Аннотация
Oats, both separately and as part of other dishes, refer to that healthy food that is regularly consumed by the broad masses of the population of various countries. Oats are a grain crop with a high level of nutrients, such as proteins, fats, minerals and vitamins. The oat grain from other cereals is distinguished by a high protein content and the unique composition of essential amino acids. The high content of β-glucan-soluble fiber allows us to consider oats as part of the complex therapy and prevention of cardiovascular diseases. There are more than 70 types of oats in the world, one of the most cultivated is Avena sativa L . The objects of the study were the samples of oat grains of the Drug [Friend], Adamo and Rysak [Trotter] varieties grown on the territory of the Russian Federation (the crop of 2017). The results of the study of the chemical composition and nutritional value of the samples of oat grains of these varieties have been provided. In the samples of the studied oat grain varieties, the content of proteins, carbohydrates and fats is not inferior to other varieties. The record content of protein, among the samples studied, is noted in the samples of oat grains of the Rysak variety, the minimum content - in the samples of oat grains of the Drug variety. The oat grains of the Adamo variety are characterized by a high content of carbohydrates, their mass fraction in this variety is 76.40%. The protein concentrate of oats grains of the Rysak variety was obtained using two methods: alkaline and acid extraction. The highest efficiency was observed in the alkaline extraction in the presence of an extracting agent - 1 M of the aqueous sodium hydroxide solution. The protein yield was 75.53% at a temperature of 40 ± 2°C, an irrigation module of 1 : 10, an active acidity of pH equal to 11.5 and a processing time of 120 ± 2 min.Ключевые слова
Oats, Nutritional value, Protein product, Alkaline extractionВВЕДЕНИЕ
Oats (Avena sativa L.) is an important grain crop which is among the top ten cultivated cereals in the world with such crops as corn, rice, wheat, barley and sorghum [1–3]. Oat grains are characterized by a high protein content, a balanced amino acid composition, they also contain fiber, carbohydrates, unsaturated fatty acids, essential micro- and other nutrients that vary depending on a variety and growth conditions [4–6]. It has been established [7] that oat proteins contain the fractions that are soluble in alkali – 78%, alcohols – 11%, salts – 2.9% and others – 4%. The process of wet grinding of oat grains results in the production of a protein concentrate with a content of 59–75%. The combined use of acetylation and succinylation improves oat protein solubility and fat fixation, the emulsifying properties, impairs water retention and foam stability, the nutritional value has not changed, and the digestibility has improved [8–10]. The study was aimed at the possibility of obtaining protein concentrates from the samples of the oat grains cultivated on the territory of the Russian Federation using two methods – acid and alkaline extraction.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
The experimental studies were carried out at the Research Institute of Biotechnology of the Kemerovo State University.
The objects of the study were the samples of common oat grains of the Drug, Adamo and Rysak varieties (the crop of 2017). The grain samples were preliminary ground in a laboratory mill so that all the ground grains passed through a sieve with a hole diameter of 1 mm when sieved.
The mass fraction of protein in oat grains was determined using the spectrophotometric method with a biuret reagent forming the complexes of peptide bonds with copper ions (II) colored in violet in an alkaline medium according to a calibration schedule. Based on the data obtained, the mass concentration of protein in the studied solution was calculated taking into account the dilutions.
Plotting a calibration graph. A standard protein solution containing 10 mg protein in 1 ml was prepared from which a number of protein solutions of the known concentration were prepared. The studied protein solution was poured into three test tubes: 1.0 ml (Sample No. 1) in the first tube, 0.5 ml in the second (Sample No. 2) and 0.25 ml in the third (Sample No. 3). Then, 0.5 and 0.75 ml of water were added to the second and third tubes, respectively (the volume of the contents should be the same in each tube and amount to 1 ml at this stage). In the second and third tubes, the analyzed protein solution diluted 2 and 4 times, respectively, was prepared. These dilutions were taken into account in the calculation.
4 ml of biuret reagent (4 ml of reagent per 1–10 mg of protein) were poured into the control tube with the samples with the known protein concentration and with the samples of the test protein solution. The contents of each tube were mixed and left at room temperature for 30 minutes to develop color.
The optical density of the solutions (extinction) was measured using a UV 1800 spectrophotometer (Shimadzu, Japan) at 540–650 nm against the control (Sample No. 1). The results obtained for the protein solutions of the known concentration were shown graphically, plotting optical density along the ordinate axis and the protein mass that corresponds to this value – along the abscissa axis. The calibration curve was constructed using the average data of standard solution colorimetric extinction (triple replication).
To recalculate the results (mg/ml) as a percentage, it is necessary to know the mass of the raw material or the product and solvent taken for protein extraction. The protein content (%) was determined using the formula:
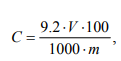
where V is the volume of the extract obtained; m is the weight of the sample; 1000 is a mg to g conversion factor; 100 is a 100 g conversion factor.
The extraction degree was calculated using the formula:
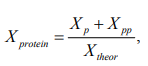
The mass fraction of carbohydrates in oat grains was determined in accordance with GOST 31683-2012. The carbohydrates contained in the grains were dissolved in a hot dilute hydrochloric acid solution, the protein substances were precipitated and the optical rotation angle of the resulting solution was measured and filtered.
The conditional grain starchiness X, %, was calculated using the formula:
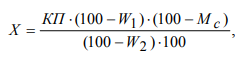
where K is a calibration factor (% of starch / a degree of the saccharimeter scale) when analyzed using a hydrochloric acid solution; П is an indication of the saccharimeter (polarimeter), scale degrees; W1 is the moisture content of the original grains, %; W2 is grinding moisture, %; Mc is the mass fraction of black dockage in the grains, %; 100 is per cent to share conversion.
The mass fraction of fiber and fat in oat grains was determined in accordance with GOST 32749-2014. The method is based on recording the reflection spectra of the analyzed samples in the near infrared region and determining the mass fractions of fat and fiber therein.
The mass fraction of the measured indicator (excluding moisture), in terms of absolutely dry matter X1, %, was calculated using the formula:
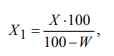
where X is the mass fraction of the measured indicator that corresponds to the indication of the device, %; W is the mass fraction of moisture and volatile substances, %.
The mass fraction of ash was determined in accordance with GOST 10847-74. The essence of the method consists in burning the sample of the ground grain followed by the quantitative determination of a noncombustible residue. The ash content (X) in percents of grain sample, in terms of dry matter, was calculated using the formula:
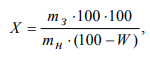
where mз is the weight of ash, g; mн is the weight of the sample of the ground grains, g; W is the moisture content of the ground grains, %.
The mass fraction of water was determined in accordance with GOST 13586.5-93. The method consists in dehydrating the sample of the ground grains in an air-heating cabinet with the fixed parameters: temperature, drying time and determining the loss of its mass.
The moisture content of the grains (%) was calculated using the formula:
![]()
where m1 is the weight of the sample of whole grains after pre-drying, g; m2 is the weight of the sample of the ground grains after drying, g.
The oat grain protein complex was studied by means of electrophoresis in polyacrylamide gel according to Laemmli. Necessary reagents for electrophoresis: THAM (tris(hydroxymethyl)aminomethane), acrylamide, bis acrylamide, SDS, glycine, hydrochloric acid, ammonium persulfate, tetramethylethylenediamine, glycerol, 2-mercaptoethanol and bromophenol blue. A sample buffer and a gel solution are prepared from the resulting solutions.
When preparing separating gel, all the solutions for preparation must be of room temperature before mixing. The gel solution was mixed to prevent the formation of bubbles and 100 μl of 10% SDS was added. A block was collected for gel pouring. First, 100 μl of 10% PSA was added to the gel solution, and then 10 μl of TMEDA was stirred without bubbles and poured into a gel block. Then we waited until the gel polymerized. A concentrated gel was prepared from the previously prepared reagents.
The excess water was removed from the block with the polymerized separating gel by flowing onto filter paper. A comb and block were prepared to pour the stacking gel. 50 μl of 10% PSA and 5 μl of TMEDA were added to the concentrated gel solution, mixed and poured into the block, and a comb was put in to form wells. Then we waited for the stacking gel to polymerize.
To conduct electrophoresis, the filled block was placed in a chamber and the required volume of an electrode buffer was prepared. To this end, a 10x electrode buffer was diluted in a ratio of 1 : 9. An electrode buffer was poured into the chamber. 10% SDS was added to the upper chamber in a volume of 0.01 of the volume of the buffer in the upper chamber.
The samples were placed in the well with the gel using a micro syringe and the camera was connected to the power unit.
The completion of electrophoresis was controlled by the approach of the front of the bromphenol blue dye to the lower mark of the gel.
The gel electropherograms were developed using bright blue Coomassie. A boiling water bath installed in a fume hood was prepared. A dye solution was prepared. The gel was placed in a beaker and filled with a five-fold volume of the dye solution. The beaker with the gel was placed on the water bath in the fume hood and boiled for 10 minutes. The dye was poured out from the beaker, the gel was washed with distilled water and filled with a 7% acetic acid. The acetic acid solution was boiled in the water bath for 5 minutes, then changed to a fresh one twice and boiled again. Adding small pieces of the filter paper that absorbs the dye to the acetic acid solution makes washing faster.
The active acidity was determined and the activity of hydrogen ions was measured using a potentiometric analyzer. To this end, the test sample was added to the beaker, an electrode was placed in a way to avoid the contact of the electrode with the walls of the beaker.
The amino acid composition was determined using an Aracus PMA GmbH automatic amino acid analyzer. The principle of the method consists in the cation-exchange separation of amino acids with a pH step gradient and post-column derivatization with ninhydrin.
The elemental composition was determined by means of capillary electrophoresis using a Kapel-105/105M capillary electrophoresis system.
The mass fraction of phospholipids was determined by HPLC with UV detection using an LC-10 liquid chromatograph (Shimadzu, Japan).
The fatty acid composition was determined in accordance with GOST 30418-96 "Vegetable oils. Method for determination of fatty acid content".
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
Oat grains are widely used both in cereals, in beverages and in bakery products. Oat protein is considered more nutritious than most of the crops consumed today [11, 12].
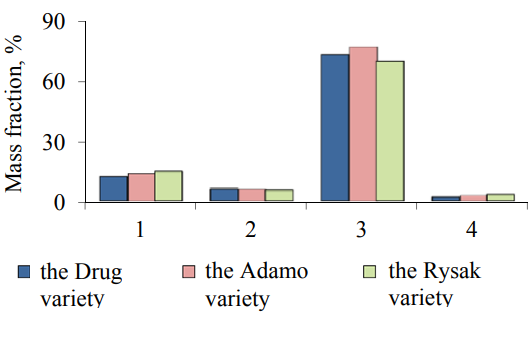
The chemical composition and nutritional value of the samples of different types of cereals vary greatly depending on growth, climatic and geographical conditions, and well as the variety of a cereal crop. Fig. 1 presents the results of determining the chemical composition of oat grains.
The highest amount of protein, carbohydrates and fat is contained in the samples of oat grains of the Rysak, Adamo and Drug variety, respectively. The lowest – in the samples of grain oats of the Drug, Adamo and Rysak variety, respectively.
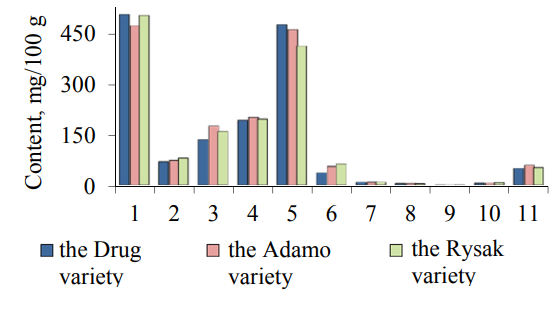
The elemental composition of common oat grains is presented in Fig. 2. The main ash elements of the samples of common oat grains are potassium and phosphorus. There is also silicon, magnesium, manganese, calcium, sulfur, iron, copper and zinc in oat grains. Phosphorus is found in oat grains in an organic and mineral state. The total phosphorus content varies from 409 to 472 mg/100 g, herewith, the content of organic phosphorus varies from 297 to 329 mg per 100 g, and the content of phosphorus in the mineral form varies from 31 to 39 g per 100 g of seeds. Organic phosphorus is found in phytin, lipid and nucleic acid complexes. The percentage of organic phosphorus in phytin reaches 62%, in the lipid complexes – up to 1%, and in the nucleic acids – from 9.5 to 10.5% of total of phosphorus compounds.
The carbohydrate composition of the samples of common oat grains was determined using the chromatography method. The results of the study are presented in Fig. 3.
Carbohydrates (Fig. 1) account for the largest part among the other substances in the samples. The carbohydrate complex of oats grains consists of starch, hemicellulose, cellulose, lignin and simple saccharides. A starch fraction predominates in common oat grains, the mass fraction of starch in the common oat grains of the Drug variety is 36.37%, and the content of starch in the oat grains of the Adamo variety is 12% higher than in the common oat grains of the Rysak variety. The mass fraction of hemicellulose in common oat grains varies from 12.6 to 14.51%, moreover, the content of this carbohydrate in the oat grains of the Drug variety is 14.18%, which is 2.3% lower than the content of hemicellulose in the oat grains of the Adamo variety. Hemicellulose is represented in oat kernels mainly by non-starchy water-soluble beta-glucan – a polysaccharide that is able to reduce a high blood cholesterol level. The presence of β-glucan causes the viscosity of aqueous solutions and the jelly-like properties of oat grain derivative products. The chromatographic analysis showed that hemicellulose is 20–80% of β-glucan, its amount depends on the variety, location and growth conditions of oats. The content of β-glucan in the oat grains of the Drug variety is 17.8% lower than in the oat grains of the Adamo variety and 2% higher than in the oat grains of the Rysak variety. Hemicellulose and fiber activate the motility of the gastrointestinal tract and promote the secretion of digestive juices in a living body. The oat grains of the Adamo variety dominates in a fiber content, the mass fraction of fiber in the common oat grains of the Adamo variety is 10.92%, and the mass fraction of fiber in the oat grains of the Drug variety is 10.19%, which is 1.2% lower than the fiber content in the oat grains of the Rysak variety.
The mass fraction of monosaccharides in the samples of common oat grains of the Adamo variety was 0.21%, and in the samples of the Drug variety – 38% lower than that in the samples of the Adamo variety, 0.16% – in the samples of the Rysak variety. According to the content of disaccharides, the oat grains of the Adamo variety (the mass fraction of disaccharides was 1.68%) dominate among the studied varieties, the lowest mass fraction of disaccharides was noted for the oat grains of the Drug variety (the mass fraction of disaccharides was 1.51%). It has also been shown that the lignin content is within the range from 3.0 to 3.4%. The maximum content of lignin has been noted for the oat grains of the Adamo variety, and the minimum mass fraction of lignin is characteristic for the oat grains of the Rysak variety.
One of the advantages of oat grains in relation to other cereal crops is a high content of fats. The mass fraction of fat in the studied varieties varies from 5.5 to 6.2% (Fig. 1). At the same time, the percentage of the lipids extracted with the help of nonpolar organic solvents accounts for up to 4.5%, bound lipids – 0.30–0.45% and tightly bound lipids – up to 0.38%. The detailed analysis of the fatty acid composition of oat grains (Fig. 4) shows that oat grains contain a palmitic, stearic, myristic and stearic saturated fatty acid. The unsaturated fatty acids include an oleic, linoleic and linolenic acid. Arachidonic, lauric and palmitoleic acids were found in trace amounts.
The vegetable oil of oat grains is characterized by a high nutritional value due to the predominance of linoleic acid in its composition, which is an essential one. The mass fraction of linolenic acid is insignificant in total of all the fatty acids. Due to this, the oxidable ability of the vegetable oil obtained from oat grains increases.
The quantitative protein content and the amino acid score in oat grains are very high and significantly exceed those for other cereals. Oats take the third place in terms of the quantitative content of protein substances being only inferior to wheat and winter rye. The mass fraction of proteins in the studied oat grains varies from 12.2% to 14.7% (Fig. 1). Herewith, the protein substances predominate, their mass fraction reaches 87–90% of the total amount of nitrogenous compounds, and the amount of non-protein substances is 10–13% in grains. It is worth noting that the proteins are distributed throughout the mass of the grains, but not evenly. Thus, the endosperm accounts for up to 51%, the aleurone layer – 56% and the embryonic axis – only 2–4% of nitrogenous substances.
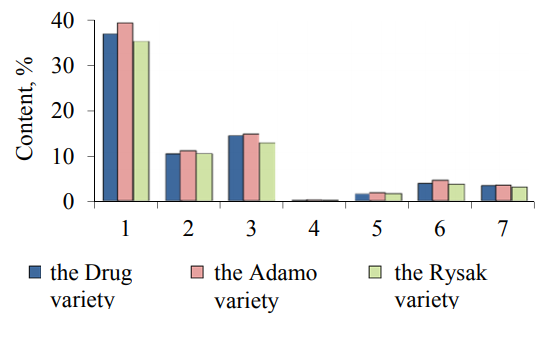
The analysis of the fractional composition of proteins of the samples of common oat grains (Fig. 5) shows that the protein complex of oat grains, regardless of a variety, consists mainly of albumins, globulins, glutelins and prolamins, moreover, the storage proteins (globulins and glutelins) make the largest part in oat grains. Thus, the content of glutelins in the common oat grains of the Drug variety was 27.98% – this is the lowest value among the studied varieties of common oat grains. The oat grains of the Rysak variety have the maximum mass fraction of glutelins (the mass fraction of globulins was 41.88%). The oat grains of the Adamo variety are characterized by a high content of glutelins (the mass fraction was 33.76%) and globulins (the mass fraction of globulins was 28.19%), which is 7% more than the content of globulins in the oat grains of the Drug variety and 13.5% less than in the oat grains of the Rysak variety. The record amount of albumins is contained in the oat grains of the Drug variety (the mass fraction of albumins was 23.37%), which is 2.1 times more than that in the protein part of the oat grains of the Rysak variety and 2.3 times more than in the oat grains of the Adamo variety.
The oat grains of the Adamo variety are characterized by a high content of prolamins, their mass fraction in this oat variety reaches 17.84%, which is 16.3% more than the content of prolamins in the oat grains of the Rysak variety and 30.4% more than the content of prolamins in the oat grains of the Drug variety. The different quantitative content of prolamins and globulins depending on an oat variety indicates the mobility of these fractions and the general biological state of oat grain tissues.
Vegetable protein is very valuable due to its amino acid composition, since amino acids are the main building elements in a living body. The scientific literature presents data on the amino acid composition of oats, however, no exact information on the amino acid composition of the proteins that make up the samples of common oat grains of the Drug, Adamo and Rysak varieties has been found. Fig. 6 presents the amino acid composition of the samples of oat grains of the varieties under study.
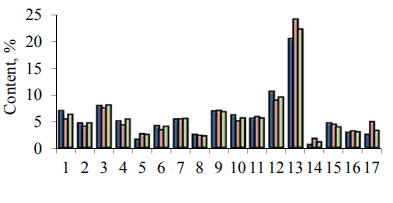
The qualitative amino acid composition of grains does not depend on the type and variety of oats. Oat varieties differ in the quantitative content of amino acids. The analysis of the results shows that the composition of oat grains includes all the eight essential amino acids and they can be referred to complete proteins. The most complete protein is a Rysak variety, the biological value of this variety is 66 in relation to the protein standard – a chicken egg. The quantitative content of essential amino acids in the oat grains of the Rysak variety is 29.4% in relation to the total amino acid content. The oat grains of the Drug variety contain 27.6% of essential amino acids in relation to the total content, and the oat grains of the Adamo variety are characterized by a low content of essential amino acids.
Oats are considered as a source of high quality proteins. The protein content varies within the range of 12–15%. There are mutagenized varieties with a high protein content of up to 24%. They have a balanced composition of amino acids and a high fat content in comparison with other cereals. Our results are consistent with the reports of other authors [13–14] and the studied samples of grains of the oats cultivated on the territory of the Russian Federation are not inferior to other varieties and growth territories in the nutritional value. The studies on the chemical composition of oat grains of the Rysak variety, oats grains of the Drug variety and oat grains of the Adamo variety showed that oat grains contain a large amount of protein, carbohydrates and lipids. The record content of protein among the studied objects has been found in the oat grains of the Rysak variety, the minimum mass fraction of protein has been noted for the oat grains of the Drug variety. The oat grains of the Adamo variety are characterized by a high content of carbohydrates, their mass fraction in this variety is 76.40%. According to the content of essential amino acids, the grains of the Rysak variety are also a leader. Therefore, the oat grains of the Rysak variety have been chosen for further studies.
Alkaline and acid extraction was used to isolate proteins from the samples of oat grains of the Rysak variety. The main parameters of an alkaline extraction process are active acidity (pH is 9.0, 9.5, 10.0, 10.5, 11.0, 11.5, 12.0, 12.5 and 13.0), temperature (35, 40, 45 and 50°C), process duration (30, 60, 90 and 120 min) and an extractable substance and extractanthydromodule ratio (1 : 05, 1 : 10, 1 : 15 and 1 : 20). The main parameters of an acid extraction process are active acidity (pH is 1.0, 2.0, 3.0 and 4.0), temperature (30, 35, 40, 45 and 50°C), process duration (30, 60, 90, 120 and 150 min) and an extractable substance and extractant-hydromodule ratio (1 : 5, 1 : 10, 1 : 15 and 1 : 20). The most common and economically sound extractants were used as alkaline solutions: a 1M potassium hydroxide solution and a 1M sodium hydroxide solution. An aqueous hydrochloric acid solution and an aqueous sulfuric acid solution – as aqueous inorganic acid solutions. The results of the studies are presented in Fig. 7–10.
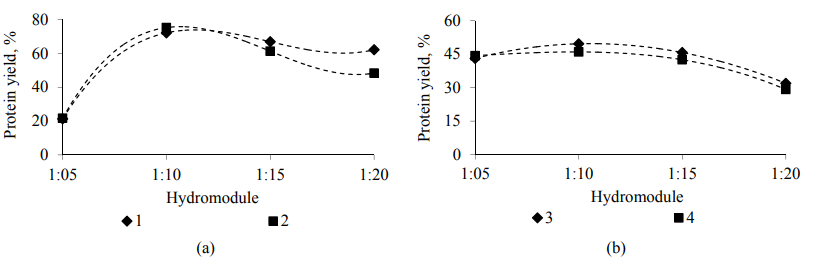
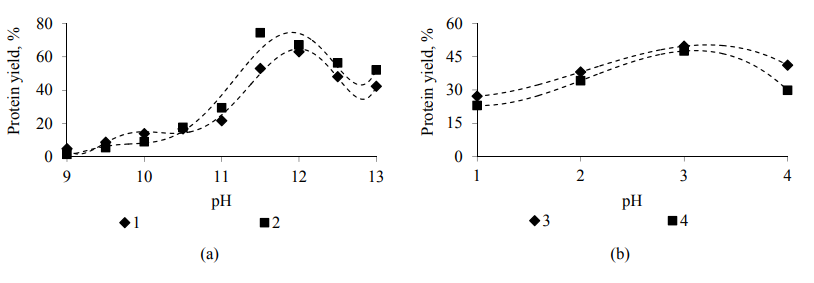


Effect of duration of the process of alkaline (a) and acid (b) extraction on the yield of protein substances from the oat grains of the Rysak variety in the presence of: 1–1 M of a potassium hydroxide solution; 2–1 M of a sodium hydroxide solution; 3 – of an aqueous hydrochloric acid solution; 4 – of an aqueous sulfuric acid solution.
Thus, in the course of the studies of the technological approach of isolating protein from the oat grains of the Rysak variety using the alkaline and acid extraction method, an extraction agent has been selected – a 1 M aqueous sodium hydroxide solution and an aqueous hydrochloric acid solution, respectively. The rational values of the technological parameters of the extraction process have determined a temperature of 40 ± 2°C and 35 ± 2°C, a hydromodule of 1 : 10 and 1 : 10, an active acidity of 11.5 and 3.0 and a duration of 120 ± 2 and 90 ± 2 min, respectively, for alkaline and acid extraction. The use of these technological regimes allows us to achieve the maximum yield of protein substances from oat grains to 75.53% and 54.60% for the studied processes.
The obtained results are in agreement with the results of the authors of [3] who reported that up to 78% of oat proteins are soluble in alkalis and the alkaline extraction with the use of a sodium hydroxide solution was more effective for the sample of oat grains of the Rysak variety.
ВЫВОДЫ
The carried out studies have confirmed that the oat varieties cultivated on the territory of the Russian Federation are not inferior to other oat varieties grown on other territories in a nutritional value. The study of the chemical composition of the samples of oat grains of the Drug, Adamo and Rysak varieties has distinguished the Rysak variety in terms of the content of proteins and essential amino acids.
The parameters of alkaline and acid extraction of protein from the oats grains of the Rysak variety have been optimized and an extraction agent and optimal technological regimes have been selected. It has been shown that the use of a 1 M aqueous sodium hydroxide solution and the selected technological regimes (the temperature is 40 ± 2°C, the hydromodule is 1 : 10, the active acidity is 11.5 and the duration is 120 ± 2 min) allows us to achieve the maximum yield of protein substances from oat grains (the yield of protein is 75.53% ). It has been established that the use of an aqueous hydrochloric acid solution and the selected technological regimes (the temperature is 35 ± 2°C, the hydromodule is 1 : 10, the active acidity is 3.0 and the duration is 90 ± 2 min) allows us to achieve the maximum yield of protein substances from oat grains (the yield of protein is 54.60%). The most effective method for obtaining a protein product from the samples of oat grains of the Rysak variety is the alkaline extraction method.
БЛАГОДАРНОСТИ
The study has been supported by the Ministry of Science and Education of the Russian Federation [Project No. 15.4642.2017/8.9].
СПИСОК ЛИТЕРАТУРЫ
- Bar-El Dadon S., Abbo S., and Reifen R. Leveraging traditional crops for better nutrition and health - The case of chickpea. Trends in Food Science and Technology, 2017, vol. 64, pp. 39-47. DOI: 10.1016/j.tifs.2017.04.002.
- Ning Y., Liu W., and Wang G.-L. Balancing Immunity and Yield in Crop Plants. Trends in Plant Science, 2017, vol. 22, no. 12, pp. 1069-1079. DOI: 10.1016/j.tplants.2017.09.010.
- Cheng A. Review: Shaping a sustainable food future by rediscovering long-forgotten ancient grains. Plant Science, 2018, vol. 269, pp. 136-142. DOI: 10.1016/j.plantsci.2018.01.018.
- Draper S.R. Amino acid profiles of chemical and anatomical fractions of oat grains. Journal of the Science of Food and Agriculture, 1973, vol. 24, no. 10, pp. 1241-1250. DOI: 10.1002/jsfa.2740241013.
- Banaś A., Debski H., Banaś W., et al. Lipids in grain tissues of oat (Avena sativa): Differences in content, time of deposition, and fatty acid composition. Journal of Experimental Botany, 2007, vol. 58, no. 10, pp. 2463-2470. DOI: 10.1093/jxb/erm125.
- Hu X.-Z., Zheng J.-M., Li X.-P., Xu C., and Zhao Q. Chemical composition and sensory characteristics of oat flakes: A comparative study of naked oat flakes from China and hulled oat flakes from western countries. Journal of Cereal Science, 2014, vol. 60, no. 2, pp. 297-301. DOI: 10.1016/j.jcs.2014.05.015.
- Lapvetelainen A. and Aro T. Protein composition and functionality of high-protein oats flour derived from integrated starch-ethanol process. Cereal Chemistry, 1994, vol. 71, no. 2, pp. 133-139.
- Ma C-Y. and Khanzada G. Functional properties of deamidated oat protein isolates. Journal of Food Science, 1987, vol. 52, no. 6, pp. 1583-1587. DOI: 10.1111/j.1365-2621.1987.tb05884.x.
- Ma C.-Y. and Wood D.F. Functional properties of oat protein modified by acylation, trypsin hydrolysis or linoleate treatment. Journal of the American Oil Chemists' Society, 1987, vol. 64, no. 12, pp. 1726-1731.
- Gilles G., Rathy P., Amiot J., Roy A., and Brisson G. Nutritional value of acylated oat protein-concentrate. Journal of Agricultural and Food Chemistry, 1998, vol. 35, pp. 589-592. DOI: 10.1007/BF02542510.
- Mäkinen O.E., Sozer N., Ercili-Cura D., and Poutanen K. Protein from Oat: Structure, processes, functionality, and nutrition. Sustainable protein sources, 2017, pp. 105-119. DOI: 10.1016/B978-0-12-802778-3.00006-8.
- Sterna V., Zute S., and Brunava L. Oat grain composition and its nutrition benefice. Agriculture and Agricultural Science Procedia, 2016, vol. 8, pp. 252-256. DOI: 10.1016/j.aaspro.2016.02.100.
- Mohamed A., Biresaw G., Xu J., Hojilla-Evangelist M.P., and Rayas-Duartec P. Oats protein isolate: Thermal, rheological, surface and functional properties. Food Research International, 2009, vol. 42, no. 1, pp. 107-114. DOI: 10.1016/j.foodres.2008.10.011.
- Sunilkumara B.A., Leonova S., Öste R., and Olssonb O. Identification and characterization of high protein oat lines from a mutagenized oat population. Journal of Cereal Science, 2017, vol. 75, pp. 100-107. DOI: 10.1016/j.jcs.2017.03.003.