Аннотация
Honey has always been seen as the main source of healthy natural food and folk medicines. It has been prized due to bioactive components that are responsible for different therapeutic effects. Phenolic compounds are one the parts of these components. It is claimed that these have been antioxidant agents. But it also has to be evaluated by different perspectives in biomechanics except antioxidative effects. A variety of diseases may be treated by the inhibition of some individual enzymes. A pharmaceutical drug and synthetic agents are used to treat and avert illness even though there is a potential risk named drug resistance. Nowadays, the most effective treatment seems to be the combined administration of natural foods. The study aims at investigating hyaluronidase (HYA), xanthine oxidase (XOD) and the urease enzyme inhibition of some chestnut honeys from different locations of Giresun and Ordu in Turkey. Moreover, the antioxidant activities of the prepared chestnut honey extracts were investigated by using different methods. The total phenolic (TP), total flavonoid (TF), FRAP, CUPRAC assays and DPPH, and ABTS inhibition potential were carried out using in vitro models. The enzyme IC50 values in the samples ranged from 0.793 to 12.639 mg/ml for HYA; from 0.029 to 0.106 g/ml for XO; from 0.002 to 0.054 g/ml for urease, respectively. In conclusion, honey extracts exhibited good potentials towards the inhibition of activities of the studied enzymes, and the samples also suggest a practical value for surveying natural inhibitors for specific clinical purposes. Moreover, all results can provide a basis of future studies on the alternative medicinal application related to honey.Ключевые слова
Chestnut honey, hyaluronidase, xanthine oxidase, urease, inhibition, antioxidantВВЕДЕНИЕ
Honey is valuable functional food that provides an important part of the energy needed by a body cell and also known as a traditional medicine source [1]. Its content is changeable and depends on a lot of factors, such as the botanical (floral or vegetable) and geographical (regional or territorial) origins and species of bee [2], although it consists of approximately 75–80% of carbohydrates among which there are such principal constituents as fructose and glucose, 17–20% of water and 1–2% of other substances [3].
Honey is commercially available and varies greatly in quality all over the world. It is largely assessed on the basis of some physico- and bio-chemical parameters such as color, moisture, HMF, diastase activity, proline, acidity, electrical conductivity, phenolic contents, and some enzyme inhibition sources are a strong indicator for the prediction of honey quality [4]. Although it is known that these quality parameters can vary only due to the botanical and geographical origins of honey, other factors are also important, including the climate, environmental conditions and processing that honey has undergone [5].
Turkey, due to its geographical location, has a wide scale relative to monofloral honeys. Chestnut honey, having high bioactivity, is part of monofloral honey. This honey with a light bitter taste is offered in the market and is often produced especially by the eastern Black Sea region beekeepers and it is seen as a source of alternative medicine for local people. It is claimed that the regular consumption of these bee products might contribute to a reduction in several forms of ROS-mediated pathological injury, notwithstanding the geographical origin of the honey [6]. Antioxidant studies can create the basis of these claims.
Enzyme inhibitors are mainly bioactive secondary metabolites that bind to an enzyme and decrease its bioactivity and catalytic activity. Moreover, blocking enzyme activity can kill a pathogen or correct a metabolic imbalance [7]. Although a lot of synthetic and chemical products are used as useful inhibitors, natural products become popular for enzyme inhibition. Since the resistance of synthetic and chemical drugs has become a major clinical and public health problem. Natural enzyme inhibitors are often mediated by its specificity and its effectiveness that designated the absorption desirable to inhibit the enzyme [7]. Honey acts as natural enzyme inhibitors. Hyaluronidase (HYA), xanthine oxidase (XOD) and urease inhibitors are especially one of them.
Hyaluronic acid (HA) is a natural biopolymer that is responsible for some biological progress (synovial fluid, eye vitreous fluid etc.) in bacteria and higher animal metabolism. β-D-glucuronidase, β-N-acetyl-hexosaminidase and especially hyaluronidases play an important part during the degradation of this natural polymer [8, 9]. Moreover, the HA substrate has to keep on metabolic control with HYAs which could change tissue permeability by accelerating their dispersion and delivery [3].
Xanthine oxidase (XOD, EC 1.17.3.2) catalyses the oxidation of xanthine or hypoxanthine to uric acid, and also creates a superoxide radical formed during the metabolic process [10]. This radical has to be neutralized by antioxidant systems and inhibitors which slow down or stop the effect of the level of the enzyme. There are some known inhibitor chemicals to inhibit XOD. Allopurinol is one of these inhibitor chemicals that can cause some negative effect, such as hepatitis, nephropathy, hypersensitivity etc. [10].
Urease (urea amidohydrolase, E.C.3.5.1.5) is a nickel containing metallo-enzyme found in plants, bacteria, fungi and soil. This enzyme is used by plants to break down urea into carbon dioxide and ammonia. The indophenols method was used to determine urease activity by measuring ammonia production [11]. Besides these given inhibitors, some natural products can act as broad-based urease inhibitors due to their bioactive contents [6].
Finally, the quality of chestnut honey is related to its bioactive levels. Some geological, geochemical, seasonal, floral source and climatic parameters are responsible for the bioactive levels such as antioxidant values and enzyme inhibition concentrations. There have recently been a lot of considerable studies on the biological activity of chestnut honey. But the work on enzymatic inhibition is also extremely limited. For this reason, the aim of this study was to investigate hyaluronidase, xanthine oxidase and urease enzyme inhibition of ten chestnut honeys from Giresun and Ordu and also to determine their antioxidant properties. Hence, the research approaches and findings presented in this paper would be useful for investigating new alternative sources, such as bee products.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Chemicals and instruments. All chemicals were of an analytical grade and were used as received without further purification. All chemicals were also purchased from Sigma Chemical Co. (St. Louis, MO, USA). In vitro spectrophotometric measurements were performed by using a Mapada UV-6100 PCS spectrophotometer (Shanghai Mapada Instruments Co., China).
Melissopalynological characteristics of floral honey. Botanical origin denomination with melissopalynological analysis is necessary for getting information about discriminatory abilities of honeys. For this reason, the samples were collected by experienced beekeepers during the harvest season of 2015 and were handled by melissopalynological analysis for sample tagging. The method was described by Louveaux et al. (1978) and the procedure progress was the same with Nair et al. (2013). For analysis, approximately 10 g of honey were dissolved in 20 ml of distilled water. This mixture was divided into two centrifuge tubes of 15 ml and centrifuged for about 5 min at 3000 rpm. Distilled water was added to the sediment again repeating the previous operation. Approximately 5 ml of glycerine-water 1 : 1 were added to the sediment and it was left for 30 min. After this time, the sample was centrifuged. The sediment was removed with the aid of a stylet embedded in glycerine jelly and deposited on a microscopic slide sealed with paraffin wax [13]. For pollen identif cation, sample materials were microscopically observed and compared with the reference slide. The frequency classes of pollen grains were attributed as predominant pollen (more than 45%). The percentage of chestnut (Castanea sativa Mill.) pollen from the samples ranged from 78% to 90%. Table 1 presents pollen analyses and honey properties.
Sample preparation. Approximately 10 g of the honey sample was extracted with 50 ml of distilled water in a flask attached to a condenser at 60°C for more than 6 h. The extract was subsequently filtered to remove particles, and the final volume was adjusted with distilled water.
Enzyme inhibition assays. All the enzyme inhibition assays were done in triplicate and given as IC50 values, were determined as the concentration of a compound that provides 50% of inhibition of maximal activity. IC50 was determined graphically from the inhibition curves by plotting enzyme activity against sample extract concentrations that were known as a natural inhibitor.
Hyaluronidase (HYA) inhibition. The slightly modified Sigma protocol was fixed for determining HYA inhibition activity [14]. Briefly, the reaction mixture consisted of 100 ml of hyaluronidase (1.67 U/mg), 100 ml of a phosphate buffer (200 mM, pH 7, 37°C) with 77 mM sodium chloride and 0.01% BSA mixed with 25 ml of a sample extract solution. After pre-incubation at 37°C for 10 min, the reaction was initiated by the addition 100 ml of a substrate solution in the form of hyaluronoic acid (0.03% in 300 mM sodium phosphate, pH 5.35). The assay mixture was incubated at 37°C for 45 min. The undigested hyaluronoic acid was precipitated with 1ml of an acid albumin solution made up of 0.1% bovine serum albumin in 24 mM sodium acetate and 79 mM acetic acid, pH is 3.75. After leaving the mixture at room temperature for 10 min, the absorbance was measured at 600 nm using a Mapada UV-6100 PCS spectrophotometer (Shanghai Mapada Instruments Co., China).
Xanthine oxidase (XOD) inhibition. The xanthine oxidase inhibitory activity was measured using the method by Ryu et al. (2012) with slight modifications. The assay mixture of XO inhibitory activity consisted of 0.5 ml of the test compound, 0.77 ml of a phosphate buffer (pH is 7.8) and 0.07 ml of bovine milk XO (Sigma–Aldrich, St. Louis, USA), which was prepared immediately before use. After pre-incubation at 25°C for 15 min, the reaction was initiated by the addition of 0.66 ml of a substrate solution into the mixture. The assay mixture was incubated at 25°C for 30 min. The reaction was stopped by the addition of 0.2 ml of 0.5 N HCl and the absorbance was measured at 295 nm.
Urease inhibition. The urease assay was explained step by step. The reaction mixtures including 100 µl of Jack Bean Urease, 400 µl of a buffer (100 mM urea, 0.01 M K2HPO4, 1 mM EDTA and 0.01 M LiCl, pH is 8.2) and 500 µl of the honey extract were incubated at room temperature for 15 min. 500 μl of a phenol reagent (1% w/v phenol and 0.005% w/v sodium nitroprusside) and 500 μl of an alkali reagent (0.5% w/v NaOH and 0.1% active chloride NaOCl) were added to each tube. The increasing absorbance at 625 nm was measured after 50 min using a UV/vis spectrophotometer [6].
Determination of antioxidant capacity.
Total phenolic content (TPC) assay. The total
phenolic content (TPC) of honey extracts was analyzed
using the Folin–Ciocalteu assay [15]. To this end, 680 ml
of distilled water, 20 ml of aquatic extracts and 400 ml of
0.2 N Folin–Ciocalteu were mixed and then vortexed.
After 2 min, 400 ml of Na2CO3 (7.5%) was added and the
mixture was incubated for 2 h at room temperature. After
incubation in the dark, the absorbance at 760 nm was
measured before distilled water. The concentration of
TPCs was calculated as mg of gallic acid equivalents
(GAE) per 100 g of a sample, using a calibration curve
determined using gallic acid standard solutions.
Total flavonoid content (TF) assay. The flavonoid compounds in honey samples were determined using the spectrophotometric method [16] and expressed as mg QE (Quercetin Equivalents)/100 g honey. Regarding this method, the extracted solutions were prepared in a different concentration (0.5 ml) and mixed with 0.1 ml of 10% aluminum nitrate, 0.1 ml of 1 mol/l potassium acetate, and 4.3 ml of 80% ethyl alcohol. The samples were kept at room temperature for 40 min and the absorbance was measured at 415 nm.
Ferric reducing antioxidant power (FRAP) assay. The ferric reducing antioxidant power is based on the reduction of the Fe3+-TPTZ complex under acidic conditions. Regarding this method, the increase in absorbance of a blue-colored ferrous form (Fe2+-TPTZ complex) is measured at 593 nm [17]. The working FRAP reagent was prepared as required by mixing 25 ml of 0.3 M acetate buffer at pH equal to 3.6 with 2.5 ml of a 10 mM/l 2,4,6-tripyridyl-S-triazine (TPTZ) solution in 40 mM/l HCl and 2.5 ml of 20 mM/l FeCl3 × 6H2O. An amount of 100 μl of the sample was mixed with 3 ml of a freshly prepared FRAP reagent. Then, the reaction mixture was incubated at 37°C for 4 min. A calibration curve was applied using an aqueous solution of ferrous sulfate FeSO4 × 7H2O.
Cupric reducing antioxidant capacity (CUPRAC) assay. The cupric reducing antioxidant capacity (CUPRAC) of the honey extracts was determined to using the method of Apak et al. (2004). Trolox was also tested under the same conditions for a standard calibration curve. 1 ml of a CuCl2 solution (0.01 M), 1 ml of a neocuproine ethanolic solution (0.0075 M) and 1 ml of an NH4-acetate buffer solution were added to a test tube and mixed; (x) ml of the sample extract followed by (1.1 – x) ml of water were added (the total volume = 4.1 ml) and shaken well. The absorbance against a reagent blank was measured at 450 nm after 30 min. CUPRAC values were expressed as a mmol Trolox equivalent per 100 g of the sample.
DPPH• and ABTS• radical scavenging activity assays. The DPPH• and ABTS•+ assay was based on the method of Brand-Williams et al. (1995) and van den Berg et al. (1999), respectively. For the DPPH method, various concentrations (0.75 ml) of compound extracts were mixed with 0.75 ml of 0.1 mM DPPH in methanol.
In addition, for the ABTS method, the stock solutions included 7 mM ABTS and 2.4 mM potassium persulfate. This stock was diluted by mixing 1 ml of an ABTS (7 mM) solution with methanol to obtain the absorbance of 0.700 ± 0.001 units at 734 nm using a spectrophotometer. 20 ml of the honey extract and 2 ml of the diluting ABTS solution volume were mixed and incubated to react for 12 h at room temperature under dark conditions.
All the results of these assays were compared to each other and were expressed as SC50, the concentration of the samples causes 50% of the scavenging of a relevant radical.
Statistical analysis. All the tests were repeated in triplicate and the data were expressed as the mean ± standard deviation. All the calculations were performed using SPSS version 17.0 (SPSS Inc., Chicago, Illinois, USA). P < 0.01 was considered as indicative of significance as compared to each group.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
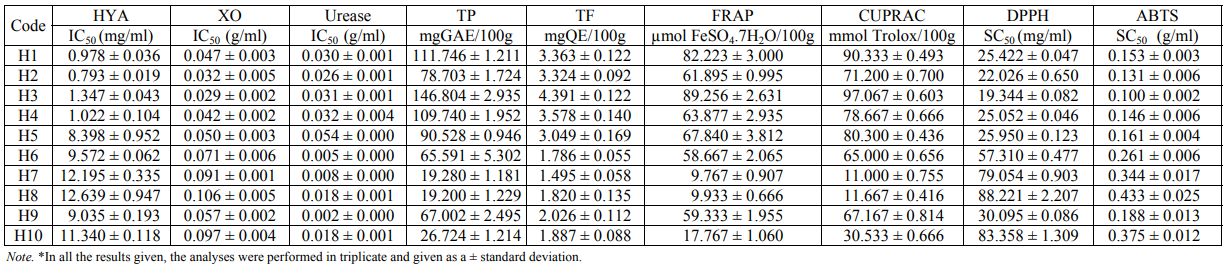
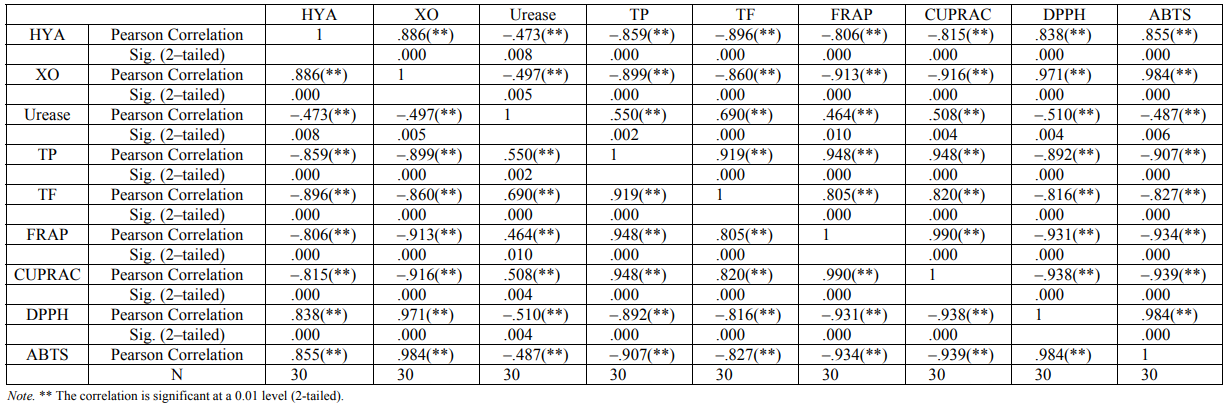
Results. In this study, in vitro inhibition results of chestnut honey based on hyaluronidase, xanthine oxidase and urease enzymes were primarily clarified. According to its content and enzyme mechanisms, honeys have been shown to have different inhibitory effects. All chestnut type honey extracts were shown to inhibit three enzymes according to IC50 values and varying concentrations (Table 2). The inhibition values determined in chestnut honeys were hyaluronidase inhibition values from 0.793 to 12.639 mg/ml, xanthine oxidase from 0.029 to 0.106 g/ml and urease from 0.002 to 0.054 g/ml. The highest inhibitory activity of urease, hyaluronidase and xanthine oxidase was determined in H2 (0.793 mg/ml), H3 (0.029 g/ml) and H9 (0.002 g/ml) tagged chestnut honeys, respectively. While the lowest urease inhibitory activity was found H5, the lowest hyaluronidase and xanthine oxidase inhibitory activity was found in H9. While a positive correlation (R2 = 0.886) between hyaluronidase and xanthine oxidase enzyme inhibition was detected, negative weak correlation (R2 = –0.473) was seen between hyaluronidase and urease enzyme inhibition (Table 3).
The polyphenolic contents of the samples were evaluated in two different ways: total phenolic contents (TPC) and total flavonoids (TF). Total phenolic contents of the honeys ranged from 19.222 to 146.804 mg GAE/100 g (Table 2). The phenolic contents were the highest in H3 honey, while H1 honey and H4 honey exhibited the highest levels according to the others. The lowest phenolic content was found in H7 and H8 samples (19.222 and 19.304 mg GAE/100 g). The total flavonoids contents of the ten honeys ranged from 1.565 to 4.431 mg QE/100 g. The highest and the lowest values were found H3 and H7 honey, respectively. The results were shown in Table 2.
In electron transfer reaction-based methods, when the oxidant is reduced, it changes color, and the degree of a color change is related to antioxidant capacity. Cu (II) ion reducing antioxidant capacity (CUPRAC) and iron (III) Reduction / Antioxidant Power (FRAP) of mainly ET-based methods were applied for our samples. When the results ranged from 9.8 to 89.3 µmol of a FeSO4 × 7H2O/100 g sample in a FRAP assay, the results for the CUPRAC method were 11.0–97.1 mmol of a Trolox/100 g sample within the range (Table 2). While the highest antioxidant activities of honey samples in both methods were found in H3 and H1 samples, the lowest activities were in H7 and H8 honey samples.
ABTS and DPPH radical scavenging methods are commonly used to measure a free radical scavenging ability in various natural products. The SC50 value was determined as the concentration of a compound that gives 50% of inhibition of the maximal activity and the low SC50 value indicates that the sample showed high radical scavenging activity. In the results of the DPPH and ABTS radical scavenging method, the SC50 values of ten chestnut honeys ranged from 19.344 to 88.221 and from 0.10 to 0.43 mg/ml (Table 2). According to both methods, while the highest radical scavenging activity was observed in H3, lower results were in H8. In all the antioxidant tests, the high linear correlation coefficient was determined using the same methodological methods: ABTS-DPPH (R2 = 0.984), FRAP-CUPRAC (R2 = 0.990), and TP-TF (R2 = 0.919) (Table 3).
When comparing enzyme inhibition and antioxidant activity, a negative correlation was found between the inhibition of xanthine oxidase and total phenolic (R2 = –0.899), total flavonoids (R2 = –0.860), FRAP (R2 = –0.913) and CUPRAC (R2 = –0 916), while using DPPH (R2 = 0.971) and ABTS (R2 = 0.984) a positive correlation was determined (Table 3). A negative correlation was between the inhibition of hyaluronidase and total phenolics (R2 = –0.859) and total flavonoids (R2 = –0.919) and FRAP (R2 = –0.806) and CUPRAC (R2 = –0.815). A positive correlation was also detected between DPPH (R2 = 0.838) and ABTS (R2 = 0.855) (Table 3). No correlation was found between the inhibition of the urease enzyme and the antioxidant tests.
Discussion. Besides the nutritional value of honey owned, it has significantly stood out in recent years to have a biological potential. Honey is a powerful antioxidant source with a rich phenolic content. It has antimicrobial, antibacterial, anti-inflammatory, anticancer and antiviral effects as well. These effects are affected by several factors, such as the floral source involved and seasonal, geographical and environmental conditions. Based on this reality, when compared with the studies in the literature it is sometimes seen as the same type of honey with a higher or lower activity. There are some available studies in the literature on chestnut, forest rose, heather, oak and flower honey. Chestnut honey used in the study is believed to be good ethno-medicine for asthma, respiratory disease and cancer [21]. In vitro and in vivo studies of health effects of chestnut honey showed that it is not only asthma, respiratory disease and cancer, but also chestnut honey that can be consulted for different health problems. De Vasconcelos et al. (2010) reported that it was used to dress chronic wounds, burns or skin ulcers due to its antibacterial activity. Alvarez-Suarez et al. (2012) studied chestnut honey for proving its cellular effect and the results showed that chestnut honey had a strong antioxidant activity and it might provide defense and promote cell functions in erythrocytes. Choi et al.’s (2012) study concerned accelerating wound healing and promoting early HO-1 protein expression in mice with chestnut honey. And these findings also indicate that chestnut honey can promote wound healing in diabetics with early HO-1 protein expression. Although it could be seen in different studies on chestnut honey that the investigation of some enzyme inhibition degrees could be evaluated as limited.
Although enzyme inhibitor activities in natural products are quite extensive when compared to other enzyme studies, their biological values have not been completely clarified. Nowadays, pharmaceutical searches focus on enzyme inhibition studies because these studies have led to the discoveries of drugs useful in a variety of physiological conditions. Enzyme inhibitors are molecules that interact in some way with an enzyme to block their activity towards natural substrates. Urease is a metalloenzyme that catalyzes the hydrolysis of urea into carbon dioxide and ammonia, its inhibitors have recently attracted great attention as potentially new anti-ulcer drugs [25]. Urease, providing an opportunity for bacteria to live at low pH in the stomach that can result in cancer plays a role in gastritis and peptic ulcer pathogenesis [6]. Urease inhibitor studies are performed for the therapy of the diseases caused by bacteria. Its inhibition is very important for the treatment of Helicobacter pylori related diseases. If that would inhibit urease, an inhibitor has been shown to inhibit an antiulcerative effect. As Helicobacter pylory cannot live in an acidic medium resulting from urease inhibition, this fact is the evidence that urease inhibition is antibacterial. In this sense, hydroxamic acids are known as the best inhibitors of the urease enzyme. The discovery of urease natural inhibitors will be important for alternative medicine for the treatment of diseases such as gastric ulcer. All ten chestnut honeys in the study inhibited urease in a manner dependent on varying IC50 values and concentrations (Table 1). Sahin (2016) found the IC50 of urease inhibition of chestnuts of a different origin between 0.010 and 0.034 g/ml.
Another important enzyme is hyaluronidase in the metabolic system. It is associated with a lot of pathological diseases and its inhibitors show an antiinflammatory, anti-allergic, anti-tumor, anti-aging, anti- rheumatoid, anti-toxin and antimicrobial effect [26, 27]. Hyaluronidase fragments are reported to be associated with cancer [26]. In addition, HA inhibitors have led to new therapeutic concepts for the treatment of throat and breast cancer associated with the hyaluronanhyaluronidase system in pathophysiological conditions [26, 28]. There was an aspect of the study by Isoyama et al. (2005) that inhibitors of hyaluronidase (HAase) might be useful as contraceptives, because they inhibit the acrosomal reaction initiated by testicular HAase. Therefore, the synthetic and natural inhibitors of hyaluronidase have recently attracted the attention of researchers [27]. In the literature, there are few studies about the effect of honeys on hyaluronidase inhibition. Kolayli et al. (2016) found that oak, heather and chestnut honeys have the highest anti-hyaluronidase activity. The chestnuts used in the present study showed a low IC50 value (inhibitory high).
In this study, the third enzyme used to examine inhibition effects of chestnut honey extracts was xanthine oxidase that is responsible for oxidative damage that causes a lot of pathological diseases such as gout, hyperuricemia, hepatitis, carcinogenesis and aging [30]. Xanthine oxidase regulation is an important means in the prevention of a lot of diseases. In the previous studies, Boumerfeg et al. (2012) investigated the antioxidant and radical scavenging effects of the Teucrium polium and its active fractions by applying various established in vitro xanthine oxidase inhibition assays. The study of natural compounds clearly indicated that Teucrium polium was a potent scavenger of O2 - . So, it could prevent the formation of ROS. Another study from Sowndhararajan et al. (2012) was carried out to evaluate the xanthine oxidase inhibitory potential of a methanol extract of Erythrina indica Lam. leaves and stem bark. The obtained results of that study showed that Erythrina indica stem bark exhibited a good XO inhibitory activity and therefore may contain bioactive constituents useful in the treatment of XO induced diseases. Besides these natural products, there were some details about bee products for xanthine oxidase inhibition in literature, too. The enzyme inhibitor potential of the respective bee products was estimated by Sahin (2016), it was designed for a comparative study on the enzyme inhibitors of some bee products. Sahin (2016) viewed the effects on the inhibition of xanthine oxidase of honey and the highest inhibitory effect was detected in chestnut honey. In this study, all the samples depending on a different floral effect showed the significant inhibition of xanthine oxidase. As a result, honey is an important inhibitor depending on a floral source with the degree of inhibition against urease, hyaluronidase and xanthine oxidase enzymes. Regular honey consumption contributes to a reduction in inflammatory injuries and strengthening the human immune system.
The molecular diversity of antioxidant substances in a sample can always interfere with the formation of a linear relationship between the results obtained with the applied methods. It is also apparent from the results of the literature: it is not appropriate to provide information about the antioxidant capacity of a sample with a single antioxidant method. In other words, there is not an antioxidant method that detects all antioxidants as a radical source, the antioxidant capacity was examined using different methods. It has also been found that there is a correlation among the methods used. Thus, each method used bridges the gap of other method’s methodologies.
It is worth mentioning that honey contains a lot of biologically active components such as polyphenols, vitamin C, organic acids, catalase, glucose oxidase, amino acids and proteins that may react with reactive oxygen species (ROS). Some studies have also proposed that these compounds may help to slow down aging due to antioxidant abilities [6, 33, 34]. Especially, the phenolic contents of honeys are directly responsible for their antioxidant degree, so they can be evaluated as natural and therapeutically products. There were a great many related studies in this area to support this reality. The phenolic content of monofloral honey of the flora of Turkey is within the range of 16–78 mg GAE/100 g [33], the total phenolic content of chestnut honey Turkey ranges from 19.05 to 108.21 mg GAE/100 g [34] and the phenolic content of chestnut honey in Black Sea was 38.90–65.30 mg GAE/100 g in a sample [6]. The average polyphenol content was 14.67 mg GAE/100 g in a sample of a different botanical origin in Southern Italy [35], while in this content from Croatia chestnuts was within the range of 18–29.2 mg GAE/100 g in a sample [36]. The total amount of phenolic chestnuts in the present study (19.2–146.8 mg GAE/100 g in a sample) is more than literature. H3 chestnut honey has the highest phenolic content in all honey samples. When compared to other studies, they reported that there was a correlation between the amount of total phenolic and biological activity of honey [33, 37].
Antioxidant, antimutagenic and free radical scavenging activities are generally found to increase with flavonoids that are parts of phenolic compounds [38]. The total flavonoid assay was used for the determination of a cumulative flavonoid ingredient in honey samples. In addition, the total flavonoid contents of the analyzed honeys were comparable to the previously reported values. Kolayli et al. (2016) reported that the total flavonoid contents in chestnut honeys in Turkey were within the range of 6.0–7.6 mg QE/100 g in a sample. In another study a high average of the flavonoid content of chestnut honeys was provided as 10.8 mg QE/100 g in a sample [33]. Perna et al. (2012) found the total amount of flavonoids in a chestnut of a different botanical origin in Italy as 7.92 mg QE/100 g in honey. In our study, the total flavonoids ranging from 1.5 to 4.4 mg QE/100 g in the sample obtained from Giresun and Ordu seems to be between the values given in the previous studies.
On the basis of the chemical reactions involved, the total antioxidant capacity assays can also be grouped into two categories: hydrogen atom transfer (HAT) based methods and single electron transfer (SET) based methods. FRAP and CUPRAC are part of SET-based methods and detect the ability of a potential antioxidant to transfer one electron to reduce any compound, including metals, carbonyls, and radicals. The FRAP method measures the ability of reduction to Fe2+from Fe3+ in antioxidants [39]. All the studied chestnut honey samples were found to have a reduced ability to ferric iron (9.8 ± 1.3 – 89.3 ± 1.4 µmol Fe (II)/100g in a sample) (Table 1). Sahin (2016) also revealed the antioxidant capacity of chestnut honey using the FRAP method that it was within the range from 3.111 ± 0.078 to 4.690 ± 0.094 µmol Fe (II)/g in a sample. In addition, Can et al. (2015) found 4.30 ± 0.13 µmol Fe (II)/g in a sample. According to the obtained results, the antioxidant capacity of honey largely depends on the botanical origin of honey and phenolic compounds. In this study, the total phenolic and flavonoid contents showed as a result of a FRAP test a positive correlation as 0.948 and 0.805, respectively. Thereby, a higher polyphenolic content indicates a higher reduction in ferric iron. One of the methods in the total antioxidant capacity is a CUPRAC test based on an electron transfer which is a method for measuring the ability of reduction to Cu+ from Cu+2 in antioxidants. High CUPRAC value indicates a high antioxidant capacity. All the honey samples in the present study had a high CUPRAC value (11.0–97.1 mmol Trolox/100 g). There were a few studies on the determination of antioxidant activity using the CUPRAC method. Kaygusuz et al. (2016) found a CUPRAC value of 23.8 ± 1.5 to 17.18 ± 1.52 µmol Trolox/g in a sample from chestnut honey with the origin of Trabzon. A high positive correlation value (R2 = 0.990) was identified between FRAP and CUPRAC antioxidant activities. That was normal because it was known that these methods were based on nearly the same redox hypothesis.
The DPPH and ABTS radical scavenging methods are commonly used to measure the free radical scavenging ability of a lot of natural products [41]. When comparing these radical scavenging activity assays, it was found that they have some different advantages and disadvantages. According to Sahah and Modi (2015), DPPH is known as stable and commercially available organic nitrogen radicals, is widely reported methods for the determination of antioxidant activity. It can also be preferred for its operational simplicity in the preparation of chemicals and a short incubation time. In addition, Sahah and Modi (2015) mentioned about the ABTS radical scavenging activity method widely reported for the measurement of antioxidant activity. Despite its frequent use, the assay has some drawbacks such as forming an unstable radical and calculating nonreproducible results. In each method, the SC50 value was defined as the concentration of a compound that gives 50% of inhibition of the maximal activity and the low SC50 value indicates that the sample showed a high radical scavenging activity. DPPH (19.344–88.221 mg/ml) and ABTS (0.10–0.43 mg/ml) results in the present study demonstrated that all honeys had an antioxidant activity. Since the reaction conditions used by other authors in the literature were different, it was difficult to make direct comparisons between the previous study data and the ABTS and DPPH values in the current study. However, we could say that our study results were compatible with the results presented by other authors [1, 21, 25]. Between the DPPH and ABTS radical scavenging methods, a positive correlation with the value R2 = 0.984 was detected. In addition, the negative correlation between the total phenolic contents as a result of DPPH and ABTS tests, respectively (R2 = –0.892 and R2 = –0.907), indicates that a high phenolic content indicates a high antioxidant capacity. There was a correlation between all the antioxidant tests used in this study.
In conclusion, a correlation between the antioxidative effects and the degree of some enzyme inhibitions based on the biochemical and nutritional substantiality of chestnut honeys can be evaluated as the evidence. As is known, the factors that affect the chestnut honey composition lead to the correlation of bioactive properties as some enzyme inhibitions and anti-oxidation degrees. The current study results claimed that all the honey samples that had the anti-oxidative levels and different inhibition concentrations of hyaluronidase, xanthine oxidase and urease, are beneficial for human consumption. Their richness of bioactive properties was found to be relatively moderate among the honey samples collected in different regions.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare that they have no conflict of interest. The authors alone are responsible for the content and writing of this paper.
БЛАГОДАРНОСТИ
The authors would like to thank Giresun University Research Fund, Bilimsel Arastirma Projeleri (BAP) Yurutucu Sekreterligi (Project FEN-BAP-A-200515-70) and TUBITAK project (114Z370) for the financial support. The authors are also grateful to all the beekeepers (especially Yusuf Külekçi and Mehmet Külekçi) and those who assisted with the collection of honey specimens.
СПИСОК ЛИТЕРАТУРЫ
- Kolayli S., Yildiz O., Sahin H., and Aliyazioglu R. Biochemistry and Pysicochemical Properties of Honey. In: Boukraa L. (ed.). Honey in Traditional and Modern Medicine. CRC Pres, Taylor & Francis Group, 2013, pp. 21–35.
- Devillers J., Morlot M., Pham-Delegue M.H., and Dore J.C. Classification of monofloral honeys based on their quality control data. Food Chemistry, 2004, vol. 86, no. 2, pp. 305–312. DOI: 10.1016/j.foodchem.2003.09.029.
- Eraslan G., Kanbur M., Silici S., and Karabacak M. Beneficial effect of pine honey on trichlorfon induced some biochemical alterations in mice. Ecotoxicology and Environmental Safety, 2010, vol. 73, no. 5, pp. 1084–1091. DOI: 10.1016/j.ecoenv.2010.02.017.
- Bogdanov S., Ruoff K., and Persano Oddo L. Physico-chemical methods for the characterisation of unifloral honey: A review. Apidologie, 2004, vol. 35, pp. 4–17. DOI: 10.1051/apido:2004047.
- Bertoncelj J., Golob T., Kropf U., and Korosec M. Characterisation of Slovenian honeys on the basis of sensory and physicochemical analysis with a chemometric approach. International Journal of Food Science and Technology, 2011, vol. 46, no. 8, pp. 1661–1671. DOI: 10.1111/j.1365-2621.2011.02664.x.
- Sahin H. Honey as an apitherapic product: its inhibitory effect on urease and xanthine oxidase. Journal of Enzyme Inhibition and Medicinal Chemistry, 2016, vol. 31, no. 3, pp. 490–494. DOI: 10.3109/14756366.2015.1039532.
- Rauf A. and Jehan N. Natural products as a potential enzyme inhibitors from medicinal plants. Chapter 7. In: Şentürk M. (ed.). Enzyme inhibitor and activators. INTECH Publ., 2017, pp. 165–177. DOI: 10.5772/67376.
- Necas J., Bartosikova L., Brauner P., and Kolar J. Hyaluronic acid (hyaluronan): a review. Veterinarni Medicina, 2008, vol. 53, no. 8, pp. 397–411. DOI: 10.12691/ajmbr-3-4-6.
- Fronza M., Muhr C., da Silveira D.S.C., et al. Hyaluronidase decreases neutrophils infiltration to the inflammatory site. Inflammation Research, 2016, vol. 65, no. 7, pp. 533–542. DOI: 10.1007/s00011-016-0935-0.
- Ryu H.W., Lee J.H., Kang J.E., Jin Y.M., and Park K.H. Inhibition of xanthine oxidase by phenolic phytochemicals from Broussonetia papyrifera. Journal of the Korean Society for Applied Biological Chemistry, 2012, vol. 55, no. 5, pp. 587–594. DOI: 10.1007/s13765-012-2143-0.
- Weatherburn M.W. Phenol-hypochlorite reaction for determination of ammonia. Analytical Chemistry, 1967, vol. 39, no. 8, pp. 971–974. DOI: 10.1021/ac60252a045.
- Louveaux J., Maurizio A., and Vorwohl G. Methods of melissopalynology. Bee World, 1978, vol. 59, no. 4, 139–157. DOI: 10.1080/0005772X.1978.11097714.
- Nair S., Meddah B., and Aoues A. Melissopalynological characterization of north Algerian honeys. Foods, 2013, vol. 2, no. 1, pp. 83–89. DOI: 10.3390/foods2010083.
- Kolayli S., Sahin H., Can Z., Yildiz O., Sahin K. Honey shows potent inhibitory activity against the bovine testes hyaluronidase. Journal of Enzyme Inhibition and Medicinal Chemistry, 2016, vol. 31, no. 4, pp. 599–602. DOI: 10.3109/14756366.2015.1054819.
- Singleton V.L. and Rossi J.L. Colorimetry of total phenolics with phosphomolybdic - phosphotungstic acid reagents. Journal of Enology and Viticulture, 1965, vol. 16, pp. 144–148.
- Chang C.C., Yang M.H., Wen H.M., and Chern J. C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis, 2002, vol. 10, no. 3, pp. 178–182.
- Benzie I.F.F. and Strain J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods in Enzymology, 1999, vol. 299, pp. 15–27. DOI: 10.1016/S0076-6879(99)99005-5.
- Apak R., Güçlü K., Ozyürek M., and Karademir S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. Journal of Agricultural and Food Chemistry, 2004, vol. 52, no. 26, pp. 7970–7981. DOI:10.1021/jf048741x.
- Brand-Williams W., Cuvelier M.E., and Berset C. Use of free radical method to evaluate antioxidant activity. LWT-Food Science and Technology, 1995, vol. 28, no. 1, pp. 25–30. DOI:10.1016/S0023-6438(95)80008-5.
- Van den Berg R., Haenen G.R., Van Den Berg H., and Bast A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chemistry, 1999, vol. 66, no. 4, pp. 511–517. DOI: 10.1016/S0308-8146(99)00089-8.
- Küçük M., Kolayli S., Karaoğlu S., et al. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chemistry, 2007, vol. 100, no. 2, pp. 526–534. DOI: 10.1016/j.foodchem.2005.10.010.
- De Vasconcelos M.C.B.M., Bennett R.N., Rosa E.A.S., and Ferreira-Cardoso J.V. Composition of European chestnut (Castanea sativa Mill.) and association with health effects: fresh and processed products. Journal of the Science of Food and Agriculture, 2010, vol. 90, no. 10, pp. 1578–1589. DOI: 10.1002/jsfa.4016.
- Alvarez-Suarez J.M., Giampieri F., González-Paramás A.M., et al. Phenolics from monofloral honeys protect human erythrocyte membranes against oxidative damage. Food and Chemical Toxicology,2012, vol. 50, no. 5, pp. 1508–1516. DOI: 10.1016/j.fct.2012.01.042.
- Choi D.S., Kim S., Lim Y.M., et al. Hydrogel incorporated with chestnut honey accelerates wound healing and promotes early HO-1 protein expression in diabetic (db/db) mice. Tissue Engineering and Regenerative Medicine, 2012, vol. 9, no. 1, pp. 36–42. DOI: 10.1007/s13770-012-0036-2.
- Amtul Z., Rahman A.U, Siddiqui R.A., and Choudhary M.I. Chemistry and mechanism of urease inhibition. Current Medicinal Chemistry, 2002, vol. 9, no. 14, pp. 1323–1348. DOI: 10.2174/0929867023369853.
- Stern R. Hyaluronidases in cancer biology. Seminars in Cancer Biology, 2008, vol. 18, no. 4, pp. 275–280. DOI: 10.1016/j.semcancer.2008.03.017.
- Sunitha K., Suresh P., Santhosh M.S., et al. Inhibition of hyaluronidase by N-acetyl cysteine and glutathione: Role of thiol group in hyaluronan protection. International Journal of Biological Macromolecules, 2013, vol. 55, pp. 39–46. DOI: 10.1016/j.ijbiomac.2012.12.047.
- Girish K.S. and Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Science, 2007, vol. 80, no. 21, pp. 1921–1943. DOI: 10.1016/j.lfs.2007.02.037.
- Isoyama T., Thwaites D., Selzer M.G., et al. Differential selectivity of hyaluronidase inhibitors toward acidic and basic hyaluronidases. Glycobiology, 2006, vol. 16, no. 1, pp. 11–21. DOI: 10.1093/glycob/cwj036.
- Mehta S.K. and Nayeem N. Natural xanthine oxidase inhibitors for management of gout: a review. Research and Reviews: Journal of Medical and Health Sciences, 2014, vol. 3, pp. 4–13.
- Boumerfeg S., Baghiani A., Djarmouni M., et al. Inhibitory activity on xanthine oxidase and antioxidant properties of Teucrium polium L. extracts. Chinese Medicine, 2012, vol. 3, no. 1, pp. 30–41. DOI: 10.4236/cm.2012.31006.
- Sowndhararajan K., Joseph J. M., and Rajendrakumaran D. In vitro xanthine oxidase inhibitory activity of methanol extracts of Erythrina indica Lam. leaves and stem bark. Asian Pacific Journal of Tropical Biomedicine, 2012, vol. 2, no. 3, pp. S1415–S1417. DOI: 10.1016/S2221-1691(12)60428-6.
- Can Z., Yildiz O., Sahin H., et al. An investigation of Turkish honeys: their pyhysico-chemical properties, antioxidant capacities and phenolic profiles. Food Chemistry, 2015, vol. 180, pp. 133–141. DOI: 10.1016/j.foodchem.2015.02.024.
- Sagdic O., Silici S., and Ekici L. Evaluation of the phenolic content, antiradical, antioxidant, and antimicrobial activity of different floral sources of honey. International Journal of Food Properties,2013, vol. 16, no. 3, pp. 658–66. DOI: 10.1080/10942912.2011.561463.
- Perna A., Simonetti A., Intaglietta I., Sofo A., and Gambacorta E. Metal content of southern Italy honey of different botanical origins and its correlation with polyphenol content and antioxidant activity. International Journal of Food Science and Technology, 2012, vol. 47, no. 9, pp. 1909–1917. DOI:10.1111/j.1365-2621.2012.03050.x.
- Šarić G., Marković,K., Vukičević D., et al. Changes of antioxidant activity in honey after heat treatment. Czech Journal of Food Sciences, 2013, vol. 31, no. 6, pp. 601–606. DOI: 10.17221/509/2012-CJFS.
- Baltrusaityte V., Venskutonis P.R., and Ceksteryte V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chemistry, 2007, vol. 101, no. 2, pp. 502–514. DOI: 10.1016/j.foodchem.2006.02.007.
- Lee J., Koo N., and Min D.B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Comprehensive Reviews in Food Science and Safety, 2004, vol. 3, no. 1, pp. 21–33. DOI: 10.1111/j.1541-4337.2004.tb00058.x.
- Rubio C.P., Hernandez-Ruiz J., Martinez-Subiela S., Tvarijonaviciute A., and Ceron J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Veterinary Research, 2016, vol. 12, no. 1, article number 166. DOI: 10.1186/s12917-016-0792-7.
- Kaygusuz H., Tezcan F., Erim F.B., et al. Characterization of Anatolian honeys based on minerals, bioactive components and principal component analysis. LWT-Food Science and Technology,2016, vol. 68, pp. 273–279. DOI: 10.1016/j.lwt.2015.12.005.
- Ahn M.-R., Kumazawa S., Usui Y., et al. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chemistry, 2007, vol. 101, no. 4, pp. 1383–1392. DOI:10.1016/j.foodchem.2006.03.045.
- Shah P. and Modi H.A. Comparative Study of DPPH, ABTS and FRAP Assays for Determination of Antioxidant Activity. International Journal for Research in Applied Science & Engineering Technology (IJRASET), 2015, vol. 3, no. 6, pp. 636–641.