Аннотация
This study featured broad/fava bean pods as by-products of food production. It assessed the chemical composition of green bean pods (Vicia faba L.) and their methanolic extract.The extract was tested in vitro for antioxidant, anti-inflammatory, antimicrobial, and anticancer activities against prostate cancer (Pc3) and liver cancer (HepG2) cells. Broad bean pods proved to be rich in carbohydrates, fiber, protein, potassium, calcium, and magnesium. The extract contained 286 mg GAE/g total phenols and 105 mg QE/g total flavonoids. The antioxidant activity of the methanolic extract was measured by 1,1-diphenyl-2-picryl hydrazyl (DPPH) assay. The highest DPPH scavenging activity belonged to the extract concentrations of 1000 μg/mL (80.5%) and 500 μg/mL (73.7%), whereas the IC50 value was 87.35 μg/mL. The methanolic extract possessed the anti-inflammatory effect as it significantly reduced the hemolysis of red blood cells. The maximal inhibition percentage reached 66.7% at 1000 μg/mL. Regarding the antimicrobial activity, the broad bean pod methanolic extract inhibited Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa, as well as Candida albicans. The extract reduced the cell viability of human hepatocarcinoma (HepG2) and prostate cancer (PC3) cells in a concentration-dependent manner. It also caused significant changes in cell shape, compared to the control.
Therefore, broad beans can be recommended for human consumption together with pods, fresh or cooked, as a potential source of bioactive substances in functional food production.
Ключевые слова
Vicia faba L., pods, anticancer effect, antioxidant activity, anti-inflammatory properties, antimicrobial ability, DPPH radicalsВВЕДЕНИЕ
Medical drugs may have side effects and are often expensive. As a result, people tend to turn to natural plant products and medicinal plants in search of nutrients and health-beneficial phytochemicals. Legumes are an important source of protein, carbohydrates, and fiber. In addition, they are low in fat [1, 2]. Legumes are introduced into human diet for numerous nutritional and health-related properties, e.g., phenolic compounds, oligosaccharides, enzyme inhibitors, phytosterols, and saponins [3, 4]. Legumes are also known to reduce the risk of cancer, cardiovascular diseases, hypertension, and diabetes [5–7].
Broad beans (Vicia faba L.) are a popular food of plant origin that belongs to the Fabaceae (Leguminosae) family and the Vicia gene cluster [8]. Broad beans are also called fava/faba beans, broad beans, Windsor beans, horse beans, tick beans, etc. In Hindi, V. faba is known as kalamatar or bakala [9]. V. faba has four subspecies that differ in the size of seeds: major (large seeds), equine (medium seeds), minor (small seeds), and paucijuga (small seeds) [10].
Broad beans are cultivated in many regions of the world, including Egypt, India, the Netherlands, Spain, Sudan, Saudi Arabia, and China. The seed coat can be white, buff (or beige), purple, green, or red. However, buff beans are the most accepted for human consumption.
Phenolic compounds are micro components that receive a lot of scientific attention due to their healthimproving qualities, e.g., antioxidant activity. Procyanidins, catechins, flavanols, isoflavones, phenolic acids, and tannins are natural antioxidants, and broad beans contain them all [11–14]. Phenolic chemicals of plant origin impede the digestion of lipids and carbohydrates, thus inhibiting their absorption. They may reduce postprandial hyperglycemia in diabetic patients and facilitate weight loss in patients with obesity [15].
The high content of flavonoids and phenolic acids renders V. faba coat antioxidant and anticancer properties [16]. The acetone extract of its seed coat revealed antioxidant, antibacterial, anti-inflammatory, and anticancer properties [17]. Mejri et al. reported that the methanolic extract of broad bean pods decreased the high levels of serum alanine aminotransferase, aspartate aminotransferase, creatinine, and uric acid in the serum of diabetic rats [18]. The methanol extract of broad bean pods also reduced oxidative stress by activating such antioxidant enzymes as catalase, glutathione peroxidase, and superoxide dismutase [18, 19]. Broad beans lowered blood sugar and total cholesterol as well as prevented heart conditions, eye diseases, various cancers, and dysfunction of kidney and liver [16, 20–25].
Egypt is one of the leading consumers of broad beans. There, they are known as ful. Stewed (ful medames) or fried broad beans (falafel) are considered the main dish of a typical Egyptian breakfast. Broad bean pods are usually cast off as wastes. However, young broad bean pods are traditionally consumed together with beans in Egyptian village cuisine.
This research tested in vitro the methanolic extract of V. faba pods for their antioxidant, antimicrobial, antiinflammatory, and anticancer properties.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Materials. Immature broad bean Vicia faba L. pods were purchased on a local market in Mansoura, Egypt.
Chemicals. All chemicals were obtained from Al-Gomhoria Company (Mansoura, Egypt), which produces medicines and medical supplies.
Permission to conduct the experiment was granted by the Scientific Research Ethics Committee of the Faculty of Specific Education, Mansoura University (No, 12-3/11/22).
Methods. Preparing pod powder. Broad beans were cleaned and thoroughly washed in water. Afterwards, the beans were separated, and the green pods were oven-dried at 40°C until constant weight, ground to a fine powder, and stored at -20°C.
Preparing methanolic extract. We soaked 250 g of pod powder in 1 L methanol, mixed, left it overnight, and filtered through filter paper. The filtrate was kept in a dark-glass bottle. After that, we took another portion of methanol, added it to the residue, shook thoroughly, left it overnight, and filtered. The new filtrate joined the previous one. Finally, the residue was resoaked in methanol overnight and filtered. The three filtrates were collected to make the methanolic extract solution. We removed the solvent by evaporating it in a rotary evaporator. The obtained extract was collected and dried in a desiccator to a constant weight, then kept in dark-glass bottles for further use.
Chemical analysis. The methods recommended by the Association of Official Analytical Chemists provided experimental data on ash, fat, fiber, protein, and moisture contents [26]. Carbohydrates were calculated as 100 – (ash + fiber + protein + water). We employed the method of inductively coupled plasma-atomic emission spectrometry (ICP-AES) (Horiba Jobin-Yvon Ultima 2 CE) to determine the mineral composition under optimal experimental conditions [27].
Phytochemical screening. The pod extract underwent phytochemical tests for the qualitative profile of glycosides, phenolics, tannins, alkaloids, flavonoids, and saponins. This part of the research followed the methods described by Trease & Evans and Harborne [28, 29].
Total phenolics and total flavonoids. We applied the Folin-Ciocalteu colorimetric method as recommended by Singleton & Rossi to define the total phenolic content at 765 nm [30]. The results were expressed as 1 mg gallic acid equivalent per 1 g pod extract (mg GAE/g). The total flavonoid content was calculated using the method described by Dehpour et al., i.e., colorimeterical- ly at 415 nm [31]. The results were represented as 1 mg quercetin equivalent per 1 g extract (mg QE/g).
Antioxidant activity. DPPH radical scavenging assay. The methanolic extract of broad bean pods was tested for its capacity to scavenge free radicals with the help of 1,1-diphenyl-2-picryl hydrazyl (DPPH). Initially, 1 mL of DPPH methanol solution (0.1 mM) was mixed with 3 mL of the pod extract at various concentrations: 3.9, 7.8, 15.62, 31.25, 62.5, 125, 250, 500, and 1000 g/mL. The mix was briskly shaken before being left to stand at room temperature for 30 min. After that, we used a UV-visible spectrophotometer to detect absorbance at 517 nm [32]. The log dosage inhibition curve made it possible to determine the IC50 value, i.e., the concentration the sample needed to block 50% of the DPPH free radical. If the absorbance was low, the free radical activity was high [33]. The percentage of the DPPH scavenging effect, %, was calculated by the following Eq. (1):
where A 0 was the absorbance of the control reaction; A1 was the absorbanc ef of the lextracted samples. 100
Ferric reducing power assay. We evaluated the antioxidant capacity of the sample extract using the reducing power as described by Debnath et al. [34]. In line with the procedure, we combined 1 mL solution with 2.5 mL of sodium phosphate buffer (0.2 mM, 6.6 pH) and 2.5 mL of 1% K3[Fe(CN)6]. The resulting mix incubated at 50°C for 20 min. To halt the reaction, we added aliquots of 10% CCl3 COOH (2.5 mL). Finally, 2.5 mL reaction mix, 2.5 mL distilled water, and 1 mL fresh 0.1% FeCl3 solution reacted at room temperature for 10 min. The absor
bance was measured at 700 nm. High absorbance corresponded with high reducing power.
In vitro anti-inflammatory assay. Preparing erythrocyte suspension. Three healthy volunteers provided blood, 3 mL each, which was collected into heparinized tubes and centrifuged at 3000 rpm for 10 min. The red blood pellets were dissolved in a volume of normal saline equal to the supernatant. Dissolved red blood pellets were measured in volume and reconstituted in an isotonic buffer solution (10 mM sodium phosphate buffer, pH 7.4) as a 40% v/v suspension to be used later as the erythrocyte suspension.
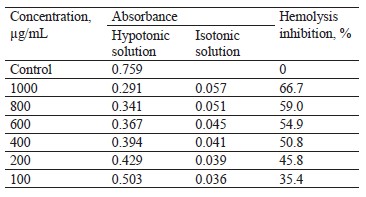
Hypotonicity-prompted hemolysis. In centrifuge tubes, we dissolved the pod extract samples in a hypotonic solution (distilled water) at concentrations of 100, 200, 400, 600, 800, and 1000 µg/mL. Isotonic solutions (5 mL) were also prepared in centrifuge tubes with 100– 1000 µg/mL of pod extracts. In addition, the vehicle control tube contained 5 mL of distilled water. Each sample received 0.1 mL erythrocyte suspension and was mixed lightly. The tubes were incubated at 37°C for 1 h and then centrifuged at 1300 g for 3 min. To determine the hemoglobin content in the supernatant, we measured the absorbance, or optical density (OD), at 540 nm.
The inhibition percentage of hemolysis, %, was calculated as follows:
where OD1 was the absorbance of the extracted sample in the isotonic solution; OD2 designated the absorbance of the extracted sample in the hypotonic solution; OD3 stood for the absorbance of control sample in the hypotonic solution.
Antimicrobial activity of broad bean pod methanolic extract. Agar well diffusion method: the agar well diffusion made it possible to assess the antibacterial activity of the pod extract. We covered the entire agar surface with microbial inoculum and diluted the extract solution to the necessary concentration. A well with a diameter of 6 to 8 mm was drilled aseptically with a sterile drill. The agar plates were incubated in the proper environment for each type of microbe. The widths of the acquired inhibition zone around the wells (mm) were measured after 16–24 h (Mucoraceae), 24 h (Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger), and 48 h (other microbial species) of incubation. Gentamicin was used as a reference standard at a concentration of 4 µg/mL. The abovementioned microbial strains could not develop in agar media due to the potent antimicrobial properties of the extract, which diffused into the medium [35].
Effect of pod methanolic extract on Pc3 and HepG2 cells. Cell viability and proliferation assay (MTT): the MTT test assessed the cytotoxic activity of the methanolic extract of broad bean pods against HepG2 and PC3 cells. The method involved a 96-well culture plate as recommended in [36].
A full monolayer sheet formed after 24 h of incubation at 37°C with 1×105 cells/mL (100 µL/well) in the 96-well tissue culture plate. After the confluent sheet of cells had developed, we decanted the growth medium from 96-well microtiter plates and washed the cell monolayer twice with wash media. Then, we prepared twofold dilutions of the extract in RPMI medium with 2% serum as a maintenance medium. Three wells served as controls and received only the maintenance medium after 0.1 mL of each dilution was poured in various wells. The plate was tested after incubation at 37°C. Cells were evaluated for any indications of toxicity, such as shrinkage, granulation, or a partial/total loss of monolayer. After adding 20 µL of MTT solution (5 mg/mL) to each well, the medium was mixed with the MTT using a shaking table at 150 rpm for 5 min. The MTT then metabolized during 1–5 h of incubation at 37°C and 5% CO2. After that, the medium was discarded, and the plate was dried with a paper towel to remove residue, if necessary. Then, we resuspended metabolic by-product of MTT, formazan, in 200 µL of dimethyl sulfoxide and agitated it at 150 rpm for 5 min to combine formazan with the solvent. The optical density was measured at 560 nm, the subtract background was determined at 620 nm. The cell count and optical density were directly connected.
Statistical analysis. The data were presented as the mean ± SD. All tests were processed using the SPSS statistical analysis program (Version 24), as described by McCormick & Salcedo [37].
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
Proximate chemical analysis of broad bean (Vicia faba L.) pods. Table 1 shows the chemical composition of the broad bean pods in their green state after ovendrying at 50°C. The dried pods contained 9.27% moisture, 8.38% protein, 0.38% fat, 7.22% ash, 14.59% fiber, and 60.16% carbohydrates. In our research, the content of carbohydrates and dietary fiber appeared to be quite high. However, Mateos-Aparicio et al. reported different data, especially for fiber and protein: 40.1% dietary fiber, 13.6% protein, 6.3% ash, and 1.3% fat on a dry weight basis [38]. Our results also differed from those published by Mejri et al., who detected a high moisture content of 79.26% on a wet weight basis, with 13.81% proteins, 18.93% carbohydrates, 0.92% lipids, and 57.46% dietary fiber [18]. In a study reported by Vernaleo et al., fava beans proved rich in dietary fiber and phytonutrients, e.g., isoflavone and plant sterols [39]. The differences in the chemical composition of broad bean pods obtained by different research teams could be attributed to the geographical location, handling, processing, or variety.
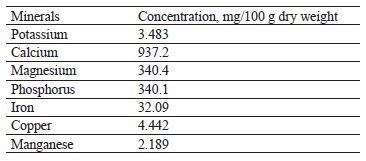
Mineral contents of broad bean pods. Table 2 demonstrates the mineral profile of broad bean pods per 100 g. Obviously, broad bean pods proved to be a good source of potassium, calcium, magnesium, and iron. Our results were in line with those by Vernaleo et al., who also revealed that broad beans were rich in phosphorus, iron, copper, manganese, calcium, magnesium, and potassium [39]. Similarly, Mateos et al., who studied broad bean pods as by-products, reported that they contained a lot of potassium, calcium, and iron [38].
Phytochemical screening of broad bean pod methanolic extract. Table 3 illustrates the results of a phytochemical screening, which revealed phenolic compounds, flavonoids, glycosides, tannins, alkaloids, and saponins in the methanolic extract of broad bean pods.
The ethanolic extract of V. faba L. was found to contain all phytochemicals except for anthracenosides, sterols, and triterpenes (Fabaceae). The aqueous extract contained less tannins, alkaloids, glycosides, sterol, triterpenes, and saponins than the ethanolic extract. Reducing sugars were present in the ethanolic extract exclusively [40]. Broad beans are known to contain polyphenols in leaves, roots, and seeds [41]. The content of cotyledons in beans was reported to exceed that in hulls. According to recent studies, broad beans and their derivatives may be included in diets against hypertension, diabetes, and cardiovascular diseases [42].
Total phenols and total flavonoids in broad bean pod methanolic extract. Table 4 shows the total phenols and flavonoids in the methanolic extract of broad bean pods. The phenol content was 286 mg GAE/g, while the total flavonoid content was 105 mg QE/g. The data obtained were higher than those reported by Mejri et al., where the total phenolic compounds in the methanol extract of broad bean pods were 115.21 mg GAE/g extract and the total flavonoids were 47.34 mg QE/g extract [18]. According to Valente et al., the total free phenols in dried pods depended on the variety and ranged from 10.87 to 26.34 mg/100 g, while the total esterified phenolics ranged from 8.76 to 26.72 mg/100 g dry weight [43]. Chan et al. reported that the methanolic extract of broad bean pods was rich in total phenolics and flavonoids, including numerous polar aglycones and flavonoid glycosides [44]. The phenolic content issue still requires more scientific attention. Chaieb et al. studied 13 genotypes of broad bean pods grown in the same area and under the same conditions [45]. Their phenol content ranged from 56.97 to 149.21 mg GAE/g whereas the total flavonoids ranged from 10.23 to 45.92 mg RE/g, depending on the genotype.
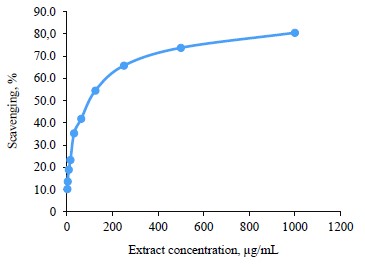
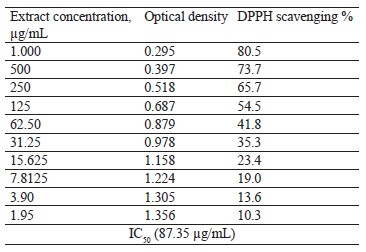
Antioxidant activity of broad bean pod methanolic extract. DPPH assay. The antioxidant activity of the broad bean pod extract was measured by 1,1-diphenyl-2-picryl hydrazyl (DPPH) assay. Table 5 and Fig. 1 show that the DPPH scavenging percentage increased together with the extract concentration. The highest value of DPPH scavenging activity reached 80.5% at the extract concentrations of 1000 µg/mL. The concentrations of 500 and 250 µg/mL also showed high levels of DPPH scavenging activity, which reached 73.7 and 65.7%, respectively.
IC50 is the concentration of the antioxidant substance needed to reduce the initial DPPH concentration by 50%. Low IC50 indicates high antioxidant activity. In our research, IC50 was quite low and equaled 87.35 µg/mL, which means that the broad bean pods had high antioxidant activity. Mateos-Aparicio et al. also reported high reducing power and free-radical scavenging activity of polyphenols extracted from broad bean pods [19]. The antioxidant activity of broad bean pods probably came from their high phenolic content [46].
Some plants are known to contain natural substances with good anticancer potential. Broad bean pods are rich in fiber, phenolic acids, and flavonoids, which can prevent the oxidation of cell membranes and protect the cells from free radicals and toxic substances. In addition, tannins in broad beans could provide hydroxyl radical scavenging activity [47]. Hypothetically, broad bean pod extract prevents the reaction of hydroxyl radicals with the hydrogen atoms of the sugar moiety of DNA and hence protects DNA from damage [48].
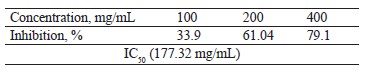
Antioxidant activity of broad bean pod methanolic extract: reducing power. Table 6 shows that the reducing power of the broad bean pod methanolic extract increased together with its concentration. The IC50 values reached 177.32 mg/mL.
Any substance with a reducing power combines with potassium ferricyanide (Fe3+) to generate potassium ferrocyanide (Fe2+), which then reacts with ferric chloride to form a ferric-ferrous complex, or Perls Prussian blue, which is absorbed at 700 nm [49]. Reductive action and antioxidant activity are connected [50]. As mentioned before, the BBP methanol extract demonstrated high antioxidant activity (60.72%). The lowest IC50 for the DPPH and ABTS assays corresponded with the highest free radical scavenging activity [18].
Anti-inflammatory activity of broad bean pod methanolic extract. We appealed to the HRBC (human red blood cells) method in vitro to study the anti-inflammatory effect of the broad bean pod extract. According to the procedure, the erythrocyte membrane and the lysosomal membrane are comparable; therefore, by stabilizing the erythrocyte membrane, the extract from broad bean pods may stabilize the lysosomal membrane.
Table 7 shows that all the extract concentrations exhibited a significant reduction in the hemolysis of red blood cells: the maximal inhibition percentage reached 66.7% at 1.000 µg/mL. The inhibition percentage decreased with lowering the extract concentration. Therefore, the pod extract indeed possessed anti-inflammatory properties in the studied models.
The hypotonic solution causes hemolysis of red blood cells because fluid accumulates in the cells, thus rupturing their membranes. The damaged red blood cells become more susceptible to lipid oxidation via free radicals. As a result, some components, e.g., protein and fluids, start entering the tissues, which is similar to inflammation [51].
The extract of broad bean pods proved able to preserve the red blood cell membranes by preventing the oxidation of lipids in them. In addition, it stabilizes the red blood cell membrane by preventing the production of lytic enzymes and active inflammatory mediators.
In this research, the broad bean pod extract proved to contain flavonoids, alkaloids, and saponin, which are known for their anti-inflammatory properties. Many studies reported the antioxidant and anti-inflammatory effects of plant flavonoids [52–54].
Plant flavonoids may owe their anti-inflammatory properties due to their ability to inhibit the enzymes of arachidonic acid metabolism, as well as the enzymes that contribute to the production of inflammatory mediators [55, 56].
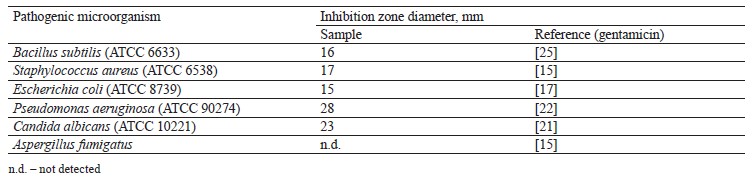
Antimicrobial activity of broad bean pod methanolic extract. The antimicrobial activity of the methanolic extract isolated from broad bean pods was assessed in vitro by the agar well diffusion method against four pathogenic bacteria strains and two kinds of fungi. The bacteria strains included two Gram-positive (Bacillus subtilis and Staphylococcus aureus) and two Gramnegative (Escherichia coli and Pseudomonas aeruginosa) samples, while the two fungi were represented by Candida albicans and Aspergillus fumigatus. Antimicrobial activity was determined by agar diffusion (100 µL), 6.0 mm disc diameter. All samples were dissolved in normal saline (0.9% NaCl), which had no antimicrobial activity against all the tested pathogenic strains.
Table 8 shows that the pod extract prevented the bacterial growth of B. subtilis, S. aureus, E. coli, and P. aeruginosa. It also inhibited fungus C. albicans. The corresponding inhibition zones were 16, 17, 15, 28, and 23 mm, respectively. However, the A. fumigatus fungus appeared resistant to the broad bean pod extract. The antimicrobial effect was more effective against S. aureus, P. aeruginosa, and C. albicans than gentamycin, which served as reference control. To some extent, these results agreed those reported by Peyvast & Khorsandi, who also registered the antimicrobial activity of broad bean seed hull ethanolic extract against E. coli, B. subtilis, and S. aureus [57].
Anticancer activity of broad bean pod methanolic extract. Liver cancer is the fourth most common cause of death in the world [58]. This type of cancer has high mortality and morbidity because hepatitis C virus infection has become wide-spread in the last decades. Hepatitis C virus is the leading cause of cirrhosis, which is one of the risk factors for liver cancer [59, 60]. Since anticancer medications have so many side effects, natural products have good prospects as a novel anticancer remedy.
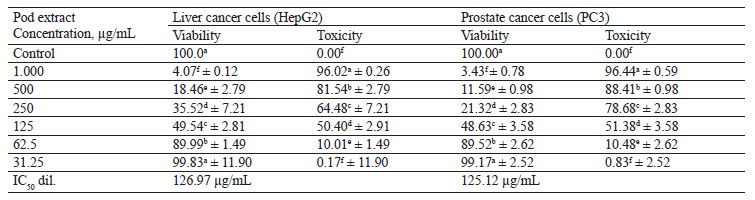
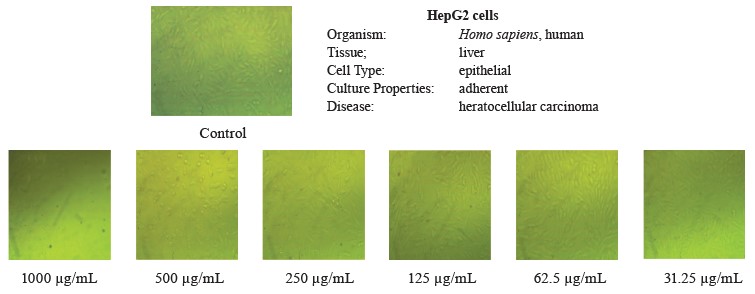
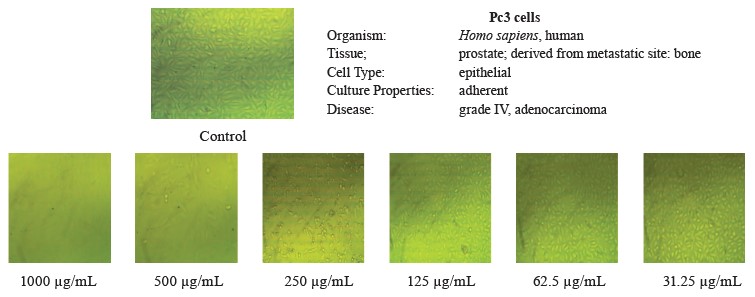
In this study, we tested the effect of methanolic extract of broad bean pods on human hepatocellular carcinoma (HepG2) and prostate cancer (PC3) cells. Table 9 and Figs. 2 and 3 show that the methanolic extract of broad bean pods reduced cell viability and increased cell toxicity of both HepG2 and PC3 in a concentration dependent manner. The low extract concentration of 31.25 had no significant effect on cell viability. However, all other concentrations caused significant decreases in the viability of the two kinds of cells, increasing their cell toxicity. In HepG2 cells, the viability for the extracts with concentrations of 125, 250, 500, and 1000 µg/mL was 49.54, 35.52, 18.46, and 4.07%, respectively; in PC3 cells, it was 48.63, 21.32, 11.59, and 3.43%, respectively. The broad bean pod methanolic extract exhibited high toxicity to HepG2 and PC3 cells: the toxicity percentage exceeded 96% for 1000 µg/mL. The IC50 values of the pod extract were observed at concentrations of 126.97 µg/mL for HepG2 cells and 125.12 µg/mL for PC3 cells, which are good results for an anticancer agent.
Figures 2 and 3 demonstrate that the methanolic extract of broad bean pods caused remarkable alterations in the cell shape, compared to the control. The changes in the cellular morphology increased together with the extract concentration. A large amount of dead and detached cells indicated a toxic effect of the pod extract on the proliferation of tumor cells after 24 h of incubation. The low concentration of 31.25 µg/mL caused no significant alterations. In contrast, high concentrations triggered substantial changes in the morphology of the tumor cells, and these changes increased together with the extract concentration.
Plant by-products burden the environment, and their utilization attracts a lot of scientific attention. For example, some fruit and vegetable wastes can be used as feed for cattle and sheep; others can be used in soil fertilization [61]. Bioactive components found in plant and vegetable wastes can become a source of antioxidant and anticancer nutraceuticals [62]. In addition, polyphenols and micronutrients found in legumes possess important biological values [63, 64]. Polyphenols are known to protect the human organism from chronic diseases, such as cardiovascular conditions, diabetes, asthma, cancer, and inflammation [65]. The anticancer activity of the broad bean pod extract is attributed to its high content of phenolic compounds
p-Coumaric and ferulic acids are present in the phenolic acid profile of broad bean pods. In other studies, they displayed anticancer activity against different types of cell lines [66].
Ceramella et al. performed DPPH and ABTS assays on extracts of broad bean pods in acetone, methanol, and 70% ethanol [67]. All three extracts demonstrated an excellent antioxidant activity, as well as a satisfactory anticancer activity against melanoma SK-Mel-28 cells.
Polyphenols were found extremely important in preventing and treating chronic inflammation-related illnesses, such as cardiovascular diseases, obesity, neurodegeneration, cancers, and diabetes [68, 69]. Polyphenols can suppress toll-like receptors and pro-inflammatory genes. The antioxidant activity of polyphenols is attributed to their ability to inhibit enzymes that contribute to the production of eicosanoids and their anti-inflammation properties. For example, they inhibit certain enzymes that produce reactive oxygen species, e.g., xanthine oxidase and NADPH oxidase. On the one hand, they boost other endogenous antioxidant enzymes, e.g., superoxide dismutase, catalase, and glutathione peroxidase. On the other hand, they inhibit phospholipase A2, cyclooxygenase, and lipoxygenase, thus reducing the production of prostaglandins and leukotrienes, as well as inflammation antagonism. These effects that polyphenols have on the immune system mitigate the syndromes of various chronic inflammatory diseases [69].
ВЫВОДЫ
Pods of broad beans (Vicia faba L.) proved to contain such bioactive substances as phenolic compounds, flavonoids, tannins, and alkaloids, not to mention dietary fiber. The methanolic extract of dried fresh green pods demonstrated a potent antioxidant activity towards DPPH radicals, as well as good anti-inflammatory properties. The pod extract also showed antimicrobial activity against some food-born pathogenic microorganisms. In addition, it possessed anticancer activity against HepG2 and PC3 cell lines. These properties belonged to phytochemicals and soluble fibers in the methanolic extract. Therefore, fresh immature broad bean pods can be recommended for human consumption, raw or cooked. Dried ripened pods can be solvent-extracted to obtain various bioactive components that may serve as additives in functional food production.
Вклад авторов
All the authors were equally involved in the research analysis and manuscript writing.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare that there is no conflict of interest regarding this publication.СПИСОК ЛИТЕРАТУРЫ
- Abdel-Aal E-SM, Hucl P. Amino acid composition and in vitro protein digestibility of selected ancient wheats and their end products. Journal of Food Composition and Analysis. 2002;15(6):737–747. https://doi.org/10.1006/jfca.2002.1094
- Tazrart K, Lamacchia C, Zaidi F, Haros M. Nutrient composition and in vitro digestibility of fresh pasta enriched with Vicia faba. Journal of Food Composition and Analysis. 2016;47:8–15. https://doi.org/10.1016/j.jfca.2015.12.007
- Bouhnik Y, Flourie B, D'Agay-Abensour L, Pochart P, Gramet G, Durand M, et al. Administration of transgalacto-oligosaccharides increases fecal bifidobacteria and modifies colonic fermentation metabolism in healthy human. The Journal of Nutrition. 1997;127(3):444–448. https://doi.org/10.1093/jn/127.3.444
- Campos-Vega R, Loarca-Piña G, Oomah BD. Minor components of pulses and their potential impact on human health. Food Research International. 2010;43(2):461–482. https://doi.org/10.1016/j.foodres.2009.09.004
- Aune D, De Stefani E, Ronco A, Boffetta P, Deneo-Pellegrini H, Acosta G, et al. Legume intake and the risk of cancer: A multisite case – control study in Uruguay. Cancer Causes and Control. 2009;20:1605–1615. https://doi.org/10.1007/s10552-009-9406-z
- Anderson JW, Major AW. Pulses and lipaemia, short- and long-term effect: Potential in the prevention of cardiovascular disease. British Journal of Nutrition. 2002;88(S3):263–271. https://doi.org/10.1079/BJN2002716
- Ranilla LG, Kwon Y-I, Genevese MI, Lajolo FM, Shetty K. Effect of thermal treatment on phenolic compounds and functionality linked to type 2 diabetes and hypertension management of Peruvian and Brazilian bean cultivars (Phaseolus vulgaris L.) using in vitro methods. Journal of Food Biochemistry. 2010;34(2):329–355. https://doi.org/10.1111/j.1745-4514.2009.00281.x
- Akpinar N, Akpinar MA, Türkoğlu S. Total lipid content and fatty acid composition of the seeds of some Vicia L. species. Food Chemistry. 2001;74(4):449–453. https://doi.org/10.1016/S0308-8146(01)00162-5
- Singh AK, Bhatt BP, Sundaram PK, Gupta AK, Singh D. Planting geometry to optimize growth and productivity faba bean (Vicia faba L.) and soil fertility. Journal of Environmental Biology. 2013;34:117–122.
- Hossain MS, Mortuza MG. Chemical composition of Kalimatar, a locally grown strain of faba bean (Vicia faba L.). Pakistan Journal of Biological Sciences. 2006;9(9):1817–1822. https://doi.org/10.3923/pjbs.2006.1817.1822
- Merghem R, Jay M, Brun N, Voirin B. Qualitative analysis and HPLC isolation and identification of procyanidins from Vicia faba. Phytochemical Analysis. 2004;15(2):95–99. https://doi.org/10.1002/pca.731
- Amarowicz R, Troszynska A, Baryłko-Pikielna N, Shahidi F. Polyphenolics extracts from legume seeds: correlations between total antioxidant activity, total phenolics content, tannins content and astringency. Journal of Food Lipids. 2004;11(4):278–286. https://doi.org/10.1111/j.1745-4522.2004.01143.x
- Siah JA, Konczak I, Agboola S, Wood JA, Blanchard CL. In vitro investigations of the potential health benefits of Australian-grown faba beans (Vicia faba L.): Chemopreventive capacity and inhibitory effects on the angiotensin-converting enzyme, α-glucosidase, and lipase. British Journal of Nutrition. 2012;108(S1):S123–S134. https://doi.org/10.1017/S0007114512000803
- Boukhanouf S, Louaileche H, Perrin D. Phytochemical content and in vitro antioxidant activity of faba bean (Vicia faba L.) as affected by maturity stage and cooking practice. International Food Research Journal. 2016;23(3):954–961.
- Ikarashi N, Takeda R, Ito K, Ochiai W, Sugiyama K. The inhibition of lipase and glucosidase activities by acacia polyphenol. Evidence-Based Complementary and Alternative Medicine. 2011;2011. https://doi.org/10.1093/ecam/neq043
- El-Feky AM, Elbatanony MM, Mounier MM. Anti-cancer potential of the lipoidal and flavonoidal compounds from Pisum sativum and Vicia faba peels. Egyptian Journal of Basic and Applied Sciences. 2018;5(4):258–264. https://doi.org/10.1016/j.ejbas.11.001.
- Troszynska A, Estrella I, Lohpez-Amohres ML, Hernahndez T. Antioxidant activity of Pea (Pisum sativum L.) seed coat acetone extract. LWT – Food Science and Technology. 2002;35(2):158–164. https://doi.org/10.1006/fstl.2001.0831
- Mejri F, Selmi S, Martins A, Benkhoud H, Baati T, Chaabane H, et al. Broad bean (Vicia faba L.) pods: a rich source of bioactive ingredients with antimicrobial, antioxidant, enzyme inhibitory, anti-diabetic and health-promoting properties. Food and Function. 2018;9(4):2051–2069. https://doi.org/10.1039/C8FO00055G
- Mateos-Aparicio I, Redondo-Cuenca A, Villanueva-Suarez M-J. Broad bean and pea byproducts as sources of fiber-rich ingredients: Potential antioxidant activity measured in vitro. Journal of the Science of Food and Agriculture. 2012;92(3):697–703. https://doi.org/10.1002/jsfa.4633
- Macarulla MT, Medina C, de Diego MA, Chávarri M, Zulet MA, Martínez JA, et al. Effects of the whole seed and a protein isolate of faba bean (Vicia faba) on the cholesterol metabolism of hypercholesterolaemic rats. British Journal of Nutrition. 2001;85(5):607–614. https://doi.org/10.1079/bjn2000330
- Rizkalla SW, Bellisle F, Slama G. Health benefits of low glycaemic index foods, such as pulses, in diabetic patients and healthy individuals. British Journal of Nutrition. 2002;88(S3):255–262. https://doi.org/10.1079/BJN2002715
- Valente IM, Cabrita ARJ, Malushi N, Oliveira HM, Papa L, Rodrigues JA, et al. Unravelling the phytonutrients and antioxidant properties of European Vicia faba L. seeds. Food Research International. 2019;116:888–896. https://doi.org/10.1016/j.foodres.2018.09.025
- Kumar A, Nidhi, Prasad N, Sinha SK. Nutritional and antinutritional attributes of faba bean (Vicia faba L.) germplasms growing in Bihar, India. Physiology and Molecular Biology of Plants. 2015;21(1):159–162. https://doi.org/10.1007/s12298-014-0270-2
- Apaydin H, Ertan S, Özekmekçi S. Broad bean (Vicia faba) – A natural source of L-dopa – Prolongs “on” periods in patients with Parkinson’s disease who have “on–off” fluctuations. Movement Disorders. 2000;15(1):164–166. https://doi.org/10.1002/1531-8257(200001)15:1%3C164::AID-MDS1028%3E3.0.CO;2-E
- Hacıseferoǧullar H, Gezer İ, Bahtiyarca Y, Menge HO. Determination of some chemical and physical properties of Sakız faba bean (Vicia faba L. Var. major). Journal of Food Engineering. 2003;60(4):475–479. https://doi.org/10.1016/S0260- 8774(03)00075-X
- Official Methods of Analysis. 17th Edition. Gaithersburg: AOAC; 2000.
- Pavlova D, Karadjova I. Chemical analysis of Teucrium species (Lamiaceae) growing on serpentine soils in Bulgaria. Journal of Plant Nutrition and Soil Science. 2012;175(6):891–899. https://doi.org/10.1002/jpln.201100046
- Trease GE, Evans WC. Pharmacognosy. 12th Edn. London; Philadelphia: Bailliere Tinadl; 1989. 856 p.
- Harborne JB. Phytochemical methods – A guide to modern techniques of plant analysis. London: Chapman and Hall; 1998. 296 p.
- Singleton VI, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. American Journal of Enology and Viticulture. 1965;16(3):144–158.
- Dehpour AA, Ibrahimzadeh MA, Fazel NS, Mohammad NS. Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Grasas y Aceites. 2009;60(4):405–412.
- Patel RM, Patel NJ. In vitro antioxidant activity of coumarin compounds by DPPH, superoxide and nitric oxide free radical scavenging methods. Journal of Advanced Pharmacy Education and Research. 2011;1:52–68.
- Koleva II, van Beek TA, Linssen JPH, de Groot A, Evstatieva LN. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochemical Analysis. 2002;13(1):8–17. https://doi.org/10.1002/pca.611
- Debnath T, Park P-J, Deb Nath NC, Samad NB, Park HW, Lim BO. Antioxidant activity of Gardenia jasminoides Ellis fruit extracts. Food Chemistry. 2011;128(3):697–703. https://doi.org/10.1016/j.foodchem.2011.03.090
- Magaldia S, Mata-Essayaga S, de Caprilesa CH, Pereza C, Colella MT, Olaizolaa C, et al. Well diffusion for antifungal susceptibility testing. International Journal of Infectious Diseases. 2004;8(1):39–45. https://doi.org/10.1016/j.ijid.2003.03.002
- Sinicropi MS, Iacopetta D, Rosano C, Randino R, Caruso A, Saturnino C, et al. N-thioalkylcarbazoles derivatives as new anti-proliferative agents: Synthesis, characterization and molecular mechanism evaluation. Journal of Enzyme Inhibition and Medicinal Chemistry. 2018;33(1):434–444. https://doi.org/10.1080/14756366.2017.1419216
- McCormick K, Salcedo J. SPSS statistics for data analysis and visualization. John Wiley & Sons; 2017. 528 p.
- Mateos-Aparicio I, Redondo-Cuenca A, Villanueva-Suárez M-J, Zapata-Revilla M-A, Tenorio-Sanz M-D. Pea pod, broad bean pod and okara, potential sources of functional compounds. LWT – Food Science and Technology. 2010;43(9):1467–1470. https://doi.org/10.1016/j.lwt.2010.05.008
- Vernaleo B. Back to basics: Why Basic Research (and the fava bean) are key to the cure (PDF). Parkinson's Disease Foundation; 2016.
- Jagessar RC. Phytochemical screening and chromatographic profile of the ethanolic and aqueous extract of Passiflora edulis and Vicia faba L. (Fabaceae). Journal of Pharmacognosy and Phytochemistry. 2017;6(6): 1714–1721.
- Baginsky C, Peña-Neira Á, Cáceres A, Hernández T, Estrella I, Morales H, et al. Phenolic compound composition in immature seeds of fava bean (Vicia faba L.) varieties cultivated in Chile. Journal of Food Composition and Analysis. 2013;31(1):1–6. https://doi.org/10.1016/j.jfca.2013.02.003
- Madar Z, Stark AH. New legume sources as therapeutic agents. British Journal of Nutrition. 2002;88(S3):287–292. https://doi.org/10.1079/BJN2002719
- Valente IM, Maiaa MRG, Malushia N, Oliveiraa HM, Papac L, Rodriguesb JA, et al. Profiling of phenolic compounds and antioxidant properties of European varieties and cultivars of Vicia faba L. pods. Phytochemistry. 2018;125:223–229. https://doi.org/10.1016/j.phytochem.2018.05.011
- Chan PT, Matanjun P, Md Yasir S, Tan TS. Antioxidant activities and polyphenolics of various solvent extracts of red seaweed, Gracilaria changii. Journal of Applied Phycology. 2015;27:2377–2386. https://doi.org/10.1007/s10811-014-0493-1
- Chaieb N, González JM, López-Mesas M, Bouslama M, Valiente M. Polyphenols content and antioxidant capacity of thirteen faba bean (Vicia faba L.) genotypes cultivated in Tunisia. Food Research International. 2011;44(4):970–977. https://doi.org/10.1016/j.foodres.2011.02.026
- Turco I, Ferritti G, Bacchetti T. Reviewof the health benefits of faba bean (Vicia faba L.) polyphenols. Journal of Food and Nutrition Research. 2016;55(4):283–293.
- Carbonaro M, Virgili F, Carnovale E. Evidence for protein-tannin interaction in legumes: Implications in the antioxidant properties of faba bean tannins. LWT – Food Science and Technology. 1996;29(8):743–750. https://doi.org/10.1006/fstl.1996.0116
- Spanou C, Stagos D, Tousias L, Angelis A, Aligiannis N, Skaltsounis A-L, et al. Assessment of antioxidant activity of extracts from unique Greek varieties of Leguminosae plants using in vitro assays. Anticancer Research. 2007;27:3403–3410.
- Choudhary DK, Mishra A. In vitro and in silico interaction of faba bean (Vicia faba L.) seed extract with xanthine oxidase and evaluation of antioxidant activity as a therapeutic potential. Natural Product Research. 2018;33(18):2689–2693. https://doi.org/10.1080/14786419.2018.1460831
- Duan X-J, Zhang W-W, Li X-M, Wang B-G. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chemistry. 2006;95(1):37–43. https://doi.org/10.1016/j.foodchem.2004.12.015
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? British Journal of Pharmacology. 2004;142(2):231–225. https://doi.org/10.1038/sj.bjp.0705776
- Middleton E, Kandaswami C. Effect of flavonoids on immune and inflammatory cell function. Biochemical Pharmacology. 1992;43(6):1167–1179. https://doi.org/10.1016/0006-2952(92)90489-6
- Read MA. Flavonoids: Naturally occurring anti-inflammatory agents. American Journal Of Pathology. 1995;147(2):235–237.
- Halliwell B, Rafter J, Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols and other phenols: Direct or indirect effects? Antioxidants or not? The American Journal of Clinical Nutrition. 2005;81(1):268S–276S. https://doi.org/10.1093/ajcn/81.1.268S
- Oweyele VB, Oloriegbe YY, Balaogun EA, Soladoye AO. Analgesis and anti-inflammatory properties of Nelsonia Canescens leaf extract. Journal of Ethnopharmacology. 2005;99(1):153–156. https://doi.org/10.1016/j.jep.2005.02.003
- Metowogo K, Agbonon A, Eklu-Gadegbeku K, Aklikokou AK, Gbeassor M. Anti-ulcer and anti-inflammtory effects of Hydro-alcohol extract of Aloe buettneri A. Berger (Lilliaceae). Tropical Journal of Pharmaceutical Research. 2008;7(1):907–912. https://doi.org/10.4314/tjpr.v7i1.14676
- Peyvast G, Khorsandi Z. Antibacterial activity of broad bean extracts on resistance bacteria. Pakistan Journal of Biological Sciences. 2007;10(3):398–402. https://doi.org/10.3923/pjbs.2007.398.402
- Villanueva A. Hepatocellular carcinoma. The New England Journal of Medicine. 2019;380(15):1450–1462. https://doi.org/10.1056/NEJMra1713263
- Yang R, Chen H, Gu Z. Factors influencing diamine oxidase activity and γ-aminobutyric acid content of fava bean (Vicia faba L.) during germination. Journal of Agricultural and Food Chemistry. 2011;59(21):11616–11620. https://doi.org/10.1021/jf202645p
- Mohamed AA, Elbedewy TA, El-Serafy M, El-Toukhy N, Ahmed W, Ali El Din Z. Hepatitis C virus: A global view. World Journal of Hepatology. 2015;7(26):2676–2680. https://doi.org/10.4254/wjh.v7.i26.2676
- L’Hocine L, Martineau-Côté D, Achouri A, Wanasundara JPD, Loku Hetti Arachchige GW. Broad bean (faba bean). In: Manickavasagan A, Thirunathan P, editors. Pulses: Processing and product development. Cham: Springer; 2020. pp. 27–54. https://doi.org/10.1007/978-3-030-41376-7_3
- Batra P, Sharma AK. Anti-cancer potential of flavonoids: recent trends and future perspectives. 3 Biotech. 2013;3:439–459. https://doi.org/10.1007/s13205-013-0117-5
- Perez-Jimenez J, Neveu V, Vos F, Scalbert A. Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database. European Journal of Clinical Nutrition. 2010;64:S112–S120. https://doi.org/10.1038/ejcn.2010.221
- Wink M. Modes of action of herbal medicines and plant secondary metabolites. Medicines. 2015;2(3):251–286. https://doi.org/10.3390/medicines2030251
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity. 2009;2(5):270–278. https://doi.org/10.4161/oxim.2.5.9498
- Anantharaju PG, Gowda PC, Vimalambike MG, Madhunapantula SV. An overview on the role of dietary phenolics for the treatment of cancers. Nutrition Journal. 2016;15. https://doi.org/10.1186/s12937-016-0217-2
- Ceramella J, la Torre C, de Luca M, Iacopetta D, Fazio A, Catalano A, et al. Exploring the anticancer and antioxidant properties of Vicia faba L. pods extracts, a promising source of nutraceuticals. PeerJ. 2022;10. https://doi.org/10.7717/peerj.13683
- Ghiringhelli F, Rebe C, Hichami A, Delmas D. Immunomodulation and anti-inflammatory roles of polyphenols as anticancer agents. Anti-Cancer Agents in Medicinal Chemistry. 2012;12(8):852–873. https://doi.org/10.2174/187152012802650048
- Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10(11). https://doi.org/10.3390/nu101116