Аннотация
Introduction. The study offers a new rational approach to processing cottage cheese whey and using it as a highly nutritional functional ingredient in food production. We proposed a scientifically viable method for hydrolyzing cottage cheese whey with enzyme preparations of acid proteases from Aspergillus oryzae with an activity of 400 units/g and a pH range of 3.0 to 5.0.Study objects and methods. Pre-concentrated whey was enzymatically hydrolyzed at 30°C, 40°C, and 50°C for 60 to 180 min (pH 4.6). Non-hydrolyzed whey protein concentrates were used as a control. The amount of enzyme preparation was determined by calculation. All hydrolysate samples showed an increase in active acidity compared to the control samples. Further, we conducted a full-factor experiment with three levels of variation. The input parameters included temperature, duration of hydrolysis, and a substrate-enzyme ratio; the output parameters were the degree of hydrolysis and antioxidant capacity.
Results and discussion. The experiment showed the following optimal parameters for hydrolyzing cottage cheese whey proteins with the enzyme preparation of proteases produced by Aspergillus oryzae: temperature – 46.4°C; duration – 180 min; and the amount of enzyme preparation – 9.5% of the protein content. The antioxidant capacity was 7.51 TE mmol/L and the degree of hydrolysis was 17.96%.
Conclusion. Due to its proven antioxidant capacity, the whey protein hydrolysate obtained in the study can be used as a functional food ingredient.
Ключевые слова
Cottage cheese whey, protein, enzymatic hydrolysis, functional ingredient, Aspergillus oryzae, concentration factorВВЕДЕНИЕ
Processing whey, including cottage cheese whey, is highly relevant today due to several urgent problems. Among them are deficiencies in nutrients and raw materials, as well as low social and environmental efficiency. According to analytical data, 59% of the whey produced in Russia is fed to livestock and only 21% is processed for further use. The remaining 20% is discharged into fields or wastewater, exacerbating the existing environmental problems [1]. When discharged into the environment, whey acts as a biochemical contaminant. It is characterized by high biological oxygen consumption (50–60 g O2 per one liter annually) and high chemical oxygen consumption (50.5–54 g O2 per one liter) [2]. Thus, whey entering sewage systems or, in emergency cases, water bodies can cause serious environmental problems. Simple calculations show that the oxidation of organic compounds contained in 25 tons of whey (an output of a medium-sized cheese factory) needs as much oxygen as the oxidation of household wastewater in a city with a population of 40000 people. Wastewater has a high concentration of readily oxidizable organic compounds. Therefore, whey can cause a decrease in dissolved oxygen concentration in water bodies. Moreover, the presence of suspended protein particles can lead to the accumulation of bottom sediments and rotting processes [3].
A positive scenario suggests a growth in production from using highly efficient technologies for the deep processing of raw materials, creating “smart” storage and logistics systems, as well as minimizing losses and waste. For this, we need to focus on the “intravital” formation of the composition and properties of raw materials. It is a prerequisite for modern food technologies and “smart” agriculture. Only this approach can lead to potential progress in technologic development and consistently contribute to positive trends in the nutrition of the population [4].
A healthy lifestyle requires new ways of increasing the nutritional value of foods. Intensive food production often leads to raw materials losing essential micronutrients at all stages of processing (refining, pasteurization, etc.) [5].
Another possible cause of nutritional deficiency is excessive consumption of medicines for chronic diseases, including gastrointestinal disorders, acute respiratory viral infections and flue epidemics, etc. These drugs cause “pharmacological” malabsorption, contributing to the deficiency of essential nutrients supplied with food [6, 7]. As a result, they affect adaptive, compensatory, and regulatory capabilities of the body, change its physiological functions, and lead to chronic diseases of not only the digestive system, but also other organs and systems. These include atherosclerosis, hypertension, type 2 diabetes, metabolic immunosuppression, alimentary obesity, autoimmune pathology, etc. Moreover, the lack of proteins, polyunsaturated fatty acids, vitamins, and minerals in the diet leads to impaired immunoreactivity and resistance to natural and anthropogenic environmental factors [8].
The current situation dictates a need for new directions and technologies for producing healthy foods, including dairy products. However, one of the problems here is introducing functional ingredients. The development of functional foods often involves enriching foods with functional ingredients and/or eliminating those substances which cause negative reactions (food hypersensitivity). For this, we need adequate scientific data on healthy nutrients used as ingredients and their effect on the product’s taste and aroma profile [9, 11].
Controlled biocatalysis with specific enzymes can undoubtedly help food formulators develop functional food products [12].
Many researchers suggest using whey protein hydrolysates as functional ingredients. Due to bioactive peptides, they enhance the beneficial effect of traditional foods on public health [13, 15]. According to many authors, milk proteins modified by enzymatic hydrolysis have both technological properties (moisture binding, emulsifying, and foaming abilities) and functional properties (antioxidant, immunomodulating, hypotensive, etc.) [16–19].
Whey protein hydrolysates are used in the production of specialized products, for example, in sports nutrition [20].
In addition, whey treated with modern methods can increase the biological value of the end-product and improve functional and technological properties of raw materials and meat systems. For example, introducing whey into the meat system can regulate certain bio- and physicochemical processes by activating the biotechnological potential of natural systems in the ingredients [21]. In one study, hydrated protein preparations of Belcon Alev I and Lactobel ED were used to produce high-quality cooked sausages with a high biological value, digestibility, and prebiotic properties [22].
Whey protein is successfully used in the production of sausages as it not only creates a gelatinous mass that replaces fat, but also retains moisture [23]. This means that the yield of end-products can be increased without reducing the content of valuable animal protein or using additives. In addition, the end-products have better functional and technological properties, as well as improved taste characteristics [24].
Another benefit of using concentrated whey proteins in meat production is improved absorption of the endproduct by the human body, which, together with a reduced calorie content, contributes to the physiological value of the product. Thus, replacing the fat component of the meat product with a protein fraction of dairy origin can be a fundamentally new solution to the global problem of obesity [25].
Cottage cheese whey is currently the main source of protein hydrolysates. Despite high volumes of whey produced in Russia and its obvious benefits, there are insufficient data on its use as a raw material for functional ingredients [10, 26].
Our study aimed to prove a possibility of using cottage cheese whey proteins subjected to biocatalysis as functional food ingredients.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
The objects of the study included cottage cheese whey and enzyme preparations – acid proteases from Aspergillus oryzae with an activity of 400 units/g and a pH from 3.0 to 5.0.
The initial samples of whey, concentrate, and hydrolysate were analyzed for active acidity (pH) potentiometrically according to State Standard 32892-2014 and for a mass fraction of total protein according to State Standard 23327-98II. The assays were performed in triplicate.
Whey protein concentrate was obtained on an AL 362 pilot ultrafiltration unit (Altair, Russia) with a concentration factor of 5.0.
Enzymatic hydrolysis was carried out as follows. An enzyme preparation was introduced into cottage cheese whey preheated to the hydrolysis temperature (namely 30°C, 40°C, and 50°C) for 60–180 min. Active acidity was 4.6. Whey protein concentrates were used as a control. The enzyme amount was calculated by the formula:
where ME is the amount of the enzyme preparation per 1 g protein;
P is the protein content in 100 g whey;
α is the required enzyme activity; and
А is the initial enzyme activity.
The hydrolysis was carried out in a thermostatic water bath with constant stirring. At the end, the samples were heated to 85°C and held for 15 min to inactivate the enzyme preparation.
The samples were then cooled and poured into sterile dishes.
The degree of hydrolysis was determined spectrophotometrically according to the method of Spencer et al. [27]. In particular, we took 2 mL of the sample into a 15 mL plastic falcon and added 10 mL of a 1% aqueous solution of sodium dodecyl sulfate. For this, we used an automatic pipette (Eppendof, Germany) with a measurement range of 500–5000 μL. The resulting reaction mixture was incubated in a water bath at 75 ± 1°C for 15 min.
A series of dilutions of L-leucine in a 1% SDS aqueous solution with concentrations of 0.15–3.0 mmol/dm3 were used as standards for determining the degree of hydrolysis according to the Spencer et al. method. In particular, the falcons with different standard concentrations were successively filled with 2 ml of 0.2125 M sodium phosphate buffer (pH 8.20) and 250 μL of a standard solution, as well as 50 μL of a hydrolysate sample in the first case, 250 μL of a UV concentrate sample in the second case, and a blank sample in the third case. In addition, 2 mL of a 0.1% solution of 2,4,6-trinitrobenzenesulfonic acid was added to all the falcons. The falcons were tightly closed and shaken. The samples were then incubated in a water bath at 50°C for one hour. At the end of the incubation, 4.0 mL of a 0.1 M hydrochloric acid solution was added to each falcon to stop the reaction. The falcons were tightly closed, shaken, and kept for 30 min at room temperature for cooling. The optical density of the solutions was determined on a Synergy 2 microplate photometer-fluorometer (BioTek, USA) at a wavelength of 340 nm.
To determine the amount of leucine equivalents, we took 0.75 mL of the hydrolysate with the maximum degree of hydrolysis (100%) and transferred it into a 5.0 mL microreaction vessel. Then, we added 0.75 mL of distilled water and 2.4 L of concentrated hydrochloric acid. The vessel was incubated in an oven at 120 ± 2°C for 23 h. After incubation, the samples were cooled for one hour at room temperature and filtered under vacuum in a funnel with a glass filter. The contents of the microreaction vessel were quantitatively transferred to the filter and rinsed with distilled water. The pH of the wash water entering the Bunsen flask was monitored using Lach-Ner universal paper. The contents of the Bunsen flask were quantitatively transferred into a 100 mL laboratory glass beaker. The active acidity of the filtrate was adjusted to 7.00 ± 0.02 pH by adding a 40% aqueous solution of sodium hydroxide. The neutralized filtrate was quantitatively transferred into a 100 mL volumetric flask and the volume was adjusted to the mark with a 1% SDS aqueous solution. The contents of the flask were thoroughly mixed.
After foam collapse, a 0.25 mL sample was taken from the volumetric flask and analyzed. The degree of hydrolysis of the hydrolysate protein was calculated according to the equation:
where is the optical density in the hydrolysate sample at 340 nm;
is the optical density in the blank sample at 340 nm;
is the optical density in the UV - concentrate sample at 340 nm;
30 is the hydrolysate dilution factor;
6 is a dilution factor for raw materials to obtain a hydrolysate;
K is the slope of the calibration graph showing the dependence of the optical density of the solution at 340 nm on the concentration of the standard in the sample (0.1733 L/mol);
is the optical density in the acid hydrolysate sample (100% hydrolysis) at 340 nm; and
133.33 is the acid hydrolysate dilution factor.
The in vitro antioxidant capacity (TEAC) was measured using the ABTS radical cation.
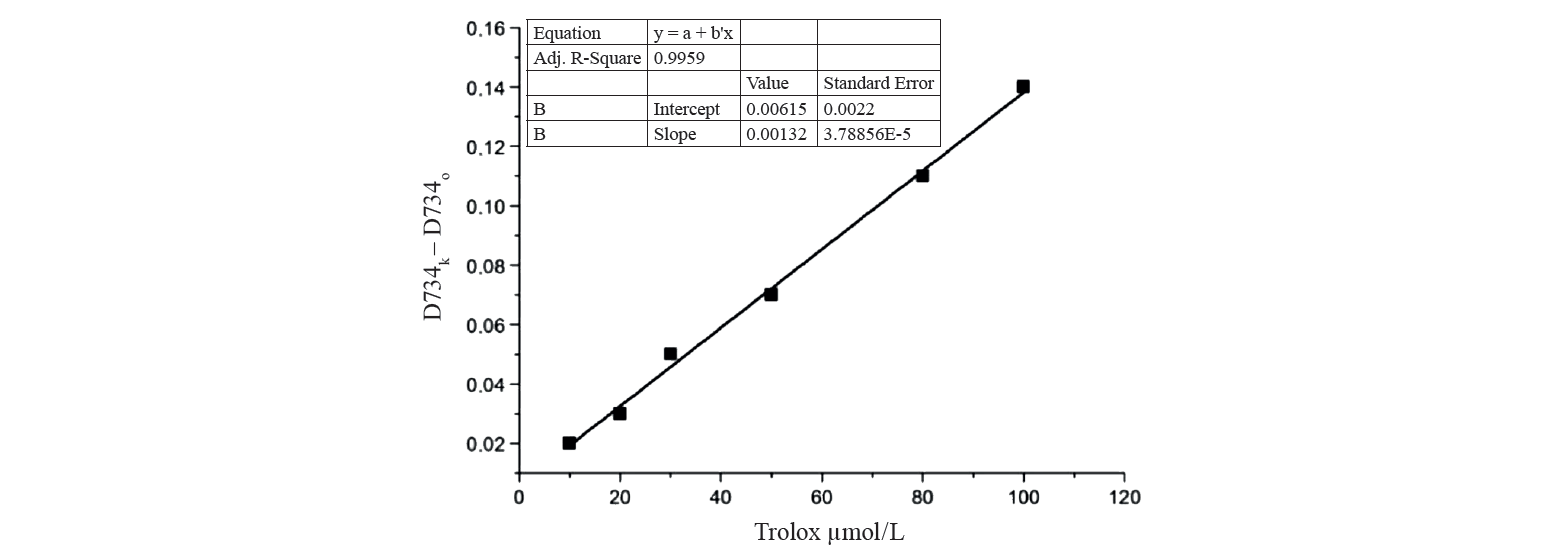
The ABTS radical cation was obtained according to the Re et al. method by incubating a solution of 7 mM ABTS and 2.45 mM potassium peroxodisulfate in the dark at room temperature for 12–18 h [28]. The concentrated solution of ABTS radical cation was diluted with а 50 mM phosphate-buffered saline (with 100 mM sodium chloride), pH 7.4, to OD734 = 0.70 ± 0.02. This value corresponded to the final concentration of ABTS radical cation = 47 μM (ε734 = 1.5×104 mol–1·L·cm–1).
To determine the antioxidant capacity (AОC), 20 μL of the test samples or a Trolox solution and 180 μL of the ABTS radical cation solution were added to the wells of 96-well non-absorbing polystyrene microplates with a flat bottom. The control was 180 μL of the ABTS radical cation solution and 20 μL of a 50 mM phosphatebuffered saline (with 100 mM sodium chloride), pH 7.4. The reaction was recorded as OD734 decreased during 40.5 min with a measurement interval of 60 s at 25°C on a Synergy 2 photometer-fluorimeter (BioTek, USA). The assays were performed in quadruplicate.
The calibration curve of decreased optical density versus Trolox concentrations varying within 1–10 μM can be seen in Fig. 1. Equivalent concentrations of antioxidants in the samples were determined in relation to the decrease in optical density of the reaction medium in the presence of the studied compounds. The AOC of the samples was expressed in μM TE. When testing the antioxidant activity of hydrolysate samples with respect to the ABTS radical cation, the working range of dilution factors for a 50 mM phosphate-buffered saline (pH 7.4) was 150.
Sensory analysis described by Spellman was used to determine bitterness in enzymatic hydrolysates [29].
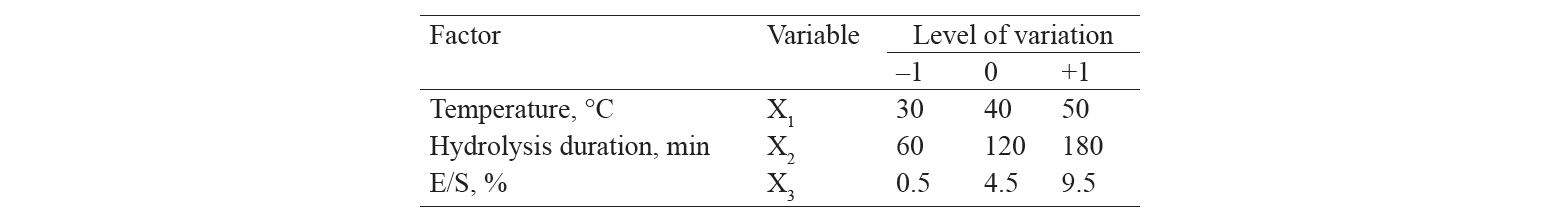
To optimize the conditions for enzymatic hydrolysis of cottage cheese whey, we conducted a full-factor experiment with three variables: temperature (X1), duration of hydrolysis (X2), and enzyme-substrate ratio E/S (X3). Each of the parameters varied at three levels (Table 1). The output parameters were the degree of hydrolysis (DH) and antioxidant capacity (TEAC).
The variation levels of independent parameters in the multifactorial experiments conducted to optimize the hydrolysis of cottage cheese whey are shown in Table 1.
The results of the multifactorial experiments were statistically processed using the DOE block of Statistica 10.0 (StatSoft Inc., USA).
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
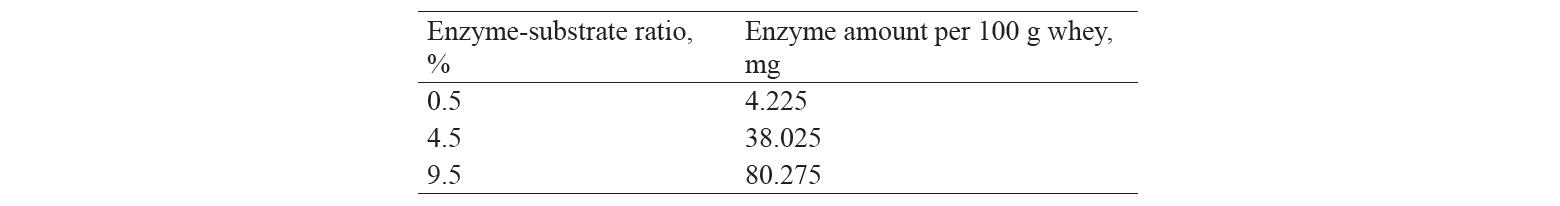
The protein content was 0.56% in the initial whey and 1.35% in the concentrate. The enzyme amounts per 1 g of protein are shown in Table 2.
The results of the full-factor experiments conducted to optimize enzymatic hydrolysis of cottage cheese whey proteins are demonstrated in Table 3.
The experiments showed an increase in the degree of hydrolysis and antioxidant activity of cottage cheese whey proteins with larger amounts of enzyme preparations and longer fermentation at 30°C and 50°C. The maximum degree of hydrolysis (19.66%) was recorded at 50°C, 180 min fermentation, and 9.5% enzyme. However, higher temperatures led to a noticeable, almost two-fold decrease in antioxidant activity in the control samples, which were not hydrolyzed. Thus, temperature had a significant effect on this indicator (Table 4).
According to sensory evaluation, the most bitter taste was registered in the sample that was hydrolyzed at 30°C with the maximum duration and enzyme amount (13.06% degree of hydrolysis). However, sample No. 30, which was obtained at the maximum temperature, duration of hydrolysis, and enzyme amount, did not taste bitter. It means that these conditions make the process more directional, producing hydrolysates that do not contain peptides with bitter amino acids at the end of the chain. At the same time, this sample had the highest degree of hydrolysis.
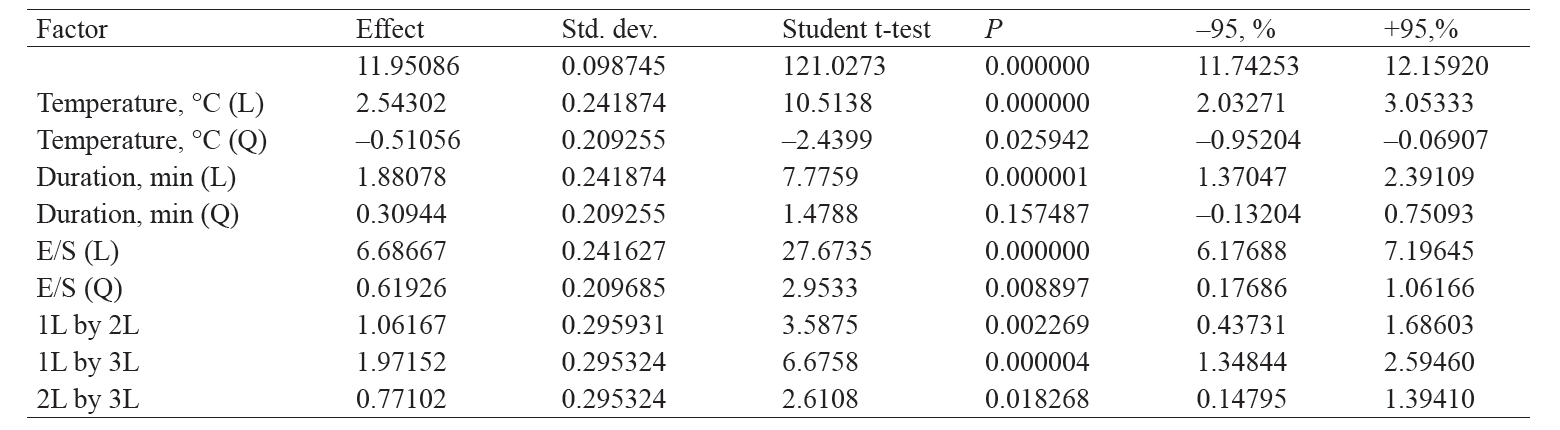
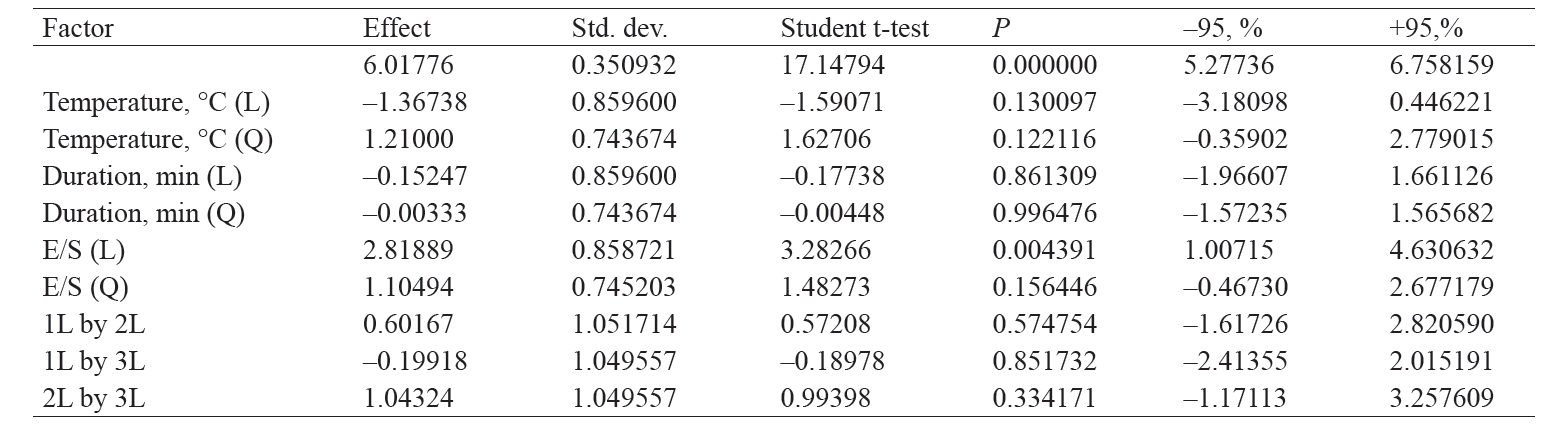
Table 4 shows the statistical analysis of effects that variable factors have on the degree of hydrolysis of cottage cheese whey proteins. As we can see, all the variable factors, except for the quadratic duration factor, have a significant (P < 0.05) effect on the degree of hydrolysis.
The relation between the degree of whey protein hydrolysis and variable parameters is graphically illustrated in Fig. 2. The graphs show no local maxima or minima, suggesting that the degree of hydrolysis rises with an increase in each of the parameters.
Table 5 shows the statistical analysis of effects that variable factors have on the antioxidant activity of cottage cheese whey proteins. As we can see, only the linear factor of enzyme amount has a significant (P > 0.05) effect on the antioxidant capacity.
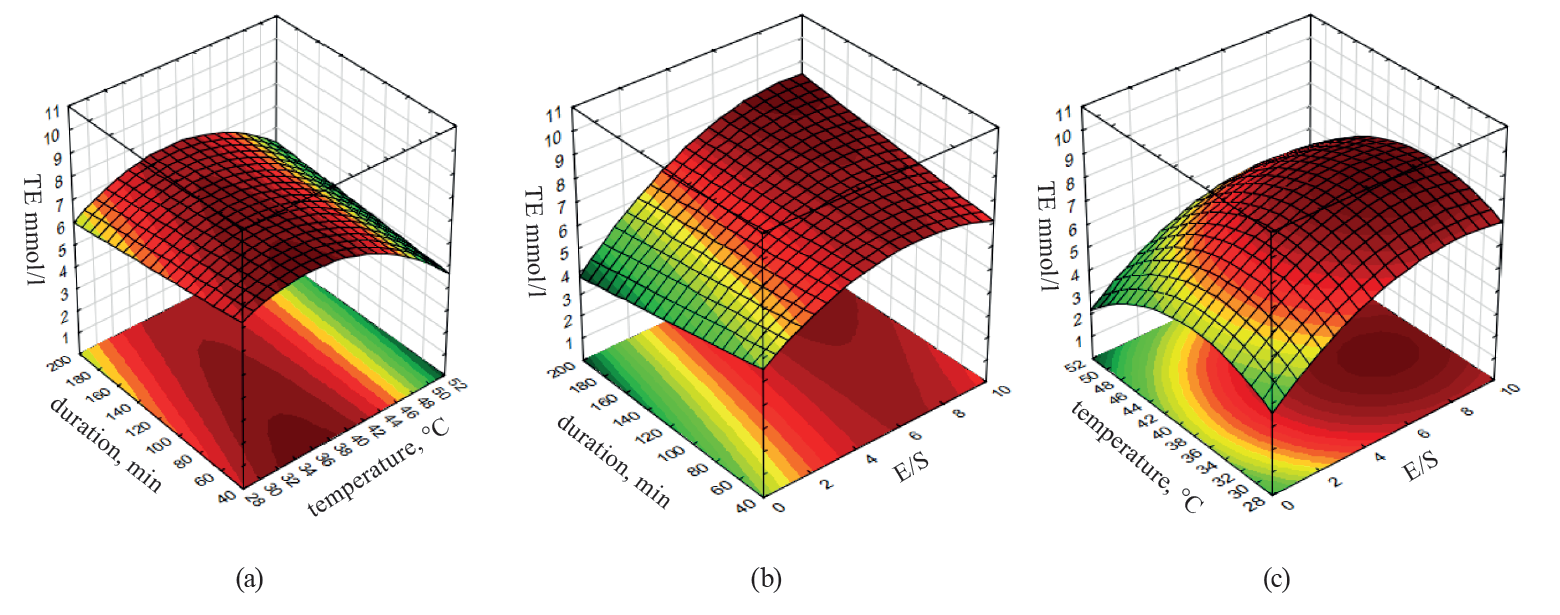
The surfaces of equal response of hydrolysates TEAC versus variable parameters of whey concentrate hydrolysis are presented in Fig. 3. We can clearly see the presence of a local maximum of antioxidant activity in Figs. 3a and 3b.
Finally, we correlated the key factors in the multifactorial experiments in order to select optimal conditions for the enzymatic hydrolysis of the UV-concentrate of cottage cheese whey using the enzyme preparation from Aspergillus oryzae.
ВЫВОДЫ
Based on the statistical analysis, we selected the following optimal conditions for the hydrolysis of cottage cheese whey proteins: temperature – 46.4°C; duration – 180 min; and the enzyme amount – 9.5% of the protein content. These conditions provided the antioxidant capacity of 7.5 TE mmol/L with a 17.96% degree of hydrolysis.
The given data open up new prospects for processing acid cottage cheese whey and using whey proteins as potential functional components with increased antioxidant activity. We showed that targeted biocatalytic conversion can make whey proteins more functional. The obtained hydrolysate of cottage cheese whey proteins can be used to develop new functional foods, including meat and dairy products.
КОНФЛИКТ ИНТЕРЕСОВ
The authors state that there is no conflict of interest.
ФИНАНСИРОВАНИЕ
This work was part of the State Assignment given within the “Program of Fundamental Scientific Research by the State Academies of Sciences for 2013-2020” (theme code 0578-2019-0023, Section 2) to “determine the relations between the parameters of biocatalytic conversion of protein-carbohydrate systems and the composition of enzyme complexes for the formation of desired functional properties”.СПИСОК ЛИТЕРАТУРЫ
- Khramtsov AG. Problema polnogo i ratsionalʹnogo ispolʹzovaniya molochnoy syvorotki v usloviyakh rynochnoy ehkonomiki [The problem of full and rational use of whey in a market economy]. News of institutes of higher education. Food technology. 1994;218–219(1–2):5–9. (In Russ.).
- Zolotaryova MS, Volodin DN, Topalov VK, Evdokimov IA, Chablin BV. O pererabotke molochnoy syvorotki i vnedrenii nailuchshikh tekhnologiy [On processing whey and introducing the best technologie]. Milk Processing. 2016;201(7):17–19. (In Russ.).
- Sviridenko YuYa, Kravshenko EF, Yakovleva OA. Milk whey application and local purification of effluents. Dairy Industry. 2008;(11):58–60. (In Russ.).
- Galstyan AG, Aksyonova LM, Lisitsyn AB, Oganesyants LA, Petrov AN. Modern approaches to storage and effective processing of agricultural products for obtaining high-quality food products. Vestnik Rossijskoj Akademii Nauk. 2019;89(5):539–542. (In Russ.). DOI: https://doi.org/10.31857/S0869-5873895539-542.
- Rogov IA, Oreshkin EN, Sergeev VN. Medical and technological aspects of the development and production of functional foods. Food Industry. 2017;(1):13–15. (In Russ.).
- Lisitsyn AB, Chernukha IM, Lunina OI. Modern trends in the development of the functional food industry in Russia and abroad. Theory and Practice of Meat Processing. 2018;3(1):29–45. (In Russ.). DOI: https://doi.org/10.21323/2414-438X-2018-3-1-29-45.
- Zobkova Z, Fedulova L, Fursova T, Zenina D, Kotenkova E. Evaluation of the adaptogenic propertries of the Quark product enriched with probiotics, polyphenols and vitamins. Potravinarstvo Slovak Journal of Food Sciences. 2019;13(1):713–719. DOI: https://doi.org/10.5219/1156.
- Sergeev VN, Bobrovnitskiy IP. Vliyanie optimizatsii ratsionov pitaniya bolʹnykh pervichnym khronicheskim gastroduodenitom i yazvennoy boleznʹyu dvenadtsatiperstnoy kishki na dinamiku osnovnykh klinicheskikh sindromov, neyroehndokrinnyy i psikhologicheskiy status [The influence of diet optimisation for patients with primary chronic gastroduodenitis and duodenal ulcer on the dynamics of the main clinical syndromes, neuroendocrine and psychological status]. Journal of restorative medicine and rehabilitation. 2010;35(1):24–29. (In Russ.).
- Lisitsyn AB, Chernukha IM, Lunina OI. Food hypersensitivity and products of animal origin resources. Theory and Practice of Meat Processing. 2017;2(2):23–36. (In Russ.). DOI: https://doi.org/10.21323/2414-438X-2017-2-2-23-36.
- Zolotaryov NA, Fedotova OB, Agarkova EYu. Curds whey hydrolyzates for curds emulsion products. Dairy Industry. 2017;(8):36–38. (In Russ.).
- Tasturganova E, Dikhanbaeva F, Prosekov A, Zhunusova G, Dzhetpisbaeva B, Matibaeva A. Research of fatty acid composition of samples of bio-drink made of camel milk. Current Research in Nutrition and Food Science. 2018;6(2):491–499. DOI: https://doi.org/10.12944/CRNFSJ.6.2.23.
- Lisitsyn AB. Perspektiva razvitiya pishchevoy biotekhnologii [The prospects of food biotechnology]. Technologies of food and processing industry of AIC – healthy food. 2013;(1):11–14. (In Russ.).
- Kharitonov VD, Pavlo VV, Pismenskaya VN. Issledovanie osnovnykh faktorov, vliyayushchikh na formirovanie kachestvennykh pokazateley novykh molochnykh produktov slozhnogo syrʹevogo sostava [A study of the main factors affecting the formation of quality indicators of new dairy products from complex raw materials]. Storage and Processing of Farm Products. 2001;(9):7–10. (In Russ.).
- Tutelʹyan VA, Knyazhev VA. Realizatsiya kontseptsii gosudarstvennoy politiki zdorovogo pitaniya naseleniya Rossii: nauchnoe obespechenie [Implementing the concept of the state policy of healthy nutrition for the population of Russia: scientific support]. Problems of Nutrition. 2000;69(3):4–7. (In Russ.).
- Novoselova MV, Prosekov AYu. Technological options for the production of lactoferrin. Foods and Raw Materials. 2016; 4(1):90–101. DOI: https://doi.org/10.21179/2308-4057-2016-1-90-101.
- Maruyama S, Mitachi H, Awaya J, Kurono M, Tomizuka N, Suzuki H. Angiotensin I-converting enzyme inhibitory activity of the C-terminal hexapeptide of αs1-casein. Agricultural and Biological Chemistry. 1987;51(9):2557–2561. DOI: https://doi.org/10.1271/bbb1961.51.2557.
- Meisel H, Bockelmann W. Bioactive peptides encrypted in milk proteins: Proteolytic activation and thropho-functional properties. Antonie van Leeuwenhoek, International Journal of General and Molecular Microbiology. 1999;76(1–4):207–215. DOI: https://doi.org/10.1023/A:1002063805780.
- Niki E, Yoshida Y, Saito Y, Noguchi N. Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochemical and Biophysical Research Communications. 2005;338(1):668–676. DOI: https://doi.org/10.1016/j.bbrc.2005.08.072.
- Prosekov AYu, Dyshlyuk LS, Milentyeva IS, Sykhikh SA, Babich OO, Ivanova SA, et al. Antioxidant and antimicrobial activity of bacteriocin-producing strains of lactic acid bacteria isolated from the human gastrointestinal tract. Progress in Nutrition. 2017;19(1):67–80. DOI: https://doi.org/10.23751/pn.v19i1.5147.
- Petrova EI, Gavrilova NB. Research of enzymatic hydrolysis of milk whey proteins and development of a bioactive component for a sports nutrition. Agrarian Bulletin of the Urals. 2013;114(8):33–35. (In Russ.).
- Shipulin VI, Kulikov YuI, Lupandina ND, Nazarova ON. Technology of sausage products using adapted components of milk whey. Meat Industry. 2013;(11):18–22. (In Russ.).
- Shipulin VI, Postnikov SI, Statsenko EN, Marchenko VV, Sudakova NV. Use of milk protein-carbohydrate mixtures in cooked sausages. Meat Industry. 2012;(6):22–25. (In Russ.).
- Miklyashevski P, Pryanishnikov VV, Babicheva EB, Ilʹtyakov AV. Ispolʹzovanie soevykh belkov v pererabotke myasa [The use of soy proteins in meat processing]. All about the meat. 2006;(3):10–13. (In Russ.).
- Omarov RS, Shlykov SN, Sycheva OV, Kravets AB. Molochnye belki v myasnykh delikatesakh [Milk proteins in meat delicacies]. Meat Technology. 2010;96(12):48–49. (In Russ.).
- Nazarova ON, Shipulin VI. Theoretical and practical aspects of biotechnology meat with micro-particle whey protein. Science. Innovations. Technologies. 2013;(1):55–62. (In Russ.).
- Volodin DN, Zolotaryova MS, Topalov VK, Evdokimov IA, Chablin BV. Osobennosti pererabotki tvorozhnoy syvorotki [Peculiarities of processing cottage cheese whey]. Milk Processing. 2017;209(3):6–9. (In Russ.).
- Spencer JFT, Spencer DM. Yeasts in natural and artificial habitats. Berlin, Heidelberg: Springer; 1997. 381 p. DOI: https://doi.org/10.1007/978-3-662-03370-8.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 1999;26(9–10):1231–1237. DOI: https://doi.org/10.1016/S0891-5849(98)00315-3.
- Spellman D, O’Cuinn G, FitzGerald RJ. Bitterness in Bacillus proteinase hydrolysates of whey proteins. Food Chemistry. 2009;114(2):440–446. DOI: https://doi.org/10.1016/j.foodchem.2008.09.067.