Аннотация
Introduction. More attention has been paid in recent decades to extracts and essential oils from various plants as natural antioxidant sources due to their positive effects on food oxidation reactions. Our study aimed to compare the antioxidant activity of aqueous and alcoholic extracts from Salvia leriifolia L. and Linum usitalissmum L.The extracts were subjected to a pulsed electric field with intensities of zero (without pretreatment), 3 and 6 kV·cm–1, and a constant pulse number of 30. For this purpose, parameters such as total phenolic compounds and antioxidant activity were investigated by DPPH and TEAC methods.Results and discussion. Our results showed that a higher intensity of a pulsed electric field pretreatment and the use of an alcoholic solvent significantly raised total phenolic compounds in the extracts and their antioxidant activity at a 95% confidence level. We found significant effects of the plant source (Linum usitalissmum and Salvia leriifolia), pretreatment (pulse electric field at intensities of 0.3 and 6 kV·cm–1), and a solvent (aqueous and alcohol) on the extracts’ antioxidant activity (P < 0.05). In addition, there was a significant correlation between the results of the DPPH and the TEAC antioxidant activities (P < 0.01 and r = 0.932).
Conclusion. The total antioxidant activity (based on both TEAC and DPPH methods) and total phenolic compounds extracted from Salvia leriifolia were higher than those from Linum usitalissmum (P < 0.05). Based on the results, the extract obtained from Salvia leriifolia with an alcoholic solvent and a pulsed electric field pretreatment (at 6 kV·cm–1 and 30 pulses) was selected as possessing desired antioxidant properties.
Ключевые слова
Antioxidant, extraction, pulsed electric field, Linum usitalissmum, Salvia leriifoliaВВЕДЕНИЕ
Lipid oxidation is one of the major chemical changes that occur during food processing, storage, and preparation. Lipid molecules are rapidly oxidized in the presence of oxygen, especially in the case of unsaturated fatty acids [1]. Antioxidants are widely used today to reduce the rate of oxidation reaction of fats in foods.
Antioxidants are molecules or compounds that act against free radicals which damage to molecules, resulting in the loss of their function. Antioxidants provide a primary defense against such oxidative degradations [2]. In industrial processes, synthetic antioxidants – such as butyl hydroxy toluene and butyl hydroxy anisol – are mainly used to increase the food’s shelf life. In this regard, nutritionists have found that these compounds can have adverse effects on the body [3].
Therefore, it is necessary to use strong antioxidants with lower toxicity and greater efficacy. In recent decades, natural antioxidants have drawn the attention of food researchers due to their safety in food formulation. These are extracts and essential oils of various plants that produce positive effects on nutrient oxidation reactions.
Pre-extraction seed treatment is one of the most essential steps to ensure high quality extraction. One of the treatment methods is the use of a pulsed electric field. It is an important non-thermal method of treating foodstuffs by placing them in a chamber between two electrodes and subjecting to high-voltage pulses for a short time. A pulsed electric field focuses mainly on the microscopic scale so that pores are created in the cell membrane, accelerating the exit of intercellular compounds. This process preserves qualitative, nutritional, and energy consumption properties, as well as increases productivity in food production [4].
Most importantly, a pulsed electric field destroys the cell wall and its membrane and increases the mass transfer rate. Indeed, when a living cell is affected by such a field, the cell wall and its membrane are naturally damaged. The inside material is easily removed and the surrounding material enters the cell, resulting in its destruction. With increased permeability of plant and animal cells, their intracellular material is extracted more easily and quickly. Therefore, this treatment can be used as a pre-processing step in the extraction of valuable cellular materials [5, 6].
Salvia leriifolia L. is one of the plants that contain antioxidant compounds. It is a native species of Lamiaceae family to Khorasan and Semnan provinces, Iran [7]. It grows in cold and semi-arid or arid regions at altitudes between 900 and 1650 meters, with an average rainfall of 80 mm. A special shape of its leathery leaves, especially white villi on both sides, and a wide growth on the surface of the soil make this plant resistant to harsh winter winds or severe heat [8].
Various studies have reported therapeutic properties of Salvia leriifolia. For example, its aqueous and alcoholic root extracts have neuroprotective properties against topical anemia in the rat brain [9]. The analgesic and sedative activity of Salvia leriifolia leaf extract in the amount of 500 mg/kg is comparable to that of diazepam in the amount of 5 mg/kg [8]. In treating chronic inflammation, the plant’s extract is similar to diclofenac [10]. Its aqueous and alcoholic leaf extracts were found to prevent gastric ulcers in rats similarly to Sucralfate [11].
In addition, the plant’s root and leaf extracts showed considerable antimicrobial activity [9]. They also have strong antioxidant properties that prevent the oxidation of oils. This property is competitive with that of antioxidants commonly used in the food industry, such as butylated hydroxy toluene and alphatocopherol. It is due to the presence of a secondary metabolite of chalcones, called butin, in this plant. Finally, Salvia leriifolia is of industrial importance. In this regard, researchers have found that its seeds contain 26% yellow oil, with a very low peroxide index and a high antioxidant index, which increases its shelf life compared to other oils [12].
Another plant with antioxidant properties is Linum usitalissmum L. It is a one-year-old plant of Linaceae family that grows in bushes. This plant has over 200 species but only Linum usitalissmum has economic importance. In addition, its seeds have several powerful antioxidants, including lignans. 100 g of Linum usitalissmum contains about 9.2 mg of vitamin E, mainly in the form of gamatocopherol [13].
The most common method for extracting compounds from plant tissues uses aqueous and ethanol solvents. Therefore, we aimed to evaluate effects of an electrical pulse pre-treatment and to compare the aqueous and alcoholic extracts of Linum usitalissmum and Salvia leriifolia seeds.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Preparation of raw materials. For this study, Linum usitalissmum L. seeds and Salvia leriifolia L. aerial limbs, leaves, and stems were obtained from a certified apothecary. We also used chemicals produced by Merck (Germany).
Extraction of aqueous and alcohol extracts from Linum usitalissmum and Salvia leriifolia seeds pretreated with a pulsed electric field. Initially, Linum usitalissmum and Salvia leriifolia seeds were cleaned and the external materials and impurities were separated and dried in an oven at 45°C. The samples were powdered in a household mill (Fama Model Cs, Germany) and passed through a 40-mesh sieve. Finally, they were packed in air- and water-proof packages and kept in a freezer at –18°C until further experiments to preserve the extract’s antioxidant and functional properties.
The aqueous and alcoholic extracts were made using the Kabiri and Seyyedlangi method [14, 15]. For this, the prepared powders were mixed with a water solvent (aqueous extract) or 80% methanol (alcoholic extract) at the ratio of 50:1.
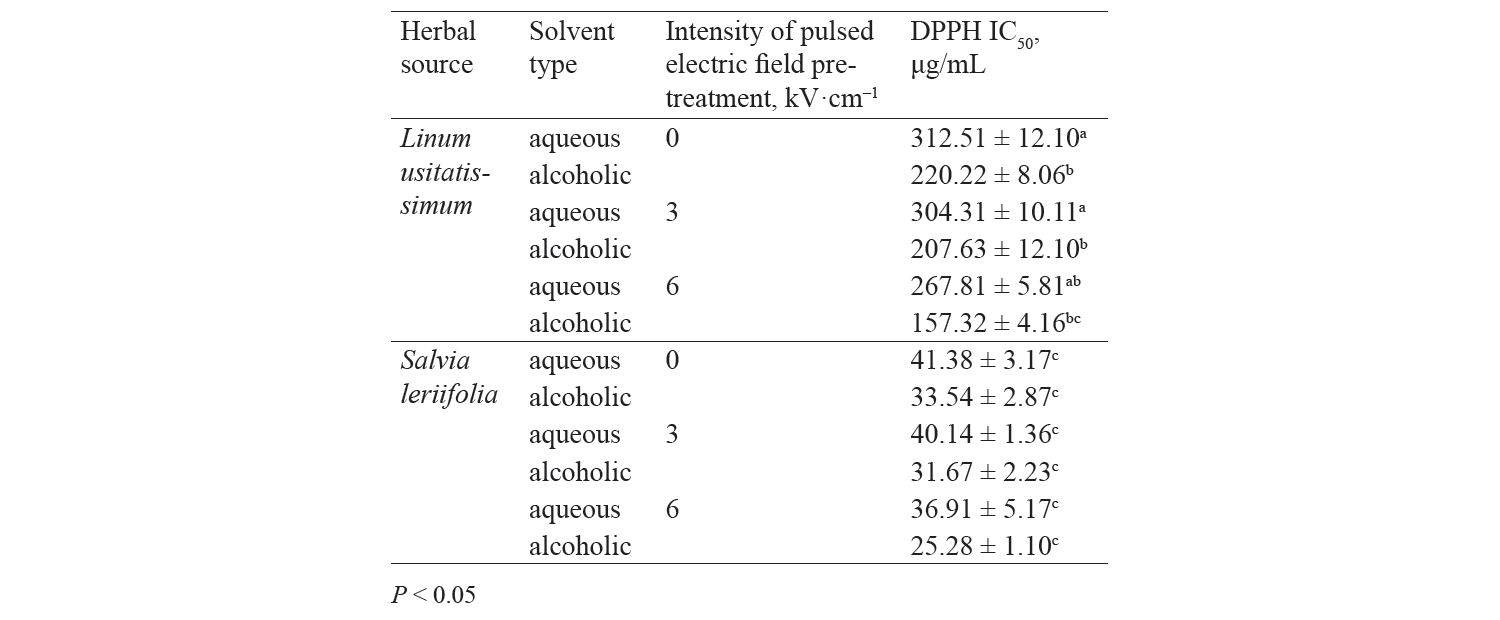
Subsequently, to apply a pulsed electric field pretreatment, each of the extracts was subjected to an alternating electric field with zero (without pretreatment), 3, and 6 kV·cm–1 intensity and a constant pulse number of 30 (Table 1). The linear electric current in this device is transmitted to a series of capacitors and the energy stored in the capacitors is discharged to the chamber containing two electrodes with a pulse switch. The discharge chamber is made of Plexiglass 1 and the distance between the two electrodes is 4 cm. These waves were applied to facilitate the extraction.
Evaluation of antioxidant properties of Linum usitalissmum and Salvia leriifolia aqueous and alcoholic extracts. Total phenolic compounds. The amount of total phenolic compounds was measured by the Folin-Ciocalteu method according to Oardoz et al. [16]. For this purpose, 10 g of extracts was first extracted with 200 mL of methanol for 24 h at room temperature using a magnetic stirrer. The extract was filtered with Whatman Paper No. 1 and the sediment was extracted again under the same conditions. The solvent was then removed by a vacuum evaporator at less than 40°C and concentrated as far as possible. Then, 0.5 mL of the extract was mixed with 2.5 mL of 0.2N Folin-Ciocalteu reagent and 2 mL of 7.5% sodium carbonate solution. The mixture was kept at room temperature for 120 min. The absorbance rate of the solution was then read by a spectrophotometer at 760 nm. The total content of phenolic compounds was expressed in mg/g of extract using the line equation drawn on the basis of gallic acid. The calibration curve was plotted as follows.
Different concentrations of gallic acid were first prepared and 0.5 mL of each was mixed with 2.5 mL of 10% Folin-Ciocalteu reagent (v/v) and 2 mL of 7.5% sodium carbonate for half to 8 min (w/v). The samples were stored at room temperature for 30 minutes and then absorbed at 760 nm [17]. Distilled water was used as a control.
Antioxidant activity by DPPH method. To extract antioxidant compounds, 10 g of aqueous and alcoholic extracts with 100 mL methanol was stirred with a magnetic stirrer at a speed of 100 rpm at 25°C for 24 h and finally filtered with Whatman filter paper. The solution was then transferred to a freezing dryer for methanol removal and, finally, the dried extract was stored at –20°C [18]. The antioxidant activity of the samples was further evaluated by the method of Brand Williams et al. [19]. 3.9 mL of DPPH stock was poured into the cell and read by a spectrophotometer at 515 nm. Then, 0.1 mL of each extract was added to the DPPH stock solution and after 90 minutes of incubation, the absorbance of the samples was read at 515 nm. The inhibition percentage of DPPH radical was calculated using Eqs. (1) and (2).
where A0 is control absorption and As is sample absorption.
The results were then expressed as IC50 (the amount of antioxidant required to reach 50% of the initial DPPH concentration). To draw a standard curve, we used a Trolox solution with a concentration of 1000–100 μmol. First, the percentage of radical neutralization activity was obtained for each sample. Then, we calculated the antioxidant activity of the samples using a standard curve in μmol of Trolox per gram dry weight (μmol/g).
Antioxidant activity by TEAC method. To extract antioxidant compounds, 10 g of the milled sample with 100 mL of methanol was mixed with a magnetic stirrer at 100 rpm and 25°C for 24 h and then filtered with a Whatman filter. Then, the methanol was transferred to a freezing dryer and, finally, the dried extract was stored at –20°C [18]. The antioxidant activity of the samples was further evaluated by the method of Yu et al. [20].
First, we made an aqueous solution of ABTS at a concentration of 1 mM to prepare the radical ABTS. Potassium persulfate was then added to this solution to reach a final concentration of 2.45 mM. The resulting solution was incubated at room temperature and darkness for 2 h. During this time, the ABTS molecule produced the ABTS•+ cation radical. Then, 4 μL of the samples was taken with a Peptide and mixed with 4 mL of the ABTS•+ solution in the cell. Its absorption at a 734 nm wavelength was verified at 6 min after mixing (for 30 s). A standard curve was plotted, corresponding to the reaction of 40 μL of Trolox (at concentrations of 50, 100, 250, 500, 750, and 1000 μM) to 4 mL of the ABTS•+ solution. The inhibition percentage of ABTS•+ of the samples was calculated according to Eq. (2). Also, the ABTS•+ radical inhibition activity was expressed based on the standard Trolox curve as the Trolox solution equivalent antioxidant capacity (mM TEAC).
Statistical design and analysis of results. The results of our study were evaluated with SPSS 16 software.
To extract the essential oil, we used a completely randomized design with a three-factor arrangement. In particular, the three factors were a plant source (Salvia leriifolia and Linum usitalissmum), a type of pretreatment (pulsed electric field at the intensity of zero (no pretreatment), 3 and 6 kV·cm–1), and a type of solvent (aqueous and alcoholic).
The samples were obtained in three replications and the means were compared by the Duncan test at a significant level of 5% (P < 0.05). Finally, Excel software was used to plot the diagrams.
where Ablank is the absorption of a control sample without the active compound and Asample is the absorption of a sample containing a distilled extract).
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
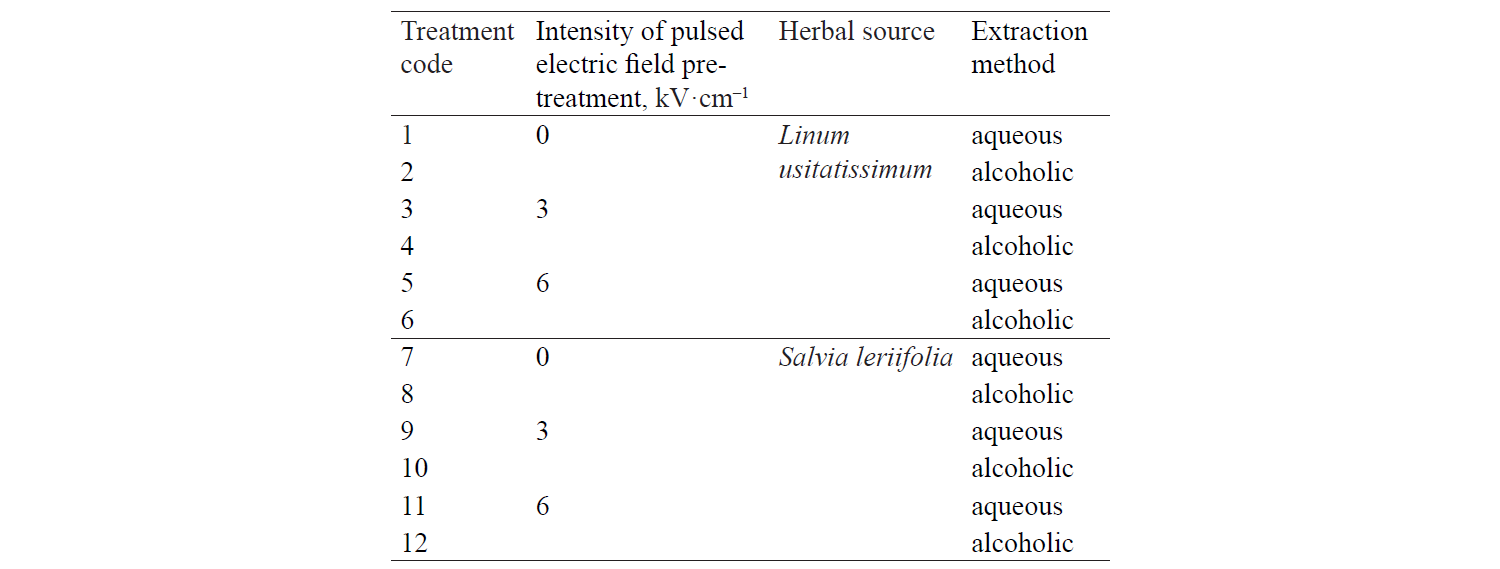
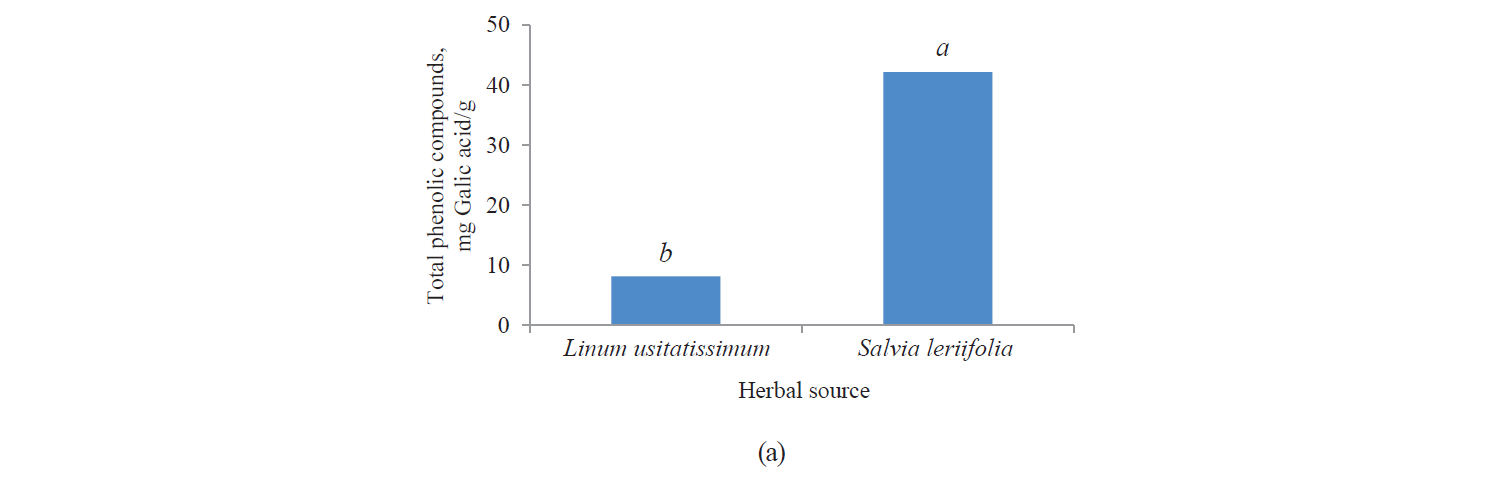
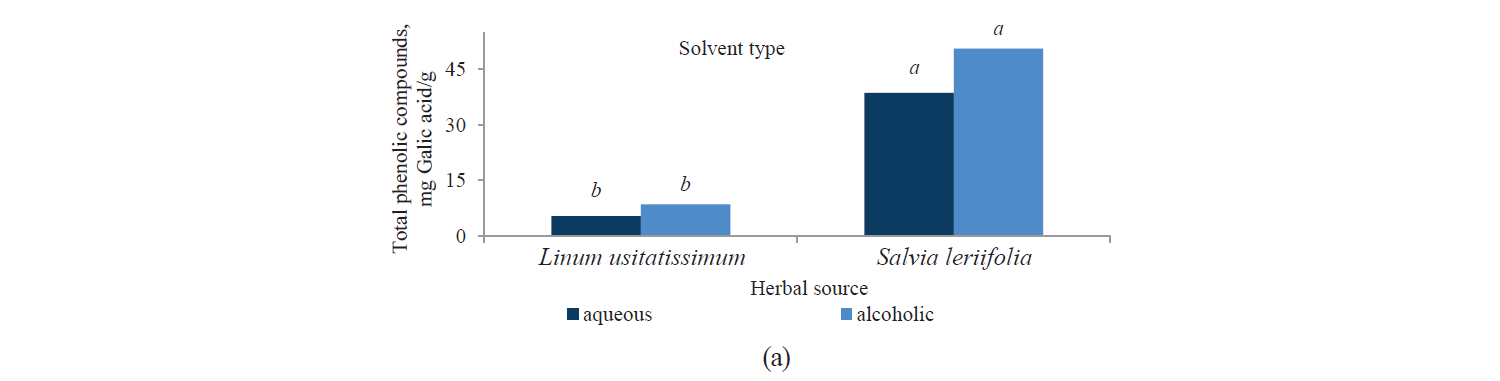
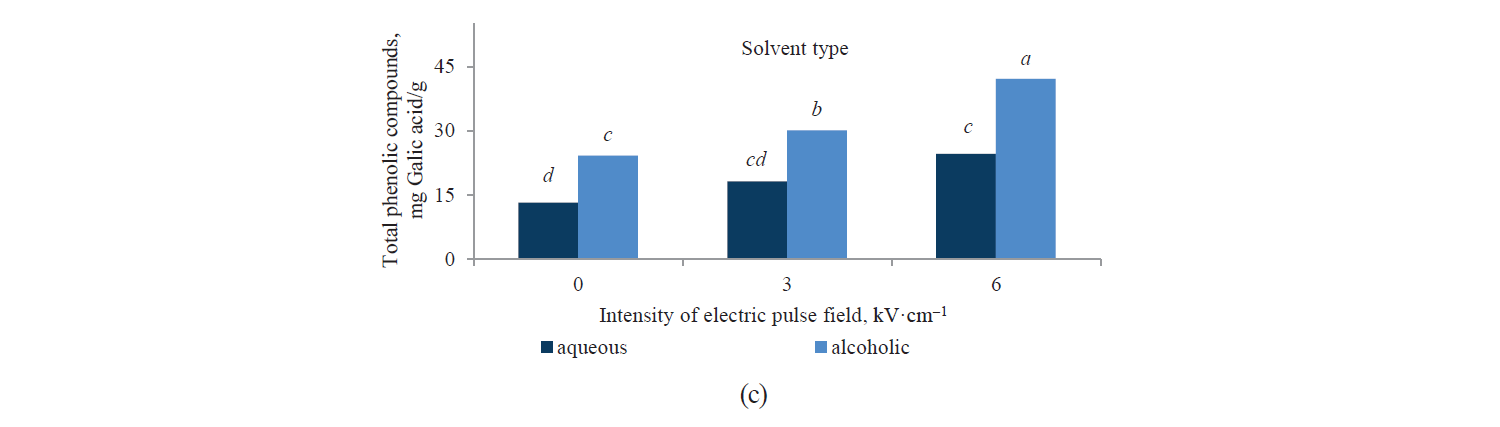
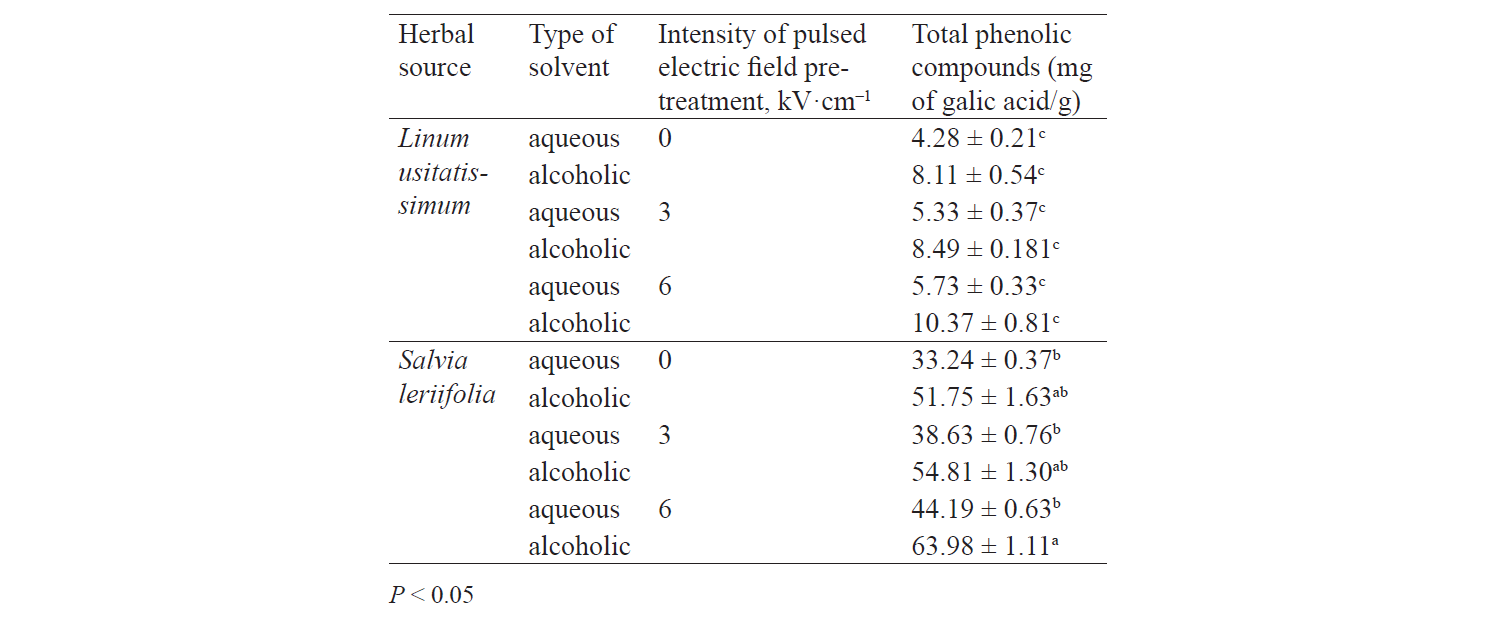
Total phenolic compounds. Fig. 1 presents independent effects of the factors, Fig. 2 shows their binary effect, while Table 3 indicates the interaction between the three factors in their effect on the content of phenolic compounds in the extracts. We found that a higher intensity of a pulsed electric field and the use of an alcoholic solvent significantly increased total phenolic compounds at a 95% confidence level. On the other hand, the content of total phenolic compounds was higher in the Salvia leriifolia L. extract, compared to Linum usitalissmum L. (P < 0.05).
Various factors, such as plant variety, harvest area, and harvest time, appear to affect the content of phenolic compounds. Different studies have found different amounts of total phenolic compounds in the Salvia leriifolia plant. For example, Hamrouni-Sellami et al., Ahmadi et al., Najafi et al., Abadi et al., and Bahadori et al. reported total phenolic compounds of 0.399–2.37, 40.47–61.32, 11.28–23.88, 12.68–83.85, and 17.3–294.9 mg of gallic acid per gram of extract, respectively [21–25]. In our study, this value reached 33.24–63.98 mg of gallic acid per gram of extract, depending on the solvent type and the use of a pulsed electric field pretreatment.
Total phenolic compounds in Linum usitalissmum oilseed have been reported by Oomah et al., Brodowska et al., and Russo and Reggiani at 8–10 mg of caffeic acid per gram of extract), 0.988 mg of catechin per gram of extract, and 4.64–9.40 mg caffeic acid per gram of extract, respectively [26–28]. In our study, their amount ranged from 4.28 to 10.37 mg gallic acid per gram of extract, depending on the solvent type and the use of a pulsed electric field pretreatment.
The studies showed that the amount of extracted phenolic compounds increased with a higher intensity of a pulsed electric field, reaching their maximum at a 6 kV·cm–1 pre-treatment. Schroeder et al. attributed this to the electrical degradation of cells and their increased permeability due to the use of a pulsed electric field [29]. In this regard, Bozinou et al. investigated the extraction of phenolic compounds and antioxidant activity of dried oak leaves with a 7 kV·cm–1 pulse electric field pretreatment [30]. They stated that the highest amount of phenolic compounds was obtained with a pulse time of 20 ms, a pulsing duration of 40 min, and a pulse interval of 100 ms.
Liu et al. examined the enhancement of extracted phenolic compounds in onion pre-treated with a pulsed electric field [31]. They stated that the optimum conditions for this purpose were a pulsed electric field of 2.5 kV, 90 pulses, and a temperature of 45°C. In these conditions, the amounts of extracted phenolic and flavonoid compounds were 86.82 mg of gallic acid per 100 g and 37.58 mg of quencherine per 100 g, respectively. These values were 2.2 times and 2.7 times as high as those in the control samples, respectively.
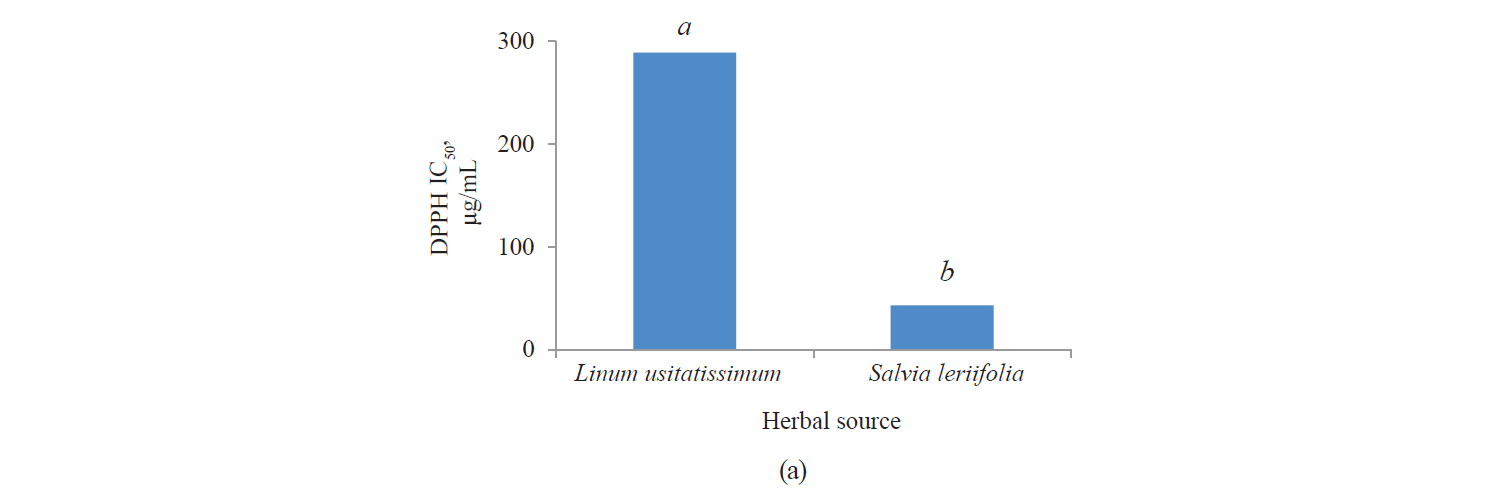
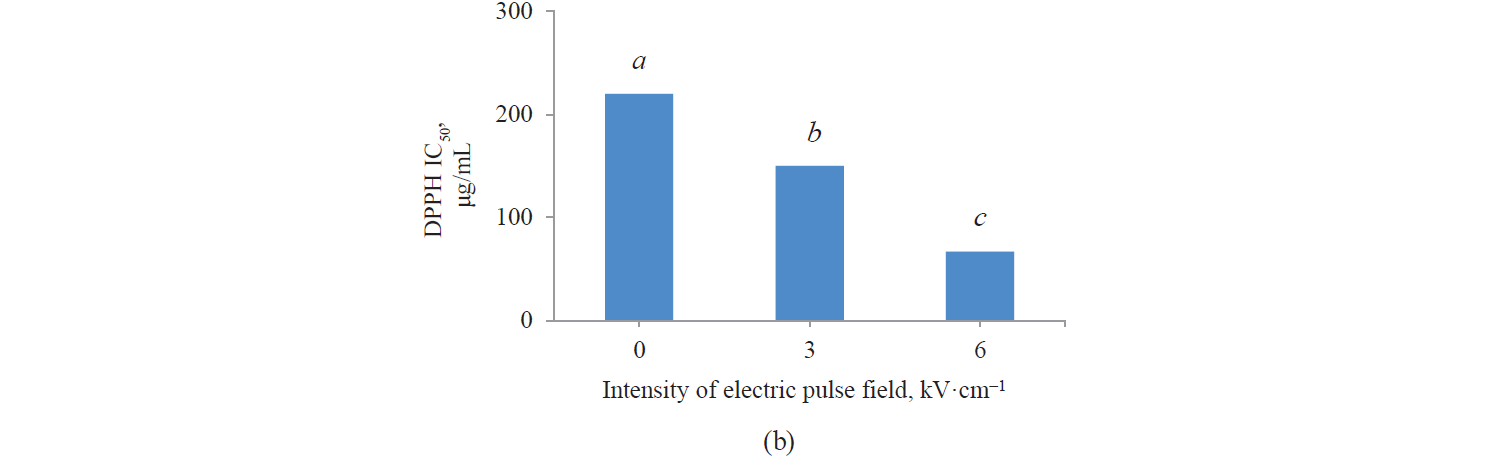
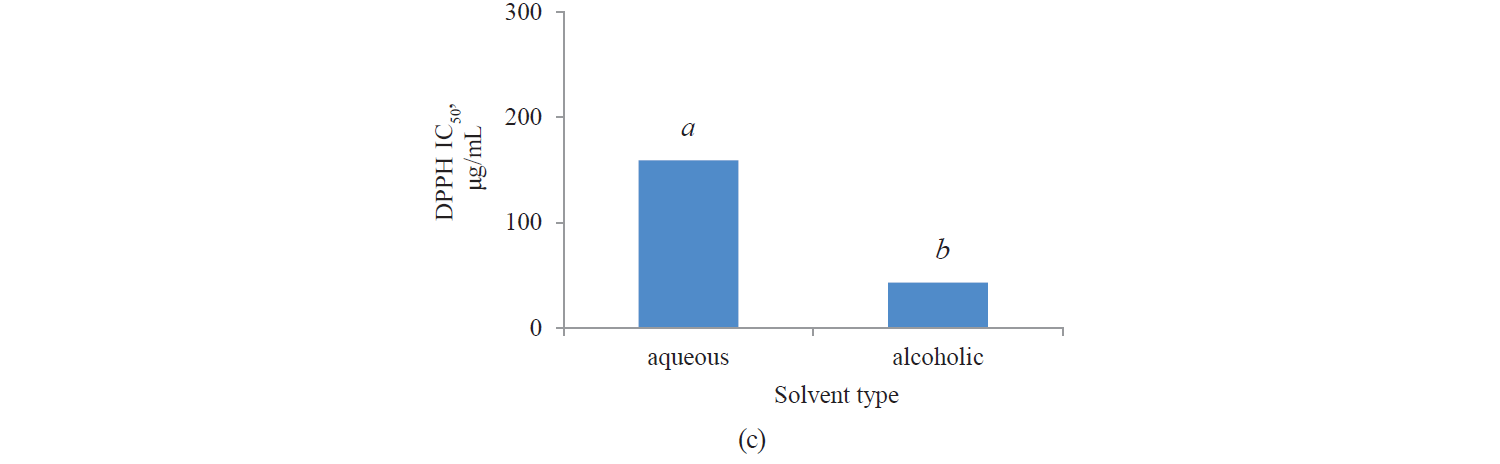
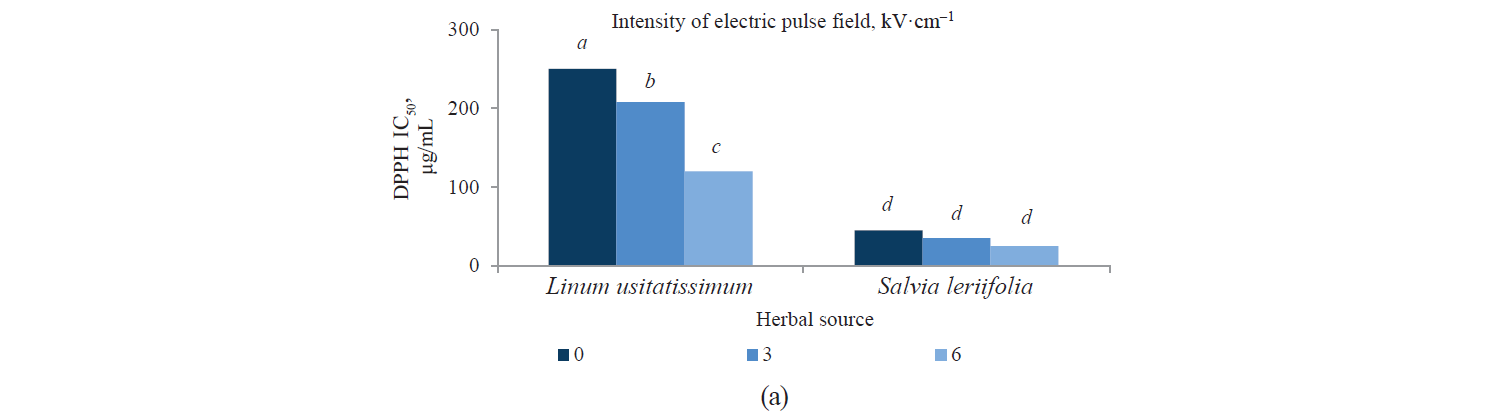
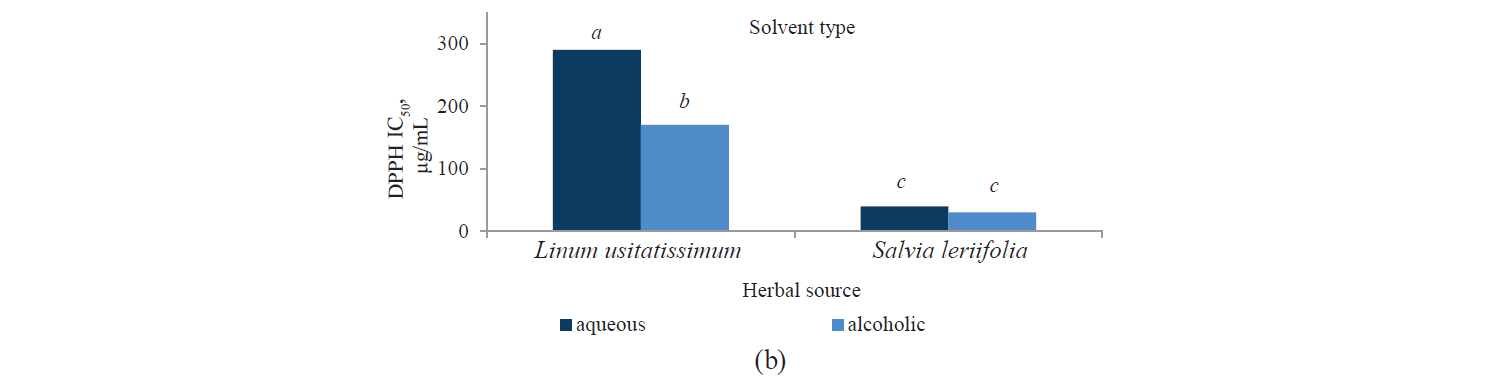
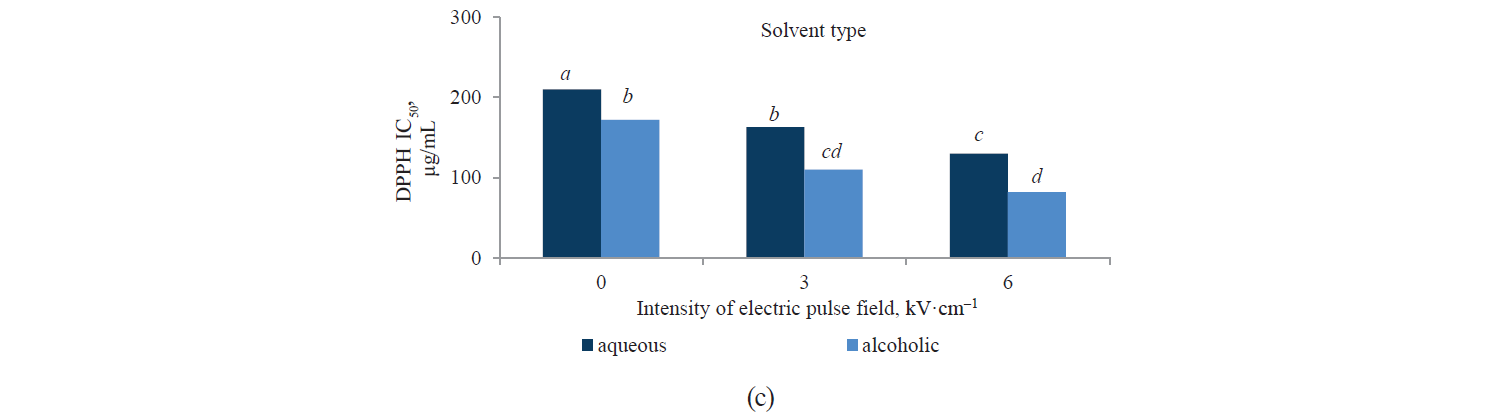
Antioxidant activity by DPPH method. Fig. 3 presents independent effects of the agents, Fig. 4 shows their binary effects, and Table 3 indicates their interaction in relation to the antioxidant activity of the extracts derived with the DPPH method. As we can see, their antioxidant activity significantly increased, at a 95% confidence level, with a higher intensity of a pulsed electric field and the use of an alcoholic solvent. At the same time, we found that the antioxidant activity of Salvia leriifolia extracts was higher than that of Linum usitalissmum (P < 0.05).
The content of phenolic compounds is not an accurate measure of antioxidant activity. Since the Folin-Ciocalteu reagent nonspecifically reacts with phenolic and other compounds, such as organic acids, sugars are also able to reduce this reagent. Therefore, it is also necessary to measure antioxidant activity in other ways, for example, by the DPPH method, which we used in our study [32].
Phenolic compounds donated hydrogen or electron to the groups exposed to oxidation [33]. Thus, the content of phenolic compounds can be used as an important indicator of antioxidant activity. As noted above, various factors, such as plant variety, harvest area, and harvest time, appear to affect the amount of phenolic compounds and, subsequently, antioxidant activity.
In our study, the antioxidant activity of extracts (IC50) from Salvia leriifolia plant extracted by the DPPH method ranged between 25.28 and 41.38 μg/mL, depending on the type of solvent and the intensity of a pulsed electric field.
We also investigated various sources of antioxidant activity (IC50) in Linum usitalissmum. This value was reported by Brodowska et al. and Alachaher et al. to reach 299.00 and 220.05 μg/mL of extract, respectively [27, 34]. In our study, the amount of total phenolic compounds extracted from Linum usitalissmum varied from 157.37 to 312.51 μg/mL, depending on the solvent type and the use of a pulsed electric field pretreatment.
On the other hand, we found that the extracts’ antioxidant activity increased with a higher pulsed electric field intensity. The highest values were observed in the samples with a 6 kV·cm–1 pretreatment. This was quite predictable from the measurement of total phenolic compounds, whose content also increased with a higher intensity of the applied electric field. In this regard, Bozinou et al. investigated the extraction of phenolic compounds and antioxidant activity of dried oak leaves with a 7 kV·cm–1 pulse electric field pretreatment [30]. They stated that the antioxidant activity was proportional to the content of total phenolic compounds: the higher the amount of phenolic compounds, the higher the antioxidant activity. In their study, phenolic compounds were highest with a pulse time of 20 ms, a pulsing duration of 40 min, and a pulse interval of 100 ms. Under these conditions, the sample’s antioxidant activity was maximum.
Liu et al. studied the enhancement of extracted phenolic compounds in onion subjected to a pulsed electric field [31]. They stated that the optimum conditions for this purpose were a pulsed electric field of 2.5 kV, 90 pulses, and a temperature of 45°C. The researchers also found that the extract’s antioxidant activity increased with a higher pulse electric field intensity and a larger number of pulses applied. Their finding also proved the correlation between the antioxidant activity and the amount of phenolic compounds.
Lopez Giral et al. also investigated a pulsed electric field pretreatment to improve the extraction of phenolic compounds from three different grape varieties (Graciano, Tempranillo, and Grenache) during two production periods [35]. The pretreatment conditions included a pulsed electric field of 7.4 kV·cm–1, a pulse width of 20 ms, and a frequency of 400 Hz. They stated that using a pulsed electric field increased the color intensity, total phenol index, anthocyanin index, and total antioxidant power. These researchers therefore introduced a pulsed electric field pretreatment as a suitable technology for extracting phenolic compounds. However, they acknowledged that the ability of the method depended on the type of grape and the initial amount of phenolic compounds.
Similarly, Minussi et al. demonstrated a positive relationship between antioxidant power and the content of total polyphenolic compounds in grape juice, particularly compounds such as gallic acid, catechin, and epi-catechin [36].
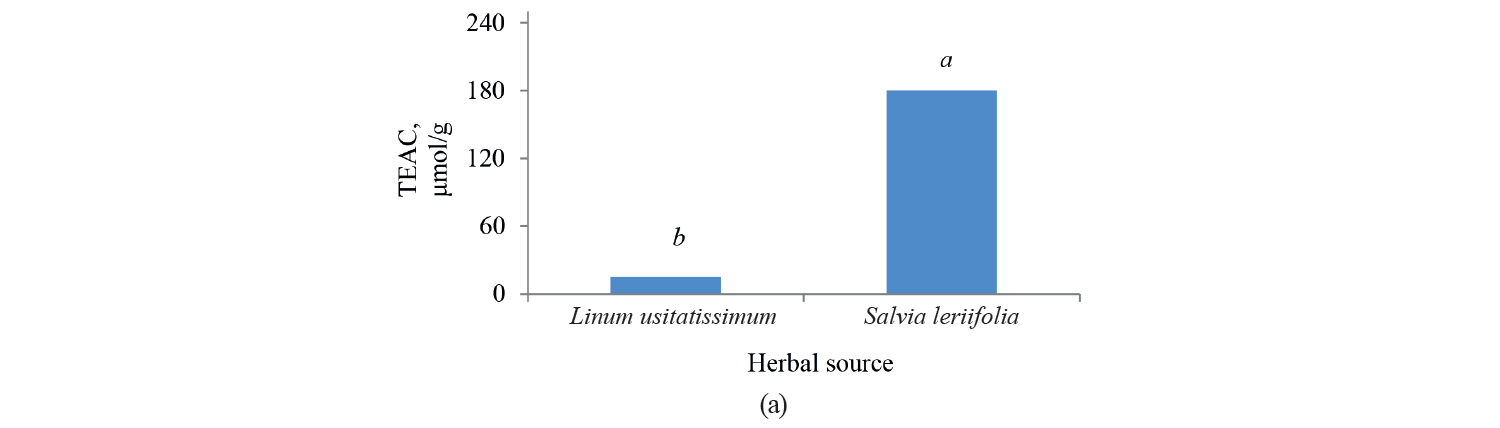
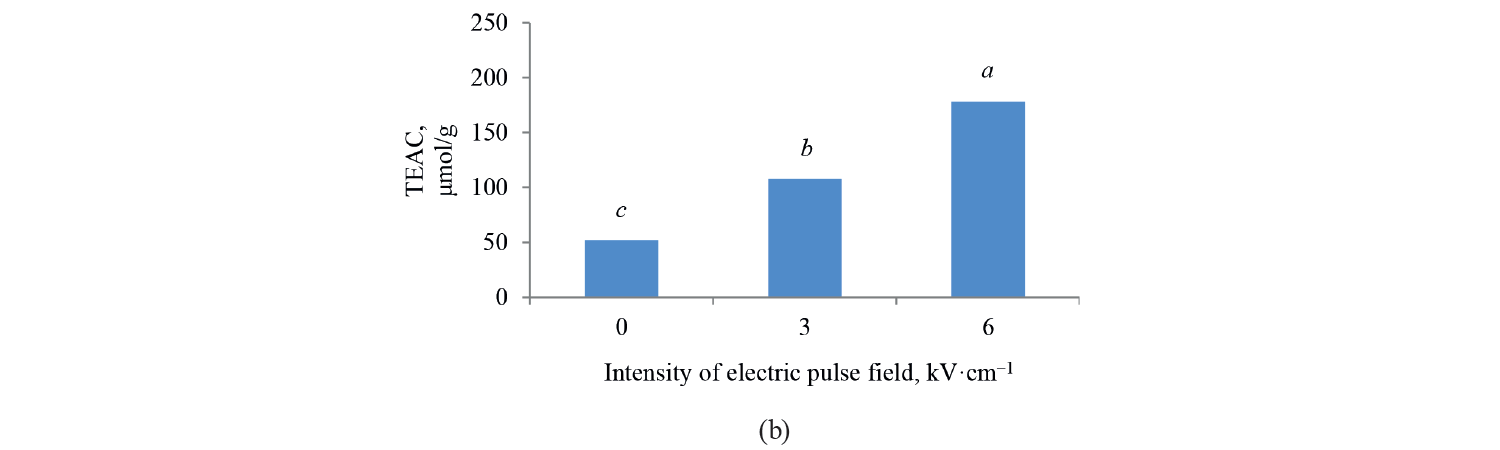
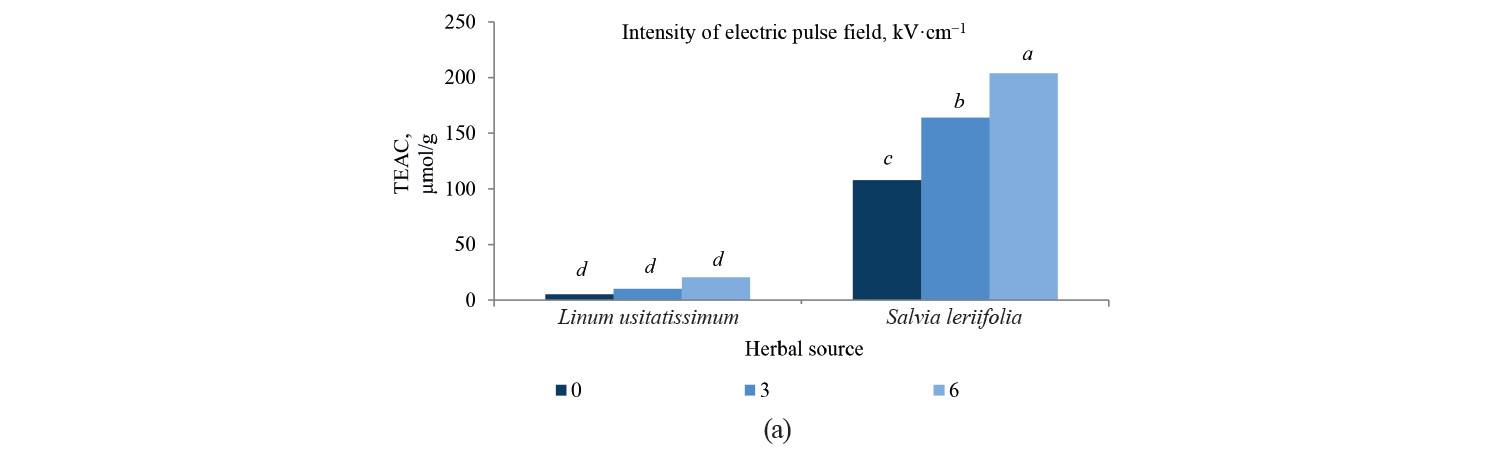
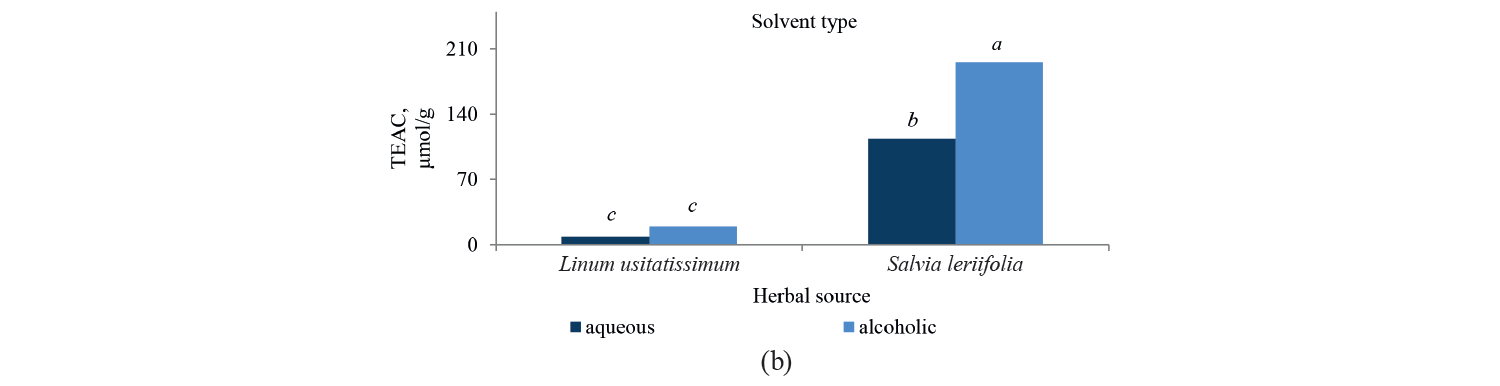
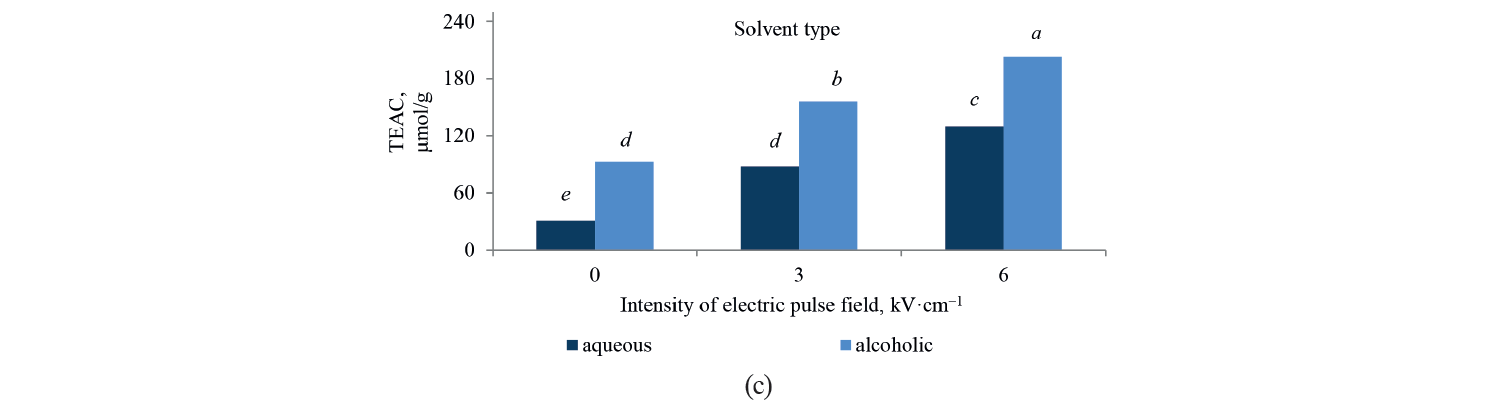
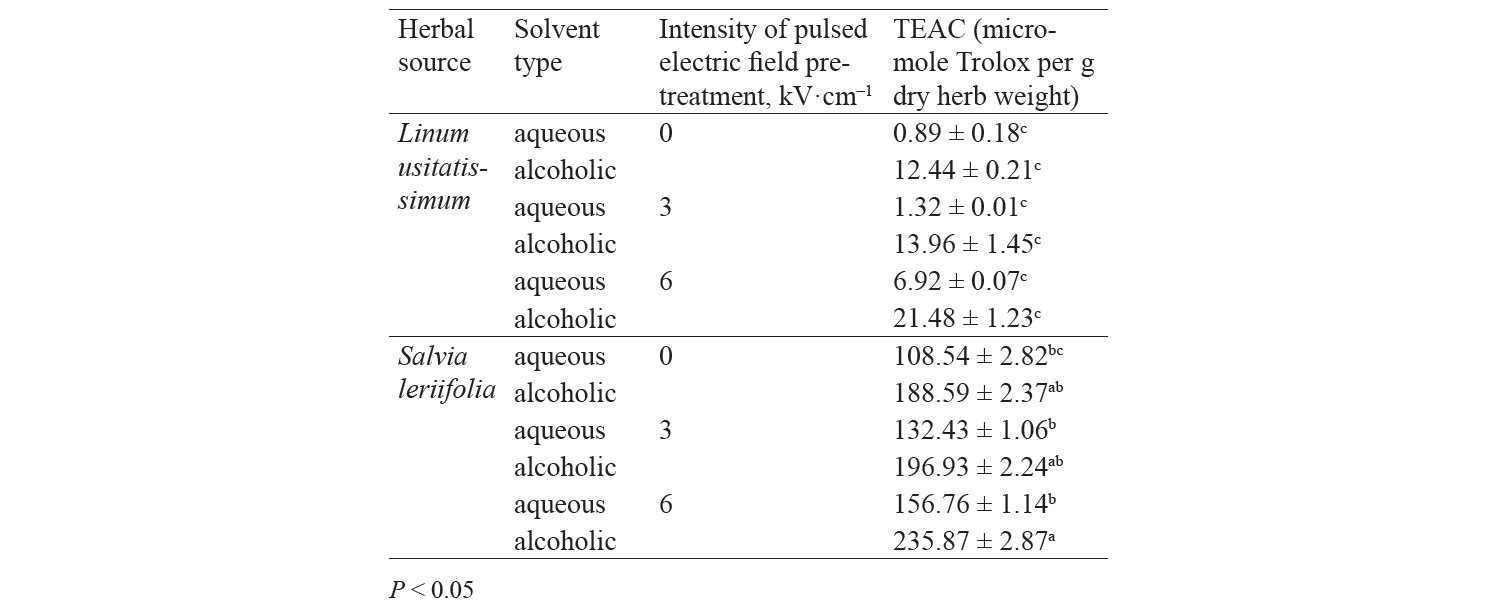
Antioxidant activity by TEAC method. Independent, binary, and combined effects of the agents on the antioxidant activity of the extracts extracted with the Trolox method are presented in Fig. 5, Fig. 6, and Table 4. As observed, a higher intensity of a pulsed electric field pretreatment and the use of an alcoholic solvent significantly increased the TEAC number of the extracts at a 95% confidence level. On the other hand, the number of TEAC extracts of Salvia leriifolia was higher than that of Linum usitalissmum (P < 0.05).
As noted earlier, the amount of phenolic compounds alone is not a precise measure for antioxidant activity. Since the Folin-Ciocalteu reagent nonspecifically reacts with phenolic and other compounds such as organic acids, sugars also can reduce this reagent. Therefore, it is also necessary to measure antioxidant activity with other methods [32]. Therefore, we used the Trolox Equivalent Antioxidant Capacity (TEAC) method to measure antioxidant activity.
There was a significant correlation between the values of antioxidant activity measured by the DPPH method and the TEAC method (P < 0.01 and r = 0.932). The Trolox equivalent antioxidant capacity test and diphenyl picryl hydrazyl are both synthetic free radicals with similar application. However, the Trolox equivalent antioxidant potential can be used to measure antioxidant activity of polar and nonpolar compounds [37].
The ABTS•+ cation radical is more active than the DPPH radical and is therefore widely used in the measurement of antioxidant activity. In this test, ABTS oxidation first occurred following the reaction with potassium persulfate. The ABTS•+ cation radical subsequently reacted with antioxidants or other hydrogen donating radicals and transformed in a reduced form [37]. Consequently, the antioxidant inhibition percentage can be measured by determining the absorption reduction rate. The radical inhibition activity in this test was reported based on the Trolox equivalent antioxidant capacity.
As noted above, various factors (plant variety, harvest area and time) appear to affect the amount of phenolic compounds and, subsequently, antioxidant activity.
We found that the antioxidant activity of Salvia leriifolia extracts measured with the TEAC method ranged between 108.54 and 235.87 μmol of Trolox per g dry plant weight, depending on the type of solvent and the intensity of pulsed electric field pretreatment.
Some studies evaluated the antioxidant activity of Linum usitalissmum with the TEAC method. Russo and Ragiani and Deng et al. reported the value of 560–860 (for oilseed Linum usitalissmum) and 22 000 μmol Trolox/g dry weight, respectively [28, 28]. In our study, the amount of total phenolic compounds extracted from Linum usitalissmum ranged from 0.89 to 21.48 μmol Trolox/g dry weight, depending on the solvent type and the intensity of pulsed electric field pretreatment.
On the other hand, the antioxidant activity of the extracts increased with a higher pulsed electric field intensity. A pre-treatment of 6 kV·cm–1 provided the highest amount of compounds. This result was predictable from the measurement of total phenolic compounds, which also increased with a higher intensity of the applied electric field. In this regard, Bozinou et al. investigated the extraction of phenolic compounds and antioxidant activity of dried oak leaves by using a pulsed electric field pretreatment at 7 kV·cm–1 [30]. They stated that the level of antioxidant activity was proportional to the amount of total phenolic compounds, so a higher content of phenolic compounds increased the antioxidant activity. In their study, the highest amount of phenolic compounds was associated with a pulse time of 20 ms, pulse duration of 40 min, and pulse interval of 100 ms. Under these conditions, the level of antioxidant activity was also maximum.
ВЫВОДЫ
Our study aimed to compare the antioxidant activity of aqueous and alcoholic extracts derived from Salvia leriifolia L. and Linum usitalissmum L. subjected to a pulsed electric field at the intensities of zero (without pre-treatment), 3 and 6 kV·cm–1 with a constant pulse of 30. We investigated such parameters as total phenolic compounds and antioxidant activity. According to our results, the Salvia leriifolia extract had more phenolic compounds and higher antioxidant activity than the Linum usitalissmum extract under the same conditions.
On the other hand, a pulsed electric field pretreatment and the use of an alcoholic solvent (methanol) for extraction increased the content of phenolic compounds and the extract’s antioxidant activity. In fact, the solubility of phenolic compounds depended on the type of solvent and their interaction. Finally, the extract derived from Salvia leriifolia with an alcoholic solvent and a pulsed electric field pretreatment (at 6 kV·cm–1 with 30 pulses) was selected as possessing desirable antioxidant properties.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare that there is no conflict of interests regarding the publication of this article.
СПИСОК ЛИТЕРАТУРЫ
- Angelo AJ, Vercelotti J, Jacks T, Legendre M. Lipid oxidation in foods. Critical Reviews in Food Science and Nutrition. 1996;36(3):175–224. DOI: https://doi.org/10.1080/10408399609527723.
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stressinduced cancer. Chemico-Biological Interactions. 2006;160(1):1–40. DOI: https://doi.org/10.1016/j.cbi.2005.12.009.
- Gao J-J, Igalashi K, Nukina M. Radical scavenging activity of phenylpropanoid glycosides in Caryopteris incana. Bioscience Biotechnology and Biochemistry. 1999;63(6):983–988. DOI: https://doi.org/10.1271/bbb.63.983.
- Asavasanti S, Ristenpart W, Stroeve P, Barrett DM. Permeabilization of plant tissue by monopolar pulsed electric field: effect of frequency. Journal of Food Science. 2011;76(1):E98–E111. DOI: https://doi.org/10.1111/j.1750-3841.2010.01940.x.
- Sale AJH, Hamilton WA. Effects of high electric fields on microorganisms: I. killing of bacteria and yeast. BBA – General Subjects. 1967;148(3):781–788. DOI: https://doi.org/10.1016/0304-4165(67)90052-9.
- Hui SW. Effect of pulse length and strength on electroporation efficiency. Method in molecular biology. 1995. 48:29–40. DOI: https://doi.org/10.1385/0-89603-328-7:29.
- Abdel-Samie MAS, Wan JJ, Huang WN, Chung OK, Xu BC. Effects of cumin and ginger as antioxidants on dough mixing properties and cookie quality. Cereal Chemistry. 2010;87(5):454–460. DOI: https://doi.org/10.1094/CCHEM-01-10-0012.
- Haralick RM, Dinstein I, Shanmugam K. Textural features for image classification. IEEE Transactions on Systems, Man and Cybernetics. 1973;3(6):610–621. DOI: https://doi.org/10.1109/TSMC.1973.4309314.
- Sadeghnia HR, Nassiri Asl M, Haddad Khodaparast MH, Hosseinzadeh H. The effect of Salvia leriifolia Benth root extracts on lipid peroxidation during global ischemic-reperfusion in rats. Journal of Medicinal Plants. 2003;3(7):19–28.
- Hosseinzadeh H, Yavary M. Anti-inflammatory effect of Salvia leriifolia Benth. leaf extract in mice and rat. Pharmaceutical and Pharmacological Letters. 1999;9(2):60–61.
- Hosseinzadeh H, Lary P. Effect of Salvia leriifolia leaf extracts on morphine dependence in mice. Phytotherapy Research. 2000;14(5):384–387. DOI: https://doi.org/10.1002/1099-1573(200008)14:5<384::AID- TR641>3.0.CO;2-F.
- Hadad Khodaparast MH, Haghdoost A, Elhami-Rad AH, Movahhed G, Karazhiyan H. Antioxidant activity and thermal Properties of Salvia leriifolia (Norozak) root extract. Proceedings of the international conference on Innovations in Food and Bioprocess Technologies; 2006; Pathumthani. Pathumthani: AIT; 2006. p. 378.
- Martinchik AN, Baturin AK, Zubtsov VV, Molofeev VY. Nutritional value and functional properties of flaxseed. Problems of Nutrition. 2012l;81(3):4–10. (In Russ.).
- Kabiri S, Sayyed-Alangi SZ. Comparison of antioxidant effect of different extracts from Melissa officinalis leaves with immersion and microwave-assisted extractions and its oxidative stability on soybean oil. Journal of Innovative Food Technologies. 2015;2(4):23–38. DOI: https://doi.org/10.22104/jift.2015.201.
- Farhoosh R, Purazrang H, Khodaparast MHH, Rahimizadeh M, Seyedi SM. Extraction and separation of antioxidative compounds from Salvia leriifolia leaves. Journal of Agricultural Science and Technology. 2004;6:57–62.
- Ordoeez AAL, Gomez JD, Vattuone MA, Isla MI. Antioxidant activities of Sechium edule (Jacq) Swartz extracts. Food Chemistry. 2006;97(3):452–458. DOI: https://doi.org/10.1016/j.foodchem.2005.05.024.
- Shahidi F, Naczk M. Phenolic in food and nutraceuticals. CRC press; 2004. 558 p.
- Pag AI, Radu DG, Draganescu D, Popa MI, Sirghie C. Flaxseed cake – a sustainable source of antioxidant and antibacterial extracts. Cellulose Chemistry and Technology. 2014;48(3–4):265–273.
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate Antioxidant activity. Food Science and Technology. 1995;28(1):25–30.
- You LJ, Zhao M, Regenstein JM, Ren JY. Changes in the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates during a simulated gastrointestinal digestion. Food Chemistry. 2010;120(3):810–816. DOI: https://doi.org/10.1016/j.foodchem.2009.11.018.
- Hamrouni-Sellami I, Rahali FZ, Rebey IB, Bourgou S, Limam F, Marzouk B. Total phenolics, flavonoids, and antioxidant activity of sage (Salvia officinalis L.) plants as affected by different drying methods. Food and Bioprocess Technology. 2013;6(3):806–817. DOI: https://doi.org/10.1007/s11947-012-0877-7.
- Ahmadi F, Sabzalian MR, Mirlohi A. Impacts of planting dates on essential oil, phenolic compounds and some morphological traits in Nuruozak. 3rd national congress on medicinal plants; 2014; Mashhad. Mashhad: National network of research and technology of medicinal plants; 2014. p. 478.
- Najafi S, Mir N, Shafeghat M. Antioxidant and antibacterial activities of six medicinally important species of the genus Salvia from north east of Iran. Journal of Genetic Resources. 2016;2(1):41–47. DOI: https://doi.org/10.22080/JGR.2016.1479.
- Abadi ZHM, Mahdavi B, Rezaei-Seresht E. Contents of aerial parts of Salvia leriifolia benth. Journal of Chemical Health Risks. 2016;6(3):185–194. DOI: https://doi.org/10.22034/JCHR.2016.544146.
- Bahadori MB, Asghari B, Dinparast L, Zengin G, Sarikurkcu C, Abbas-Mohammadi M, et al. Salvia nemorosa L.: A novel source of bioactive agents with functional connections. LWT – Food Science and Technology. 2017;75: 42–50. DOI: https://doi.org/10.1016/j.lwt.2016.08.048.
- Oomah BD, Kenaschuk EO, Mazza G. Phenolic acids in flaxseed. Journal of Agricultural and Food Chemistry. 1995;43(8):2016–2019. DOI: https://doi.org/10.1021/jf00056a011.
- Brodowska K, Catthoor R, Brodowska AJ, Symonowicz M, Łodyga-Chruścińska E. A comparison of antioxidant properties of extracts from defatted and non-defatted flax (Linum usitatissimum) seeds. Albanian Journal of Agricultural Science. 2014;13(2):16–23.
- Russo R, Reggiani R. Phenolics and antioxidant activity in flax varieties with different productive attitude. International Food Research Journal. 2015;22(4):1736–1739.
- Schroeder S, Buckow R, Knoerzer K. Numerical simulation of pulsed electric field (pef) processing for chamber design and optimization. 7th international conference on CFD in the minerals and process industries; 2009; Melbourne. Melbourne: CSIRO; 2009.
- Bozinou E, Karageorgou I, Batra G, Dourtoglou VG, Lalas SI. Pulsed electric field extraction and antioxidant activity determination of Moringa oleifera dry leaves: A comparative study with other extraction techniques. Beverages. 2019;5(1). DOI: https://doi.org/10.3390/beverages5010008.
- Liu ZW, Zeng XA, Ngadi M. Enhanced extraction of phenolic compounds from onion by pulsed electric field (PEF). Journal of Food Processing and Preservation. 2018;42(9). DOI: https://doi.org/10.1111/jfpp.13755.
- Mohsen SM, Ammar ASM. Total phenolic contents and antioxidant activity of corn tassel extracts. Food Chemistry. 2009;112(3):595–598. DOI: https://doi.org/10.1016/j.foodchem.2008.06.014.
- Cuvelier M-E, Richard H, Berset C. Comparison of the antioxidative activity of some acid-phenols: structure-activity relationship. Bioscience, Biotechnology and Biochemistry. 1992;56(2):324–325. DOI: https://doi.org/10.1271/bbb.56.324.
- Alachaher FZ, Dali S, Dida N, Krouf D. Comparison of phytochemical and antioxidant properties of extracts from flaxseed (Linum usitatissimum) using different solvents. International Food Research Journal. 2018;25(1):75–82.
- López-Giral N, González-Arenzana L, González-Ferrero C, López R, Santamaría P, López-Alfaro I, et al. Pulsed electric field treatment to improve the phenolic compound extraction from Graciano, Tempranillo and Grenache grape varieties during two vintages. Innovative Food Science and Emerging Technologies. 2015;28:31–39. DOI: https://doi.org/10.1016/j.ifset.2015.01.003.
- Minussi RC, Rossi M, Bologna L, Cordi L, Rotilio D, Pastore GM, et al. Phenolic compounds and total antioxidant potential of commercial wines. Food Chemistry. 2003;82(3):409–416. DOI: https://doi.org/10.1016/s0308-8146(02)00590-3.
- Arnao MB. Some methodological problems in the determination of antioxidant activity using chromogen radicals: a practical case. Trends Food Science and Technology. 2000;11(1):419–421. DOI: https://doi.org/10.1016/S0924-2244(01)00027-9.
- Deng QC, Yu X, Ma FL, Xu JQ, Huang FH, Huang QD, et al. Comparative analysis of the in-vitro antioxidant activity and bioactive compounds of flaxseed in China according to variety and geographical origin. International Journal of Food Properties. 2018;20:S2708–S2722. DOI: https://doi.org/10.1080/10942912.2017.1402029.