Аннотация
In the production crystallization of glucose, there are special problems at the nucleation stage, which requires seed crystals. The need for them reaches 10–15% of the weight of the solution, reducing the productivity of equipment. In this paper, the results of the studies on the identification and creation of effective seed crystals for the nucleation of anhydrous glucose in the presence of surface active agents (SAA) have been described. The nucleation process was controlled according to a change in the transparency of solutions and microscopy. The following have been tested as seeds: small (< 60 microns) commercial anhydrous glucose crystals and the same crystals wet with propanol; large (> 200 μm) and small (< 60 μm) hydrate glucose crystals wet with propanol. The large and small hydrate glucose crystals preliminarily wet with propanol or another aliphatic alcohol is recognized as the best of the tested seeds. When these crystals were mixed with a supersaturated glucose solution at a temperature of 60°C, they rapidly (within 15–30 min) disintegrated into a lot of uniform tiny particles with a size of 1–5 μm, became crystallization centers and began to grow rapidly in the form of anhydrous glucose. A similar phenomenon was also observed when nucleating with the hydrate crystals wet with propanol and at a temperature below 50°C. Based on the tests, new types of seed crystals and a method for preparing thereof in the form of alcohol suspensions of ground anhydrous glucose crystals and crystals of any sizes of hydrate glucose have been proposed.Ключевые слова
Glucose, nucleation, seed crystals, surface active agents, alcohol suspensions of crystalsВВЕДЕНИЕ
Crystalline glucose is a valuable food product and medicine. Glucose is used in healthcare, the food and pharmaceutical industries and veterinary medicine. It refers to strategic products, ensuring the health of the nation and the national security of the country. It is vital to use it as solutions for high blood losses, heart failure, shock and other severe conditions of the body. At present, due to lack of domestic production, Russian health care is supplied with crystalline glucose and injection solutions from imports. Improving the crystalline glucose production technology to organize its production in Russia in the order of import substitution is an urgent task.
On an industrial scale, glucose is obtained in a hydrate ɑ-form with crystallization at a temperature below 50°C, in the form of anhydrous ɑ-glucose – within the temperature range of 50–108°C and an anhydrous β-form at a temperature above 108°C. This article is devoted to the study of anhydrous ɑ-glucose crystallization.
In the production of crystalline glucose, the process of its crystallization is the most complex and responsible, since the technological mode of crystallization with high yield and quality of crystals depends on a dozen different physical and technological parameters [1].
In theory, the process of crystallization is conditionally divided into two stages: nucleation and crystal growth. The emergence of a small volume consisting of 10 or 100 molecules of a new phase in a supersaturated mother liquor, from which a viable crystalline embryo of a critical size capable of further growth is formed, is understood by nucleation. The number of molecules or ions forming a nucleus of a critical size is not the same for different substances, and the conditions for existence and growth are also different. The general regularities of the existence of a critical nucleus for various substances were formulated by J. Gibbs, M. Volmer and other researchers [2, 3, 4]. Herewith, there are two types of nucleation: homogeneous – with the self-adherent formation of nuclei and heterogeneous – with the use of seed crystals.
In the process of glucose crystallization, the stage of nucleation of crystals is the most complex, since it is less studied, more difficult to control, the regime disturbance is practically not amenable to correction, and the final result thereof is usually determined onlyat the end of crystallization.
In the production of glucose in anhydrous form, crystals are seeded using a "shock" method – a conventionally homogeneous one and a method for applying the total amount of seed crystals – a heterogeneous one [1]. The "shock" method is used in the crystallization of anhydrous glucose under isothermal conditions (with boiling massecuite in a vacuum apparatus). The nucleation of crystals occurs rapidly in the labile zone of supersaturations of the solution and requires a low expenditure of seed crystals (15–20 g per ton of syrup). This nucleation process can be referred to conditionally homogeneous ones, since the violent nucleation over the whole volume of the solution is caused by a relatively small amount of seed crystals relative to the volume of the solution. The problem of the method consists in the visual, approximate determination of the amount of the new crystallization centers that are formed, which disturbs the optimum crystallization regime, the production of massecuite crystals with inhomogeneous crystals, and the presence of conglomerates [5, 6]. The heterogeneous nucleation method is used in the crystallization of anhydrous glucose under polythermal conditions (with cooling massecuite in a crystallizer), at which the formation of nuclei is much slower and leads not only to the inhomogeneity of the crystals, but also to an increase in the duration of the crystallization process [7, 8]. Due to this, the need for seed crystals significantly increases (up to 5–10% by weight of syrup) in the conditions of industrial crystallization.
The problem of production nucleation is urgent in the preparation of a lot of crystalline substances, since the crystallization process has general basic regularities. In this regard, scientific achievements in the development of new methods for accelerating nucleation in the adjacent production of fructose, lactose, pharmaceutical preparations, aerosols and other chemicals are of interest [9–16].
In the experiments of fructose nucleation [9], the effect of the initial supersaturation of the solution on nucleation has been shown. With the supersaturation coefficients characteristic for the metastable zone, the obtained crystals were comparatively large, but inhomogeneous in size. With higher supersaturations, the crystals were smaller, but more homogeneous. The yield of crystals increased with an increase in supersaturation, reached its maximum, and then decreased because of the problems with the separation of small crystals from the mother liquor. In the experiments with the crystallization of ibuprofen [10], the boundaries of the metastable crystallization zone were determined from the control of the onset of nucleation. At the same time, the boundary concentration of the solution was determined at the time of the appearance of the nuclei fixed visually. In the experiments with a seed, the nucleation was detected immediately near the saturation boundary. In the experiments without a seed, the induction period was long.
In the production of lactose, the acceleration of nucleation and the growth of crystals was achieved by mixing due to the vibrations of the solution [11], bubbling the solution with cold air with short cyclic temperature fluctuations and a total decrease therein in each cycle [12], passing the supersaturated solution flow through a hole controlled by Re = 1000 [13].
In [14] the original methods of homogeneous nucleation of paracetamol under continuous conditions without a seed and traditional solution cooling through the heat exchange surface have been considered. The main means of nucleation activation are the mixing of jets of counterflows of hot (66°C) and cold (22°C) solutions in a ratio of 8 : 1 and the use of radial and coaxial mixing of the combined flows in a mixer. The nucleation activation in this case is due to fluctuations in temperature, concentration and supersaturation in solutions [15]. An important condition for the successful formation of nuclei is the degree of supersaturation of the hot solution and the velocity of jets.
To activate the nucleation of a lot of substances, the ultrasonic treatment of solutions is successfully applied [16–18]. The installation of an ultrasonic generator on a pipe with a continuous flow of the solution accelerated the nucleation of paracetamol and provided the formation of homogeneous nuclei, excluding the secondary nucleation [16]. The authors [17, 18] studied the ultrasonic effect on the sedimentation of particles of glycerine aerosols. The coagulation of aerosol particles and the acceleration of their sedimentation upon the application of an ultrasonic field have been shown. Such an effect of the ultrasonic field is also observed during the coagulation and precipitation of aerosols of dust and smoke particles. The acceleration of coagulation and sedimentation of particles is promoted by the introduction of the additional coagulation centers. In the experimental hydrate glucose crystallization [19], the ultrasound treatment of solutions also accelerated the process of homogeneous nucleation, but did not affect the growth of the nuclei obtained. The small nuclei did not grow to technical sizes with the subsequent propagation, which would allow them to separate from the viscous mother liquor during centrifugation. Therefore, the method was never applied in practice.
Of scientific and practical interest is the activation of nucleation with the help of surface active agents (SAA) due to the fact that according to the theory of crystallization the work of nucleation (Аз) and, correspondingly, the radius of the critical nucleus (rk) depend on the surface tension of solutions, which follows from the known equations for sphericalshaped nuclei [2, 3, 20]:
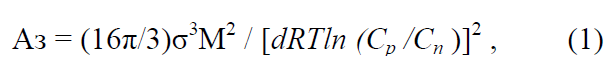
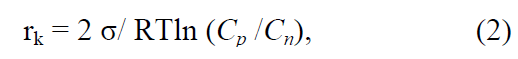
It follows from the equations that the work of formation and the radius of the critical nucleus are directly dependent on the surface tension of the solution, namely, the lower the surface tension of the solution, the sooner the nucleus is formed. Numerous studies confirm the effect of SAA [21–28] on the rate of nucleation, the size and shape of crystals of various substances during condensation or dispersion. The use of a nonionic SAA (KDPG-100) during phosphogypsum crystallization promoted the enlargement of its crystals, an increase in their homogeneity and the improvement of filtration conditions [21]. The experiments [22] show an increase in the number of particles of n-butyl methacrylate during its polymerization, depending on the concentration of SAA of sodium dodecyl sulfate. With the critical SAA concentration that corresponds to colloidal stability, the number of particles increased in proportion to the third degree of SAA concentration. In the next paper [23], as a result of the numerical simulation of the process of nucleation and the growth of nanoparticles in the presence of SAA, it has been revealed that the adsorption of SAA molecules on the surface of nanoparticles slows down their growth in supersaturated solutions. With an increase in SAA concentration, the average size of nanoparticle decreases; at the critical concentration of SAA, when their molecules completely cover the surface of nanoparticles, the growth of the latter completely ceases. In the crystallization of goethite [24], the effect of SAA was expressed not only in an increase in the fraction of fine (2–5 μm) particles, but also in the formation of SAA and goethite compounds
In our experiments [25], under the effect of SAA (distilled acetylated monoglycerides), there was a decrease in the rate of nucleation and the growth of glucose crystals, and the effect of SAA in the form of aliphatic alcohols on glucose nucleation was positive: the surface tension of glucose solutions decreased, the nucleation and crystal growth accelerated. Such a positive effect of the use of aliphatic alcohols is promising for improving the seeding method in glucose production in order to reduce the need for seed crystals and increase the efficiency of equipment.
This article is devoted to the search for effective seeding methods for accelerating the nucleation of glucose using SAA, the creation of new types of seed crystals with a long period of use to simplify the nucleation of crystals.
The study purposes included:
– the tests of various types of seeds with SAA and the selection of the most effective ones for the acceleration of nucleation;
– the determination of the technological mode of preparation, shelf life and use of seed crystals of anhydrous and hydrate glucose with the use of SAA;
– the determination of the excessive concentration (ΔC) and temperature of syrup, most favorable for seeding and nucleation.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
The studies of the nucleation of anhydrous glucose with the use of seeds with SAA were carried out within the temperature range of 55–75°С typical for the mode of glucose production under production conditions [6, 7] of most favorable for seeding and nucleation.
A rotary evaporator with a flask capped to prevent the evaporation of water and alcohol and experimental horizontal crystallizers (0.6 l) equipped with a ribbontype mixer and a water "jacket" was used as experimental units.
The formation of crystal nuclei was determined from the measurement of the transparency of the solution using a FEK-56 photocolorimeter [26]. The transparency of solutions was measured in a 10 mm cuvette with a length of a light filter wave of 580 nm in comparison with the initial solution. The size and shape of the crystals were monitored microscopically using Leica DMLM and MBI-4 microscopes.
The crystallization process was monitored according to a change in the solids of the intercrystalline solution and massecuite [27].
The difference between the amounts of dissolved glucose in the supersaturated and saturated solution (g/100 g of water) was taken as the excess concentration (ΔC):
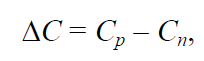
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
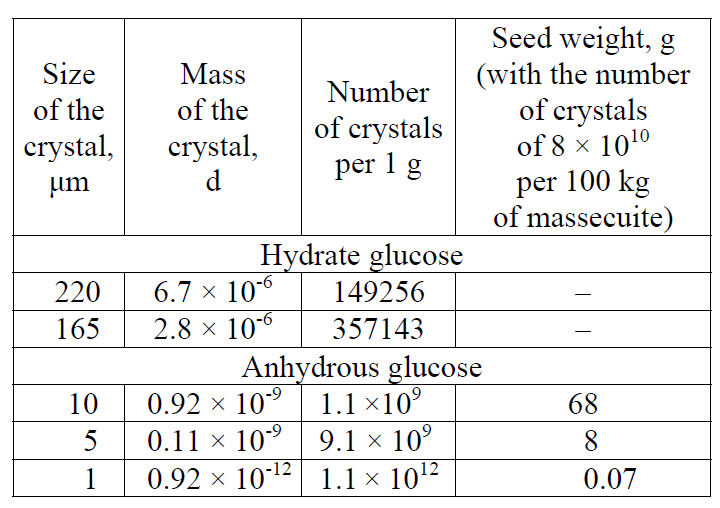
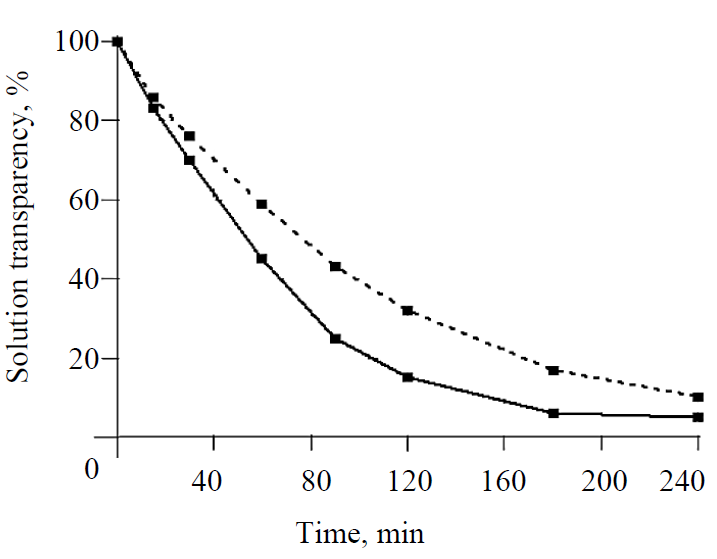
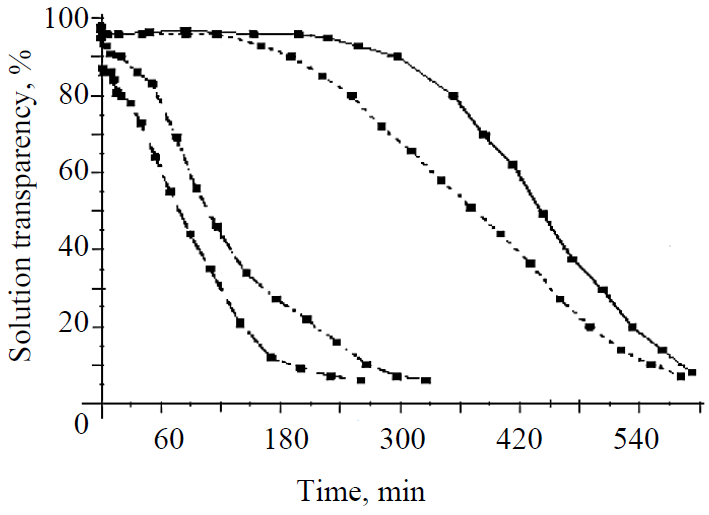
Effect of SAA on the kinetics of glucose nucleation. The basis for carrying out the studies presented in the article was the found accelerating effect of SAA in the form of aliphatic alcohols on glucose nucleation. This set of experiments was the most instructive at 40°C in the form of hydrate glucose. It slowly crystallizes, which allowed the most reliable detection of the effect of SAA on glucose nucleation. Fig. 1 shows the nucleation of glucose nuclei according to a change in the transparency of solutions over time, depending on SAA supplements and seed crystals. First of all, attention is drawn to the difference in the duration of the induction periods, characterized by the unchanged transparency of the solution, equal to about 210 minutes for a pure solution, and the reduction thereof by half for the solution with propanol and practically the complete absence thereof for the solution with a seed and propanol. A crystalline seed has the largest accelerating effect on nucleation, and the presence of propanol significantly enhances it. Such activation is explained by a decrease in the work costs for the formation of a new phase as a result of the introduction of the finished solid phase and propanol, which reduces the surface tension of the solution, into the solution.
Selection of effective seeds for glucose crystallization. The large (> 200 μm) and small (< 60 μm) crystals of commercial hydrate and anhydrous glucose and the same crystals wet in alcohol were tested as seeds. The dosage of a seed was 0.15% by weight of syrup. The nucleation process was controlled according to a change in the transparency of solutions over time.
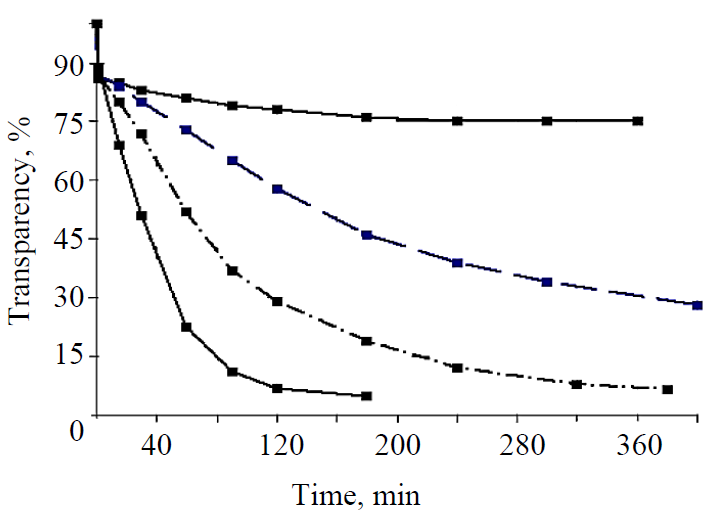
Fig. 2 presents the curves of changes in the transparency of solutions with different seeds.
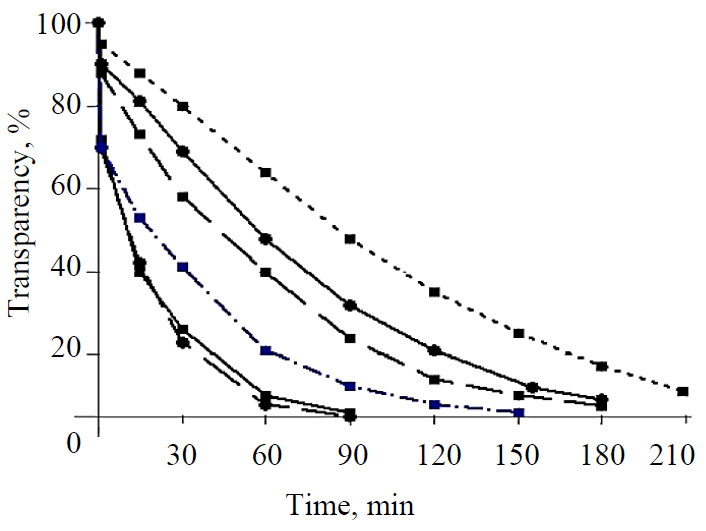
The upper curve with the highest transparency of solutions characterizes the nucleation of glucose with a seed from large crystals of hydrate glucose. It follows from the figure that when the seed crystals were mixed with the solution, the transparency of the solution gradually decreased over time. The microscopic control established the gradual dissolution and destruction of large crystals into inhomogeneous crystalline fragments with a size from 100 to 5 μm and less, which led to a decrease in the transparency of solutions. The following two transparency curves relate to the nucleation of crystals with seeds of commercial anhydrous glucose (no propanol) and small crystals of anhydrous glucose, moistened with propanol. The arrangement of the curves indicates that the nucleation of nuclei with the seed crystals wet with propanol was much faster, which is confirmed by the lower (by 20–30%) transparency of solutions for seeds with propanol. The small seed crystals of commercial anhydrous glucose clumped when they were mixed together with concentrated syrup, they could not be completely divided into separate particles, so that each of them became a separate, independent crystallization center. Otherwise, the coalesced particles join together, forming conglomerates. The best results on nucleation were obtained with the seeds from large-size hydrate glucose crystals wet with alcohol, which is confirmed by the lowest transparency of solutions. When these crystals were introduced into the syrup, they disintegrated into small homogeneous particles of 1–5 μm within 15 to 30 minutes, became crystallization centers and rapidly grew in the form of anhydrous glucose crystals. The same thing happened with the small crystals of hydrate glucose, wet with propanol.
Fig. 3 clearly demonstrates the splitting of large hydrate glucose crystals (a) into small particles with the transformation thereof into anhydrous glucose nuclei (b).
The mechanism of transformation of alcohol wet crystals of hydrate glucose to the centers of crystallization of anhydrous glucose can be explained by several concomitant processes. One of the main such processes is the influence of high temperature of the solution, not peculiar to the conditions of existence of hydrate glucose crystals, which causes the weakening of the connection of crystallization water with glucose molecules in crystals, their decay and dissolution; the second equally active process is the interaction of the alcohol film of the crystal with the solution, namely, when the alcohol is dissolved in the water of the glucose solution in the film surrounding the crystal, the interphase tension decreases, the temperature rises, the volume of the film is compressed, contributing to the rapid destruction of the crystal to the smallest homogeneous particles [28]. Then, as the dispersion and partial dissolution of the crystal are concerned, the temperature of the solution near the surface of the crystals decreases and the supersaturation coefficient increases, which leads to the violent nucleation of anhydrous glucose. The low nucleation heat of anhydrous glucose nuclei is also promoted by the low heat of its crystallization – 2.36 kcal/mol compared to hydrate glucose, which has a heat of crystallization two times higher – 4.72 kcal/mol. Dispersing various crystalline substances using SAA is also known in the derivation of other substances [29, 30].
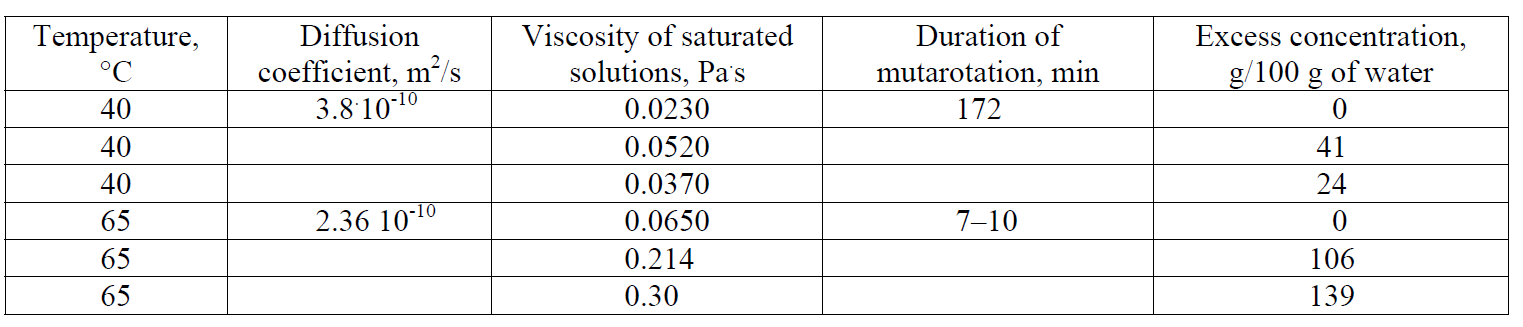
For clarity, Table 1 shows the effect of quantitative conversion during the nucleation of the hydrate glucose crystals wet with propanol into anhydrous glucose nuclei by the example of large crystals (220 μm). According to Table 1, their number in 1 g of hydrate glucose with a crystal size of 220 μm is 149, 256. When these crystals are dispersed in a solution to 1–5 microns in size, the number of the last particles increases to 9.1 × 109 – 1 × 1012, becoming the centers of crystallization of anhydrous glucose. Theoretically, due to this, the seed mass that corresponds to the total demand in crystallization centers – 8 × 1010 per 100 kg of massecuite [1], can be reduced by 1000 times.
Preparation of alcohol seed suspensions. The preserved intactness of glucose crystals in the medium of absolute alcohols [25] served as a basis for the creation of the alcohol suspensions of seed crystals for a long-term use. The method is particularly relevant for the finely ground crystals subject to caking in a conventional way of bulk storage, which makes them unsuitable for seeding. When mixed with a viscous solution, they cannot be divided into separate particles, which leads to the formation of conglomerates. The alcohol suspensions of seed crystals, in contrast to them, are well distributed as separate particles when mixed with a glucose solution.
For glucose production, alcohol seeding suspensions from both anhydrous and hydrate glucose are of interest depending on a method for seeding crystals.
Due to the fact that anhydrous glucose crystals when mixed with a solution are not subjected to dispersion, they should be ground to obtain alcohol suspensions. We obtained the alcohol suspensions of seed crystals with a size of 1–10 microns from anhydrous glucose by mechanically grinding crystalline anhydrous glucose with the crystals of any size by means of a mill, for example PDI-70, with a filter to classify particles by size. The obtained crystals were poured into a container and filled with absolute alcohol in a ratio of 3 : 2. The container with the suspension was sealed and stored at room temperature for the subsequent use as a seed (at any time during a year of storage). One of the following was used as alcohols: propanol, butanol, isopropanol and isobutanol.
The seed crystals of hydrate glucose when mixed with syrup at a temperature above 50°C are dispersed, so there is no need for their mechanical grinding and long-term storage in alcohol. Commercial hydrate glucose crystals, in the absence of signs of caking, and large crystals, for example, grits after glucose sifting, should only be wet with absolute alcohol immediately before the use of them as a seed. Small seed crystals (< 30 microns) require alcohol storage due to their high caking. Before feeding the alcohol suspensions of crystals to the glucose syrup, they were previously separated from alcohol, filled with syrup, mixed and fed into a vacuum apparatus or crystallizer as a seed.
Determination of the effect of excess concentration and solution temperature on glucose nucleation. The nucleation of crystals and their subsequent growth largely depends on the temperature and degree of supersaturation of syrup at the time of seeding nuclei.
In the experiments, anhydrous glucose crystals with a size of 60 μm wet with propanol with a dosage of 0.15% by weight were used as seeds.
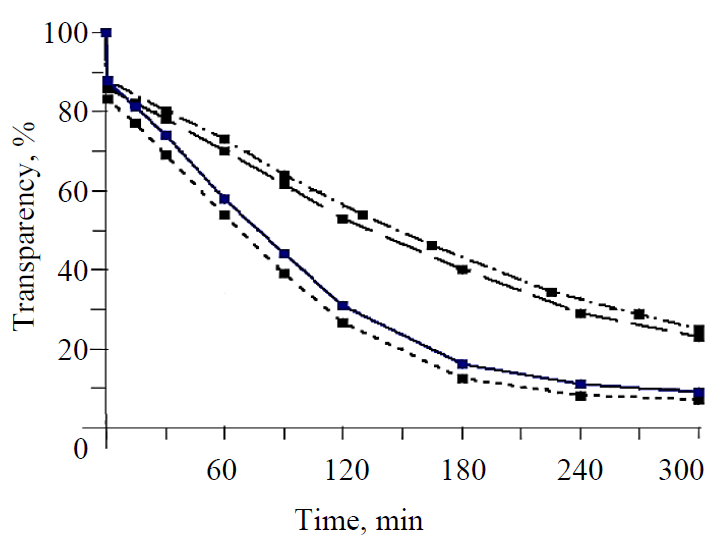
Effect of excess concentration on glucose nucleation. Fig. 4 shows the dependence of the transparency of the solution on the excess concentration of solutions. It follows from the figure that as the excess concentration increases, the transparency of solutions decreases as a result of the formation of nuclei and their growth simultaneously with the growth of the introduced seed crystals. With the excess concentration of 15 g/100 g of water, the transparency of the solution slowly decreases with time, which is due to the growth of the introduced seed crystals. At the same time, there is no formation of new nuclei. With the excess concentration of 44 g/100 g of water, the crystal growth accelerates, and with the excess concentration of 59 g/100 g of water, the pattern of the curve at the initial moment, that sharply decreases, along with the growth of seed crystals, demonstrates the formation of new nuclei and indicates a transition from the metastable concentration zone to the labile zone. With the excess concentration of 88 g/100 g of water, the transparency of solution drops sharply within 80 min, which indicates the predominance of nucleation over the growth of nuclei, characteristic of the labile zone of supersaturations.
Studies at a temperature above 50°С. Temperature plays a special role in glucose crystallization. In the studies on the effect of temperature on the rate of growth of crystals, a sharp increase in the rate of growth with an increase in temperature, which follows from the general theses of the theory of crystallization, has been proved [15]. In case of the crystallization of substances under the conditions limited by the diffusion mode, with an increase in temperature the diffusion coefficient increases and the thickness of the film decreases because of a decrease in the viscosity of the solution with an increase in temperature [31].
When the kinetic crystal-chemical stage is limiting, with an increase in temperature the size of a twodimensional crystalline nucleus decreases, and, consequently, so does the work of its formation, which also increases the crystal growth rate [15]. In addition, a certain role is played by a decrease in the hydration of ions or molecules in the solution [32], which also accelerates the transition of a substance from a partially arranged layer to the crystal face. This is confirmed by the fact that in the area of low temperatures, the kinetics of crystal growth is often determined precisely by the rate of the crystallochemical stage, at the same time the diffusion stage is limiting for the same salts at high temperatures.
Determining the effect of temperature on glucose crystallization is not an easy task. It develops as an accelerating or delaying effect on a whole set of the reactions and processes involved in crystallization: tautomerism, diffusion, the surface reaction of the integration of molecules into the crystal lattice, the type of crystals, the solubility of glucose, the viscosity of solutions, the hydration of crystals. Consequently, the effect of temperature on the rate of crystallization of glucose develops as the combined effect of these factors. The rate of crystallization of anhydrous glucose is known to be much higher than that of hydrate glucose.
Fig. 5 shows a change in the transparency of the solution depending on temperature for two values of excess concentration. It follows from the figure that when the temperature decreases from 70 to 55°C, the rate of nucleation and crystal growth increases. This is confirmed by a decrease in the transparency of solutions with a decrease in temperature. With the excess concentration of 40 g/100 g of water, the flat pattern of the transparency curves indicates the predominant growth of the introduced seed crystals over time.
With the excess concentration of 60 g/100 g of water, the introduction of a seed intensified the nucleation to a higher degree. A sharp decrease in the transparency of solutions demonstrates the predominance of nucleation over crystal growth and leads to rapid saturation in the solution. A decrease in the rate of crystallization of anhydrous glucose with an increase in temperature was also determined in another paper [33]. Thus, with an increase in temperature from 55 to 75°С, the crystallization rate decreased from 4.01 to 3.52 mg/g min, or by 12%, which is explained by an increase in the viscosity of saturated glucose solutions with an increase in temperature.
Thus, the nucleation of glucose crystals in the presence of SAA accelerates with an increase in the excess concentration of solutions and with a decrease in temperature. At the same time, the concentration of the upper boundary of the metastable zone decreases from 82 to 80%.
On the basis of the obtained results, the technological mode of crystallization of anhydrous glucose under isothermal conditions with a seed from the large hydrate crystals wet with propanol has been tested in the amount of 0.01–0.015% by weight of syrup. This amount was sufficient to meet the total demand of the process in crystallization centers. A feature of the nucleation process in the presence of SAA (propanol) was the introduction of a seed into the syrup in the metastable supersaturated zone (at a temperature of 70°C and the concentration of solids of 79–80% in the syrup). The process of nucleation during the crystallization of anhydrous glucose without SAA is carried out at the concentration of solids of syrup of 81–82% [1]. The tests showed that in order to meet the total demand for crystallization centers, the dosed mass of seed crystals should be increased by a factor of 1.5–2 compared to the calculated one (Table 1).
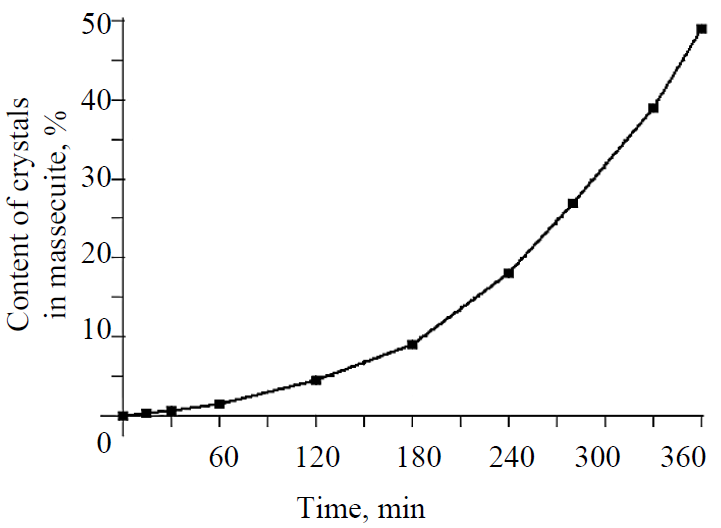
It can be assumed that some of the hydrate particles dissolved when mixed with the syrup. The crystalline mass continued to grow for 6 hours (Fig. 6). The massecuite was of high quality, it contained about 50% of homogeneous crystals with a size of 0.15–0.20 mm and was easily divided into crystals and intercrystal molasses, which is the evidence in favor of the proposed seed types.
Studies at a temperature below 50°С. Of theoretical and practical interest are the studies on the detection of conditions of the nucleation and growth of anhydrous glucose crystals using SAA at temperatures below 50°C, especially when hydrate glucose crystals are used for seeding.
The temperature intervals of the formation of crystals of hydrate and anhydrous glucose were established by V.B. Newkirk [34–36]. Hydrate crystals (С6Н1206 × Н2О), as a stable solid phase, are formed at a temperature from –5.3 to +50°С. At temperatures above 50°C, a stable solid phase is anhydrous glucose (С6Н1206). Later on, J. Vaschatko and A. Smelik [37] specified the temperature of the transition of hydrate glucose to anhydrous glucose and the boundaries of the metastable zone for hydrate and anhydrous glucose. According to their data, the crystals of anhydrous glucose are spontaneously formed at a temperature of 52.2°C and higher; the crystals present in the solution can also grow with a decrease in temperature to 34.55°C.
Below are the results of the studies of nucleation of anhydrous glucose in the temperature zone of crystallization of hydrate glucose (at a temperature of 45–48°C) with the help of the seed crystals of hydrate glucose with a size of 200 microns wet with propanol.
Fig. 7 presents the results of the experiments at a temperature of 48°C. It follows from the figure that after introducing the crystals of hydrate glucose wet with propanol into the supersaturated solution, the transparency of solutions drops. This indicates the beginning of nucleation. The microscopic analysis showed that 30 minutes after the beginning of the experiment, the large hydrate crystals completely disintegrated to the smallest particles (as shown in the micrographs of Fig. 3) and began to grow rapidly in the form of anhydrous glucose crystals, although the form of a seed from hydrate crystals and the temperature zone should stimulate the growth of hydrate glucose crystals. The activation of nucleation of anhydrous glucose under the studied crystallization conditions is in the same way as in the experiments with a temperature above 50°C. The growth of nuclei with a high rate characteristic of anhydrous glucose continues with a decrease in temperature to about 36°C. And then, at a temperature close to 35°C, the crystals dissolve almost instantaneously until a pure solution is obtained. This is consistent with the data of J. Vaschatko and A. Smelik, who determined the lower temperature boundary of 34.55°C for the existence of anhydrous glucose crystals. According to some tests, nucleation occurs within the temperature range from 50 to 38°C.
The obtained data on the nucleation of anhydrous glucose at a temperature below 50°С with a seed from the hydrate glucose crystals wet with alcohol, were a prerequisite for the further improvement of the technological mode of crystallization of anhydrous glucose under polythermal conditions.
Tests of the technological mode of crystallization of anhydrous glucose at a low temperature. As already mentioned above, anhydrous glucose crystallizes under industrial conditions within the temperature range of 75–55°С [6, 7].
In laboratory conditions, the technological modes for crystallization of anhydrous glucose by reducing the temperature of massecuite from 60–65 to 37–38°С have been tested.
Fig. 8 shows the graph of crystallization of anhydrous glucose with a decrease in temperature from 60 to 38°C. The nucleation of crystallization centers was carried out with the help of the seed crystals of hydrate glucose with a size of 200 μm, wet with propanol in the amount of 0.015% by weight of syrup. During the nucleation, the concentration of solids in the syrup was 78% at a temperature of 60°C. The growth of the crystalline mass lasted for 13 hours.
The large crystals (200 microns) wet with alcohol, introduced into the syrup, disintegrated into small particles, became nuclei and grew in the form of anhydrous glucose. With the further mixing and gradual cooling, the nuclei grew to technical sizes of 0.10–0.15 mm by the end of crystallization and were homogeneous. The content of crystals in massecuite reached 43–45% after 12–14 hours; the crystals were easily separated from the mother liquor during centrifugation. The mother liquor was depleted from the initial concentration of solids of 78% to 62% in the crystallization period. The obtained results confirmed the positive experience of using seeds from hydrate glucose. The amount of crystals introduced (0.015% by weight of syrup) was enough to provide the complete demand for crystallization centers. Seeding the syrup with a full number of nuclei allows for the nucleation stage in the metastable supersaturated zone, reducing the initial temperature and concentration of the syrup.
The tested technological mode has significant advantages in comparison with others, for example, [8], where the initial temperature of the syrup exceeds 80°C, and its concentration is 83–84% during the nucleation period. In this case, seeding the crystals lasts for about 4 hours, which leads to the formation of heterogeneous crystals, the deterioration of the quality of glucose and an increase in the duration of crystallization. The technological mode within the temperature range 60–38°С is more preferable, it is gentle for glucose subject to thermal decomposition, it prevents the accumulation of glucose decay products. The proposed nucleation method is more effective, requires less (by weight) seed crystals of hydrate glucose of any size to provide the complete demand in crystallization centers, and the resulting massecuite is characterized by high homogeneity crystals and the absence of conglomerates. The viscosity of the initial syrup and the intercrystalline solution is lower. The advantage of the tested technological mode is also saving technical water for the cooling of massecuite and drinking water for washing crystals in a centrifuge.
A related question arose during the study: why are the heterogeneous nucleation and growth of hydrate glucose crystals to technical sizes are slow, for 24–50 hours, under the temperature conditions (below 50°C) of their existence and growth, and why do the crystals of the same hydrate glucose wet with propanol behave differently under the same temperature conditions; do they turn into the nuclei of anhydrous glucose within half an hour and grow with a high (5 times faster) rate characteristic of anhydrous glucose?
As is known, the driving force of crystallization is the supersaturated state of the solution, due to which the nuclei of a critical size are formed in the solution and their further growth takes place. To realize the crystal growth, the diffusion inlet of the molecules or ions of the crystallized substance to the crystal surface and the kinetic reaction of integrating them into the crystal lattice are needed. The rate of crystallization is limited by the slowest of these two stages. We established [38] that the kinetic surface reaction is limiting in the crystallization of hydrate glucose, while the diffusion component is, on the contrary, limiting in the crystallization of anhydrous glucose. Analyzing the conditions of the nucleation process of anhydrous glucose (Table 2) at a temperature > and < 50°C, we find that: the diffusion coefficient at a temperature of 40°C is 1.5 times higher (3.8.10–10 m2/s) than at a temperature of 65°C (2.36.10-10 m2/s). This is due to a decrease in the viscosity of saturated glucose solutions with a decrease in temperature, the value of which at a temperature of 40°C is 2.6 times lower than the viscosity of saturated solutions at a temperature of 65°C. For supersaturated solutions in the metastable zone, the difference between the values of viscosity at the analyzed temperatures is even higher. In addition, the reduction in the thickness of the film surrounding the crystal and the acceleration of the diffusion of molecules through it to the surface of the crystal contributes to the improvement of crystallization conditions at a temperature of 40°C.
An important role in the crystallization of glucose is played by the tautomer reaction of the interconversion of α- and β-glucose [39, 40]. The faster the conversion of β-glucose into the α-form, the higher the crystallization rate of α-glucose. According to Table 2, the duration of tautomeric conversions at a temperature of 40°C is 172 minutes, at a temperature of 65°C – 7–10 minutes, which should accelerate the crystallization process at a temperature of 65°C.
Based on the analysis of the data of Table 2, the high rate of crystallization of anhydrous glucose at a temperature of < 50°C in comparison with hydrate glucose becomes clear. An increase in the diffusion coefficient and a decrease in the thickness of the film the crystal is enclosed in, create favorable conditions for the crystallization of anhydrous glucose in the diffusion mode. The delayed tautomerism is apparently compensated by the acceleration of diffusion.
Further on, the analysis of the conditions of the crystallization of hydrate glucose is provided. As already mentioned above, the main stage that inhibits the process of crystallization of hydrate glucose is the kinetic reaction of the integration of molecules into the crystal lattice. At temperatures below 50°C, to crystallize hydrate glucose solutions with a concentration of 70–72%, in which glucose molecules have a high degree of hydration, are used [32]. Hydration makes it difficult for a molecule to jump from the solution to the crystal lattice. To do this, the glucose molecule must first get rid of water molecules, namely to break chemical bonds with water molecules, get rid of the intercrystalline solution and spend additional energy to include one water molecule in the crystal lattice. The use of SAA accelerates the nucleation of hydrate glucose, as shown in Fig. 1, but this effect is less distinct at the low rate of crystallization of hydrate glucose compared with anhydrous glucose. O.D. Linnikov [41] established that in the case of the kinetic mode of crystallization (NaCl and KCl), the basic energy of nucleation and growth of crystals is spent precisely on dehydration, since the diffusion component is comparatively less significant. The large energy costs for the nucleation and growth of hydrate glucose crystals are also confirmed by the heat of crystallization of hydrate glucose which is twice as high in comparison with anhydrous glucose.
ВЫВОДЫ
As a result of the studies, new data have been obtained for the theory and practice of glucose production. The activating effect of SAA – aliphatic alcohols on the nucleation of glucose has been established. The tests of seed crystals of anhydrous and hydrate glucose, dry and wet with alcohol for nucleation, showed the advantages of crystals wet with alcohol. The seed crystals (of any size) of hydrate glucose wet with alcohol that make it possible to improve the homogeneity of crystals and to reduce significantly the required mass of seed crystals, have been recognized as the most effective. A method for producing seed crystals of anhydrous and hydrate glucose with a long shelf life has been proposed. The activating effect of the hydrate crystals wet with alcohol on the nucleation of anhydrous glucose at a temperature below 50°C has been established. In the experimental crystallization at a temperature of about 35°C, the crystals dissolved almost instantaneously. With positive results, the technological mode of crystallization of anhydrous glucose under polythermal conditions has been tested with a decrease in temperature from 60 to 38°С. The data obtained are recommended for further testing and use in the development of technological regulations for the production of crystalline glucose.
СПИСОК ЛИТЕРАТУРЫ
- Andreev N.R. and Khvorova L.S. Glucose anhydrous: manufacturing technology and use. Pharmacy, 2012, no. 3, pp. 43–45. (In Russian).
- Gibbs J.V. Termodinamicheskie raboty [Thermodynamic work]. Moscow-Leningrad: State Publishing House of Technical and Theoretical Literature, 1950. 492 p.
- Volmer M. Kinetik der phasenbildung. Dresden und Leipzig, T. Steinkopff, 1939. 220 p. (Russ. ed.: Volmer M. Kinetika obrazovaniya novoy fazy. Мoscow: FIZMATLIT Publ., 1986. 208 p.).
- Melikhov I.V., Kozlovskaya E.D., Kutepov A.M., et al. Kontsentrirovannye i nasyshchennye rastvory [Concentrated and saturated solutions]. Мoscow: Nauka Publ., 2002. 456 p.
- Khvorova L.S. Analiz tekhnologicheskikh skhem kristallizatsii glyukozy [Analysis of technological schemes of glucose crystallization]. Sbornik dokladov IV mezhdunarodnoy konferentsii-vystavki «Vysokoeffektivnye pishchevye tekhnologii. metody i sredstva ikh realizatsii» [Collection of reports of the 1V international conference-exhibition "Highly effective food technologies, methods and means of their implementation"]. Part 1. Мoscow, 2006, pp. 145–147.
- Andreev N.R., Khvorova L.S., and Zolotukhina N.I. The kinetics of anhydrous glucose nucleation in isothermal conditions. Sugar, 2010, no. 12, pp. 55–58. (In Russian).
- Andreev N.R., Khvorova L.S., and Selezneva O.S. Crystallization of Anhydrous Glucose under Polythermal Conditions. Storage and processing of farm products, 2014, no. 1, pp. 13–14. (In Russian).
- Fidanko, Staliyanov, and Sretenov. Metod kristallizatsii angidridnoy glyukozy [Method of crystallization of anhydrous glucose]. Certificate of authorship of Bulgaria, no. 15427, 1974.
- Laila U., Pudjiraharti S., and Kosasih W. Crystallization of D-fructrose Anhydride III (DFA III) in Batch Cooling Crystallization System: the Influence of Initial Supersaturation. International Journal of Engineering Research and Applications (IJERA), 2013, vol. 3, no. 1, pp. 676–679.
- Rashid A., White E.T., Howes T., et al. The metastability and nucleation thresholds of ibuprofen in ethanol and Water-ethanol mixtures. International Journal of Chemical Engineering, 2015, vol. 8, DOI: 10.1155/2015/560930.
- Teixeira G.A., Brito A.M., Alves M.R., et al. Studi of Supersaturation, Vibration Intensyti and Time of Crystallization Variables in the Vibrated Bed Lactose Monohydrate Production Process. Chemical Engineering Transactions, 2013, vol. 32, pp. 2191–2196. DOI: 10.3303/CET 1332366.
- Slavorosova E.V., Kulenko V.G., Shevchuk V.B., and Fialkova E.A. Intensification of lactose crystallization in condensed whey. Dairy Farming Journal, 2016, no. 2 (22), pp. 109–116. (In Russian).
- Shaffer K.R., Patersonm A., Davies C.E., and Hebbink G. Nucleation of lactose using continuous orifice flow. International Dairy Journal, 2016, vol. 61, no. 6, pp. 148–154. DOI: 10.1016/j.idairyj.2016.06.001.
- Jiang M, Li Y.E.D., Tung H.-H., and Braatz R.D. Effect of jet velocity on crystal size distribution from antisolvent and cooling crystallizations in a dual impinging jet mixer. Chemical Engineering and Processing: Process Intensification, 2015, vol. 97, pp. 242–247. DOI: 10.1016/j.cep.2015.09.005.
- Kozlova O.G. Rost i morfologiya kristallov [Growth and morphology of crystals]. Moscow: MSU Publ., 1980. 357 p.
- Jiang M., Papageorgiou C.D., Waetzig J., et al. Indirect Ultrasonication in Continuous Slug-Flow Crystallization. Crystal. Crystal. Growth Desing, 2015, vol. 15, no. 5, pp. 2486–2492. DOI: 10.1021/acs cgd.5b00263.
- Kudryashova O.B., Antonnikova A.A., and Korovina N.V. On Mechanisms of Ultrasonic Sedimentation of Fine Aerosols. Russian Physics Journal, 2015, vol. 58, no. 2, pp. 271–277. DOI: 10.1007/s11182-015-0492-y. (In Russian).
- Antonnikova A.A., Korovina N.V., and Kudryashova O.B. Ultrasonic sedimentation of fine aerosols. Bulletin of the Tomsk Polytechnic University. Engineering of geo-resources, 2014, vol. 324, no. 2, pp. 57–62. (In Russian).
- Zharova E.Ya. Primenenie fizicheskikh metodov pri kristallizatsii glyukozy [Application of physical methods for glucose crystallization]. Moscow: TSNIITEI Pishcheprom Publ., 1965. 25 p.
- Volkov V.A. Kolloidnaya khimiya. Poverkhnostnye yavleniya i dispersnye sistemy [Colloid chemistry. Surface phenomena and dispersed systems]. St. Petersburg: Lan Publ., 2015. 659 p.
- El-Shall H., Abdel-Aal E.A., and Moudgil B.M. Effect of surfactants on phosphogypsum crystallization and filtration during wet-process phosphoric acid production. Separation Science and Technology, 2000, vol. 35, no. 3, pp. 395–410. DOI 10.1081/SS.100100164.
- Krishnan S., Klein A., El-Aasser M.S., and Sudol E.D. Effect of surfactant concentration on particle nucleation in emulsion polymerization of n – butyl methacrylate. Macromolecules, 2003, vol. 36, no. 9, pp. 3152–3159. DOI: 10.1021/ma021120p.
- Bol’shagin E.Y. and Roldughin E.I. Kinetics of nucleation and growth of metal nanoparticles in the presence of surfactants. Colloid Journal, 2012, vol. 74, no. 6, pp. 649–654. DOI: 10.1134/S1061933X12060038.
- Antonov A.N., Novakova A.A., and Gendler T.S. The influence of surface-active substances on the crystallization process and magnetic properties of goethite nanoparticles. Moscow University Physics Bulletin, 2012, vol. 67, no. 2, pp. 230–232. DOI: 10.3103/S002713491202004X.
- Khvorova L.S., Andreev N.R., and Lukin D.N. Effect of surfactants on kinetics of dextrose crystallization. Food Processing: Techniques and Technology, 2017, vol. 44, no. 1, pp. 81–86. (In Russian).
- Khvorova L.S. and Shleina L.D. Method of seed control during glucose crystallizatio. Food Processing Industry, 1990, no. 4, pp. 58–60. (In Russian).
- Lukin N.D., Ananskikh V.V., Lapidus T.V., and Khvorova L.S. Tekhnologicheskiy kontrol' proizvodstva sakharistykh krakhmaloproduktov [Technological control of the production of sugary starch products]. Мoscow: Rosselkhozakademiya Publ., 2007. 261 p.
- Stabnikov V.N., Reuter I.M., and Protsyuk T.B. Etilovyy spirt [Ethanol]. Мoscow: Food Processing Industry Publ., 1976. 272 p.
- Demidova M.G., Beketova D.I., Arymbaeva A.T., and Bulavchenko A.I. Effect of surfactant additives on the size and morphology of ammonium and potassium nitrate particles obtained by crystallization from reverse micellar solutions of tergitol NP-4. Journal of Inorganic Chemistry,2013, vol. 58, no. 10, pp. 1214–1219. DOI: 10.1134/S0036023613100069.
- Muraviev E.V., Pavlenko A.A., Akhmadeev I.R., et al. Researches of processes of dispergating of the compacted powders. Polzunovsky Vestnik, 2016, vol. 1, no. 4, pp. 64–67. (In Russian).
- Mullin J.W. Crystallization (4th ed.). Butterworth-Heinemann: Oxford, 2001. 594 p.
- Grabovska E.V. Rozvitok naukovikh osnov udoskonalennya tekhnologiy tsukristikh krokhmaleproduktiv [Development of the science of the fundamentals of the technologies of fermented starch products]. Abstract of Diss. Dr. Sci. (Eng.). Kiev, 2006. 43 p.
- Khvorova L.S., Tishchenko-Romanchenko G.V., and Tregubov N.N. Dependence of the rate of crystallization of anhydrous glucose on the basic physicochemical factors. Sugar industry, 1981, no. 8, pp. 56–58. (In Russian).
- Newkirk W.B. Manufacture and uses Refined Dextrose. Industrial & Engineering Chemistry, 1924, vol. 16, no. 11, pp. 1173–1175. DOI: 10.1021/ie50179a028.
- Newkirk W.B. Development and Production of Anhydrous Dextrose. Industrial & Engineering Chemistry, 1936, vol. 28, no. 7, pp. 760–766. DOI: 10.1021/ie50319a003.
- Newkirk W.B. Hydrate Dextrose. Industrial & Engineering Chemistry, 1939, vol. 31, no. 1, pp 18–22. DOI: 10.1021/ie50349a003.
- Vaschatko J. and Smelik A. Die Kristallisacion der Sacharoze, D-Glucose und D-Fructose aus ubersattigten Lösungendes des metastabielen Gebiete. Zucker, 1968, no.6, pp. 144–151.
- Khvorova L.S. and Kovalenok V.A. Mathematical modeling of glucose crystallization kinetics. Storage and processing of farm products, 2008, no. 5, pp. 45–48. (In Russian).
- Kuznetsov V.D. Kristally i kristallizatsiya [Crystals and crystallization]. Moscow-Leningrad: State Publishing House of Technical and Theoretical Literature, 1954. 411 p.
- Khvorova L.S. and Baranova L.V. Polyarimetricheskie issledovaniya v teorii i praktike glyukoznogo proizvodstva [Polarimetric studies in the theory and practice of glucose production].Materialy V mezhdunarodnoy konferentsii «Pishchevye innovatsii i biotekhnologii» [Proc. of the V Intern. Conf. “Food Innovation and Biotechnology”]. Kemerovo, 2017, pp. 129–131.
- Linnikov O.D. Relations between activation energies for nucleation and of growth of crystals. Nanosystems: Physics, Chemistry, Mathematics, 2014, vol. 5, no. 4, pp. 546–552.