Аннотация
Meat technological processing has a significant effect on the dynamics and depth of the oxidative processes. The study on the antioxidative activity of the extracts, obtained from the salted meat samples before and after the thermal treatment, was carried out in order to establish a mechanism of action of the main technological processes (meat salting and thermal treatment) on the oxidative changes. The subjects of the research were the samples of pork Longissimus dorsi muscles, which were minced and salted with sodium chloride in the amount of 0.0, 2.0, 3.5 and 5.0%. The antioxidative activity was determined by the rate of oxidation of reduced by oxygen form of 2.6-Dichlorophenolindophenol. The catalase activity was measured spectrophotometrically by the rate of hydrogen peroxide decomposition; the superoxide dismutase activity was measured by the rate of inhibition of the pyrogallol autoxidation; the glutathione peroxidase activity was measured by the rate of NADPH decomposition. The research was carried out at V.M. Gorbatov Federal Research Center for Food Systems of RAS (Russia). According to the study results, meat salting initiated a decrease in the meat antioxidative activity by 9.2-18.0% depending on the sodium chloride concentration. Meat salting with sodium chloride in the amount of 5.0% led to a decrease in the activity of glutathione peroxidase by 24.6%, catalase by 60.1% and superoxide dismutase by 33.7%. The correlation dependence between the antioxidative activity and catalase activities, as well as between superoxide dismutase and glutathione peroxidase activity was revealed: the absolute values of the correlation coefficients were 0.97, 0.99 and 0.94 respectively. In the conducted research a decrease in the meat antioxidative activity by 22.9-28.3% (р < 0.05) was recorded under the action of high temperatures (72 ± 2°С) as a result of catalase inactivation and catalase partial inactivation of superoxide dismutase and glutathione peroxidase. The thermal treatment neutralized the sodium chloride negative effect on the antioxidative activity and activity of the meat antioxidative enzymes (р > 0.05). The obtained results on a decrease in the antioxidative activity in the meat salting process justify the necessity to develop approaches that allow reducing the sodium chloride content in meat products in order to retard the oxidative changes without deterioration of product consumer characteristicsКлючевые слова
Antioxidative activity, glutathione peroxidase, catalase, superoxide dismutase, sodium chlorideВВЕДЕНИЕ
The fat oxidation processes have long been a subject of fundamental and applied research in terms of studying the mechanisms, process dynamics, methods of short-stop, effect on the product quality etc. A comprehensive interest in the problem of fat oxidation in meat products is due to significant influence of the lipids chemical modifications on the formation of the quality and safety of meat and meat products, including nutritional value and consumer characteristics (color, taste, smell, consistency) [1–3]. Prevention of fat oxidation is of paramount importance for meat industry, boosting production of quality products and increase in their shelf life.
Antioxidative system of meat is presented by non-enzymatic components (transferrin, vitamins A, E, K etc.) and by antioxidative enzymes (superoxide dismutase, catalase, glutathione peroxidase etc.) [4–6]. Fat-soluble bioantioxidants (phospholipids, tocopherols, vitamin A, carotenoids, ubiquinone, vitamin K complex, steroid hormones) carry out their protective function in biological membranes; water-soluble bioantioxidants (ascorbic, citric, nicotinic and benzoic acids; sulfurcontaining compounds: cysteine, homocysteine, lipoic acid; phenolic compounds: polyphenols, flavonoids; ceruloplasmin, transferrin, lactoferrin, albumin, urea, uric acid) carry out their protective function in the cytosol of cells, inter-cellular fluid, blood plasma and lymph [7]. However, the total antioxidative activity of meat raw materials depends on the amount of antioxidants and their interaction, as well as on the presence of substances, which themselves do not have anti- or pro-oxidative effect, but can intensify or retard the effect of antioxidants.
A number of technological factors influence on the dynamics and depth of oxidative processes course: moisture mass fraction, heat treatment, light, type of packaging etc. [8–10]. Food ingredients, used in the production of meat products, also play a significant role in the formation of oxidation products [11, 12]. Sodium chloride, food phosphates, citrates, ascorbates etc. should be noted among such components. However, if salts of phosphoric, citric and ascorbic acids are known for their antioxidative properties, then the available data, concerning the nature of the sodium chloride action on the lipids hydrolysis and oxidation, as well as concerning the mechanisms of these changes, are quite controversial [13, 14].
It is known that oxidative processes are often due to the atmospheric oxygen action and can be suppressed or significantly retarded due to the natural complex of antioxidative defense of an organism, which includes a number of enzymes and native hydrophobic and hydrophilic biologically active compounds; in this context, the objective of this work was to establish the dynamics of impact of technological doses of sodium chloride on antioxidative activity of meat with the case study of Longissimus dorsi. This involved the study of the antioxidative activity and the activity of antioxidative enzymes (catalase, superoxide dismutase and glutathione peroxidase) in supernatant, extracted from meat samples.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
The objects of the study were the samples of pork Longissimus dorsi muscles of the big white female 2nd-category-pork of 2 years old. Taking into account the technological doses of sodium chloride in sausage and meat products recipes, the range of sodium chloride concentration was chosen from 2.0 to 5.0% for research. In order to distribute salt in meat raw materials regularly, meat was minced with a mincing machine through the lattice holes of 2–3 mm and was salted with sodium chloride according to State Standard R 51574 in the amount of 0.0; 2.0; 3.5 and 5.0%. Taking into account the small degree of meat mince, the prepared samples were kept during 24 h at a temperature of 4 ± 2°C, after which they were packed in vacuum and subject to thermal treatment until the temperature is 72 ± 2°C. Determination of antioxidative activity and the activity of antioxidative enzymes was carried out before and after thermal treatment.
The extraction was performed according to the method, described by Hernandez et al., with some modification [15]. The minced pork samples were subject to extraction with laboratory dispersing equipment (LDE) (Labotex, Russia) with the use of 0.05 M phosphate buffer (pH 7) as an extractant during 3 minutes, at the volumes ratio of the extracted sample and extractant solution of 1 : 5, temperature of 4–5°C and the stirring speed of 5000 rpm. The extract was separated by centrifugation at a speed of 15.000 rpm during 15 min at a temperature of 4.0°C with the centrifuge Sigma 3K30 (Germany). The supernatant was filtered through the glass wool Sigma-Aldrich (Germany).
To obtain supernatant from the thermally treated raw material, it was minced with a mincing machine through the lattice holes of 2–3 mm, then it was subject to extraction with LDE (Labotex, Russia) with the use of 0.05 M phosphate buffer (pH 7) as an extractant during 3 minutes, at the volumes ratio of the extracted sample and extractant solution of 1 : 5, temperature of 4–5°C and the stirring speed of 5000 rpm. The extract was separated by centrifugation at a speed of 15.000 rpm during 15 min at a temperature of 4.0°C with the centrifuge Sigma 3K30 (Germany). The supernatant was filtered through the glass wool Sigma-Aldrich (Germany).
Determination of antioxidative activity was based on the registration of the oxidation rate of reduced 2.6-Dichlorophenolindophenol (2.6-DCPIP) by oxygen, dissolved in reaction medium; herewith, colorless leuco form of 2.6-DChPhIPh turned to colored form with maximum absorption at 600 nm [16]. The optical density was measured with the photometer BioChem SA (HTI, USA). Inhibition coefficient (IC) with supernatant of autoxidation 2.6-DCPIP was the indicator of antioxidative activity [16].
Determination of the catalase activity was performed according to the method, described by Jin G. et al. [17] with some modification. 0.1 ml of supernatant was mixed with 2.9 ml 11 Mm of hydrogen peroxide in phosphate buffer (pH 7.0) at room temperature (22 ± 2°C). The resultant mixture was stirred thoroughly, transferred into the cuvettes with the distance between the active faces of 1 cm, and the decrease in optical density of the test samples was measured immediately after the start of the reaction and after 3 min of incubation at a wavelength of 240 nm with the photometer KFK-3-01 “ZOMZ” (Zagorsk, Russia). The calculation of the decrease in the hydrogen peroxide concentration was produced taking into account the extinction coefficient of 39.4 M-1cm-1. As a catalase activity unit, the amount of hydrogen peroxide in mmol was taken, which is decomposed during 1 min while adding the supernatant, obtained by extraction of 1 g of meat; the results were expressed in U/g of meat.
Determination of the superoxide dismutase activity was performed by the method, described by Gatellier P. et al. [18] by measuring the pyrogallol autoxidation inhibition. 75 μl of supernatant were mixed with 75 μl 10 Mm of pyrogallol solution in 2850 μl 50 Mm of phosphate buffer (pH 8.2). The resultant mixture was stirred thoroughly, transferred into the cuvettes with the distance between the active faces of 1 cm, and the decrease in optical density of the test samples was measured immediately after the start of the reaction and after 2 min of incubation at a wavelength of 340 nm with the photometer KFK-3-01 “ZOMZ” (Zagorsk, Russia). The pyrogallol autoxidation was determined in the blank sample in the same reaction mixture, adding, instead of the supernatant, the same volume of distilled water (blank sample). As a superoxide dismutase activity unit, the ability of the sample to inhibit 50% of the reaction was taken; the results were expressed in U/g of meat.
Determination of the glutathione peroxidase activity was performed according to the method, described by Jin G. et al. [17] with some modification. The reaction mixture was prepared, containing 1 ml 75 mM of phosphate buffer (pH 7.0), 10 μL 150 mM of reduced glutathione, 10 μL 46 E/mL of glutathione reductase, 30 μL 25 mM of EDTA, 30 μL 5 mM of NADPH, 200 μL of supernatant and 10 μL of 20%-TritonX-100. The volume of the ready mixture was 1.5 ml. Addition of 50 μL of 7.5 mM H2O2 initiated the reaction. Conversion of NADPH into NADP + was registered with the photometer KFK-3-01 “ZOMZ” (Zagorsk, Russia) at a wavelength of 340 nm during 3 min taking into account the extinction coefficient of 6220 M-1cm-1. As a glutathione peroxidase (E) activity unit, the amount of mol of NADPH was taken, which is decomposed during 1 min while adding the supernatant, obtained by extraction of 1 g of meat; the results were expressed in U/g of meat.
All experiments were carried out in triple replication. Statistical processing of the results, determination of the Pearson correlation coefficients and the approximation values of reliability were performed using the programme MS Excel. Assessment of the statistical significance of the differences between parameters was performed using the Student's t-test.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
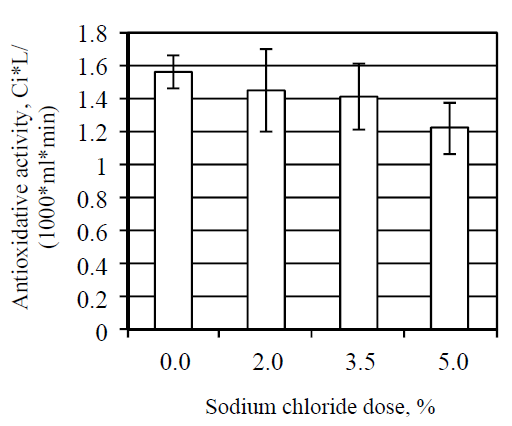
Meat salting had a significant influence on the meat antioxidative activity. Statistical processing of the obtained data showed that the change in the antioxidative activity with increase in salt concentration occurred in a linear fashion.
According to the obtained results (Fig. 1), addition of sodium chloride in the amount of 2.0, 3.5 and 5.0% led to a decrease in the meat antioxidative activity by 9.2% (p < 0.05), 13.3% (p < 0.05) and 18.0% (p < 0.05) respectively.
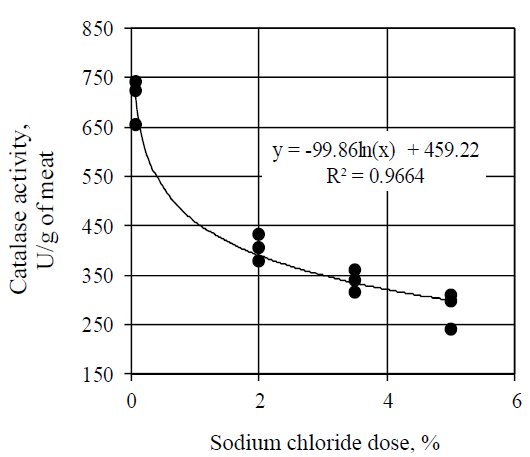
Taking into account the significant role of antioxidative enzymes as a natural antioxidative protection, the identified regularity of changes in the antioxidative activity of the meat supernatant, depending on the sodium chloride concentration, required a more detailed study of the main components of the meat antioxidative system: of catalase, which catalyzes decomposition of hydrogen peroxide with formation of water and oxygen; of superoxide dismutase, which initiates the conversion of superoxide into oxygen and hydrogen peroxide, and of glutathione peroxidase, which catalyzes the recovery of peroxides due to tripeptide-glutathione [19].
It is known that the antioxidative activity indicates the total protection of meat system from peroxidation, which is toxic for the structures of cell membranes and for the functional activity of proteins-enzymes. The increase in the antioxidative activity indicates a high ability to withstand the effects of factors, which activate free radical oxidation of lipids; decrease, on the contrary, indicates a reduction of antioxidative defense. Thus, salting weakens the natural defense of the meat system from oxidation and thereby initiates the oxidative changes in meat.
Catalase is a heme-containing enzyme, which decomposes hydrogen peroxide in meat and meat products. As a result, decrease in the catalase activity in meat initiates lipids peroxidation. According to the research results, sodium chloride inhibited the catalase activity (Fig. 2). Addition of sodium chloride in the amount of 2.0, 3.5 and 5.0% led to a decrease in the catalase activity by 42.6% (p < 0.05), 52.2% (p < 0.05) and 60.1% (p < 0.05) respectively.
Increase in doses of sodium chloride led to a decrease in the catalase activity by the logarithmic law. The obtained data are consistent with the research results of Jin G. et al., who identified the inhibitory influence of sodium chloride on the catalase activity in a dry-cured ham [17].
On the contrary, Lee et al. stated that there was no significant effect of sodium chloride in the amount of 2.0% on the pork catalase activity in the process of freezing, which is obviously due to the leveling effect of salt on the catalase activity as a result of its stabilization at low subzero temperatures [20].
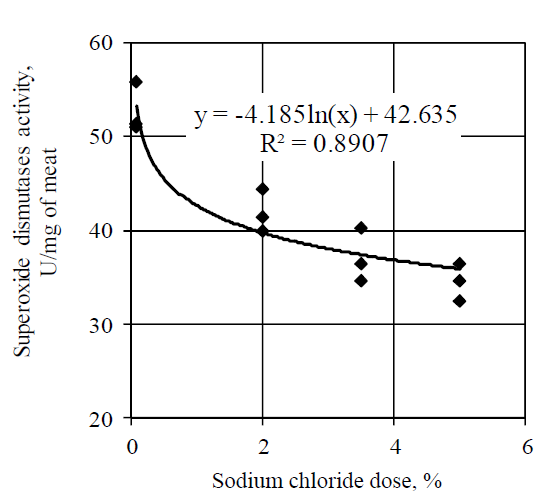
Similar tendency was observed while studying the effect of salt on the superoxide dismutase activity (Fig. 3). Increase in doses of salt decreased the superoxide dismutase activity by the logarithmic law. According to the obtained results, addition of sodium chloride in the amount of 2.0, 3.5 and 5.0% led to a decrease in the superoxide dismutase activity by 20.8% (p < 0.05), 28.6% (p < 0.05) and 33.7% (p < 0.05) respectively.
Similar results were obtained in the works of Jin G. et al., according to the conclusions of which addition of sodium chloride in the amount of 5.0% led to a decrease in the superoxide dismutase activity in comparison with the samples of dry-cured ham, salted in 1.0% of sodium chloride. Lee et al. found out the inhibition of the superoxide dismutase activity under the action of salt in minced and frozen pork.
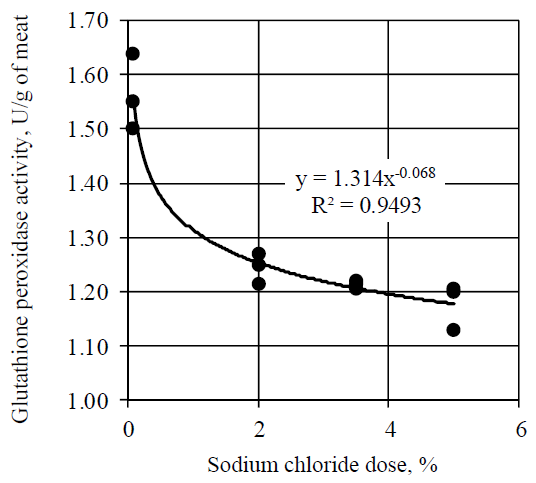
Sodium chloride had also an inhibitory effect on the glutathione peroxidase (Fig. 4). Decrease in the glutathione peroxidase activity, depending on the sodium chloride dose, occurred by the power law.
Addition of sodium chloride in the amount of 2.0, 3.5 and 5.0% led to a decrease in the glutathione peroxidase activity by 20.4% (p < 0.05), 22.4% (p < 0.05) and 24.6% (p < 0.05) respectively.
According to the results, obtained in the present experiment, sodium chloride had a less pronounced effect on the glutathione peroxidase activity in comparison with its effect on catalase and superoxide dismutase. Thus, meat salting with sodium chloride in the amount of 5.0% led to a decrease in the activity of glutathione peroxidase by 24.6% in comparison with the decrease in the catalase activity by 60.1% and in superoxide dismutase by 33.7%.
The obtained research results were partially consistent with the ones of Jin G. et al. [17] and of Lee et al. [20], who determined a negative correlation between the salt concentration and the activity of meat antioxidative enzymes. However, the research results of Jin G. et al. showed the smallest decrease in the superoxide dismutase activity at salting in comparison with the rest antioxidative enzymes, while Lee et al. determined the maximum decrease in the superoxide dismutase activity upon addition of 0.5–2.0% of sodium chloride in comparison with the catalase and glutathione peroxidase activity. According to the data, obtained by Gheisari H. R. and Eskandari M., the addition of sodium chloride slows down the inhibition of the glutathione peroxidase activity while keeping in salting in comparison with the unsalted meat [21].
Such contradictory data are obviously due to the methodological inconsistency of studies and the different selection of experiment objects. Thus, Jin G. et al., for example, studied the impact of sodium chloride on the meat antioxidative activity in the process of production of dry-cured bacon. Lee et al. to determine the antioxidative enzymes activity used sodium chloride not in the process of meat salting, but added it in the previously prepared supernatant, isolated from unsalted meat.
It is known that thermal treatment causes denaturation of enzymes. Thus, the catalase activity decreases at a temperature above 35°C and is completely inhibited at incubation at 65°C during 5 min [22]. Glutathione peroxidase denaturation is observed after incubation at a temperature of 70°C during 40 min [23], while superoxide dismutase is inactivated under at a temperature of 80–100°С [24].
Taking into account the fact that thermal stability of the protein components can be influenced by ionic strength [25], the effect of sodium chloride on the antioxidative activity and the antioxidative enzymes activity in thermal-treated meat products was studied.
According to the obtained data, the antioxidative activity of the studied samples after thermal treatment decreased by 22.9–28.3% (p < 0.05) (Fig. 5).
Nevertheless, the addition of salt had no significant effect on the value of the meat antioxidative activity (p > 0.05).
The research results showed that thermal treatment up to 72 ± 2°C did not lead to complete destruction of superoxide dismutase, the activity of which, however, decreased by 74.1–90.0% (p < 0.05) in comparison with the values before thermal treatment. The obtained research results were consistent with the ones of Sharapov M. G. at al., according to which at a temperature of 75°С there was a decrease in the superoxide dismutase activity by ~ 80% in comparison with its activity at 25°C [26]. The superoxide dismutase high thermostability is associated with a large number of sulfhydryl groups in its structure [27].
The catalase activity in meat raw materials after thermal treatment was not detected.
Thermal treatment with a temperature of up to 72 ± 2°C influenced the glutathione peroxidase activity to a lesser extent, its decrease was 24.6–31.8% (p < 0.05) of the initial value before the thermal treatment (Fig. 6), which is obviously due to the manifestation of the maximum efficiency of glutathione peroxidase at 37–40°С [28] and its negligible activity at the determined during the experiment temperatures of the samples - at 4 ± 2°C (before thermal treatment) and after reaching 72 ± 2°C. The obtained research results were consistent with the ones of Hoac T. et al., who stated, based on the study of temperature impact on the poultry antioxidative enzymes activity, that at a temperature of 60 C, 20% of glutathione peroxidase were destroyed, and at a temperature increase up to 80 C ~ 80% of enzyme were denatured [29].
It should be noted that the salt content had no significant effect on the activity of superoxide dismutase and glutathione peroxidase of the meat samples after thermal treatment (p > 0.05 in comparison with unsalted meat).
Partial preservation of the samples antioxidative activity after thermal treatment, apparently, is explained by the content of non-enzymatic components in the meat antioxidative system, which are involved in the compensation of oxidative processes and which also retain partial activity of superoxide dismutase and glutathione peroxidase. Besides, it is known that glutathione peroxidase has a greater affinity with hydrogen peroxide in comparison with catalase [27]. Therefore, this enzyme plays a significant role in the formation of antioxidative defense. In this regard, preservation of up to 68.2% of the glutathione peroxidase activity, resulted in thermal treatment, obviously contributed significantly to the formation of the antioxidative activity of thermally treated meat raw material.
It should be noted that still the researches have not reached a consensus on the interrelation of the antioxidative enzymes activity and the oxidative changes of meat and meat products. Thus, according to the results of the works of Hernandez et al. [15] and Jin G. et al. [17], a negative correlation between the antioxidative enzymes and the formation of secondary oxidation products was determined. However, Sаrraga C. et al. found that a higher level of the glutathione peroxidase activity during the addition of sodium chloride was accompanied by an increase in the amount of malonic aldehyde in dry-cured pork products. Scientists explain the presented results by the sodium chloride ability to stabilize muscle tissue, which decreases the importance of antioxidative enzymes for inhibition of oxidative processes [30]. In addition, meat is a multi-component system, containing biologically active substances that can have an indirect impact on pro- and antioxidative effect of sodium chloride. Therefore, the mechanism of impact of meat salting on the dynamics of oxidative changes in animal raw materials is still the subject of further study.
The results of this research contribute significantly to the study of influence of sodium chloride on oxidative changes. It is stated that addition of sodium chloride decreases the meat antioxidative activity as a result of inhibition of the antioxidative enzymes activity: of catalase, superoxide dismutase and glutathione peroxidase. The data analysis confirmed the existence of correlation dependence between the total antioxidative activity and the catalase, superoxide dismutase and glutathione peroxidase activity in meat raw materials: the absolute values of the Pearson correlation coefficients were 0.97, 0.99 and 0.94, respectively. The use of thermal treatment neutralized the negative impact of sodium chloride on the antioxidtive enzymes activity. Taking into account the fact that decrease in the amount of sodium chloride in meat products will result in deterioration of its taste and technological characteristics, it is necessary to use approaches allowing developing technological recommendations for varying doses of sodium chloride through the use of its substitutes in production of meat products.
СПИСОК ЛИТЕРАТУРЫ
- Niki E., Yoshida Y., Saito Y., and Noguchi N. Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochemical and Biophysical Research Communications, 2005, vol. 338, no. 1, pp. 668-676. DOI: 10.1016/j.bbrc.2005.08.072.
- Min B. and Ahn D.U. Mechanism of lipid peroxidation in meat and meat products - a review. Food Science and Biotechnology, 2005, vol. 14, pp. 152-163.
- Summo C., Caponio F., Paradiso V.M., Pasqualone A., and Gomes T. Vacuum-packed ripened sausages: Evolution of oxidative and hydrolytic degradation of lipid fraction during long-term storage and influence on the sensory properties. Meat Science, 2010, vol. 84, no. 1, pp. 147-151. DOI: 10.1016/j.meatsci.2009.08.041.
- Hernandez P., Zomeno L., Arino B., and Blasco A. Antioxidant, lipolytic and proteolytic enzyme activities in pork meat from different genotypes. Meat Science, 2004, vol. 66, pp. 525-529. DOI: 10.1016/S0309-1740(03)00155-4.
- Arthur J.R. The glutathione peroxidases. Cellular and Molecular Life Science, 2000, vol. 57, pp. 1825-1834. DOI: 10.1007/PL00000664.
- Pradhan A.A., Rhee K.S., and Hernández P. Stability of catalase and its potential role in lipid oxidation in meat. Meat Science, 2000, vol. 54, pp. 385-390. DOI: 10.1016/S0309-1740(99)00114-X.
- Kormosh N.G. Physiological role of a reactive oxygen species (subcellular level) - clinician viewpoint. Russian Journal Biotherapy, 2011, vol. 10, no. 4, pp. 29-35. (In Russian).
- Jin G., Zhang J., Yu X. et al. Lipolysis and lipid oxidation in bacon during curing and drying-ripening. Food Chemistry, 2010, vol. 123, no. 2, pp. 465-471. DOI: 10.1016/j.foodchem.2010.05.031.
- Neethling N.E., Hoffman L.C., and Britz T.J. An investigation regarding use of carbon monoxide for colour stability and inhibition of lipid and protein oxidation in meat. 59th International Congress of Meat Science and Technology, Izmir, Turkey, 2013, pp. 5-17.
- Cobos A., Veiga A., and Diaz O. Chemical and lipid composition of deboned pieces of dry-cured pork forelegs as affected by desalting and boiling: The effects of vacuum packaging. Food Chemistry, 2008, vol. 106, no. 3, pp. 951-956. DOI: 10.1016/j.foodchem.2007.07.007.
- Nuñez De Gonzalez M.T., Boleman R.M., Miller R.K., Keeton J.T., and Rhee K.S. Antioxidant properties of dried plum ingredients in raw and precooked pork sausage. Journal of Food Science, 2008, vol. 73, no. 5, pp. 63-71. DOI: 10.1111/j.1750-3841.2008.00744.x.
- Kiliç B., Şimşek A., Claus J.R., and Atilgan E. Encapsulated phosphates reduce lipid oxidation in both ground chicken and ground beef during raw and cooked meat storage with some influence on color, pH, and cooking loss.Meat Science, 2014, vol. 97, no. 1, pp. 93-103. DOI: 10.1016/j.meatsci.2014.01.014.
- Kristensen L. and Purslow P.P. The effect of processing temperature and addition of mono- and di-valent salts on the heme- nonheme-iron ratio in meat. Food chemistry, 2001, vol. 73, no. 4, pp. 433-439. DOI: 10.1016/S0308- 8146(00)00319-8.
- Min B., Cordray J.C., and Ahn D.U. Effect of NaCl, myoglobin, Fe(II), and Fe(III) on lipid oxidation of raw and cooked chicken breast and beef loin. Journal of Agricultural and Food Chemistry, 2010, vol. 58, no. 1, pp. 600-605. DOI: 10.1021/jf9029404.
- Hernández P., Park D., and Rhee K.S. Chloride salt type/ionic strength, muscle site and refrigeration effects on antioxidant enzymes and lipid oxidation in pork. Meat Science, 2002, vol. 61, no. 4, pp. 405-410. DOI: 10.1016/S0309-1740(01)00212-1.
- Kondrakhin I.P. (ed.) Metody veterinarnoy klinicheskoy laboratornoy diagnostiki: Spravochnik [Methods of veterinary clinical laboratory diagnostics: Reference book]. Moscow: KolosS Publ., 2004. 520 p.
- Jin G., He L., Yu X., Zhang J., and Ma M. Antioxidant enzyme activities are affected by salt content and temperature and influence muscle lipid oxidation during dry-salted bacon processing. Food Chemistry, 2013, vol. 141, no. 3, pp. 2751-2758. DOI: 10.1016/j.foodchem.2013.05.107.
- Gatellier P., Mercier Y., and Renerre M. Effect of diet finishing gmode (pasture of mixed diet) on antioxidant status of Charolais bovine meat. Meat Science, 2004, vol. 67, pp. 385-394. DOI: 10.1016/j.meatsci.2003.11.009.
- Makhanova R.S. On the problem of peroxide lipid oxidation. Izvestiya Orenburg State Agrarian University, 2011, vol. 1, no. 29-1, pp. 231-234. (In Russian).
- Lee S.K., Mei L., and Decke E.A. Influence of Sodium Chloride on antioxidant enzyme activity and lipid oxidation in frozen ground pork. Meat Science, 1997, vol. 46, no. 4, pp. 349-355. DOI: 10.1016/S0309-1740(97)00029-6.
- Gheisari H. R. and Eskandari M. Effect of curing on camel meat lipid oxidation and enzymatic activity during refrigerated storage. Veterinarski arhiv, 2013, vol. 83(5), pp. 551-562.
- Nadeem S.M.S., Khan J.A., Murtaza B.N., Muhammad K., and Rauf A. Purification and properties of liver catalase from water buffalo (Bubalus bubalis). South Asian Journal of Life Sciences, 2015, vol. 3(2), pp. 51-55. DOI: 10.14737/journal.sajls/2015/3.2.51.55.
- Shulgin K., Popova T., and Rakhmanova T. Isolation and purification of glutathione peroxidase. Applied Biochemistry and Microbiology, 2008, vol. 44, no. 3, рp. 247-250. DOI: 10.1134/S0003683808030034.
- Lubarev A.E. and Kurganov B.I. Izuchenie neobratimoy teplovoy denaturatsii belkov metodom differentsial'noy skaniruyushchey kalorimetrii [The study of the irreversible thermal denaturation of proteins by differential scanning calorimetry]. Uspekhi biologicheskoy khimii [Biological chemistry successes], 2000, vol. 40, pp. 43-84. (In Russian).
- Tunieva E.K. and Dederer I. Study of sodium, potassium, and calcium salts influence on protein stability by differential scanning calorimetry Theory and practice of meat processing, 2016, vol. 1, no. 1, pp. 19-24. DOI: 10.21323/2114-441X-2016-1-19-24. (In Russian).
- Sharapov M.G., Novoselov V.I., and Ravin V.K. Construction of a fusion enzyme exhibiting superoxide dismutase and peroxidase activity. Biochemistry, 2016, vol. 81, no. 4, pp. 571-579. DOI: 10.1134/S0006297916040131. (In Russian).
- Chesnokova N.P., Ponukalina E.V., and Bizenkova M.N Molecular-cellular mechanisms of free radical inactivation in biological systems. Advances in current natural sciences, 2006, no. 7, pp. 29-36. (In Russian).
- Al-Helaly Luay A., Al-kado Obeda A. Partial Purification and Some Kinetic studies of Glutathione Peroxidase (GPx) in Normal Human Plasma and Comparing with Primary Infertility Female. Tikrit Journal of Pure Science, 2013, vol. 18, pp. 36-44.
- Hoac T., Daun C., Trafikowska U., Zackrisson J., and Åkesson B. Influence of heat treatment on lipid oxidation and glutathione peroxidase activity in chicken and duck meat. Innovative Food Science and Emerging Technologies, 2006, vol. 7, no. 1-2, pp. 88-93. DOI: 10.1016/j.ifset.2005.10.001.
- Sárraga C., Carreras I., and García Regueiro J.A. Influence of meat quality and NaCl percentage on glutathione peroxidase activity and values for acid-reactive substances of raw and dry-cured Longissimus dorsi. Meat Science, 2002, vol. 62, no. 4, pp. 503-507. DOI: 10.1016/S0309-1740(02)00039-6.