of toxic pollutants into the environment. In this study, we aimed to determine effects of heavy metals on some reproductive parameters of the mountain hare.Female mountain hares (n = 41) were hunted in the reference and industrially polluted areas of Krasnoyarsk Krai during four seasons. Their skeletal muscles, liver, and kidneys were subjected to atomic absorption spectrometry to determine concentrations of lead, cadmium, and mercury.
The contents of lead, cadmium, and mercury were significantly higher in the hares from the contaminated areas compared to the reference sites. According to the results, the exposure to lead, cadmium, and mercury had an impact on the reproductive potential of the female mountain hares. In particular, we established correlations between numbers of embryos and corpora lutea and contents of lead in the kidneys and liver, as well as cadmium in the kidneys. The number of corpora lutea and embryonic losses in the female hares from the contaminated areas were higher than those in the hared from reference areas. However, the numbers of embryos did not differ significantly between the compared areas.
Our study showed that about 40% of the liver samples and 100% of the muscle tissue samples obtained from the hares in the impact zone contained high concentrations of lead and cadmium. Therefore, hunting in these industrially polluted areas may pose a toxic hazard to the indigenous peoples living there. Further research is needed to assess potential and actual fertility, offspring survival, and other important parameters of mountain hare populations exposed to different levels of chemical pollution.
Ключевые слова
Heavy metals, cadmium, lead, mercury, reproduction, warm-blooded animals, corpus luteum, embryo, liver, kidneys, skeletal musclesВВЕДЕНИЕ
Species can only sustain their natural populations if they are able to reproduce under unfavorable conditions, especially in the areas of anthropogenic impact [1]. The development of industry and farming has led to environmental pollution, with toxic elements entering the food chains [2, 3]. Anthropogenic pollutants include a wide range of synthetic organic compounds and heavy metals. Commonly found in low concentrations, they can accumulate in feed, water, and air, inevitably harming herbivores and other animals. While a one-time intake of chemicals may have a low toxic load and therefore not cause any physiological abnormalities, some compounds accumulate over time, producing a cumulative effect. Moreover, pollutants can additively disrupt body processes and functions, often targeting reproductive organs. Numerous studies point to the complexity of effects that chemical compounds can have on the endocrine system of mammals. As a result, scientists find it difficult to identify general patterns and extrapolate the data.
Under certain conditions, anthropogenic pollutants can have a significant impact on the reproductive system of animals, reducing their reproductive potential. Studies have identified potential effects of some toxic pollutants on warm-blooded animals. However, there is insufficient information on the factors that determine the rate of these effects or the impact of combined pollutants on physiological systems. These factors are key to identifying risks to the productivity and well-being of populations. Heavy metals, particularly cadmium, lead, and mercury are among the most dangerous pollutants, or ecotoxicants.
Cadmium is a common environmental pollutant from industrial and agricultural activities throughout the world. It enters the body of animals through contaminated feed and water, as well as through cadmium-contai-ning smoke or dust. Humans absorb cadmium from food in relatively low concentrations (3–5%). However, once cadmium reaches the kidneys, it can remain there for 10 to 30 years, causing necrosis of tubular cells [4, 5]. In the liver, cadmium provokes necrosis, apoptosis of hepatocytes, and cessation of autophagy [6]. Intoxication with cadmium salts can cause a disease called “itai-itai”, which originated in Japan. This disease was caused by the contamination of the Jinzu River and its tributaries with cadmium salts and other heavy metals from mining waste dumped into the river. Those residents who consumed fish from this river and used contaminated water for drinking or irrigation developed pathologies, including joint pain, hypotension, muscle hypotrophy, bone fractures and deformations, anemia, pneumonia, as well as gastrointestinal and kidney diseases [7, 8].
Cadmium poses a serious public health risk. It is especially dangerous for the reproductive system due to its impact on the hormonal status. This is why cadmium is called an endocrine disruptor [9, 10]. In particular, it disrupts the rate of steroidogenesis in the ovaries and placenta, interfering with the secretion of female sex hormones [11].
Cadmium can cause spontaneous abortion in early pregnancy. High levels of this metal in the placenta increase the expression of metallothioneins. These are low-molecular-weight proteins with a high cysteine content that prevent toxic metals from penetrating the placenta [12]. Cadmium reduces the bioavailability of zinc and can replace it in some fetal tissues, affecting normal growth and development of the fetus, as well as disrupting cell division and differentiation [13]. Moreover, cadmium reduces the synthesis of leptin, increases the concentration of corticosterone, and interrupts the production of progesterone in the placenta [14].
High concentrations of cadmium were studied in the reproductive organs of rats, rabbits, and Japanese quails [15–17]. The administration of cadmium to laboratory animals caused stromal proliferation in the ovaries, an increase in the number of atretic follicles, and degeneration of the corpus luteum [18, 19]. The effect of cadmium on ovarian follicles is associated with changing levels of gonadotropin hormones and lower levels of follicle-stimulating and luteinizing hormones.
Exposure to cadmium significantly induced oxidative stress in the ovaries of rats. This resulted in increased levels of malondialdehyde (a lipid oxidation product) coupled with decreased levels of the antioxidant enzyme catalase [20]. Agarwal et al. also reported that cadmium can cause hormonal imbalance or oxidative stress, which can provoke miscarriage [21].
At moderate or high concentrations, cadmium affects the synthesis of steroids in the reproductive organs [22]. At low concentrations, cadmium can act like estrogen or androgen by binding directly to their receptors [23]. Johnson et al. found that cadmium can cause disturbances in the reproductive system of some wild animals and contribute to hormone-related cancer [24].
Lead is one of the most dangerous toxic metals that disrupts the functioning of all organs and systems, especially the kidneys and hematopoietic, nervous, and reproductive systems. Lead has a toxic effect on the developing fetus. High concentrations of lead cause acute poisoning in humans, while chronic lead-poisoning promotes severe consequences and death [25]. Lead enters the body through inhalation of lead-containing dust or fumes, as well as through the oral route. It enters the bloodstream and accumulates in the bones and soft tissues, mainly in the brain, liver and kidneys, for a long time, often for life. Bones are believed to contain up to 95% of all lead in the body [26]. Pregnancy increases the need for calcium. Accumulated in the bones, lead can replace calcium and circulate in the bloodstream, becoming an endogenous cause of poisoning [27, 28]. Through the bloodstream, lead penetrates the placenta and disrupts the development of the fetus [29–32]. In pregnant women, lead intoxication can cause spontaneous abortion, premature birth or rupture of fetal membranes, arterial hypertension, preeclampsia, or gestational diabetes mellitus. Moreover, it slows down the growth and development of the fetus and promotes the birth of underweight babies [33–40].
Lead exposure is associated with hormonal imbalances that cause disruption in the reproductive system. Its accumulation has an adverse effect on the functioning of the endocrine glands. In particular, it affects the hypothalamic-pituitary axis, causing changes in the secretion of thyroid-stimulating hormone, growth hormone, as well as follicle-stimulating and luteinizing hormones. Nkomo et al. reported that women with an increased level of lead in the blood had higher concentrations of follicle-stimulating and luteinizing hormones [41].
An experiment with rats showed that higher concentrations of lead promoted persistent vaginal estrus after a period of normal estrus, the development of ovarian follicular cysts, and a decrease in the number of corpora lutea [42]. Chronic doses of lead administered orally caused atresia at all stages of folliculogenesis and decreased the development of follicles [43]. High doses of lead induced more noticeable changes such as swelling and necrosis of the ovarian follicles [44]. An in vitro study showed the effects of lead on the total antioxidant status and superoxide dismutase activity [45]. Vigeh et al. reported adverse effects of lead acetate at a concentration of 10 μg/dL on the reproductive parameters of men [46].
Mercury is a natural trace element that can leach from geological sediments into aquatic ecosystems or is emitted into the air from volcanic eruptions, forest fires, or hot springs [47]. Mercury is also a by-product of human activities such as gold mining, fuel combustion, cement production, and chemical industry [47–49]. Volatile mercury can be transported through the air to locations distant from its sources [50]. This makes mercury pollution a global problem. While natural emissions of mercury into the atmosphere have remained relatively stable over the past 150 years, its anthropogenic emissions have increased sharply. This has led to higher stress, especially in aquatic ecosystems [47]. Exposure to mercury varies depending on its concentrations in the environment and in different foods [25, 51].
Mercury is most toxic when converted to methylmercury, primarily by bacteria in marine and freshwater ecosystems. Methylmercury bioaccumulates in the tissues of organisms and passes from one trophic level to another. As a result, predators are exposed to the highest concentrations of methylmercury and suffer from its negative neurological, immunological, and reproductive effects. This is most pronounced in aquatic food systems [47, 48]. Elemental mercury, like organic mercury, penetrates the placental barrier and causes fetal development defects [52].
Mercury and its compounds cause a wide range of toxic effects depending on their chemical form, dose, and level of exposure [53]. In women, mercury can accumulate in the ovaries and cause reproductive problems, including infertility. High doses of mercury in experimental animals have increased the potential incidence of reproductive disorders, such as infertility, stillbirth, congenital malformations, or spontaneous abortions [48]. Exposure to mercury causes imbalance in the female hormonal system, causing infertility. The progesterone/ estrogen ratio changes in favor of estrogens, inhibiting the release of follicle-stimulating and luteinizing hormones from the anterior pituitary gland [54]. Mercury can also cause infertility by increasing the secretion of prolactin. This is similar to the effect of dopamine on the pituitary gland and midbrain, which affects lactopoiesis and reproductive organs [55].
Ma et al. observed a positive relationship between ovarian mercury accumulation and the incidence of follicular atresia in laying hens [56]. In their study, progesterone levels decreased significantly in all mercurytreated groups. On the other hand, the levels of folliclestimulating and luteinizing hormones showed an inverse correlation with mercury doses. The experimental groups also showed a significant decrease in the activity of catalase, superoxide dismutase, and glutathione reductase, as well as in the content of glutathione.
In a study by Lundholm, poultry treated with daily doses of methylmercury (5 mg for 6 days and 1 mg for 50 days) experienced significant thinning and deformation of the eggshell, as well as a decrease in egg production [57]. The in vitro assays demonstrated the inhibitory effect of mercury on gastrointestinal calcium absorption and/or bone marrow mobilization.
Altunkaynak et al. determined the effects of exposure to mercury vapor on the reproductive parameters of rats [58]. In their study, the rats exposed to higher concentrations of mercury exhibited prolonged estrous cycles and morphological changes in the corpus luteum. Their ovaries had various histomorphometric changes, with a significant increase in the volume of atretic follicles. The authors concluded that exposure to mercury vapor alters the estrous cycle but does not have a significant effect on ovulation, implantation, or pregnancy.
Another in vitro study determined the effect of mercury on the secretion activity of progesterone and insulin-like growth factor-I (IGF-I) by analyzing porcine ovarian granulosa. The study confirmed the direct effect of mercury on the release of the steroid hormone progesterone, as well as the interference of mercury in steroidogenesis and apoptosis [59].
Koli et al. studied the effect of mercury on myometrial activity in Wistar rats treated with 5, 50, and 500 μg/L of mercury chloride in drinking water for 28 days [60]. The authors concluded that low doses of mercury had a detrimental effect on myometrial activity by altering calcium entry into smooth muscle and/or calcium release from intracellular stores [60]. Their study also confirmed that mercury has a concentration-dependent uterotonic effect. Nakade et al. reported inflammation of the endometrium and myometrium in the animals exposed to mercury [61].
Most bioavailable compounds of lead, cadmium, and mercury are not destroyed in the soil, water, plants, and animals, exposing them to their toxic, carcinogenic, and mutagenic effects. The progressive emission of heavy metals into the atmosphere, soil, and water can increase their concentrations in animals and humans to critical values, with potentially irreversible consequences for both individual species and whole populations [25, 40]. Most researchers see the highest risk in the toxic effects of heavy metals on reproduction [1].
The above fully applies to mammals such as lagomorphs that live in the conditions of chemical environmental pollution. Studies have shown that heavy metals can have a significant negative impact on the reproductive systems of humans and laboratory or farm animals, as well as the development of their offspring. However, we lack scientific data on their effects on the reproduction processes in wild mammals and birds. This is due to certain difficulties in conducting field studies of free-living populations compared to laboratory experiments, where dose-related toxic effects can be accurately assessed. Field studies should consider a number of additional factors, including the movement of animals along a pollution gradient, the mosaic nature of impact fields, diet variability, etc. [1].
Literature lacks information on effects of cadmium, lead, and mercury on the natural populations of mountain hares [62–66]. Therefore, more research is needed to manage these populations in pessimal environmental conditions more effectively and to ensure safety of resulting food products.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Materials were collected in 2011–2014 in Krasnoyarsk Krai (Russia), particularly in the middle reaches of the Agapa river (71°638611 north, 87°881650 east) (n = 22) contaminated by industrial activities, as well as in the reference areas near Kheta village, Kresty village (the Khatanga river), and Kataryk village (the Kheta river) (71°319090 north, 99°312443 east) (n = 19).
In particular, we used internal organs (ovaries, liver, kidneys) and skeletal muscles (forelimb muscles undivided into separate muscles) of adult female mountain hares (n = 41) selected in the late spring-summer period, from the 20th of May to the 20th of June. The hares were hunted with snares by local hunters from among the indigenous peoples of the North, who are allowed to hunt in the spring and summer season by law (Article 19 of Russian Federal Law No. 209-FZ of July 24, 2009 “On hunting and conservation of hunting resources”).
To obtain biomaterial, linear routes (at least 10 km long), or trapping paths, were laid in the floodplains of the rivers in the reference and contaminated areas. This method is highly efficient and provides intact organs and tissues. In addition, it avoids contamination with lead, unlike shooting with lead-containing bullets.
The captured animals were marked with tags placed around their necks. The tags had been prepared and provided to the hunters in advance. The carcasses were frozen in an icebox at –18°С and packaged separately in new food-grade plastic bags.
Once every ten days, the carcasses were transported by air to a veterinary laboratory in the city of Dudinka. There, they were measured and weighed by the authors in partnership with veterinary experts from the Russian Federal Service for Veterinary and Phytosanitary Surveillance (Rosselkhoznadzor) in Krasnoyarsk Krai. Next, the samples of organs and tissues were taken for microelement analysis. Also, we counted the number of corpora lutea in the ovaries and the number of embryos in the uterus of every animal.
The material was then packaged in food-grade polyethylene and delivered to a chemical laboratory at the Rosselkhoznadzor Reference Center in Krasnoyarsk. There, Sollar (TJA Solution, USA) and Varian (Agilent Technologies, USA) atomic absorption spectrophotometers were used to determine the contents of lead, cadmium, and mercury in the muscles, liver, and kidneys expressed in terms of natural moisture (69–73, 74–78, and 70–73%, respectively).
The number of corpora lutea of pregnancy was an indicator of potential fertility, while the number of viable embryos characterized actual fertility. The viability of embryos was assessed visually based on morphometric characteristics, linear dimensions, and the absence of signs of tissue resorption or degeneration.
Total embryonic losses were calculated as a difference, %, between the number of corpora lutea of pregnancy and the number of viable embryos, according to [1]. Female hares with more corpora lutea than viable embryos were considered to have embryonic losses. Females with identical numbers of corpora lutea and viable embryos were considered to have no embryonic losses.
The number of lost embryos, or a simple difference between corpora lutea and viable embryos, was also considered in comparative analysis. The percentage of female hares with embryonic losses was calculated in relation to all the females in the sample. Average fertility rates were based on the data for all the females that had corpora lutea and/or embryos.
All the females in the sample were of reproductive age, with their precise age not determined. Neither was it possible to determine the stage of embryogenesis, since there has been little research into the reproductive processes of mountain hares. Moreover, their rutting season is quite lengthy.
The statistical analysis was carried out with standard methods using MS Excel (Office 2019) and Statgraphics (19-X64) [67]. The samples were described by calculating the mean (M), error of the mean (m), standard deviation (SD), median (Med), as well as 25 and 75% percentiles.
Since the distribution of some part of the sample differed from normal distribution, nonparametric analysis was used in addition to the standard methods of variability statistics. In particular, the Mann-Whitney test (U) and the Kruskal-Wallis test (H) were employed to compare the significance of differences, while the Spearman rank correlation method was used to determine relationships between different parameters. Statistical significance was set at p ≤ 0.05.
According to the Ministry of Ecology and Rational Management of Natural Resources in Krasnoyarsk Krai, the man-made impact on the environment has remained the same over the last few years [68]. Therefore, our experimental data are still quite relevant and can be used for comparative environmental monitoring in the study areas both in the short and long terms.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
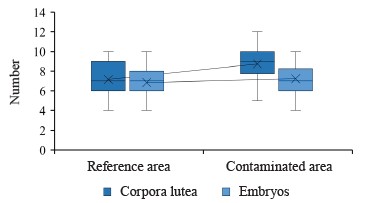
First, we determined the number of corpora lutea in the ovaries of, and the number of embryos in, female mountain hares sampled from the reference and contaminated areas (Fig. 1).
Correlational analysis established a strong positive correlation between the number of corpora lutea and the number of embryos in the female hares, both in the reference (r = 0.95, p = 0.00) and contaminated (r = 0.76, p = 0.00) areas.
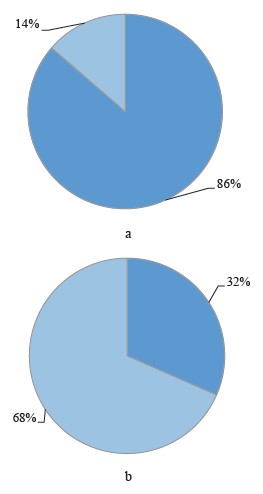
Figure 2 shows the proportion of female hares with embryonic losses in the contaminated and reference areas.
The contents of heavy metals in the organs and tissues of mountain hares were previously reported by us in [66].
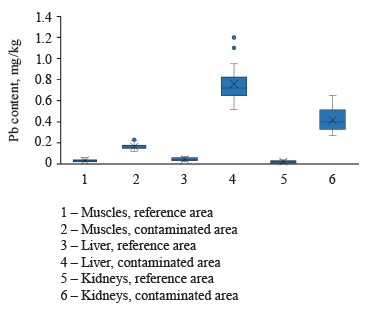
The average concentrations of lead in the muscles, liver, and kidneys of female hares in the contaminated areas were 0.16 ± 0.03, 0.75 ± 0.16, and 0.41 ± 0.10 mg/kg, respectively (Fig. 3). These values were significantly (p = 0.00) higher than those for female hares in the reference areas, namely 5.33, 18.75, and 20.5 times as high, respectively.
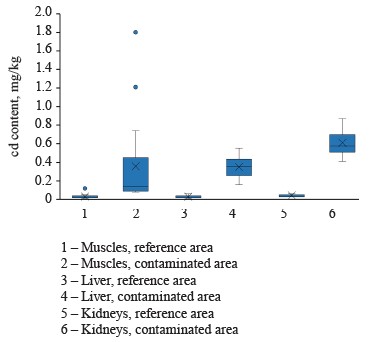
The average concentrations of cadmium in the muscles, liver, and kidneys of female hares in the contaminated areas were 0.35 ± 0.46, 0.35 ± 0.10, and 0.61 ± 0.11 mg/kg, respectively (Fig. 4). These values were significantly (p = 0.00) higher than those for female hares in the reference areas, particularly 11.66, 17.50, and 15.25 times as high, respectively.
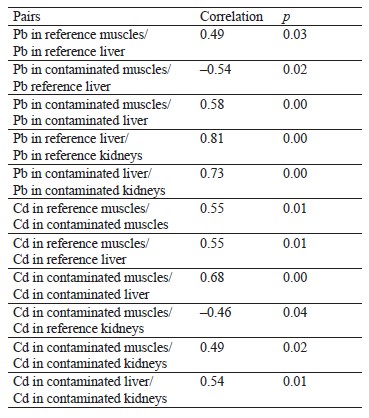
Having performed correlational analysis, we found a close positive correlation between lead concentrations in the liver and kidneys of the hares from the reference (r = 0.81, p = 0.00) and contaminated (r = 0.73, p = 0.00) areas, an average positive correlation between lead concentrations in the muscles and liver in the reference (r = 0.49, p = 0.03) and contaminated (r = 0.58, p = 0.00) areas, and an average negative correlation between lead concentrations in the liver of the hares from the reference areas and in the muscles of the hares from the contaminated areas (r = –0.54, p = 0.02) (Table 1).
As for cadmium, we established a positive correlation between its concentrations in the muscles and liver of the hares from the contaminated (r = 0.68, p = 0.00) and reference (r = 0.55, p = 0.01) areas. We also found an average positive correlation between cadmium concentrations in the liver and kidneys (r = 0.54, p = 0.01), as well as in the muscles and kidneys (r = 0.49, p = 0.02), in the contaminated areas. Finally, there was an average negative correlation between cadmium concentrations in the muscles of the hares from the contaminated areas and in the kidneys of the hares from the reference areas (r = –0.46, p = 0.04) (Table 1).
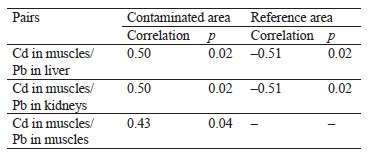
Table 2 shows correlations between lead and cadmium concentrations in the organs and tissues of the hares in the areas with different levels of contamination. In both the reference and contaminated areas, we found a correlation between cadmium concentrations in the muscles and lead concentrations in the liver (r = –0.51, p = 0.02 and r = 0.50, p = 0.02, respectively), as well as between cadmium concentrations in the muscles and lead concentrations in the kidneys (r = –0.51, p = 0.02 and r = 0.50, p = 0.02, respectively). In the contaminated areas, an average correlation was established between cadmium and lead concentrations in the muscles (r = 0.43, p = 0.04).
The content of mercury in the muscles, liver, and kidneys of the hares in the contaminated areas varied between 0.01 and 0.025 mg/kg and was significantly higher than in the reference areas.
Next, we analyzed the relationships between reproductive indicators and the contents of heavy metals in the internal organs of the mountain hares in the contaminated areas. As a result, average positive correlations were established between the number of embryos and the lead concentration in the kidneys (r = 0.43, p = 0.04), the number of corpora lutea and the lead concentration in the liver (r = 0.50, p = 0.01), the number of corpora lutea and the cadmium concentration in the kidneys (r = 0.44, p = 0.04), as well as the number of corpora lutea and the lead concentration in the kidneys (r = 0.46, p = 0.03). However, there was no correlation between reproductive indicators and mercury concentrations in the hares’ organs and tissues.
The liver and kidneys of mammals are known to accumulate lead, cadmium, and mercury. Therefore, these organs can be used to determine concentrations of these metals in the body. Although skeletal muscles are not as indicative of metal concentrations, they are quite significant in environmental and toxicological studies due to a high nutritional value [69].
Our results showed a clear relationship between the environment and the contents of heavy metals in the mountain hares. The sampling area is affected by the Norilsk industrial region, with its large metallurgical and mining enterprises accounting for 1.9% of the Russian GDP. About 2.5 million tons of pollutants are released into the atmosphere of Norilsk annually, according to the Federal Statistics Service and the Ministry of Ecology and Rational Management of Natural Resources in Krasnoyarsk Krai. A significant proportion of these pollutants are heavy metals [68]. Moreover, many of them spread through the air over a distance of 250–400 km from the point of release, polluting water sources, bottom sediments, and soil, as well as causing vegetation degradation [70–72]. Emissions from the enterprises in the cities of Kayerkan and Norilsk spread in the northern and northwestern directions, accelerating during winter snowstorms [73, 74]. Accumulating in depressions, contaminated snow delivers large amounts of trace elements to shrubs and herbaceous plants during the growing season, as well as contaminating their shoots and bark. And these plants are what mountain hares feed on.
Previous studies have reported the impact of chemical pollution on wild animals in the north of Krasnoyarsk Krai. Increased concentrations of lead, cadmium, and mercury were detected in the organs and tissues of the wild reindeer, mountain hare, and ptarmigan [66, 75, 76]. Histological and morphological studies found abnormal changes in the main body systems of micromammals, such as mouse-like rodents, living in the Norilsk industrial region [77]. Numerous pathologies were identified in the organs and tissues of small mammals, including the hematopoietic organs and endocrine system. This undoubtedly affects the reproductive potential of the local animals. Obviously, similar processes can occur in other plant-feeders in these and adjacent areas.
In our study, the average lead contents in the liver and kidneys of mountain hares in the contaminated area (Krasnoyarsk Krai) were 0.75 ± 0.16 and 0.41 ± 0.10 mg/kg, respectively. These values were significantly higher in the internal organs of lagomorphs inhabiting polluted areas in Pakistan and Turkey, but only slightly higher in the liver of the brown hare (Lepus europaeus L.) in Serbia and Poland [78–81]. Yet, the lead contents in our study were similar to those found in the liver and kidneys of mountain hares in Norway, Finland, and the Kirov region in Russia [62–65]. The concentrations of lead in the reference area in Krasnoyarsk Krai were similar to those for lagomorphs in uncontaminated regions of Europe [82–84].
The highest concentrations of cadmium (averaging 0.61 ± 0.11 mg/kg) were detected in the kidneys of mountain hares at a contaminated landfill in the north of Krasnoyarsk Krai. However, these values were significantly lower than similar indicators in the industrial and mining regions of Canada, Poland, Turkey, and Serbia [79–81, 85, 86]. Yet, they were comparable to cadmium concentrations in lagomorphs and other species from non-industrial areas [80, 83, 87–92]. Also, the contents of cadmium in the liver and kidneys of mountain hares in the reference area of Krasnoyarsk Krai were lower than similar indicators for lagomorphs in European non-industrial regions. This may be because the main sources of cadmium in the environment are agricultural, energy, and transport enterprises.
Thus, the contents of lead and cadmium in the female mountain hares from the reference areas of Krasnoyarsk Krai appeared quite low compared to these indicators for lagomorphs from other countries. At the contaminated landfills in Krasnoyarsk Krai, these contents were comparable to the values for contaminated areas in other regions. Our study did not detect abnormally high concentrations of these heavy metals, unlike numerous studies of wild animals in other industrialized countries. However, a significant part of the liver samples and all the samples of hare meat obtained in the middle reaches of the Agapa river exceeded the hygienic standards for lead and cadmium concentrations (The unified sanitary, epidemiological, and hygienic requirements for goods that are subject to sanitary and epidemiological supervision (control)). This undoubtedly indicates an increased toxic impact on the population of mountain hares and its well-being.
The numbers of corpora lutea in the ovaries of female mountain hares (potential fertility) in the contaminated and reference areas of Krasnoyarsk Krai were 8.72 and 7.15, respectively, with a significant (p = 0.00) difference of 19.16%. The number of embryos was also larger in the contaminated areas. In particular, the average number of viable embryos per female (maximum actual fertility) was 7.22 in the contaminated areas, compared to 6.84 in the reference areas. Yet, the difference between them of 5.27% was not significant. However, the survival rate of embryos was higher in the reference areas than in the contaminated areas (95.6 and 82.8%, respectively).
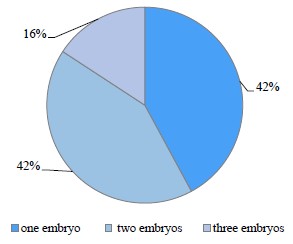
The female hares with embryonic losses accounted for 31.57% in the reference areas, which is close to the average for other species. This indicator reached 86.36% in the contaminated areas. Noteworthily, the females in the reference areas lost only one embryo, while those in the contaminated areas, an average of 1.73 embryos (p = 0.02). According to Fig. 5, 42% of the latter lost one embryo, 42% lost two embryos, and 16% lost three embryos.
Many researchers consider the average number of corpora lutea per female as an indicator of potential fertility. Its natural increase is one of the adaptation mechanisms enabling the population to survive in unfavorable conditions. Toxic pollution appears to stimulate potential fertility, which partially compensates for losses induced by chemical exposure. We can also assume that the frequency of embryo resorption may increase in contaminated areas, especially under harsh climatic conditions.
Previous studies have shown changes in the reproductive parameters of female small rodents and other species caused by increasing toxic load. In particular, the density, biomass, and diversity of micromammals in Murmansk region and the Middle Urals increased with distance from the impact zone around large metal works [93–96]. The proportion of pregnant red-backed voles (Clethrionomys rufocanus) was significantly associated with a distance from the source of pollution [97]. The embryo resorption near the source of pollution was 7.7 times as high as in the control group. However, the embryo survival rate was 96.4–95.8% in the reference areas, 92.0–81.9% in the buffer zone, and just over 60% in the impact zone, i.e., 4 km away from the source of pollution [98]. Considering the level and nature of contamination, similar values are typical for the mountain hare in the north of Krasnoyarsk Krai. However, our study showed no decrease in the actual fertility of the hares, compared to this indicator for mouse-like rodents in the above studies.
Mukhacheva studied the reproduction of the bank vole (Clethrionomys glareolus) in an industrial pollution gradient [99]. The author reported significantly reduced levels of toxicants in the organs, tissues, and developing embryos due to the system of blood-tissue barriers in the female bodies. This decrease in toxicants was detected alongside their increased contents in the contaminated feeding areas. At certain development stages, the embryos are highly resistant to the effects of heavy metals. The study showed no significant effects of their contents on potential fertility or embryonic death during intrauterine development. Noteworthily, all breeding females in the reference and contaminated areas had similar numbers of corpora lutea of pregnancy. However, corpora lutea were more numerous in the group of sexually mature females in the impact zone, which is consistent with our results. Also, the number of viable embryos was higher in the bank voles in contaminated areas [99].
It should be noted that the number of living embryos does not indicate the quality of the offspring. Studies rarely identify deviations from normal development related to the location of the placenta, the size or weight of the embryo, etc. However, chronic exposure to heavy metals during pregnancy can cause up to 30 types of serious pathological changes in the embryos of small mammals. These pathologies can lead to the birth of weakened offspring or its death [100–102].
Weight is the most important indicator of offspring’s life potential. Benitez et al. established an inverse relationship between the weight of embryos and/or newborns and the concentrations of toxicants in the placenta [103]. According to Salomeina and Mashak, chronic exposure to pollutants during pregnancy and lactation can decrease the weight of embryos by 6–10%, and in some cases by up to 25%, compared to the control group [101].
A study in the Middle Urals found that increased exposure to lead and cadmium in bank vole embryos by the end of the prenatal period caused weight loss, compared to the embryos from reference areas [1]. This inevitably weakened the offspring and increased their mortality in the early postnatal period. There is an opinion that the weight of embryos is more indicative of changes in the reproductive potential of a population than the level of embryonic losses.
A number of objective reasons prevented us from establishing relationships between the sex, age, and size of a population and its reproduction in a chemically polluted area [1]. Therefore, this should become the object of further research.
ВЫВОДЫ
Our study showed significantly higher concentrations of lead, cadmium, and mercury in the liver, kidneys, and muscles of mountain hares in the industrially polluted areas compared to the reference sites.
The relationships between the reproductive indicators and the contents of heavy metals in the internal organs of the mountain hare revealed an average positive correlation between the number of embryos and the concentration of lead in the kidneys (r = 0.43, p = 0.04), the number of corpora lutea and the concentration of lead in the liver (r = 0.50, p = 0.01), the number of corpora lutea and the concentration of cadmium in the kidneys (r = 0.44, p = 0.04), and the number of corpora lutea and the concentration of lead in the kidneys (r = 0.46, p = 0.03). The significant correlations between individual metals in the organs and tissues may indicate common sources of their entry into the environment of the study area.
The average number of corpora lutea per female mountain hare was 8.72 in the contaminated areas, which was 19.16% higher than in the reference areas (p = 0.00). However, the numbers of embryos did not differ significantly in the reference and contaminated areas.
The proportion of female hares with embryonic losses was 31.57% in the reference areas, which is close to the average for other species, and 86.36% in the contaminated areas. Moreover, they lost only one embryo in the reference areas and an average of 1.73 embryos (p = 0.02) in the contaminated areas. The females that lost one, two, and three embryos in the contaminated areas accounted for 42, 42, and 16%, respectively.
According to our results, the levels of cadmium, mercury, and lead detected in the bodies of the mountain hares in the north of Krasnoyarsk Krai do not pose a risk of acute poisoning. Although significant differences were found in the potential fertility of female hares in the reference and contaminated areas, their actual fertility was not affected. The increase in corpora lutea of pregnancy, or potential fertility, apparently compensated for greater embryonic losses induced by exposure to pollutants.
Environmental pollutants caused by anthropogenic activities enter the food chain. Due to their toxicity, these pollutants, even at low concentrations, can have an adverse effect on animals in case of prolonged exposure. This has been shown by previous studies on numerous species of vertebrates in the European part of Russia [104–109]. Pollutants affect the structure of animal communities, their territorial distribution, aggregation, population density, and reproductive indicators (fertility, survival, embryonic mortality, etc.). This was confirmed by our study, where we compared the samples from reference and industrially contaminated areas in the north of Central Siberia.
Our results are also indicative of the well-being of the mountain hare population in Krasnoyarsk Krai, mainly its ability to produce healthy offspring at the required level.
Further research is needed to assess potential and actual fertility, offspring survival, and other important parameters of mountain hare populations exposed to chemical pollution compared to those in reference areas.
Our study showed that about 40% of the liver samples and 100% of the muscle tissue samples obtained from the hares in the impact zone contained high concentrations of lead and cadmium. Therefore, hunting in these industrially polluted areas may pose a toxic hazard to the indigenous peoples living there.
Вклад авторов
All the authors contributed equally to the study and are equally responsible for the information published in this article.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare that there is no conflict of interest regarding the publication of this article.
ФИНАНСИРОВАНИЕ
The study was conducted in 2023 at the Professor Zhitkov Russian Research Institute of Game Management and Fur Farming (VNIIOZ) as part of the state assignment on “Advancing scientific foundations for sustainable use, assessment, monitoring, and forecasting of hunting bioresources” (No. FNWS-2022-0001) within the Fundamental Research Program for 2021–2030 (Russian Government Decree No. 3684-r of December 31, 2020).СПИСОК ЛИТЕРАТУРЫ
- Mukhacheva SV, Bezel' VS. Heavy metals in the mother-placenta-fetus system in bank voles under conditions of environmental pollution from copper plant emissions. Russian Journal of Ecology. 2015;(6):444–453. https://doi.org/10.7868/S0367059715060128; https://elibrary.ru/UIMJKZ
- Satarug S, Gobe GC, Vesey DA, Phelps KR. Cadmium and lead exposure, nephrotoxicity, and mortality. Toxics. 2020;8(4):86. https://doi.org/10.3390/toxics8040086
- Kaledin AP, Stepanova MV. Bioaccumulation of trace elements in vegetables grown in various anthropogenic conditions. Foods and Raw Materials. 2023;11(1):10–16. https://doi.org/10.21603/2308-4057-2023-1-551
- Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A, et al. The toxicity of cadmium and resulting hazards for human health. Journal of Occupational Medicine and Toxicology. 2006;1:22. https://doi.org/10.1186/1745-6673-1-22
- Satarug S, Boonprasert K, Gobe GC, Ruenweerayut R, Johnson, DW, Na-Bangchang K, et al. Chronic exposure to cadmium is associated with a marked reduction in glomerular filtration rate. Clinical Kidney Journal. 2018;12(4):468–475. https://doi.org/10.1093/ckj/sfy113
- Duan Y, Zhao Y, Wang T, Sun J, Ali W, Ma Y, et al. Taurine alleviates cadmium-induced hepatotoxicity by regulating autophagy flux. International Journal of Molecular Sciences. 2023;24(2):1205. https://doi.org/10.3390/ijms24021205
- Kumar S, Sharma A. Cadmium toxicity: Effects on human reproduction and fertility. Reviews on Environmental Health. 2019;34(4):327–338. https://doi.org/10.1515/reveh-2019-0016
- Nishijo M, Nakagawa H, Suwazono Y, Nogawa K, Kido T. Causes of death in patients with Itai-itai disease suffering from severe chronic cadmium poisoning: A nested case – control analysis of a follow-up study in Japan. BMJ Open. 2017;7(7):e015694. https://doi.org/10.1136/bmjopen-2016-015694
- Ali W, Bian Y, Zhang H, Qazi IH, Zou H, Zhu J, et al. Effect of cadmium exposure during and after pregnancy of female. Environmental Pollutants and Bioavailability. 2023;35(1):2181124. https://doi.org/10.1080/26395940.2023.2181124
- de Angelis C, Galdiero M, Pivonello C, Salzano C, Gianfrilli D, Piscitelli P, et al. The environment and male reproduction: The effect of cadmium exposure on reproductive function and its implication in fertility. Reproductive Toxicology. 2017;73:105–127. https://doi.org/10.1016/j.reprotox.2017.07.021
- Manna PR, Stetson CL, Slominski AT, Pruitt K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine. 2016;51:7–21. https://doi.org/10.1007/s12020-015-0715-6
- Kutyakov VA, Salmina AB. Metallothioneins as sensors and controls exchange of metals in the cells. Bulletin of Siberian Medicine. 2014;13(3):91–99. (In Russ.). https://doi.org/10.20538/1682-0363-2014-3-91-99; https://elibrary.ru/SXRMYP
- Espart A, Artime S, Tort-Nasarre G, Yara-Varón E. Cadmium exposure during pregnancy and lactation: Materno-fetal and newborn repercussions of Cd(II), and Cd–metallothionein complexes. Metallomics. 2018;10:1359–1367. https://doi.org/10.1039/c8mt00174j
- Gundacker C, Hengstschläger M. The role of the placenta in fetal exposure to heavy metals. Wiener Medizinische Wochenschrift. 2012;162(9–10):201–206. https://doi.org/10.1007/s10354-012-0074-3
- Massanyi P, Bárdos L, Oppel K, Hluchý S, Kovácik J, Csicsai G, et al. Distribution of cadmium in selected organs of mice: Effects of cadmium on organ contents of retinoids and beta-carotene. Acta Physiologica Hungarica. 1999;86(2):99–104.
- Massanyi P, Toman R, Valent M, Cupka P. Evaluation of selected parameters of a metabolic profile and levels of cadmium in reproductive organs of rabbits after an experimental administration. Acta Physiologica Hungarica. 1995;83(3):267–273.
- Nad P, Massanyi P, Skalicka M, Koréneková B, Cigankova V, Almášiová V. The effect of cadmium in combination with zinc and selenium on ovarian structure in Japanese quails. Journal of Environmental Science and Health, Part A. 2007;42(13):2017–2022. https://doi.org/10.1080/10934520701629716
- Nasiadek M, Danilewicz M, Klimczak M, Stragierowicz J, Kilanowicz A. Subchronic exposure to cadmium causes persistent changes in the reproductive system in female Wistar rats. Oxidative Medicine and Cellular Longevity. 2019;2019:6490820. https://doi.org/10.1155/2019/6490820
- Wang Y, Wang X, Wang Y, Fan R, Qiu C, Zhong S, et al. Effect of cadmium on cellular ultrastructure in mouse ovary. Ultrastructural Pathology. 2015;39(5):324–328. https://doi.org/10.3109/01913123.2015.1027436
- Ruslee SS, Zaid SSM, Bakrin IH, Goh YM, Mustapha NM. Protective effect of Tualang honey against cadmium-induced morphological abnormalities and oxidative stress in the ovary of rats. BMC Complementary Medicine and Therapies. 2020;20:160. https://doi.org/10.1186/s12906-020-02960-1
- Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: A review. Reproductive Biology and Endocrinology. 2012;10:49. https://doi.org/10.1186/1477-7827-10-49
- Zhang W, Pang F, Huang Y, Yan P, Lin W. Cadmium exerts toxic effects on ovarian steroid hormone release in rats. Toxicology Letters. 2008;182(1–3):18–23. https://doi.org/10.1016/j.toxlet.2008.07.016
- Takiguchi M, Yoshihara S. New aspects of cadmium as endocrine disruptor. Environmental Sciences: An International Journal of Environmental Physiology and Toxicology. 2006;13(2):107–116.
- Johnson MD, Kenney N, Stoica A, Hilakivi-Clarke L, Singh B, Chepko G, et al. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nature Medicine. 2003;9:1081–1084. https://doi.org/10.1038/nm902
- Massányi P, Massányi M, Madeddu R, Stawarz R, Lukác N. Effects of cadmium, lead, and mercury on the structure and function of reproductive organs. Toxics. 2020;8(4):94. https://doi.org/10.3390/toxics8040094
- Rădulescu A, Lundgren S. A pharmacokinetic model of lead absorption and calcium competitive dynamics. Scientific Reports. 2019;9:14225. https://doi.org/10.1038/s41598-019-50654-7
- Gulson B, Taylor A, Eisman J. Bone remodeling during pregnancy and post-partum assessed by metal lead levels and isotopic concentrations. Bone. 2016;89:40–51. https://doi.org/10.1016/j.bone.2016.05.005
- Zhang B, Xia W, Li Y, Bassig BA, Zhou A, Wang Y, et al. Prenatal exposure to lead in relation to risk of preterm low birth weight: A matched case-control study in China. Reproductive Toxicology. 2015;57:190–195. https://doi.org/10.1016/j.reprotox.2015.06.051
- Disha, Sharma S, Goyal M, Kumar PK, Ghosh R, Sharma P. Association of raised blood lead levels in pregnant women with preeclampsia: A study at tertiary centre. Taiwanese Journal of Obstetrics and Gynecology. 2019;58(1): 60–63. https://doi.org/10.1016/j.tjog.2018.11.011
- Soomro MH, Baiz N, Huel G, Yazbeck C, Botton J, Heude B, et al. Exposure to heavy metals during pregnancy related to gestational diabetes mellitus in diabetes-free mothers. Science of the Total Environment. 2019;656:870–876. https://doi.org/10.1016/j.scitotenv.2018.11.422
- Taylor CM, Golding J, Emond AM. Adverse effects of maternal lead levels on birth outcomes in the ALSPAC study: A prospective birth cohort study. BJOG: An International Journal of Obstetrics and Gynaecology. 2015;122(3):322–328. https://doi.org/10.1111/1471-0528.12756
- Yoon J-H, Ahn Y-S. The association between blood lead level and clinical mental disorders in fifty thousand lead-exposed male workers. Journal of Affective Disorders. 2016;190:41–46. https://doi.org/10.1016/j.jad.2015.09.030
- Bede-Ojimadu O, Amadi CN, Orisakwe OE. Blood lead levels in women of child-bearing age in sub-Saharan Africa: A systematic review. Frontiers in Public Health. 2018;6. https://doi.org/10.3389/fpubh.2018.00367
- Borja-Aburto VH, Hertz-Picciotto I, Rojas Lopez M, Farias P, Rios C, Blanco J. Blood lead levels measured prospectively and risk of spontaneous abortion. American Journal of Epidemiology. 1999;150(6):590–597. https://doi.org/10.1093/oxfordjournals.aje.a010057
- Cheng L, Zhang B, Huo W, Cao Z, Liu W, Liao J, et al. Fetal exposure to lead during pregnancy and the risk of preterm and early-term deliveries. International Journal of Hygiene and Environmental Health. 2017;220(6):984–989. https://doi.org/10.1016/j.ijheh.2017.05.006
- Huang S, Xia W, Sheng X, Qiu L, Zhang B, Chen T, et al. Maternal lead exposure and premature rupture of membranes: A birth cohort study in China. BMJ Open. 2018;8:e021565. https://doi.org/10.1136/bmjopen-2018-021565
- Kumar S. Occupational and environmental exposure to lead and reproductive health impairment: An overview. Indian Journal of Occupational and Environmental Medicine. 2018;22(3):128–137. https://doi.org/10.4103/ijoem.IJOEM_126_18
- Rahman A, Kumarathasan P, Gomes J. Infant and mother related outcomes from exposure to metals with endocrine disrupting properties during pregnancy. Science of the Total Environment. 2016;569–570:1022–1031. https://doi.org/10.1016/j.scitotenv.2016.06.134
- Tyrrell JB, Hafida S, Stemmer P, Adhami A, Leff T. Lead (Pb) exposure promotes diabetes in obese rodents. Journal of Trace Elements in Medicine and Biology. 2017;39:221–226. https://doi.org/10.1016/j.jtemb.2016.10.007
- Chekhoeva AN, Khamitsaev ZA, Kozaeva AEh, Kadokhova LA. Comprehensive analysis of the impact of heavy metals on obstetric pathology. Medicine. Sociology. Philosophy. Applied research. 2019;(2);31–37. (In Russ.). https://elibrary.ru/INJWUS
- Nkomo P, Richter LM, Kagura J, Mathee A, Naicker N, Norris SA. Environmental lead exposure and pubertal trajectory classes in South African adolescent males and females. Science of the Total Environment. 2018;628–629:1437–1445. https://doi.org/10.1016/j.scitotenv.2018.02.150
- Hilderbrand DC, Der R, Griffin WT, Fahim MS. Effect of lead acetate on reproduction. American Journal of Obstetrics and Gynecology. 1973;115(8):1058–1065. https://doi.org/10.1016/0002-9378(73)90554-1
- Dhir V, Dhand P. Toxicological approach in chronic exposure to lead on reproductive functions in female rats (Rattus norvegicus). Toxicology International. 2010;17(1):1–7.
- Kolesarova A, Roychoudhury S, Slivkova J, Sirotkin A, Capcarová M, Massanyi P. In vitro study on the effects of lead and mercury on porcine ovarian granulosa cells. Journal of Environmental Science and Health, Part A. 2010;45(3):320–331. https://doi.org/10.1080/10934520903467907
- Capcarová M, Kolesarova A, Lukac N, Sirotkin A, Roychoudhury S. Antioxidant status and selected biochemical parameters of porcine ovarian granulosa cells exposed to lead in vitro. Journal of Environmental Science and Health, Part A. 2009;44(14):1617–1623. https://doi.org/10.1080/10934520903263678
- Vigeh M, Smith DR, Hsu P-C. How does lead induce male infertility? Iranian Journal of Reproductive Medicine. 2011;9(1):1–8.
- Pollet IL, Leonard ML, O’Driscoll NJ, Burgess NM, Shutler D. Relationships between blood mercury levels, reproduction, and return rate in a small seabird. Ecotoxicology. 2017;26:97–103. https://doi.org/10.1007/s10646-016-1745-4
- Bjørklund G, Chirumbolo S, Dadar M, Pivina L, Lindh U, Butnariu M, et al. Mercury exposure and its effects on fertility and pregnancy outcome. Basic and Clinical Pharmacology and Toxicology. 2019;125(4):317–327. https://doi.org/10.1111/bcpt.13264
- Global mercury assessment 2013: Sources, emissions, releases, and environmental transport. Geneva: United Nations Environment Programme; 2013. 44 p.
- Cossa D, Heimbürger L-E, Lannuzel D, Rintoul SR, Butler ECV, Bowie AR, et al. Mercury in the Southern Ocean. Geochimica et Cosmochimica Acta. 2011;75(14):4037–4052. https://doi.org/10.1016/j.gca.2011.05.001
- Dix-Cooper L, Kosatsky T. Blood mercury, lead and cadmium levels and determinants of exposure among newcomer South and East Asian women of reproductive age living in Vancouver, Canada. Science of the Total Environment. 2018;619–620:1409–1419. https://doi.org/10.1016/j.scitotenv.2017.11.126
- Zheng N, Wang S, Dong W, Hua X, Li Y, Song X, et al. The toxicological effects of mercury exposure in marine fish. Bulletin of Environmental Contamination and Toxicology. 2019;102:714–720. https://doi.org/10.1007/s00128-019-02593-2
- Sadripour E, Mortazavi MS, Mahdavi Shahri N. Effects of mercury on embryonic development and larval growth of the sea urchin Echinometra mathaei from the Persian Gulf. Iranian Journal of Fisheries Sciences. 2013;12(4):898–907.
- Chen YW, Huang CF, Tsai KS, Yang RS, Yen CC, Yang CY, et al. Methylmercury induces pancreatic β-cell apoptosis and dysfunction. Chemical Research in Toxicology. 2006;19(8):1080–1085. https://doi.org/10.1021/tx0600705
- Sukhn C, Awwad J, Ghantous A, Zaatari G. Associations of semen quality with non-essential heavy metals in blood and seminal fluid: data from the environment and male infertility (EMI) study in Lebanon. Journal of Assisted Reproduction and Genetics. 2018;35:1691–1701. https://doi.org/10.1007/s10815-018-1236-z
- Ma Y, Zhu M, Miao L, Zhang X, Dong X, Zou XT. Mercuric chloride induced ovarian oxidative stress by suppressing Nrf2-Keap1 signal pathway and its downstream genes in laying hens. Biological Trace Element Research. 2018;185:185–196. https://doi.org/10.1007/s12011-018-1244-y
- Lundholm CE. Effects of methyl mercury at different dose regimes on eggshell formation and some biochemical characteristics of the eggshell gland mucosa of the domestic fowl. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology. 1995;110(1):23–28. https://doi.org/10.1016/0742-8413(94)00081-K
- Altunkaynak BZ, Akgül N, Yahyazadeh A, Altunkaynak ME, Türkmen AP, Akgül HM, et al. Effect of mercury vapor inhalation on rat ovary: Stereology and histopathology. Journal of Obstetrics and Gynaecology Research. 2016;42(4):410–416. https://doi.org/10.1111/jog.12911
- Roychoudhury S, Massanyi P, Slivkova J, Formicki G, Lukac N, Slamecka J, et al. Effect of mercury on porcine ovarian granulosa cells in vitro. Journal of Environmental Science and Health, Part A. 2015;50(8):839–845. https://doi.org/10.1080/10934529.2015.1019805
- Koli S, Prakash A, Choudhury S, Mandil R, Garg SK. Calcium channels, rho-kinase, protein kinase-c, and phospholipase-c pathways mediate mercury chloride-induced myometrial contractions in rats. Biological Trace Element Research. 2019;187:418–424. https://doi.org/10.1007/s12011-018-1379-x
- Nakade UP, Garg SK, Sharma A, Choudhury S, Yadav RS, Gupta K, et al. Lead-induced adverse effects on the reproductive system of rats with particular reference to histopathological changes in uterus. Indian Journal of Pharmacology. 2015;47(1):22–26. https://doi.org/10.4103/0253-7613.150317
- Pulliainen E, Lajunen LHJ, Itamies J, Anttila R. Lead and cadmium in the liver and muscles of the mountain hare (Lepus timidus) in northern Finland. Annales Zoologici Fennici. 1984;21(2):149–152.
- Kålås JA, Ringsby TH, Lierhagen S. Metals and selenium in wild animals from Norwegian areas close to Russian nickel smelters. Environmental Monitoring and Assessment. 1995;36(3):251–270. https://doi.org/10.1007/BF00547905
- Venäläinen E-R, Niemi A, Hirvi T. Heavy metals in tissues of hares in Finland, 1980–82 and 1992–93. Bulletin of Environmental Contamination and Toxicology. 1996;56:251–258. https://doi.org/10.1007/s001289900038
- Zarubin BE, Ekonomov AV, Kolesnikov VV, Shevnina MS, Sergeev AA. The resources of mountain hare in the Kirov region and their use. Far East Agrarian Bulletin. 2021;60(4):87–102. (In Russ.). https://doi.org/10.24412/1999-6837-2021-4-87-102; https://elibrary.ru/WGXKTC
- Kochkarev PV, Koshurnikova MA, Sergeyev AA, Shiryaev VV. Trace elements in the meat and internal organs of the mountain hare (Lepus timidus L., 1758) in the north of the Krasnoyarsk Region. Food Processing: Techniques and Technology. 2023;53(2):217–230. (In Russ.). https://doi.org/10.21603/2074-9414-2023-2-2436; https://elibrary.ru/YORYWG
- Ivanter EhV, Korosov AV. Biology of chemical elements. Petrozavodsk: PetrGU; 2010. 104 p. (In Russ.). https://elibrary.ru/QKOJXR
- The official report “On the state and protection of the environment in Krasnoyarsk Krai in 2022”. Krasnoyarsk, 2022. 321 p. (In Russ.).
- Potthast K. Residues in meat and meat products. Fleischwirtsch. 1993;73:432–434.
- Lobkovsky VA, Lobkovskaya LG. The ecological situation around the arrangement of the enterprises of the polar branch of the MMC Norilsk nickel: Current state and forecast. Regional Environmental Issues. 2015;(5):40–43. (In Russ.). https://elibrary.ru/VOGNRN
- Bazova MM, Koshevoi DV. The assessment of the current state of water quality in the Norilsk industrial region. Arctic: Ecology and Economy. 2017;27(3):49–60. (In Russ.). https://doi.org/10.25283/2223-4594-2017-3-49-60; https://elibrary.ru/ZHQXJT
- May IV, Kleyn SV, Vekovshinina SA, Balashov SYu, Chetverkina KV, Tsinker MYu. Health risk to the population in Norilsk under exposure of substances polluting ambient air. Hygiene and Sanitation, Russian Journal. 2021;100(5):528–534. (In Russ.). https://doi.org/10.47470/0016-9900-2021-100-5-528-534; https://elibrary.ru/DCTLGR
- Lezhenin AA, Raputa VF, Yaroslavtseva TV. Numerical analysis of atmospheric circulation and pollution transfer in the environs of Norilsk industrial region. Atmospheric and Oceanic Optics. 2016;29(6):467–471. (In Russ.). https://doi.org/10.15372/aoo20160603; https://elibrary.ru/VZJPDL
- Onuchin AA, Burenina TA, Zubareva ON, Trefilova OV, Danilova IV. Pollution of snow cover in the impact zone of enterprises in Norilsk industrial area. Contemporary Problems of Ecology. 2014;21(6):1025–1037. (In Russ.). https://elibrary.ru/TAKBWH
- Kochkarev PV, Mikhailov VV. Complex analysis of heavy metals content in bodies and tissues of the wild reindeer (Rangifer tarandus L. 1758). Bulletin of KSAU. 2016;119(8):21–27. (In Russ.). https://elibrary.ru/WCYUSN
- Skugland T, Baskin LM, Ehspelien IS, Strand U. Contents of heavy and radioactive metals in different reindeer populations. Lomonosov Geography Journal. 1997;(6):19–24. (In Russ.).
- Kireeva AV, Kolenchukova OA, Peretiatko OV, Savchenko AP, Temerova VL, Emelyanov VI. Morphological assessment of organs and tissues of small mammals living in the industrial area of Norilsk. Contemporary Problems of Ecology. 2023;30(3):330–342. (In Russ.). https://elibrary.ru/GISADM
- Ahmed MS, Azam MA, Ahmed KS, Ali H. Accumulation of some heavy metals in selected tissues of cape hare, Lepus capensis from Pakistan. Pakistan Journal of Wildlife Archives. 2016;7(2):11–20.
- Demirbaş Y, Erduran N. Concentration of selected heavy metals in brown hare (Lepus europaeus) and wild boar (Sus scrofa) from Central Turkey. Balkan Journal of Wildlife Research. 2017;4(2):26–33. https://doi.org/10.15679/bjwr.v4i2.54
- Beukovi´ D, Vukadinovi´ M, Krstovi´ S, Polovinski-Horvatovi´ M, Jaji´ I, Popovi´ Z, et al. The European hare (Lepus europaeus) as a biomonitor of lead (Pb) and cadmium (Cd) occurrence in the agro biotope of Vojvodina, Serbia. Animals. 2022;12(10):1249. https://doi.org/10.3390/ani12101249
- Wajdzik M. Contents of cadmium and lead in liver, kidneys and blood of the European hare (Lepus europaeus Pallas) in Malopolska. Journal Acta Scientiarum Polonorum Silvarum Colendarum Ratio et Industria Lignaria. 2006;5(2):135–146.
- Fidalgo L, de la Cruz B, Goicoa A, Espino L. Accumulation of zinc, copper, cadmium and lead in liver and kidney of the iberian hare (Lepus granatensis) from Spain. Research and Reviews. Journal of Veterinary Sciences. 2016;2(1):15–20.
- Massányi P, Tataruch F, Slameka J, Toman R, Jurík R. Accumulation of lead, cadmium, and mercury in liver and kidney of the brown hare (Lepuseuropaeus) in relation to the season, age, and sex in the West Slovakian Lowland. Journal of Environmental Science and Health, Part A. 2003;38(7):1299–1309. https://doi.org/10.1081/ese-120021127
- Kramárová M, Massányi P, Slamecka J, Tataruch F, Jancová A, Gasparik J, et al. Distribution of cadmium and lead in liver and kidney of some wild animals in Slovakia. Journal of Environmental Science and Health, Part A. 2005;40(3):593–600. https://doi.org/10.1081/ESE-200046605
- Pedersen S, Lierhagen S. Heavy metal accumulation in arctic hares (Lepus arcticus) in Nunavut, Canada. Science of the Total Environment. 2006;368(2–3):951–955. https://doi.org/10.1016/j.scitotenv.2006.05.014
- Halecki W, Gąsiorek M. Wajdzik M, Pająk M, Kulak D. Population parameters including breeding season of the European brown hare (Lepus europaeus) exposed to cadmium and lead pollution. Fresenius Environmental Bulletin. 2017;26(4):2998–3004.
- Petrović Z, Teodorović V, Djurić S, Milićević D, Vranić D, Lukić M. Cadmium and mercury accumulation in European hare (Lepus europaeus): Age-dependent relationships in renal and hepatic tissue. Environmental Science and Pollution Research. 2014;21:14058–14068. https://doi.org/10.1007/s11356-014-3290-0
- Shore RF, Douben PET. The ecotoxicological significance of cadmium intake and residues in terrestrial small mammals. Ecotoxicology and Environmental Safety. 1994;29(1):101–112. https://doi.org/10.1016/0147-6513(94)90035-3
- Kottferová J, Korénedová B. Distribution of Cd and Pb in the tissues and organs of free-living animals in the territory of Slovakia. Bulletin of Environmental Contamination and Toxicology. 1998;60:171–176. https://doi.org/10.1007/s001289900607
- Ehykhler V. Poisons in our diet. Moscow: Mir; 1985. 214 p. (In Russ.).
- Alonso ML, Benedito JL, Miranda M, Castillo C, Hernández J, Shore RF. Arsenic, cadmium, lead, copper and zinc in cattle from Galicia, NW Spain. Science of the Total Environment Journal. 2000;246(2–3):237–248. https://doi.org/10.1016/s0048-9697(99)00461-1
- Miranda M, Lopez-Alonso M, Castillo C, Hernández J, Benedito JL. Effect of sex on arsenic, cadmium, lead, copper and zinc accumulation in calves. Veterinary and Human Toxicology. 2000;42(5):265–268.
- Kataev GD. Monitoring of populations of small mammals micromammalia in north taiga of Fennoscandia. Bulletin of Moscow Society of Naturalists. Biological Series. 2015;120(3):3–13. (In Russ.). https://elibrary.ru/VEBWPB
- Bezel’ VS, Mukhacheva SV. Geochemical ecology of small mammals at industrially polluted areas: is there any effect of reduction in the emissions? Geochemistry International. 2020;65(8):823–832. (In Russ.). https://doi.org/10.31857/S0016752520070043; https://elibrary.ru/OQCKJD
- Mukhacheva SV. Changes of small mammals communities around а nickel-copper smelter (Harjavalta, Finland). International Journal of Applied and Fundamental Research. 2013;(8–2):145–148. (In Russ.). https://elibrary.ru/QZGTMB
- Mukhacheva SV. Long-term dynamics of small mammal communities in the period of reduction of copper smelter emissions: 1. Composition, abundance, and diversity. Russian Journal of Ecology. 2021;(1):66–76. (In Russ.). https://doi.org/10.31857/S0367059721010108; https://elibrary.ru/JKTMOH
- Kataev GD, Suomela J, Palokangas P. Densities of microtinae rodents along a pollution gradientfrom a copper-nickel smelter. Oecologia. 1994;97:491–498. https://doi.org/10.1007/BF00325887
- Kataev GD. The impact of industrial emissions of copper-nickel smelter complex on the status of populations and communities of small mammals in the Kola Peninsula. Nature Conservation Research. 2017;2(S2):19–27. (In Russ.). https://doi.org/10.24189/ncr.2017.033; https://elibrary.ru/ZDTXCH
- Mukhacheva SV. Reproduction of the bank vole population Clethrionomys glareolus (Rodentia, Cricetidae) along the gradient of industrial environmental pollution. Zoologicheskiy Zhurnal. 2001;80(12):1509–1517. (In Russ.).
- Moskvitina NS, Kuranova VN, Savel'ev SV. Abnormalities of embryonal development of vertebrates under the conditions of technogenic environmental pollution. Contemporary Problems of Ecology. 2011;18(4):487–495. (In Russ.). https://elibrary.ru/THJQWB
- Salomeina NV, Mashak SV. Structural bases of mother-fetus relations at chemical interaction during embryogenesis. Journal of Siberian Medical Sciences. 2012;(1). (In Russ.). https://elibrary.ru/PBZZDZ
- Mirzoev EB, Kobyalko VO, Gubina OA, Frolova NA. Response of the rat organism to the chronic exposure of cadmium low doses during the antenatal period. Toxicological Review. 2014;127(4):29–33. (In Russ.). https://elibrary.ru/ZCORTR
- Benitez MA, Mendez-Armenta M, Montes S, Rembao D, Sanin LH, Rios C. Mother-fetus transference of lead and cadmium in rats: involvement of metallothionein. Histology and Histopathology. 2009;24:1523–1530. https://doi.org/10.14670/HH-24.1523
- Vorobeychik EL, Sadykov OF, Farafontov MG. Ecological standardization of terrestrial ecosystems technogenic pollution (local scale). Yekaterinburg: Nauka; 1994. 280 p. (In Russ.).
- Lebedeva NV. Ecotoxicology and biogeochemistry of geographic bird populations. Moscow: Nauka; 1999. 199 p. (In Russ.). https://elibrary.ru/RXMSTZ
- Davletov IZ. Ecology of the beaver in an urban landscape. Kirov: VNIIOZ; 2005. 116 p. (In Russ.).
- Ivanter EhV, Medvedev NV. Ecological toxicology of natural populations of birds and mammals in the North. Moscow: Nauka; 2008. 228 p. (In Russ.).
- Ermakov VV, Tyutikov SF, Safonov VA. Biogeochemical indication of microelementoses. Moscow: Russian Academy of Sciences; 2018. 386 p. (In Russ.). https://elibrary.ru/YNHZUT
- Dvornikov MG, Domskiy IA, Shiryaev VV. Sergeev AA. Ecology, conservation, and use of commercial mammal resources in North-East Europe. Kirov: VESI; 2021. 291 p. (In Russ.). https://elibrary.ru/QOWDLH