Аннотация
Lactic acid bacteria (LAB) dominate the spoilage populations of vacuum-packaged emulsion-type sausages and other processed meats stored at refrigeration temperatures. An experimental investigation was carried out to evaluate the effectiveness of two in-package pasteurization treatments to prevent microbial spoilage in emulsion vacuum packaged sausages during refrigerated storage. D-values at 60 and 68°C for two isolated dominant LAB ( Lactobacillus sakei and Lactobacillus plantarum ) were determined in vitro and thermal treatments aimed to achieve a 4 log reduction in LAB. Sixty three sausage packs were divided into 3 groups: untreated packs (Control), treated at 60°C for 120 s (PAST 1) and treated at 68°C for 12 s (PAST 2). Microbial composition, pH values and sensorial changes were monitored in two-week intervals. In-package pasteurization resulted in an immediate 3.5-4.2 log CFU/g reduction in the population of LAB in PAST 1 and PAST 2 and remained at an acceptable level to the end of the experiment. On the contrary, during 84 days of cold storage, the LAB count increased significantly in the control samples and reached 9 log CFU/g. The control samples were also considered as unacceptable spoiled products after 28 days by sensorial aspects. All the pasteurized treatments also resulted resulted a significant (p < 0.05) reduction of psychrotrophic and the total mesophilic bacteria compared to the control ones. The data obtained showed that in both pasteurized groups none of the sensorial parameters were rated higher than the consumer-rejection threshold within 84 days of the study. No significant (p > 0.05) differences were observed in the number of spores, yeasts and molds between the pasteurized and control samples. It has been concluded that in-package pasteurization is an effective method without undesirable effects to prevent the spoilage caused by LAB growth and extend the emulsion vacuum packaged sausages shelf life to more than 3 months.Ключевые слова
In-package pasteurization, LAB, spoilage, shelf lifeВВЕДЕНИЕ
It is considered that vacuum packaging combined with thermal treatment and refrigerated storage promotes extending the shelf life of meat products by reducing microbial growth. Vacuum packaging can inhibit the growth of the food-borne pathogens and spoilage bacteria frequently present in meat due to the restriction of the typical spoilage bacteria growth such as Pseudomonas in the absence of oxygen [1, 2]. Consequently, the environment conditions shift the bacterial flora to anaerobic psychrotrophic microorganisms dominated mainly by Lactic Acid Bacteria (LAB) which recontaminates the cooked products during handling and slicing prior to packaging. The spoilage caused by LAB metabolic activity appears in the form of sourness, offflavors, off-odors, milky exudates, slime production, the swelling of package through gas production and discoloration and shortens shelf-life [2–4]. The characteristics of these bacteria is that they complicate the control of the proliferation of LAB during refrigerated storage, as they are psychrotrophic microaerophilic substances that tolerate nitrite and salt [4, 5]. Different approaches have been used to promote a reduction in the risk of post processing contamination, including chemical treatments, irradiation and thermal processes such as post-package pasteurization by submerging in hot water [5, 6]. In-package pasteurization is not a new approach; however, most of the early literature is used as a means of controlling Listeria monocytogenes in fully cooked meat products [6]. To the best of our knowledge, there is lack of investigation on the impact of in-package pasteurization of sausages on controlling spoilage microorganisms, primarily LAB and limited to Franz et al. [7] who applied in-package pasteurization processes for Vienna sausages and reported increasing in shelf-life from 7 days for non-pasteurized samples to 67, 99 and 119 days for the samples of three pasteurization treatments at 8°C storage.
This investigation was therefore undertaken to verify the impact of in-package pasteurization at 60 and 68°C in preventing microbial spoilage focused on LAB and extending emulsion vacuum packaged sausages shelf life during refrigerated storage (4°C).
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
To study the impact of in-package pasteurization in preventing microbial spoilage and extending the shelf life of emulsion vacuum packaged sausages, the experiments were prepared as described below.
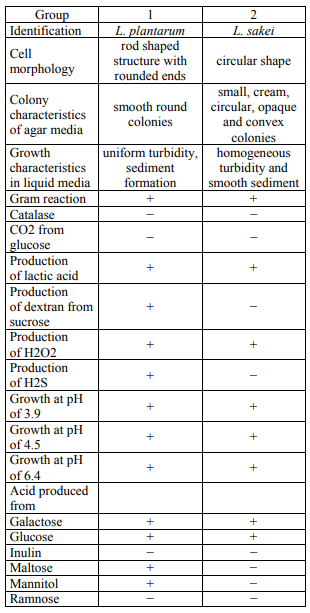
Isolation, characterization and identification of strains. Vacuum-packaged emulsion sausages were obtained from Demes Meat processing Company, Shiraz, Iran. Ten grams of samples were suspended in 90 ml sterile normal saline (0.9% w/v) and treated for 2 min in a stomacher. Then, the appropriately diluted samples were cultured on MRS (de Man Rogosa Sharp) agar (Merck, Darmstadt, Germany) and incubated at 30°C for 48 h under anaerobic conditions using Anaerocult A gas packs (Merck, Darmstadt, Germany). From each plate of MRS agar, the colonies of catalase negative, gram positive rods or cocci were supposed to be LAB. were picked up and maintained on MRS agar at 4°C. The biochemical characterization of isolates was determined using the method described by Jafarpour et al. (Table 1) [8].
Lactobacillus sakei and Lactobacillus plantarum were determined as the dominant bacterial groups associated with the spoilage in refrigerated vacuum-packaged emulsion sausages according to biochemical tests and the previous study performed with vacuumpackaged emulsion sausages [8]. The long-term storage of isolates was done at –80°C in MRS broth with 30% glycerol.
Preparation of bacterial cultures. Each strain was cultured separately in MRS broth at 37°C for 24 h aerobically to reach the early logarithmic growth stage (O.D. 550 nm ca 0.3–0.5). The inoculum density was adjusted to 1 × 107CFU/ml using a ringer solution (Oxoid). The cells were centrifuged (14000 rpm for 5 min) and washed twice with 0.1 mol/l phosphate buffer. The cells were resuspended in ringer solution and vortexed for 1 min to disrupt cell clumps.
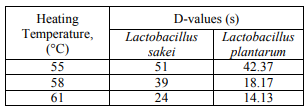
Calculation of D- and z-values. The cell suspensions (1 × 107CFU/ml determined on MRS agar using the platecount method) were sealed in glass capillary tubes and heat inactivated using the previously described methods [7, 9]. Five replicate capillary tubes were heated for each of six time intervals (ranging from 0 to 125 s) at temperatures of 55, 58 or 61°C in a thermostat controlled circulating water bath. A set of five capillary tubes not subjected to heat treatment was considered as the control. The survived bacteria at each heating temperature and heating interval were enumerated on MRS agar. All the experiments were carried out in triplicate. The D-values expressed in seconds were determined from the linear portion of the survival curves by a linear regression analysis by means of Microsoft Excel 2010 software. The z-values were determined by computing the linear regression of the mean log D-values versus their corresponding heating temperatures by means [7].
Thermal treatment/ microbiological analysis. The thermal treatment aimed to achieve a 4 log reduction of the more heat (Lactobacillus sakei) based on the D-value results obtained in Table 2. These target temperatures were also set in order to correspond to the in-package pasteurization of this type of sausage and package, since they were low/short enough not to detrimentally affect packaging material or product quality and cause undesirable changes in sausage appearance (obtained in pilot studies).
The vacuum-packaged emulsion sausages were manufactured according to a standard procedure at Demes Meat processing Company, Shiraz, Iran. The emulsion sausages contained meat and binding component emulsion, 2.2% (w/w) sodium chloride, 150–200 ppm ascorbic acid and less than 100 ppm of residual nitrite which was filled into artificial casings. Eight slices were packaged into 400 g and 17 × 12 × 3 cm3 packs.
The packs from the production line were divided into 3 groups: untreated packs (Control), the packs which were subjected to in-package pasteurization at 60°C for 120 s (PAST 1) and the packs which were subjected to in-package pasteurization at 68°C for 12 s (PAST 2). The vacuum-packaged samples were simultaneously submerged into a water bath (Precision, model 186, Precision Scientific Incorporated, Chicago, USA). The sausage core temperatures were monitored as meat industry standard approach by inserting thermocouples into the core of a sausage located in the middle of a pack for each replication and then sealed. To reduce the time reaching to treatment temperatures, the water bath temperature was set at higher values than those mentioned in PAST 1 and 2 (71°C). Once the core reached the target temperatures, the samples were held for 120 s in PAST 1 group and 12 s for PAST 2, then removed from the water bath and rapidly cooled by immersing in ice-water slurry for 60 s to prevent further cell death, then stored in a refrigerator at 4°C until further analysis. Both the pasteurized and non-pasteurized samples were tested on day 0 and in 2 weeks intervals on day 14, 28, 42, 56, 70 and 84 for microbial and sensorial analysis. On every sampling day, the packages were opened aseptically and 10 grams of sample was homogenized with 90 ml sterile 0.1% peptone water by placing meat into a sterile bag and stomached for 2 min (Seward Stomacher 400 Circulator, Seward Inc., Norfolk, U.K.). The homogenates were then serially diluted and appropriate serial dilutions were pour plated in duplicate. The following microbial parameters were determined: The total mesophilic bacteria plated on PCA agar plates (Merck, Germany) and incubated at 37°C for 48 h, Lactic Acid Bacteria (LAB) grown MRS agar (Merck 5413, Darmstadt, Germany) and incubated anaerobically using an anaerobic gas pack system (Anaerocult A, Merck, Germany) at 30°C for 48 h, Enterobacteria were enumerated on MacConkey agar (Merck 5465, Darmstadt, Germany) after incubation at 37°C for 24 h.
The psychrotrophic bacteria were enumerated using plate count agar (PCA) (Merck, Germany) after 10 days stored in 7°C. The spores were counted on (PCA) (Merck, Germany) after heat treatment at 80°C for 10 min and incubated at 37°C for 48 h.
The yeasts and molds were used cultured on Sabaraud Dextrose agar (SDA) enriched with an antibiotic to inhibit bacterial growth using a spreadplate technique and incubated at 25°C for 5 days [10].
pH measurement. The pH of the samples was determined by blending 25 g of the product with 225 ml of Ringer's solution, for 2 min, in a homogenizer (DI18B, Germany). The pH values were measured using a standardized electrode attached to a digital pH meter (CG824, Germany).
Sensory analysis. The sensory study, which was performed on each sampling day, evaluated the following parameters: the presence of gas, slime production (as a milky exudate), the presence of sour odor and yellow color. The sensory analysis was carried out according to the methodology described by Diez et al. [11]. A trained panel (a minimum of 5 panelists) scored the samples using a nine-point scale, on which one corresponded to the complete absence and nine to the maximum intensity of each parameter. A value of over 4 for all of the parameters under consideration was taken as the consumer rejection threshold.
Statistical analysis. In order to determine the difference among the treatments, an Analysis of Variance (ANOVA) was used and when differences were detected, a Duncan’s multiple comparison test was used to differentiate the treatment means. The analysis was carried out using SPSS (version 19, SPSS Inc) at a significance level of 0.05.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
D- and z-values for lactic acid bacteria. Table 2 presents the D-values of Lactobacillus sakei and Lactobacillus plantarum at 55, 58 and 61°C. The z-values were measured at 8 and 7.68°C for Lactobacillus sakei and Lactobacillus plantarum, respectively, by computing the linear regression of the mean log D-values [7]. D60 and D68 for Lactobacillus sakei (the target LAB) calculated 30 and 3 s, respectively, considering D and z-values. The results from the heat resistance pattern of two main LAB in this study clarified that they are not able to tolerate the cooking process of 70 min to reach an internal of 72°C, therefore the recontamination after cooking was recognized as the source of spoilage LAB.
It is well known that common spoilage which appears as the souring, sliming and swelling of the pack and mostly occurs before the sell-by date is highly associated with lactic acid bacteria. This type of spoilage has become a major problem of the meat industry in recent years because the undesirable sensorial effect causes rejection by consumers [4, 12]. Several studies suggested recontamination after the heating process during slicing, chilling and packaging as the main causes of spoilage. Spoilage strains originating from raw materials may spread to slicing room via air, workers and equipment [13, 14].
Therefore, in the current study we aimed to extend vacuum packaged sausage shelf life and delay the spoilage time by the in-package pasteurization of a product. To achieve this aim, heating the final packaged product for a 4 log reduction in the LAB population was used as an approach.
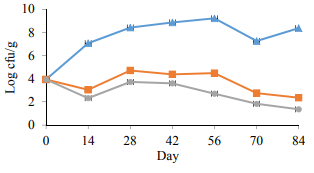
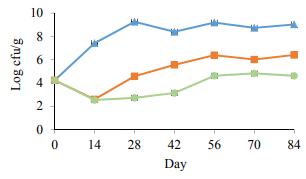
Microbiological analysis. Fig. 1 illustrates the LAB count in PAST 1 and PAST 2. The results demonstrated an obvious decline (p < 0.05) on the first sampling day after heat treatment (day 14), on the contrary, there was a significant (p < 0.05) increase in the LAB population of the control group. During 84 days of cold storage, the LAB count increased significantly in the control samples and reached to 9.2 log CFU/g. On day 70, a significant (p < 0.05) decrease was recorded in the LAB population of the control samples which were ascended to 8.3 log CFU/g at the end of the experiment. The heat treatment led to a significant decrease in both PAST 1 and PAST 2 for the whole period of the experiment. Although there was a slow growth of LAB in the heat treated samples, particularly on day 28, the count remained at an acceptable level to the end of the experiment. The LAB count in the PAST 2 group was significantly (p < 0.05) lower compared to the PAST 1 group. At the end of the trial the count of LAB, in the control group, PAST 1 and PAST 2 reached 8.3, 2.3 and 1.3 log CFU/g, respectively.
Throughout 84 days of the experiment, the population of LAB increased sharply in the control samples. Korkeala et al. [14] indicated that there was a strong correlation between the LAB count and the pH value of the product. They mentioned LAB produced lactic and acetic acid during logarithmic growth and especially at the stationary phase. They also demonstrated that whenever the counts of LAB reach 5 × 107 CFU/g, the lactic acid concentration increases sharply.
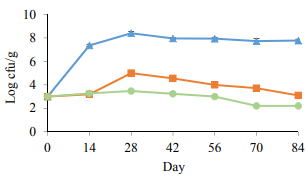
Fig. 2 shows the total mesophilic population for the control and pasteurized samples. The initial bacterial counts for the control samples were 3 log CFU/g and increased up to 7.36 log CFU/g during the first 2 weeks of storage and remained around 8 log CFU/g over time. The results showed the in-package pasteurization of vacuum packed sausages in both groups with a significant (p < 0.05) increase in the inhibition of the total mesophilic count compared to the control samples throughout the storage period. When comparing the heat treated samples in PAST 1 and PAST 2, it was seen that, although the inhibitory effect of heating mesophilic bacteria was similar in the PAST 1 and PAST 2 samples, from day 14 on, the population of mesophilic bacteria in the PAST 2 samples remained lower (p < 0.05) than in the samples pasteurized at 60°C (PAST 1). Comparing the population of the total mesophilic bacteria in this experiment with the LAB count signifies that LAB might constitute the predominant portion of mesophilic bacteria (Fig. 1 and 2).
Fig. 3 illustrates the number of psychrotrophic bacteria. The initial count of psychrotrophic bacteria was 4.2 log CFU/g on day 0. An immediate significant (p < 0.05) increase in psychrotrophic population of the control samples was observed on day 14. On the contrary, the numbers of psychrotrophic bacteria decreased from 4 log CFU/g to 2.5 log CFU/g in both pasteurized treated groups after heating. The results showed a significant increase in the psychrotrophic counts of the control samples during 84 days of cold storage which reached 9.2 log CFU/g on day 28 and stabilized between 8 and 9 log CFU/g over time. Although there were no significant (p > 0.05) differences in the population of psychrotrophic bacteria between the PAST 1 and PAST 2 samples up to day 14, from day 28, the number of these bacteria was significantly (p < 0.05) higher in the PAST 1 samples compared to PAST 2. According to Fig. 1 and Fig. 3, the psychrotrophic bacteria growth pattern is similar to the LAB growth curves. These results show that the counted psychrotrophic bacteria in Fig. 3 were probably the spoiling LAB which grew on MRS agar due to their ability to grow at temperatures below 10°C. Although the presence of nitrite inhibits most psychrotrophs from contaminating products after they are cooked, this is not the case with psychrotrophic lactic acid bacteria, whose growth continues and eventually causes spoilage [7, 15, 16]. Additionally, in the anaerobe conditions and in the presence of salt and nitrite, most psychrotrophic bacteria such as pseudomonas spp. are suppressed and the bacterial flora is gradually selected toward by LAB [16–18].
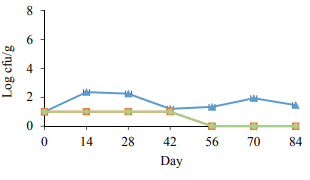
Figure 4 represents an enterobacteria count in the samples. The number of enterobacteria was 1 log CFU/g in all the groups at the beginning of the study. The enterobacterial number decreased in the PAST 1 and PAST 2 groups after heat treatment and remained at low levels to the end of the experiment. The enterobacteria showed a slight grow in the control group compared to the pasteurized samples. There were no significant differences in the enterobacterial count between PAST 1 and PAST 2. The inability of Enterobacteriaceae and yeasts to dominate the spoilage ecology of nonpasteurized and pasteurized sausages in this study also agrees with the previous studies of vacuum-packaged emulsion-type sausages [16, 19, 20]. This inability was contributed to the inhibition of Enterobacteriaceae and yeast by producing microbial lactic acid and a pH decrease in the product during the growth of lactic acid bacteria, as well as inefficient substrate utilization [20– 22]. According to Fig. 4, although the preservation methods in the cooked vacuum packaged sausages inhibit an enterobacterial growth, the effect of inpackage pasteurization on suppressing these bacteria in the PAST 1 and PAST 2 samples is considerable.
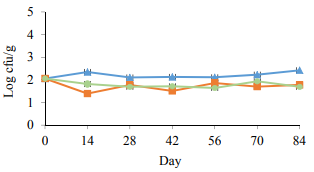
Fig. 5 presents the result of spore counts. The numbers of spores in all the experimental groups were around 2 log CFU/g on day 0. No significant changes (p > 0.05) in the spore growth pattern of the experimental groups were seen during 84 days of cold storage and the values were stabilized over time. No significant differences (p > 0.05) were seen in the spore numbers between the control and pasteurized samples. However, it was reported in the previous study [16] that in-package pasteurization may increase the risk of clostridium prevalence; no significant spore growth in the current study was seen in the pasteurized samples compared to the control ones.
According to the results, there were no yeasts and molds detected in any group. No significant differences (p > 0.05) in the yeasts and mold count were observed between the groups over the whole storage period. As it was expected, the hurdle concepts used in products such as cooking, an anaerobe condition, chemical preservation and the presence of competitive bacterial flora prevent a yeasts and molds growth and no notable differences were observed between the control and pasteurized samples.
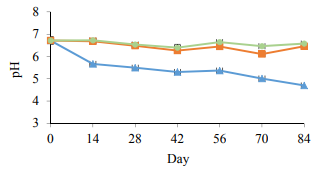
pH measurement. The initial pH of vacuum packaged sausages in this study was approximately 6.7. The pH value showed a significant drop (p < 0.05) in the control samples during 84 days of storage and reached the lowest level of 4.7 on day 84. No significant (p > 0.05) change in pH of the PAST 1 and PAST 2 samples was seen during the experimental period. pH values in the PAST 1 and PAST 2 samples were around 6.5 on day 84 which was similar to day 0. There were no significant differences (p > 0.05) in pH values between PAST 1 and PAST 2 over time. Throughout 84 days of storage, the decrease in pH in the control samples was relative to the LAB growth (Fig. 6). Several studies described that the samples are considered spoiled when pH falls below 5.8 to 5.9 and there is special organoleptic spoilage when pH values reach 4.6 to 5.5 [14, 15]. The same was in the current study, from day 28, that pH reached 5.5 and the LAB population counted to 108 CFU/g, the typical signs of spoilage were observed. As it is shown in Table 3, the panelists rated the control samples as unacceptable spoiled products from day 28 considering the presence of slime, sour odor and the production of gas, Korkeala et al. [14] also indicated that when the LAB population reached 1.4 × 107 CFU/g, the samples could be considered unacceptable for human consumption from organoleptic aspects. On the contrary, the pH value in the PAST 1 and PAST 2 samples had no significant changes during the storage period and remained above 6 throughout the experiment. The obtained results in pH values are well correlated to the LAB population and sensorial analysis which demonstrated in both pasteurized groups that none of the sensorial parameters were rated higher than the consumer-rejection threshold of 4 throughout 84 days of the study. The low LAB counts in the pasteurized samples proved during 84 days of cold storage that in-package pasteurization is an applicable approach in LAB growth control.
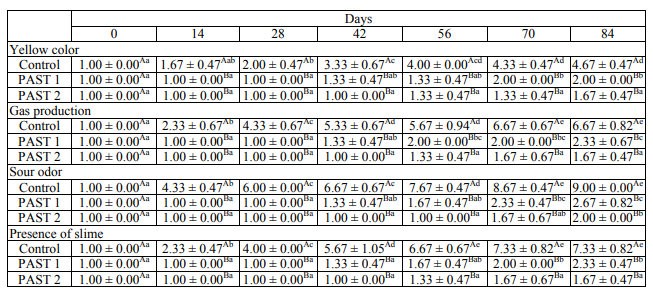
Sensory evaluation. Table 3 shows the results of a sensory analysis. At least one of the sensory parameters exceeded the threshold of 4 in the control samples on day 14. Significant signs of spoilage (P < 0.05) with particularly sour odor appeared in the control samples on day 14. The other signs of spoilage were appeared from day 28. From day 28, all the spoilage parameters, such as sour odor, the presence of slime and gas production have been considered unacceptable (P < 0.05) by the panelists. The yellow color was the last indication of spoilage which appeared from day 56. The results demonstrated that sour odor reached an unacceptable level of 9 at the end of the experiment. On the contrary, in both pasteurized groups, none of the sensorial parameters were rated higher than the consumerrejection threshold of 4 throughout 84 days of the study. The result of the current study agrees with other investigators findings [14], as it is shown in Fig. 1 and Fig. 6, from day 14, that when the LAB count in the control samples reached 107 CFU/g, an immediate drop in pH was observed. The pH value in the control samples decrease from 6.7 on day 0 to 5.6 on day 14. The result from the sensorial analysis is well correlated with pH values. It was observed that on day 14, sour odor exceeded the threshold of 4 in the control samples.
Different small letters indicate statistically significant differences in columns (p < 0.05). Different capital letters indicate statistically significant differences between days in each sample (p < 0.05).
Throughout 84 days of experiment, in-package pasteurization at 60 and 68°C had no undesirable effects such as changing color, cooking loss and package damage in PAST 1 and PAST 2 samples and extend the shelf life of PAST 1 and PAST 2 samples to more than 3 months, while the control samples were considered as spoiled products 2–3 weeks after manufacturing, on the contrary, in-package pasteurization. The results of the current study suggest in-package pasteurization that can be used as a decontamination method for preventing spoilage microorganism growth and extending product shelf life.
In summary, we consider in-package pasteurization as an effective method for preventing spoilage caused by LAB growth and extending emulsion vacuum packaged sausages shelf life. Although no substantial differences in the bacterial population or organoleptic analysis were seen between PAST 1 and PAST 2, heating at 68°C may be more practical in the modern, high volume meat industry due taking less time achieving 4 log reductions in LAB counts. Future research of other food models with different temperature and a diverse analysis in bacterial metabolic activity will contribute to clarify in-package pasteurization method effectiveness.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare no potential conflict of interest.БЛАГОДАРНОСТИ
We would like to thank Demes Meat processing Company for providing vacuum-packaged emulsion sausages and Mr. G. Niknia for his technical assistance.СПИСОК ЛИТЕРАТУРЫ
- Perez-chabela M.D.L., Totosaus A., and Gurrero I. Evaluation of thermotolerant capacity of lactic acid bacteria isolated from commercial sausages and the effects of their addition on the quality of cooked sausages. Ciencia e Tecnologia de Alimentos, 2008, vol. 28, no. 1, pp. 132-138. DOI: 10.1590/S0101-20612008000100019.
- Miranda J.M., Samuel A., Nebot C.G., et al. Technological characterization of lactic acid bacteria isolated from beef stored on vacuum-packaged and advanced vacuum skin packaged system. Journal of Food Processing & Technology, 2014, vol. 5, no. 6, p. 338. DOI: 10.4172/2157-7110.1000338.
- Diez A.M., Björkroth J., Jaime I., and Rovira J. Microbial, sensory and volatile changes during the anaerobic cold storage of morcilla de Burgos previously inoculated with Weissella viridescens and Leuconostoc mesenteroides. International Journal of Food Microbiology, 2009, vol. 131, no. 2-3, pp. 168-177. DOI: 10.1016/j.ijfoodmicro.2009.02.019.
- Nerbrink E. and Borch E. Evaluation of bacterial contamination at separate processing stages in emulsion sausage production. International Journal of Food Microbiology, 1993, vol. 20, no. 1, pp. 37-44. DOI: 10.1016/0168-1605(93)90058-O.
- Gande N. and Muriana P. Prepackage Surface Pasteurization of Ready-to-Eat Meats with a Radiant Heat Oven for Reduction of Listeria monocytogenes. Journal of Food Protection, 2003, vol. 66, no. 9, pp. 1623-1630.
- Murphy R.Y., Duncan L.K., Johnson E.R., Davis M.D., and Smith J.N. Thermal inactivation D- and z-Values of Salmonella Serotypes and Listeria innocua in chicken patties, chicken tenders, franks, beef patties, and blended beef and turkey patties. Journal of Food Protection, 2002, vol. 65, no. 1, pp. 53-60. DOI: 10.4315/0362-028X-65.1.53.
- Franz C.M.A.P. and von Holy A. Thermotolerance of meat spoilage lactic acid bacteria and their inactivation in vacuum-packaged Vienna sausages. International Journal of Food Microbiology, 1996, vol. 29, no. 1, pp. 59-73. DOI: 10.1016/0168-1605(95)00022-4.
- Jafarpour D., Shekarforoush S.S., Eskandari M.H., Niakosari M., and Hosseini A. Antifungal activity of native lactic acid bacteria strains isolated from the natural environments against fungi contaminating Iraninan white cheeses. BMC Microbiology, 2017. (Under Review).
- Juneja V.K., Bari M.L., Inatsu Y., Kawamoto S., and Friedman M. Thermal Destruction of Escherichia coli O157:H7 in Sous-Vide Cooked Ground Beef as Affected by Tea Leaf and Apple Skin Powders. Journal of Food Protection, 2009, vol. 72, no. 4, pp. 860-865.
- Beuchat L.R. Media for detecting and enumerating yeasts and moulds. International Journal of Food Microbiology, 1992, vol. 17, no. 2, pp. 145-158. DOI: 10.1016/0168-1605(92)90112-G.
- Diez A.M., Santos E.M., Jaime I., and Rovira J. Application of organic acid salts and high-pressure treatments to improve the preservation of blood sausage. Food Microbiology, 2008, vol. 25, no. 1, pp. 154-161.
- Borch E., Kant-Muemans M.L., and Blixt Y. Bacterial spoilage of meat and cured meat products. International Journal of Food Microbiology, 1996, vol. 33, no. 1, pp. 103-120. DOI: 10.1016/0168-1605(96)01135-X.
- Santos E.M., Diez A.M., González-Fernández C., Jaime I., and Rovira J. Microbiological and sensory changes in “morcilla de Burgos” preserved in air, vacuum and modified atmosphere packaging. Meat Science, 2005, vol. 71, no. 2, pp. 249-255. DOI: 10.1016/j.meatsci.2005.03.028.
- Korkeala H.J. and Björkroth K.J. Microbiological Spoilage and Contamination of Vacuum-Packaged Cooked Sausages. Journal of Food Protection, 1997, vol. 60, no. 6, pp. 724-737. DOI: 10.4315/0362-028X-60.6.724.
- Blickstad E. and Molin G. The microbial flora of smoked pork loin and frankfurter sausage stored in different gas atmospheres at 4°C. Journal of Applied Bacteriology, 1983, vol. 54, no. 1, pp. 45-56. DOI: 10.1111/j.1365-2672.1983.tb01299.x.
- Franz C.M.A.P., Dykes G.A., and von Holy A. Effect of in vitro pH and temperature changes on meat spoilage lactic acid bacteria. African Journal of Food Science, 1991, vol. 3, no. 1, pp. 59-62.
- Hamasaki Y., Ayaki M., Fuchu H., Sugiyama M., and Morita H. Behavior of psychrotrophic lactic acid bacteria isolated from spoiling cooked meat products. Applied and Environmental Microbiology, 2003, vol. 69, no. 6, pp. 3668-3671. DOI: 10.1128/AEM.69.6.3668-3671.2003.
- Björkroth K.J. and Korkeala H.J. Evaluation of Lactobacillus sake Contamination in Vacuum-Packaged Sliced Cooked Meat Products by Ribotyping. Journal of Food Protection, 1996, vol. 59, no. 4, pp. 398-401. DOI: 10.4315/0362-028X-59.4.398.
- Zurera-Cosano G., Rincon-Leon F., Moreno-Rojas R., and Pozo-Lora R. Microbial growth in vacuum packaged frankfurters produced in Spain. Food Microbiology, 1998, vol. 5, no. 4, pp. 213-218. DOI: 10.1016/0740-0020(88)90020-2.
- Von Holy A., Cloete T.E., and Holzapfel W.H. Quantification and characterization of microbial populations associated with spoiled, vacuum-packed Vienna sausages. Food Microbiology, 1991, vol. 8, no. 2, pp. 95-104. DOI: 10.1016/0740-0020(91)90002-J.
- Santos E.M., Jaime I., Rovira J., et al. Characterization and identification of lactic acid bacteria in morcilla de Burgos. International Journal of Food Microbiology, 2005, vol. 97, no. 3, pp. 285-296. DOI: 10.1016/j.ijfoodmicro.2004.04.021.
- Von Holy A., Meissner D., and Holzapfel W.H. Effects of pasteurization and storage temperature on vacuumpackaged Vienna sausage shelf-life. South African Journal of Science, 1991, vol. 87, pp. 387-390.