Аннотация
Feeds, feed preparations and high-protein feed supplements are complex multicomponent compositions that, when stored, used, processed and transported, can change their physicochemical properties, microbiological indicators and toxicological properties. In this regard, it is very important to study the above properties and quality indicators. The paper describes a study of the peptide profile, physico-chemical and toxicological properties of the enzymatic hydrolysates of feather-down raw materials obtained during a multifactorial experiment to optimize the conditions for enzymatic hydrolysis of feather-down raw materials. The raw materials were previously subjected to a short-term hydrothermal treatment to improve the course of enzymatic hydrolysis. The enzymes obtained from bacterial organisms were used for the enzymatic hydrolysis of raw materials: Protolade B and Protease 2630#2256. The following results were obtained for the physicochemical properties of enzymatic hydrolysates: the content of protein and solids in the enzymatic hydrolysates of feather-down raw materials varied in the range of 1.80-3.00% and 2.6-4.4%, respectively. In this case, the mass fraction of protein was 67.7-70.6% in terms of the mass fraction of moisture. The mass fraction of ash, in terms of the mass fraction of moisture, did not exceed 0.43% in all the samples of enzymatic hydrolysates, the mass fraction of fat was 0.74%, the mass fraction of crude fiber was 1.28%, the mass fraction of sodium chloride was 1.92% the mass fraction of the impurities insoluble in hydrochloric acid was 0.77%. It has been shown that enzymatic hydrolysates corresponded to TU 9219-094-23476484-09 “Hydrolyzed feed flour. Feather protein concentrate” for their microbiological properties (QMAFAnM, coliforms, pathogenic microorganisms, toxin-forming anaerobic bacteria and bacteria of the genus Proteus) and chemical safety parametersКлючевые слова
Feather, enzymatic hydrolysis, biosafety, physical and chemical properties, feed supplementВВЕДЕНИЕ
The main condition at the present stage of society is the development of technological progress by introducing energy and resource-saving processing lines into production and industry that allow to supply to the production of high-quality products and reduce the negative environmental consequences of production. This trend can be referred to the production of feed supplements [3, 11, 15].
Feeds, feed preparations and high-protein feed supplements are complex multicomponent compositions that, when stored, used, processed and transported, can change their physicochemical properties, microbiological indicators and toxicological properties. In this regard, it is very important to study the above properties and quality indicators [6, 11, 12].
The production of poultry products is the leading branch of agriculture for today and has a significant effect on the production of food products of other industries. Much attention is paid to the safety of feeds for poultry [10, 11].
The creation of an extensive fodder base is one of the main factors in the production of animal products. An important task that scientists have in the development of new feed supplements or feeds is the development of maximum quality control and safety of feed supplements and feeds [2, 12, 13].
The present stage of the studies aims at studying the physico-chemical properties of the enzymatic hydrolysates of feather-down raw materials obtained during a multifactorial experiment on optimizing the conditions of the enzymatic hydrolysis of feather-down raw materials at a laboratory level.
Feather-down raw materials were used after a shortterm hydrothermal treatment as feather raw materials for enzymatic hydrolysates. The use of a preliminary short-term hydrothermal treatment allows for grinding feather raw materials simultaneously with reducing the density of a keratin pack partially breaking hydrogen bonds and reducing hydrophobic interactions. The resulting substrate is more easily subjected to an enzymatic attack.
The sanitary quality of such products has an enormous effect on the reproductive ability, productivity and health of animals, as well as a direct effect on their biological value. The sanitary quality of feeds is determined by the amount of the pathogenic microorganisms contained therein [5, 16]. Most attention in the sanitary evaluation of feed supplements and feeds is paid to such indicators as the total bacterial content, the presence of pathogenic microorganisms and toxins. The high amount of such pathogenic microorganisms in feeds as salmonella, clostridia, etc., leads to an infection and the development of diseases in animals and birds, their early death, and, consequently, to high economic losses [7, 16, 14].
Microbiological studies of animal and vegetable feeds are carried out in the department of veterinary and sanitary examination for the presence of: the total bacterial content, Salmonella, Escherichia coli, Proteus and toxin-forming anaerobic bacteria [8, 19]. The presence of salmonella in feeds and feed supplements is the cause of infectious salmonellosis. Salmonellosis affects young stock, including poultry, animals and humans. Salmonellosis is followed by a fever and profuse diarrhea, and may also cause lung diseases. In pregnancy, salmonellosis can be a cause of spontaneous abortion [9, 14]. Intestinal bacillus (lat. Escherichia coli) is a species of gram-negative rod-shaped bacteria, widely distributed in the lower intestine of warm-blooded animals. Most strains of E. coli are harmless, but the serotype O157:H7 can be a cause of severe food poisoning in humans and animals [4, 15, 17].
The content of microorganisms in poultry feeds is the main cause of poisoning and the emergence of infectious agents.
Equally important is the observance of the conditions of storage of feeds and also their transportation. Mixed feeds must be transported in compliance with sanitary standards, in dry, clean, uninfected vehicles without foreign odors. When loading, transporting and unloading mixed feeds should be protected from atmospheric precipitation. Mixed feeds are stored in dry, clean, uninfected, wellventilated warehouses. If mixed feed was stored incorrectly in an unprepared and unadapted room, it is highly likely to be very dangerous to the life and health of animals [1, 12, 19].
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
The following was used as the study object: the enzymatic hydrolysates from the poultry feather-down raw materials of Kuzbass Broiler, LLC (Kemerovo region, Russia).
When analyzing the obtained enzymatic hydrolysates of feather-down raw materials using a multienzyme composition, the following methods were used:
The mass fraction of crude protein was determined by ashing with sulfuric acid in the presence of a catalyst, followed by the alkalinization of the reaction product, the distillation and titration of the released ammonia according to GOST 32044.1–2012 “Feeds, mixed feeds and raw material. Determination of mass fraction of nitrogen and calculation of mass fraction of crude protein”.
The mass fraction of the ash insoluble in hydrochloric acid was determined in accordance with GOST 13496.14–87 “Mixed fodder, raw mixed fodder, fodder. Method for determination of ash insoluble in hydrochloric acid”.
The mass fraction of moisture was determined according to GOST 17681–82 “Flour of animal origin. Test methods”.
The mass fraction of whole protein was studied using the Dumas method with the use of a RAPID N Cube protein nitrogen analyzer.
The mass fraction of fat was determined in accordance with GOST 32905–2014 “Feeds, mixed feeds and raw material. Method for determination of fat content”.
The mass fraction of crude fiber in enzymatic hydrolysates was determined in accordance with the requirements of GOST 13496.2–91 “Fodders, mixed fodders and mixed fodder raw material. Method for determination of raw cellular tissue”.
The mass fraction of sodium chloride in enzymatic hydrolysates was determined using an ionometric method in accordance with the requirements of GOST 13496.1–98 “Mixed fodder and raw mixed fodder. Methods for sodium and sodium chloride determination”.
The mass fraction of calcium in enzymatic hydrolysates was determined using a complexometric method in accordance with GOST 26570–85 “Fodder, mixed fodder and mixed fodder raw material. Methods for determination of calcium”.
The mass fraction of the mineral impurities insoluble in hydrochloric acid in enzymatic hydrolysates was determined using a water flotation method in accordance with GOST 25555.3–82 “Fruit and vegetable products. Methods for determination of mineral impurities”.
The mass fraction of phosphorus in enzymatic hydrolysates was determined in accordance with GOST 26657–97 “Fodders, mixed fodders, mixed fodder raw materials. Methods for determination of phosphorus content”.
The number of mesophilic aerobic and facultative anaerobic microorganisms (QMAFAnM) was determined in accordance with GOST 10444.15–94 "Food products. Methods for determination of quantity of mesophilic aerobes and facultative anaerobes" and GOST 25311–82 “Feeding flour of animal origin. Methods of bacteriological analysis”.
The number of coliforms (coliform bacteria) was determined in accordance with GOST 30518–97 “Food products. Methods for detection and quantity determination of coliforms” and GOST 26670–91 “Food products. Methods for cultivation of microorganisms”.
The presence of pathogenic and toxin-forming microorganisms was determined in accordance with GOST 25311–82 “Feeding flour of animal origin. Methods of bacteriological analysis”, the presence of salmonella was determined in accordance with GOST 30519–97 “Food products. Method for detection of Salmonella”.
The presence of bacteria of the genus Proteus was determined according to GOST 28560–90 “Food products. Method for detection of bacteria of Proteus, Morganella, Providencia genera”.
The content of arsenic in the samples studied was determined in accordance with GOST 26930–86 “Raw material and food-stuffs. Method for determination of arsenic”.
The content of toxic elements was determined:
– for lead - according to GOST 26932–86 “Raw material and food-stuffs. Methods for determination of lead”, cadmium - in accordance with GOST 26933–86 “Raw material and food-stuffs. Methods for determination of cadmium”,
– strontium-90 - in accordance with 32163-2013 “Foodstuffs. Method for strontium Sr-90 content determination”,
– pesticides - according to GOST 32194–2013 “Feeds, compound feeds. Determination of organochlorine pesticides residues by gas chromatographic method”,
– mercury - in accordance with GOST R 53183–2008 “Foodstuffs. Determination of trace elements. Determination of mercury by cold-vapour atomic absorption spectrometry (CVAAS) method after pressure digestion”.
– copper - in accordance with GOST 26931–86 “Raw material and food-stuffs. Methods for determination of copper”,
– zinc - in accordance with GOST 26934–86 “Raw material and food-stuffs. Method for determination of zinc”,
– aflatoxin B1 - according to GOST 31653–2012 “Feedstuffs. Method of immunoenzyme mycotoxin determination”,
– cesium-137 in accordance with GOST 32161–2013 “Foodstuffs. Method for cesium Cs-137 content determination”.
The molecular weight distribution of enzymatic hydrolysates of keratin-containing raw materials was estimated using the method of exclusion chromatography. The chromatographic system included a Varian ProStar HPLC chromatograph (USA), a PS210 SDM pump, a PS410 Autosampler and a BioSep-SEC-S 2000 (7.8 x 300 mm) column from Phenomenex (USA). This kind of column is used for the analytical separation of low molecular weight proteins and peptides by gel filtration. The column was calibrated for standard water-soluble proteins and peptides from GE Healthcare (USA), Serva (Germany) and Sigma (USA) in the range of molecular weights from 451 to 440,000 Da covering its operating range. The optical density was registered using a streaming current detector with a photodiode array (Varian 335 PDA) in the range of 190–330 nm with a base wavelength of 214 nm. A 50 mM Na-phosphate 0.15 M NaCl buffer, pH 6.8, was used as an aluent. The elution rate was 1 ml/min. The volume of the sample applied to the column was 20 μl. The sample preparation included double centrifugation at 60000 g for 40 min. The chromatograms obtained were integrated with the calculation of the relative content of the high molecular weight protein fraction (MW > 10 kDa), the midmolecular oligopeptide fraction (MW is 3–10 kDa) and the low molecular weight fraction (MW < 3 kDa) containing peptides and free amino acids.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
Enzymatic treatment involves the hydrolysis of the protein that splits into smaller fragments, including peptides and free amino acids. The functional and technological characteristics of protein hydrolysates, such as the dissolubility capacity, the moistureretaining capacity, emulsification and others, and the biological activity of hydrolysates are determined by the depth of the hydrolysis of the initial substrate.
To increase the degree of enzymatic conversion, the source feather raw materials were subjected to a shortterm hydrothermal treatment. The samples of the hydrothermally treated keratin-containing rawmaterials were obtained in accordance with the following technological parameters of hydrothermal hydrolysis:
– the initial feather moisture - 55%;
– the heating temperature - 190–200ºС;
– the heating duration - 90 sec.
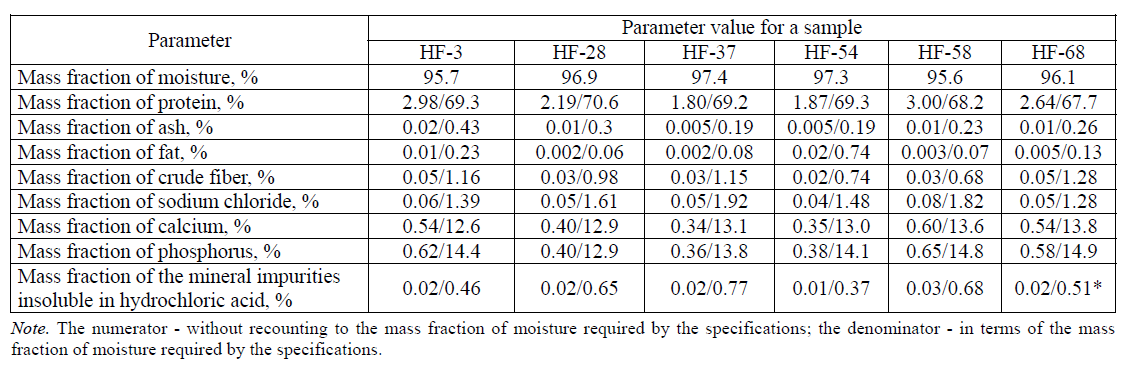
The obtained sample of the hydrothermally treated feather raw materials (feather) was characterized according to physicochemical parameters. The physicochemical properties include density, hygroscopicity, mass the fraction of moisture, the mass fraction of fat, the mass fraction of ash, the mass fraction of protein, the mass fraction of crude fiber, the mass fraction of sodium chloride, the mass fraction of calcium, the mass fraction of the mineral impurities insoluble in hydrochloric acid. Table 1 presents the results of a study of the physicochemical properties of the source feather raw materials after hydrothermal treatment.
As it can be seen from the data presented in Table 1, the feather-down raw materials subjected to hydrothermal treatment meet the requirements of TU 9219–094–23476484–09 “Hydrolyzed feed flour. Feather protein concentrate” for the physicochemical parameters.
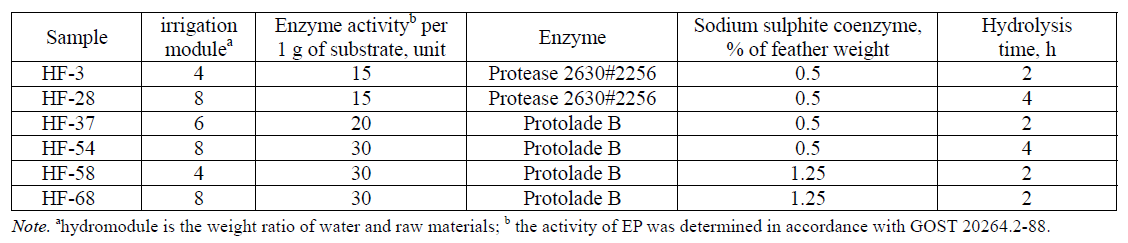
The feather, pretreated hydrothermally, was subjected to enzymatic hydrolysis during a multifactor experiment to optimize the conditions of enzymatic hydrolysis of feather-down raw materials (HF) at a laboratory level. The samples of the hydrolysates shown in Table 2 showed the most satisfactory results (% of protein, the yield of solids, the anion-exchange capacity of feather hydrolyzates in relation to peroxyl hydrolyzate) and were selected for a further study.
Further on, we studied the physico-chemical properties of six samples of enzymatic hydrolysates (Table 2) of the hydrothermally pre-treated featherdown raw materials obtained in the course of a multifactorial experiment to optimize the conditions for the enzymatic hydrolysis of feather-down raw materials at a laboratory level. Table 3 presents the results obtained.
The data presented in Table 3 show that the content of protein and solids in the enzymatic hydrolysates of the feather-down raw materials varies in the range of 1.80–3.00% and 2.6–4.4%, respectively. The mass fraction of protein in terms of the mass fraction of moisture is 67.7–70.6%. The mass fraction of calcium in terms of the mass fraction of moisture in the test samples varies in the range from 12.6 to 13.8%, the mass fraction of phosphorus ranges from 12.9 to 14.9%. The mass fraction of ash in terms of the mass fraction of moisture in all the samples of enzymatic hydrolysates does not exceed 0.43%, the mass fraction of fat - 0.74%, the mass fraction of crude fiber - 1.28%, the mass fraction of sodium chloride - 1.92% and the mass fraction of the impurities insoluble in hydrochloric acid - 0.77%. It also follows from Table 1 that the minimum protein content in terms of the mass fraction of moisture is in the hydrolysates with an irrigation module equal to 4. The content of solids and protein in the enzymatic hydrolysates of feather-down raw materials correlate with each other. This fact is apparently due to a high relative fraction of protein in the solids of enzymatic hydrolysates of feather-down raw materials. The content of moisture and ash of the enzymatic hydrolysates of feather-down raw materials correspond to the value range of these parameters for other animal hydrolysates.
The comparison of the data in Table 3, in terms of the mass fraction of moisture, testifies that the enzymatic hydrolyzates of the feather-down raw materials have satisfactory parameters for the mass fraction of ash, fat, crude fiber, sodium chloride, calcium, phosphorus and mineral impurities. As for the protein content, there is no such correspondence, due to which the further investigations within the framework of this study will be aimed at cleaning and degreasing the enzymatic hydrolysates of feather-down raw materials in order to increase the mass fraction of protein.
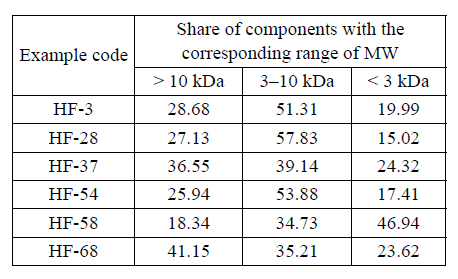
The analysis of the molecular mass distribution of enzymatic hydrolysates of keratin-containing raw materials of hydrolysates with the maximum yield of solids or the highest antioxidant capacity in relation toa peroxyl radical (HF-3, HF-28, HF-37, HF-54, HF-58, HF-68) was performed according to the procedure given. Table 4 presents the results obtained.
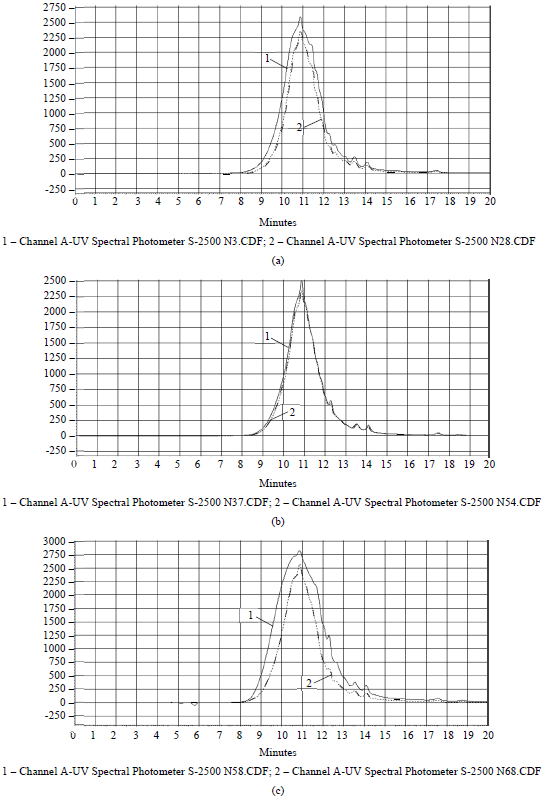
Figure 1 presents the profiles of hydrolysate elution.
The elution profiles of the hydrolysates obtained when incubating the keratin-containing raw materials in a medium without the addition of an enzyme and sodium sulfite (Fig. 1) are characterized by the presence of well-marked peaks for 11 min of elution. In addition, there are peaks of low intensity that correspond to low-molecular compounds (13–14 min). The HF-58 sample, obtained when hydrolyzing the keratin-containing raw materials with a sodium sulfite content of 1.25% for 2 hours, with the hydromodule 4 and the activity of EP for Protolad B of 30 U/g, had the highest fraction (46.94%) of low-molecular compounds of the range < 3 kDa. The increase in the hydromodule to 8 (the HF-68 sample) resulted in a shift in the size of peptide molecules towards the range of molecular weights > 10 kDa. There is predominance in the content of peptides of an average molecular weight of 3-10 kDa (39–57%) in the samples HF-3, HF-28, HF-37 and HF-54.
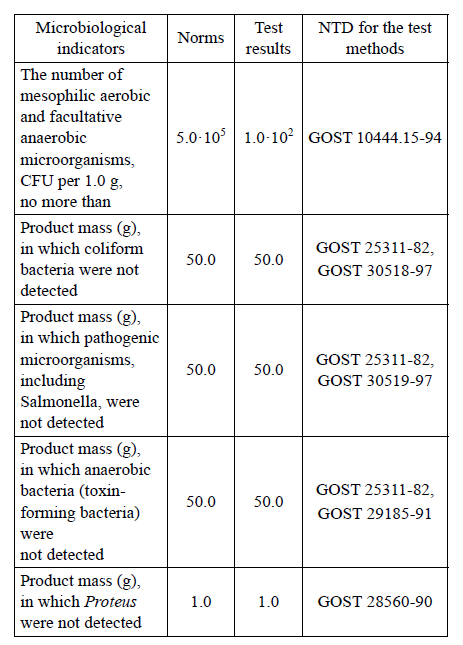
Further on, the microbiological parameters of the hydrothermally treated feather-down raw materials (feather) were examined. Table 5 presents the results of these studies.
As it can be seen from the data presented in Table 4, the feather-down raw materials meets the requirements of TU 9219-094-23476484-09 “Hydrolyzed feed flour. Feather protein concentrate” for their microbiological properties (QMAFAnM, coliforms, pathogenic microorganisms, toxin-forming anaerobic bacteria and bacteria of the genus Proteus) and chemical safety parameters.
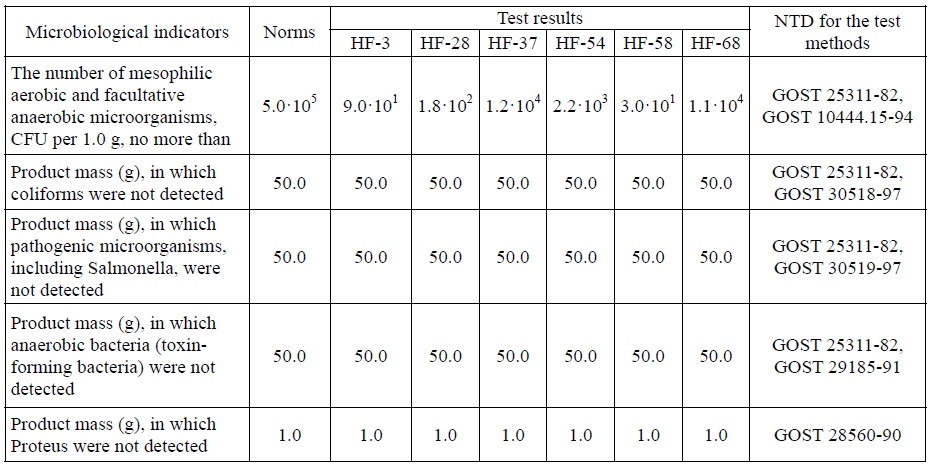
The microbiological properties of six samples of enzymatic hydrolysates were also studied. Table 6 presents the obtained results.
The data of Table 6 show that, according to microbiological properties, the samples of enzymatic hydrolysates of the pre-treated feather-down raw materials, obtained at a laboratory level, correspond to the current hygienic standards for the bacteriological safety of feeds and feed ingredients.
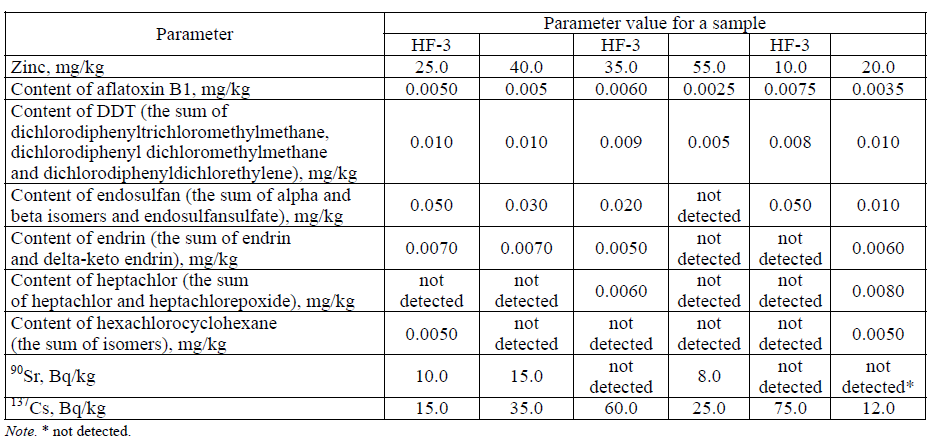
Within the framework of this study, the chemical safety indicators of the hydrothermally treated feather-down raw materials (feather) were studied. The study considered the content of lead, cadmium, arsenic and mercury, copper, zinc, aflatoxin B1, dichlorodiphenyltrichloromethylmethane, endosulfan, endrin, heptachlor and hexachlorocyclohexane, as well as the content of the 90Sr and 137Cs radionuclides, in the raw materials. Table 7 presents the study results.
The results of the studies presented in Table 7 show that the feather-down raw materials meet the requirements of TU 9219–094–23476484–09 “Hydrolyzed feed flour. Feather protein concentrate”for the chemical safety parameters.
Table 8 presents the results of the study of the physico-chemical indicators of six samples of enzymatic hydrolysates of the hydrothermally pretreated feather-down raw materials obtained in the course of a multifactorial experiment to optimize the conditions for the enzymatic hydrolysis of featherdown raw materials at a laboratory level.
It follows from Table 8 that the content of toxic elements and radionuclides in the enzymatic hydrolysates of feather-down raw materials, obtained under optimal conditions of hydrolysis at a laboratory level, corresponds to the safety parameters specified in TU 9219–094–23476484–09 "Hydrolyzed feed flour. Feather protein concentrate".
Thus, the following results were obtained in the course of this study:
(1) The physico-chemical properties of the enzymatic hydrolysates of feather-down raw materials obtained during a multifactorial experiment on optimizing the conditions of the enzymatic hydrolysis of featherdown raw materials have been studied at a laboratory level. The content of protein and solids in the enzymatic hydrolysates of feather-down raw materials varies in the range of 1.80–3.00% and 2.6–4.4%, respectively. The mass fraction of protein, in this case, is 67.7–70.6% in terms of the mass fraction of moisture. The mass fraction of ash in terms of the mass fraction of moisture in all the samples of enzymatic hydrolysates does not exceed 0.43%, the mass fraction of fat - 0.74%, the mass fraction of crude fiber - 1.28%, the mass fraction of sodium chloride - 1.92% and the mass fraction of the impurities insoluble in hydrochloric acid - 0.77%.
(2) The molecular mass distribution of the enzymatic hydrolysates of keratin-containing raw materials of hydrolysates with the maximum yield of solids or the highest antioxidant capacity in relation to a peroxy radical has been analyzed. The elution profiles of the hydrolysates obtained when incubating the keratincontaining raw materials in a medium without the addition of an enzyme and sodium sulfite were characterized by the presence of well-marked peaks for 11 min of elution. In addition, there were peaks of low intensity that corresponded to low-molecular compounds (13–14 min). The HF-58 sample, obtained when hydrolyzing the keratin-containing raw materials with a sodium sulfite content of 1.25% for 2 hours, with the hydromodule 4 and the activity of EP for Protolad B of 30 U/g, had the highest fraction (46.94%) of lowmolecular compounds of the range < 3 kDa. The increase in the hydromodule to 8 (the HF-68 sample) resulted in a shift in the size of peptide molecules towards the range of molecular weights > 10 kDa. There was predominance in the content of peptides of an average molecular weight of 3-10 kDa (39–57%) in the samples HF-3, HF-28, HF-37 and HF-54.
(3) The microbiological properties of the enzymatic hydrolysates of feather-down raw materials obtained during a multifactorial experiment on optimizing the conditions of the enzymatic hydrolysis of featherdown raw materials have been studied at a laboratory level. It has been shown that all the samples of enzymatic hydrolysates of the pretreated feather obtained at a laboratory level correspond to the parameters of microbiological safety specified in TU 9219–094–23476484–09 “Hydrolyzed feed flour. Feather protein concentrate” for their microbiological properties (QMAFAnM, coliforms, pathogenic microorganisms, toxin-forming anaerobic bacteria and bacteria of the genus Proteus).
(4) The chemical safety properties of the enzymatic hydrolysates of feather-down raw materials obtained during a multifactorial experiment on optimizing the conditions of the enzymatic hydrolysis of featherdown raw materials have been determined at a laboratory level. It has been shown that the experimental samples of enzymatic hydrolysates of feather-down raw materials completely correspond to the parameters of chemical safety in accordance with TU 9219–094–23476484–09 “Hydrolyzed feed flour. Feather protein concentrate” for the chemical and radiation safety parameters.
БЛАГОДАРНОСТИ
The study was carried out within the framework of a comprehensive project funded by the Ministry of Education and Science of the Russian Federation in the framework of the Government Decision No. 218 “Organization of high-technology production of highprotein feed additives and biofertilizers based on complex processing of downy raw materials and other low-value poultry waste” under State Contract No. 02.G25.31.0151 dated 01.12.2015. General contractor: Federal State Budget Educational Institution of Higher Education “Kemerovo Institute of Food Science and Technology (University)”.
СПИСОК ЛИТЕРАТУРЫ
- Grozina A.A. Gut microbiota of broiler chickens influenced by probiotics and antibiotics as revealed by T-RFLP AND RT-PCR. Agricultural Biology, 2014, no. 6, pp. 46-58. (In Russian).
- Djavadov E.D. Diagnosis and prevention new nfectious diseases of birds. Farm Animals, 2013, no. 2(3), pp. 69-75. (In Russian).
- Dolgov V.A. and Lavina S.A. Methodological aspects of veterinary-sanitary expertise of food raw materials and food products. Problems on Veterinary Sanitation, Hygiene and Ecology, 2016, no. 3(19), pp. 11-19. (In Russian).
- Dorozhkin V.I., Butko M.P., Gerasimov A.S., Poskonnaya T.F., and Belousov V.I. Tasks for maintenance of veterinary-sanitary safety in the manufacture and sale of products of animal origin to the Russian Federation. Problems on Veterinary Sanitation, Hygiene and Ecology, 2016, no. 1, pp. 6-16. (In Russian).
- Kononenko S.I. Actual problems in organization of feeding in modern conditions. Polythematic online scientific journal of Kuban State Agrarian University, 2016, no. 115, pp. 951-980. (In Russian).
- Kononenko S.I. Highly efficient method for productivity increase. Journal of proceedings of the Gorsky SAU, 2016, vol. 53, no. 1, pp. 67-70. (In Russian).
- Okolelova Т.М. and Korolev A.V. An alternative to antibiotic growth promoters. Ptitsevodstvo [Poultry], 2016, no. 8, pp. 24-26. (In Russian).
- Oliva T.V. and Nikolaeva I.V. Opyt primeneniya netraditsionnykh kormovykh dobavok [Experience of application of non-traditional feed additives]. Advances in current natural sciences, 2007, no. 12, pp. 228. (In Russian).
- Smirnova I.R., Satyukova L.P., and Shopinskaya M.I. Organoleptic evaluation of poultry meat when using protein hydrolyzate-based feedstuff''s. Problems on Veterinary Sanitation, Hygiene and Ecology, 2016, no. 4(20), pp. 6-10. (In Russian).
- Kairov V.R., Gazzaeva M.S., Khugaeva S.V., and Levanov D.T. Economic and biological indicators of meat poultry and pigs when using biologically active preparations for feeding. Polythematic online scientific journal of Kuban State Agrarian University, 2014, no. 102, pp. 485-498. (In Russian).
- Blake J.P., Cook M.E., and Miller C.C. Dry extrusion of рoultry processing plant wastes and poultry farm mortatalities. Sixth international symposium on agricultural and food processing wastes. St. Josept, 1990, pp. 123-125.
- Costa J.C., Barbosa S.G., and Sousa D.Z. Effects of pre-treatment and bioaugmentation strategies on the anaerobic digestion of chicken feathers. Bioresource technology, 2012, vol. 120, pp. 114-119. DOI: 10.1016/j.biortech.2012.06.047.
- Keohane P.P., Grimble G.K., and Brown B. Influence of protein composition and hydrolysis method on intestinal absorption of protein in man. Gut, 1985, vol. 26, no. 9, pp. 907-913. DOI: 10.1136/gut.26.9.907.
- Lasekan A., Abu Bakar F., and Hashim D. Potential of chicken by-products as sources of useful biological resources. Waste management, 2013, vol. 33, no. 3, pp. 552-565. DOI: 10.1016/j.wasman.2012.08.001.
- Milenteva I., Dyshlyuk L., Prosekov A., Babich O., and Shishin M. Deriving biologically active peptides and study of their qualities. Science Evolution, 2016, vol. 1, no. 2, pp. 20-33. DOI: 10.21603/2500-1418-2016-1-2-20-33.
- Mukherjee A.K., Rai S.K., and Bordoloi N.K. Biodegradation of waste chicken-feathers by an alkaline betakeratinase (Mukartinase) purified from a mutant Brevibacillus sp strain AS-S10-II. International biodeterioration & biodegradation, 2012, vol. 65, no. 8, pp. 1229-1237. DOI: 10.1016/j.ibiod.2011.09.007.
- Pedersen M.B., Plumstead P. S.Yu, and Dalsgaard S. Comparison of four feed proteases for improvement of nutritive value of poultry feather meal. Journal of Animal Science, 2012, vol. 90, no. 4, pp. 350-352. DOI: 10.2527/jas.53795.
- Piskaeva A.I., Sidorin Yu.Yu., Dyshlyuk L.S., Zhumaev Yu.V., and Prosekov A.Yu. Research and influence of silver clusters on decomposer microorganisms and E. Coli bacteria. Foods and Raw Materials, 2014, no. 1, pp. 62-66. DOI: 10.12737/4136.
- Rad Z.P., Tavanai H., and Moradi A.R. Production of feather keratin nanopowder through electrospraying. Journal of Aerosol Science, 2012, vol. 51, pp. 49-56. DOI: 10.1016/j.jaerosci.2012.04.007.
- Surkov I.V., Prosekov A.Yu., Ermolaeva E.O., et al. Evaluation and preventing measures of technological risks of food production. Modern Applied Science, 2015, vol. 9, no. 4, pp. 45-52. DOI: 10.5539/mas.v9n4p45.