Аннотация
Expanding the scope of margarine products requires the search for new technological solutions when creating stable food emulsions for various specified purposes: hard, soft and liquid margarines with various fat content for bakery, for use in confectionery production, for frying in public catering networks and as spreads for direct consumption. The article systematizes the materials on the surfactants used in the technology of food emulsion products, in particular, margarines and spreads. The analysis of emulsifiers and their mixtures used in manufacturing emulsion products with various fat content has been carried out. The results of the studies of the composition and effect of individual and complex emulsifiers on the properties of the direct and reverse type of the obtained emulsions have been summarized. The article considers the theoretical issues and practical aspects of creation of food emulsion products with the use of various emulsifiers. A step-by-step system for choosing the optimal mixture of emulsifiers for a particular product based on the hydrophilic-lipophilic balance of surfactants has been provided. A number of examples of complex emulsifiers for emulsion products for various specified purposes have been presented. It has been shown that a change in the amount and type of an emulsifier makes it possible to produce various emulsions with the required structural and rheological characteristics and with the specified composition and properties. The aspects of manufacturing margarine products with various fat content have been presented.Ключевые слова
Food emulsifiers, emulsion products, margarine, spread, mono- and diglycerides of fatty acids, hydrophilic-lipophilic balance, iodine indexВВЕДЕНИЕ
Of a variety of fat-and-oil products, a group of solid emulsion fat-and-oil products can be emphasized, including margarines, vegetable-cream and vegetable-fat spreads. These products were initially developed as an alternative to butter, however, their scope of application has significantly expanded at this stage of development of the food industry. It should be noted that the structure of consumption of solid fat-and-oil products has recently changed with a decrease in the proportion of consumed butter, margarines and spreads as edible products. The reason for these changes is due to a more attentive attitude of the population towards health and the fulfillment of the recommendations of the health authorities to reduce the consumption of fats, in particular, saturated fats. Despite this fact, the consumption of solid fat-and-oil products on the whole continues to grow. To a certain degree, this is due to the fact that the scope of margarines and spreads is not limited to direct consumption as a sandwich type product. Margarine products are widely used in public catering and in the HoReCa sector, as well as in the confectionery, bakery, canning and other food industries.
According to the Russian technical regulations (TR TS 029/2011) margarine is an emulsion fat-andoil product with a fat content of at least 20%. The formulation of margarine can include both natural and modified vegetable oils, water, milk and its derivative products, as well as various food supplements. The mass fraction of fat in margarines varies within quite a wide range, usually from 60 to 82%.
Spreads – a relatively new group of emulsion fat products with a mass fraction of fat of 39% and the melting point of the fat phase not higher than 36°C – are the most similar to butter in properties. Spreads, in comparison with margarines, have a more plastic consistency. Cream-vegetable and vegetablecream spreads are made of natural and modified vegetable oils, milk fat, cream and butter. Vegetable-fat spreads are obtained from natural or modified vegetable oils with or without the addition of food supplements and other ingredients that make it possible to obtain a stable emulsion [10].
Being homogeneous in appearance, emulsions consist of two liquids that are practically insoluble in each other. The complete or partial insolubility of the disperse phase in the dispersion medium is a necessary condition for the formation of an emulsion. In fact, all emulsions contain water as one of the phases. The other phase is organic, non-polar, and it is conventionally called "oil."
Food emulsions, which include margarines and spreads, are disperse systems formed by two mutually insoluble liquids (oil and water), one of which is dispersed in the other in the form of tiny spherical droplets. The substance of drops is considered a disperse, discrete or internal phase. The substance that constitutes the surrounding liquid is called a dispersive, continuous or external medium.
As a rule, both the aqueous and the oil phase in emulsions are complex systems and are characterized by a multicomponent structure. Thus, the oil phase, or fatty base, in a margarine emulsion is a mixture of transesterified triacylglycerols different in origin and the melting point of hydrogenated fats (soybean, sunflower, rapeseed, etc.) and liquid vegetable oils (sunflower, soybean, etc.). The aqueous phase, which determines organoleptic properties in margarines, includes flavoring agents, preservatives, citric or lactic acid to enhance microbiological resistance, thickeners, structure-forming agents (especially for low-calorie margarines), antioxidants and dyes.
There are two types of emulsions: oil-in-water (or first-kind) and water-in-oil (or second-kind). In oilin-water emulsions, the oil phase is dispersed and the aqueous phase remains continuous. They are denoted as O-W. For example, milk, cream and mayonnaise refer to oil-in-water emulsions. In water-in-oil emulsions, on the contrary, the aqueous phase is dispersed and the fat base remains continuous. They are denoted as W-O. Margarines are one of the examples of water-in-oil emulsions. Some conditions can lead to an inversion, when there is a transition of one type of emulsion to another. For example, the prolonged mechanical treatment of an emulsion can lead to the coalescence of disperse phase droplets, and the liquid of the dispersion medium is crushed into droplets and dispersed in a newly formed dispersion medium by itself [9].
In addition, emulsions are classified according to the concentration of the disperse phase. In dilute emulsions, the proportion of the disperse phase is up to 0.1%. They belong to highly-dispersive emulsions, the droplets of the disperse phase in these emulsions have a spherical shape and their diameter is about 100 nm. In concentrated emulsions, the amount of the disperse phase is from 0.1 to 74. In the case when the concentration of the disperse phase is from 25 to 50% (low-calorie margarines, spreads and mayonnaises), its viscosity does not practically differ from the viscosity of the dispersion medium, therefore, in order to increase the aggregative stability of such emulsions and imparting certain rheological properties, the substances that increase viscosity (the so-called "thickeners") are added to the dispersion medium. In emulsions with the specified concentration, the maximum content of spherical undeformed droplets is possible. In highly concentrated emulsions, the concentration of the disperse phase is not below 74%. They usually have deformed droplets, in which case the disperse phase therein can often turn into thin layers. In some cases, with a high degree of polydispersity in highly concentrated emulsions, the spherical shape of particles remains unchanged. Due to polydispersity, small droplets fill the spaces between large spherical particles. It is possible to create mixed emulsions [10].
In food production, concentrated and highly concentrated emulsions that need an increase in the aggregative stability for a long time are as a rule obtained. This is achieved by adding special emulsifying agents which are various natural or synthetic compounds. Most often, these are the substances that are soluble in one of the phases. Insoluble solids are also used in a finely disperse form. The first group of emulsifying substances is more extensive and is more often used in practice, surfactants hold a unique position therein [1, 2, 3].
The process of formation of an emulsion and its end-use properties depend on such factors as the surface tension of the phases and the interfacial tension of the heterogeneous system. Surface tension is the most important parameter that determines the stability of an emulsion. The decrease in surface tension provides an increase in the stability of the system [6].
Surface energy can be reduced with the preservation of the interfacial surface only by reducing the interfacial tension value. The ability of substances to change the surface tension of the solvent during dissolution is called surface activity. Ana the substances that are adsorbed at the interface between the two phases and decrease surface tension are called surfactants.
It is known that the decrease in interfacial tension is a consequence of a special surface phenomenon called adsorption. During adsorption, the emulsifier should easily transfer from the volume to the surface layer and, moreover, retain and concentrate in the surface layer. Low molecular substances called diphilic or amphiphilic ones that consist of various functional parts usually have such properties. The irreversible character of adsorption causes the high stability of emulsions.
The emulsifying ability of a surfactant is specified by the presence of hydrophobic and hydrophilic functional groups.
The hydrophilic part of an emulsifier molecule is usually hydroxyl, carboxyl, phosphatide, nitrogen, ester and other groups that have a significant dipole moment and are capable of forming hydrogen bonds with water molecules.
The hydrophobic part in a surfactant molecule is a hydrocarbon radical which does not usually contain the atoms capable of forming hydrogen bonds and is insoluble in water. Most often, these are the hydrocarbon radicals of fatty acids, usually palmitic C16 : 0, stearic C18 : 0 or other saturated high molecular weight (long-chain) fatty acids. Medium-chain fatty acids (such as lauric C12 : 0 and myristic C14 : 0) are undesirable in the composition of emulsifiers due to their low resistance to hydrolysis, which can lead to an unpleasant foreign taste of the finished product. The presence of unsaturated fatty acids (oleinic C18 : 1, linoleic C18 : 2 and linolenic C18 : 3) in an emulsifier is also undesirable since they are easily oxidized.
The common emulsifiers, the proportion of which in the total consumption of food emulsifiers exceeds 70%, are monoacylglycerols. Phospholipids (phosphatidylcholine, phosphatidylserine, dimethylethanolamine, phosphatidylethanolamine, phosphatidic acids, etc.) also have good emulsifying properties due to their diphilic molecules. Emulsifying properties are also characteristic of proteins the molecules of which include hydrophobic and hydrophilic fragments.
Obtaining an oil-in-water or water-in-oil emulsion depends on the type of emulsifier and a method for its addition. When obtaining an oil-in-water emulsion, the oil phase is introduced into the aqueous phase in small portions. The emulsifier is dissolved in the aqueous or in the oil phase before application. Waterin-oil emulsions are obtained by adding the aqueous phase to the oil solution of the emulsifier. This condition is only feasible when adding a small amount of the disperse phase, otherwise the phases can be reversed and the emulsion can be stratified [5].
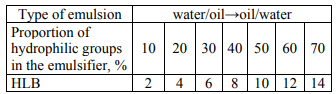
The hydrophilic-lipophilic balance number (HLB number) of an emulsifier can be a measure of its affinity with oil or water. The term "hydrophiliclipophilic balance" was first proposed by Clayton and related to the ratio of the hydrophilic and hydrophobic (lipophilic) parts of a molecule of a diphilic surfactant. The hydrophilic properties are determined by the interaction of the polar group of an emulsifier with water, and the lipophilic properties are determined by the interaction of a non-polar surfactant fragment with oil. Since the determination of the HLB number is a simpler method than the relatively accurate determination of the geometric and surfactant distribution factors in the miscible phases, the HLB number is currently an important factor in choosing an emulsifying system for a certain type of emulsion. Table 1 shows the dependence of the type of emulsion being formed on the proportion of hydrophilic groups in an emulsifier molecule.
An important factor to determine the type of emulsion being formed is the solubility or dispersibility of an emulsifier in the fat or aqueous phase. According to the Bancroft rule, fat-soluble, (or dispersible in fat) emulsifiers, the HLB values of which range from 2 to 6, form water-in-oil emulsions. Water-soluble (or water dispersible) emulsifiers, having high HLB values – from 11.0 and above, form oil-in-water emulsions.
To manufacture fat-and-oil products, both separate emulsifiers (monoglycerides and polyglycerol and ricinoleic acid esters (E 476), distilled monoglycerides (E 471) and lecithins (E 322)) and their complexes (E 471 : Е 322 and Е471 : Е476) are used. The use of these emulsifiers makes it possible to form the mixtures of immiscible phases – oil and water – and keep them uniform. An important property that differs emulsifiers from the other classes of food supplements is surface activity that can be developed in different ways. The most demanded functions of surfactants are: emulsification which allows us to obtain qualitative, stable emulsions and aerating and stabilizing whipped systems by interacting with other food substances, such as proteins and carbohydrates. In addition, surfactants make it possible to intensify the process of oil and fat crystallization being crystallization centers and controlling fat particle agglomeration. The emulsifying compositions that comprise phospholipids contribute to an increase in the thermal stability of fat-and-oil products for baking and frying, preventing splashing. Having antioxidant properties, phospholipids also provide the high resistance of fat-and-oil products during storage. In addition to the main purpose – to stabilize an emulsion, emulsifiers help to improve the plasticity of margarines and spreads, and in the production of margarines for bakery they provide some specific properties of products, for example, they increase crumb porosity and the volume of the finished product.
The features of the food system being created and the technological tasks set determine the choice of a specific emulsifier. Preference should be given to an emulsifying supplement the technological functions of which will provide the best technological effect with the minimal risk of its application.
The domestic industry produces a wide range of mono- and diglycerides that differ from each other in a number of indicators, the main of which are the melting point and the iodine index that characterize an unsaturation degree.
The technology of manufacturing emulsion fatand-oil products, including low-fat products, includes obtaining a highly-dispersive water-in-oil emulsion by emulsifying a mixture of vegetable oils and fats with dairy and other raw materials. In this case, the product must have a uniform, plastic and dense consistency, a clear, pronounced flavor or the flavor of the used filler. The presence of free vegetable oil or water in a product worsens not only its consumer characteristics, but also reduces the microbiological resistance of a product during storage.
The fat base of fat-and-oil emulsion products (as well as spreads and margarines) is a multicomponent mixture of natural or modified fats and oils with various physicochemical properties: the content of solid triglycerides, the melting point and hardness. It is these factors that determine the structural and rheological characteristics of the finished product. Margarine is considered a water-in-oil emulsion because of the predominance of the fat phase, the mass fraction of which is on average from 60 to 82%. In fact, it is not so much an emulsion as a dispersion of water droplets in a semi-solid fat-and-oil phase containing liquid oil and fat crystals [6]. The margarine emulsion production process requires considerable energy to reduce the size of disperse phase droplets in order to increase the interface between the two phases: aqueous and fat.
A margarine emulsion is left in the liquid state for a short time to be treated using full-time margarine production lines only at the stages before entering the cooler (votator or combinator) where the fat base is simultaneously crystallized and emulsified. A margarine emulsion does not require high resistance to coalescence since water droplets are fixed in a semisolid fat phase upon cooling. The size of water phase droplets affects the organoleptic and microbiological indicators. Thus, a finely-dispersed emulsion (the droplet size is 2–4 μm) promotes the inhibition of mold growth. It should be borne in mind that the presence of larger droplets (more than 10 μm) improves the perception of a product taste. The uniformity of droplet size distribution is also affected by the nature of the emulsifier used. Its role is to reduce the interfacial tension between the fat and aqueous phases, which usually leads to a decrease in the size of water droplets, as well as a more uniform distribution of droplets in size. For this purpose, lipophilic emulsifiers are usually used: distilled monoglycerides containing highmolecular fatty acids (C16 : 0–C18 : 0) in combination with refined soya lecithin.
Depending on the purpose, margarines are divided into solid, soft and liquid ones. Solid margarines are used in bakery, culinary and confectionery production, in puff pastry production and for making creams, fillings, soufflé, sweets, etc. Soft margarines are mainly used in home cooking and in public catering. Liquid ones – for baking, in home cooking, for making bakery and confectionery products, as well as for frying in fast food stores.
Standard solid margarines containing more than 80% of fat are relatively stable under normal conditions and keep their shape at a temperature of 20 +/– 2°C; to produce them, the minimum dosage of lecithin and / or mono- and diglycerides is required in addition to the number of milk proteins usually specified in the formulation.
In the production of special margarine, i.e. for making cakes or for frying, and especially in manufacturing products with a low fat content, the emulsifiers should be chosen that meet the requirements of this scope in terms of the functional properties of fat products [2].
Soft liquid margarines and bar margarines with a low fat content, having a soft plastic consistency at a temperature of 10 +/– 2°C, are usually produced using the same fat base as standard consumer margarine. As a rule, other used ingredients also coincide with the standard margarine formulation, except for some cases.
Liquid margarine is an emulsion product with a liquid consistency that keeps its uniformity at the temperatures specified for specific liquid margarine. As a rule, it is used for frying in public catering establishments. In this case, it is especially important that it does not splash. Splashing is caused by the fact that when melting margarine, the emulsion is destroyed, the water drops merge and, under the action of gravity, form an aqueous film covered with molten fat. When the boiling point is reached, the increased vapor pressure breaks through the fat layer, which makes the water phase to splash, sometimes with an explosion.
To prevent splashing, it is desirable to gradually evaporate water from small droplets with the formation of a thin golden brown sediment that does not adhere to the frying surface. An important part therein is played by the composition of margarine and the treatment methods used.
The typical formulation of liquid margarine contains about 82% of the fat phase based on soy or sunflower oil. At high pH values (about 6), the presence of salt and milk has a positive effect, while sugars and starches increase the splashing tendency. The correct choice of the type of emulsifier and its dosage can significantly improve the functional properties of frying margarine, while the emulsifiers have two functions. The first of them is an emulsifying one: thus, the use of citric acid ester and monoglycerides in combination with soy lecithin provides a stable aqueous dispersion with moderate splashing during frying. There are also some other emulsifiers that individually or together with lecithin help to prevent water phase droplets from merging during frying. The second function of an emulsifier is to prevent the oil separation during storage and to obtain a homogeneous product with a low viscosity.
In the case if it is not frying margarine, only distilled monoglycerides can be added. The aqueous phase of liquid margarine contains a certain proportion of dry skim milk and salt, as well as potassium sorbate as a preservative. The flavor supplements are added into both phases.
Particularly great is the role of emulsifiers in the production of vegetable-cream and vegetable-fat spreads. The choice of the type and dosage of an emulsifier in each specific case depends on the factors that include: the ratio of vegetable oil and milk fat, the total amount of fat in the product; the presence of an emulsifier in the used milk fat substitute; the features of technology and equipment [2].
Low-fat spreads are the only growing sector in the gradually declining spread market. With the exception of spreads with a very low fat content, they are water-inoil emulsions, the mass fraction of fat therein is from 39 to 72%. In the production of low-fat spreads, it is necessary to balance the stability of an emulsion and the mouthfeel that depends both on the composition of the product and on the mode of production. Since the disperse (aqueous) phase may exceed by volume the continuous (fat) phase in this group of fat-and-oil products, the problems with product stability, its melting characteristics and taste perception are possible [2].
In high-fat spreads, milk proteins improve the mouthfeel and taste of the product, and also act as hydrophilic stabilizers for oil-in-water emulsions. However, their use in low-fat products, along with the need for significant energy costs, can cause phase reversal.
These problems can be overcome with the correct choice of a combination of formulation components and treatment methods. The important factors in the development of formulations are the melting characteristics of the fat mixture, type and dosage of an emulsifier and the addition of such thickeners as gelatin, sodium alginate, pectin and carageenan to the aqueous phase. It is possible to use small amounts of milk whey proteins, which improves the perception of taste, and also reduces the pH value of the aqueous phase, since milk whey proteins, unlike casein, do not precipitate at low pH values. The increase in acidity, as is known, contributes to an increase in shelf life. The treatment time, i.e., the rate of emulsion output from the coolant or its productivity, and the temperature of the product at the outlet also have a significant effect on the stability of spread.
Spreads that are oil-in-water emulsions have some
advantages over water-in-oil spreads:
the structure of the product does not depend on the
type of fat used;
any fat content from 39 to 50% or more is possible;
the high dosages of whey protein are possible;
such products are more economical and easier to
manufacture;
the taste and aroma are easier to perceive [14].
The main disadvantage of such products is an insufficiently low pH value, which leads to the need for UHT treatment and, if possible, aseptic packaging to provide a shelf life comparable to that of traditional spreads.
Oil-in-water spreads are still not widely used in the fat-and-oil product market. This is most likely due to the problem of microbiological spoilage, which can be solved by increasing the dosages of preservatives. Unlike water-in-oil emulsions, emulsifiers with high hydrophilic-lipophilic balance (HLB) values can be used in such systems to stabilize an emulsion with a continuous aqueous phase.
As a result of the predominant hydrophilicity of short-chain emulsifiers with less than 8 carbon atoms, they are well soluble in the aqueous phase and do not concentrate in the surface layer. Conversely, the longchain emulsifiers with a carbon chain length more than 18 and the predominant lipophilic properties dissolve well in the oil phase and do not create the surface layer of an emulsifier either. For a good emulsifying effect, a relative balance of the hydrophilic and lipophilic properties is necessary, with some imbalance in favor of the nonpolar or polar parts depending on the type of emulsion.
The system for selecting an emulsifier based on
HLB includes three steps:
the determination of the optimal HLB value for the
planned product;
the determination of the best types of emulsifiers;
the final HLB adjustment.
As for this method, the emulsifiers and mixtures
thereof with the HLB values beyond the specified
range can be discarded to reduce the number of trials
using the trial-and-error method. The determination of
the best HLB value includes the following steps:
(1) Choosing a suitable pair of emulsifiers (one is
lipophilic, the other is hydrophilic) with the known
values; for example, mono- and diglycerides with the
HLB value of 2.8 are lipophilic surfactants, and
polysorbate 60 with the HLB value of 14.9 is a
hydrophilic surfactant.
(2) Preparing a series of experimental emulsions with
the selected emulsifiers, which are mixed in such a
way as to obtain various HLB values beginning with a
completely lipophilic and ending with a completely
hydrophilic substance. For the two emulsifiers
selected at the first stage, the range of HLB values will
be from 2.8 to 14.9. The mixture of emulsifiers should
be used in an excessive amount or be about 10–12%
of the fat content in the final product.
(3) Estimating the obtained series of mixtures of
emulsifiers using the appropriate methods for
estimating functional efficiency based on the
requirements for the product. Using one or more
mixtures of emulsifiers, better emulsions will be
obtained than with the others, but if all the mixtures
prove to be good, a series of tests with a lower dosage
of an emulsifier mixture should be repeated. If all the
mixtures give bad results, it is necessary to increase
the dosage and repeat the series of experiments.
(4) The last stage of testing a mixture of emulsifiers
should, with an accuracy of 2 units, determine the
interval of hydrophilic-lipophilic balance that will be
the best for this final product. If necessary, a more
accurate HLB value can be determined using the
following series of tests with the HLB values falling
within this interval.
The appropriate chemical type of surfactants is as important as an HLB value. Once the HLB value is fixed, it is necessary to determine whether any other emulsifier mixture will work better and whether it will be more efficient or more economical with the same HLB value. The purpose of these tests is to select several pairs of emulsifiers that cover a fairly wide variety of chemical compounds. The estimates of the functional efficiency of these mixtures are the basis for choosing the ideal mixture of emulsifiers for this application.
As noted above, the concept of hydrophiliclipophilic balance makes it possible to obtain a lot of useful information, as well as to calculate the HLB values for the mixtures of emulsifiers and to compile the tables of the experimentally obtained HLB values. For example, Table 2 shows the HLB values of individual surfactants and their mixtures.
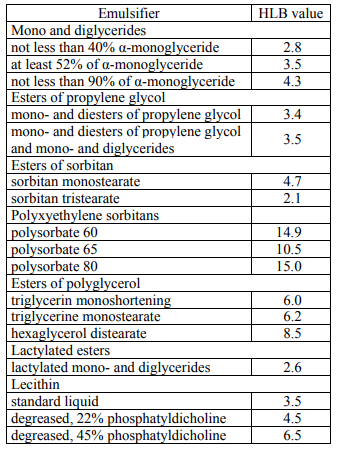
At the same time, the HLB method is not the only condition for selecting surfactants, since other important factors, including molecular weight, temperature changes and dissolution conditions, are not taken into account. To determine the value of HLB for some common food emulsifiers is rather difficult (for example, for phospholipids), and no information on the crystallization properties of monoglycerides and their derivatives can be obtained therefrom. Nevertheless, the concept of hydrophilic-lipophilic balance has proved to be a useful tool for the formation of a general idea of the likely properties of emulsifiers and their mixtures [14].
In addition to the hydrophilic-lipophilic balance, it is necessary to consider a lot of factors when choosing emulsifiers for fat products, including both the expected properties of the final product and the features of its manufacturing process. Of great importance are the type and properties of an emulsion system, the possible interaction of an emulsifier with other formulation ingredients, as well as its effect on the organoleptic properties of the product, including taste, aroma and sensory mouthfeel when the product is used for food. The technological parameters of mixing, homogenization, whipping and transferring by pumping also affect the choice of an emulsifier.
Distilled monoglycerides of fatty acids (E 471), with a mono-ester content of at least 90%, are widely used for manufacturing a large assortment of margarine products. These emulsifiers differ from each other in the type of a fatty acid in the composition of a monoglyceride molecule. It can be stearic C18 : 0, oleic C18 : 1 and other fatty acids. The monoglycerides of different fatty acids differ in the iodine index, melting point and other physicochemical indicators. The iodine index of an emulsifier depends on the degree of saturation of fatty acids in the composition of a monoglyceride molecule. The monoglycerides of unsaturated fatty acids have a higher iodine index, while they have a lower melting point and stronger lipophilic properties. Sometimes the mixtures of monoglycerides and diglycerides are used to produce margarines and spreads. It should be noted that in this case, in order to obtain the desired stability of an emulsion, an increase in the dose of an emulsifier is necessary, in comparison with the use of only distilled monoglycerides.
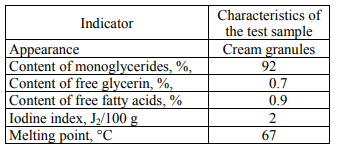
The physicochemical indicators of various emulsifiers are normalized by the supplier, depending on the tasks that a particular supplement is to fulfill. Below, as an example, are the results of the study of composition and technological properties of two samples of distilled monoglycerides of different types.
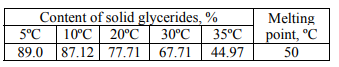
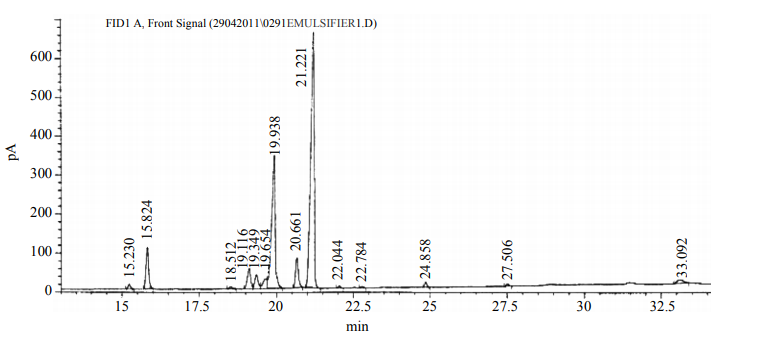
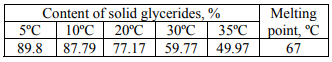
The content of solid glycerides in the first sample of an emulsifier and its melting point are shown in Table 4 and in Fig. 1, the fatty acid composition is shown in Fig. 2.
Table 5 presents the physicochemical parameters of the second emulsifier.
Table 6 and Fig. 3 present the content of solid glycerides and the melting point in the second emulsifier sample.
The second sample of an emulsifier is also distilled monoglycerides. However, it is characterized by a low iodine index and a higher melting point. Such an emulsifier can be recommended to produce margarine emulsions with a fat content of 72–82%. Since not so much the emulsifying ability is marked in the emulsifiers of this type as the ability to form a stable crystal lattice is, and thus to prevent the leakage of liquid vegetable oil.
To produce high-fat margarines and spreads, it is recommended to use the distilled monoglycerides of fatty acids of palm and other oils with a mono-ester content of 90%, with an iodine index of up to 2 mg J2/100 g and a melting point within the range of 65–72°C. These emulsifiers are easily dosed and dissolved in oil, hydrogenated fat or any other form of fat. With a properly formed fat base, the introduction of 0.1% of this emulsifier and 0.2% of lecithin into the formulation is enough to produce high-fat margarines and spreads packed in packs or plastic cups.
In the production of spreads using the lines for pasteurizing an emulsion, it is possible to recommend the use of a complex emulsifier, which is a mixture of monoglycerides E471 and special lecithin. The presence of lecithin in a complex emulsifier makes it possible to increase the spreadability and plasticity of the final product.
To produce margarines and spreads with a mass fraction of fat of 60% or less, monoglycerides with a higher iodine index of 45 to 60 g J2/100 g are used. We recommend using distilled monoglycerides (E 471) with an iodine index of 60 g J2/100 g and a melting point of 57°C in a dosage of 0.2 to 0.4% in combination with 0.2% of lecithin.
The recommended dosage of an emulsifier may also depend on the type of equipment used to make margarine or spread. When using standard highvolume production lines, such as Kemtek, Johnson and Schroeder, it is enough to add from 0.2 to 0.3% of the emulsifier E 471 to the formulation. If no qualitative emulsification or homogenization of an emulsion is provided on the line before cooling, the amount of an emulsifier should be increased to 0.4%. It should also be borne in mind that the introduction of milk proteins in the formulation of margarine is a destabilizing factor [8, 11]. Therefore, the amount of an emulsifier can also be higher for milk margarines and especially spreads.
This issue is very important in the development of spreads with the addition of a large amount of dried milk [7, 11]. In manufacturing such combined products using milk lines, the amount of distilled monoglycerides (E 471) has to be often increased to 0.4–0.5%.
In the case when the water content in the formulation of margarines and spreads is still higher, mono- and diglycerides with an iodine index of 80 mg J2/100 g and a melting point of 50°C or lower should be used. Lecithins are not used in such margarines, as this can cause phase reversal and emulsion stratification. When obtaining margarines with a 50% fat content using standard lines, it is enough to use (E 471) with an iodine index from 80 to 105 g J2/100 g. It should be borne in mind that margarines and spreads with such a fat content cannot be packaged in packs. This is due to a number of physical laws, and it is impossible to obtain a quality product with such a fat content packaged in packs by replacing an emulsifier.
In the production of margarines with a fat content of 40%, the amount of the aqueous phase is higher than that of the fat phase and monoglycerides alone are not enough to obtain a water-in-oil emulsion with sufficient plasticity. To produce margarines with a fat content of 40%, it is recommended to use the combination of monoglycerides and the esters of polyglycerol and ricinoleic acid (E 476). The emulsifier E 476 works well in the case of the combined introduction thereof with monoglycerides, the introduction thereof in the formulation alone does not give the desired result. Distilled monoglycerides (E 471) in the amount of 0.4–0.5% in combination with 0.1–0.2% of polyglycerol polyricinoleate (E 476) are usually used to produce 40% fat margarine. The introduction of this emulsifier into margarine makes it possible to obtain a very stable emulsion that cannot be destroyed even if heated for long.
The production of margarines with a fat content of 20% is possible when an emulsifier for the fat phase and a stabilizer for water-milk phase are combined in the formulation. Distilled monoglycerides in the amount of 0.5–0.6% and polyglycerol polyricinoleate at a dosage of 0.3–0.4% are used as emulsifiers, just as in the case of 40% fat margarines. As a stabilizer for the aqueous phase, it is recommended to use pectin. The use of a stabilizer also allows us to make margarines more flavorful. This is especially important for margarines with a mass fraction of fat of 20 to 40% with a small amount of dried milk (0.1–0.2%) in the formulation. For non-milk margarines, the introduction of pectins into the formulation allows us to make the taste of the aromatizing agent in the aqueous phase more pronounced.
In the production of margarine for frying with a salt content of 0.3% and higher, it is enough to use 0.1–0.2% of distilled monoglycerides and 0.2% of lecithin in the formulation. It is also recommended to use an emulsifier that is a mixture of monoglycerides E471 and special lecithin E322. The use of this complex emulsifier in the amount of 0.3–0.4% in the margarine formulation allows, when frying, for the formation of fine-mesh foam on the frying surface, which does not allow margarine to splash and improves the color of the crust of the product being fried.
In the production of margarine for frying with a fat content of 60%, it is necessary to introduce the stronger emulsifier E479 into the formulation, which is thermally oxidized soybean oil in combination with mono- and diglycerides. It is capable of reducing the splashing tendency of margarine during frying, even with a large amount of water in the margarine formulation.
To produce liquid margarines in a customer-size package for household use, the combination of emulsifiers E479 (a mixture of monoglycerides and triglycerides) in the amount of 0.5–2.0% and E492 (sorbitan tristearate) at a dosage of 0.1% are used. The use of these emulsifiers makes it possible to obtain a stable liquid water-in-oil emulsion and prevent the release of liquid oil from a margarine emulsion during storage.
Sorbitan tristearate (E492) is also recommended for use in all types of margarines to prevent margarine defects – mealiness and crumbiness [9]. This is especially important when working with fatty acids of the same type in the fat base: sunflower oil and hydrogenated sunflower fat.
If margarine is used for baking whipped sponge cakes or for making confectionary creams [13], it is necessary to introduce a special emulsifier into the formulation to grip when whipping and to keep air in the mass of dough or cream. To this end, it is necessary to introduce, along with distilled monoglycerides (E471) at a dosage of 0.2–0.3%, an emulsifier in the margarine formulation – the esters of polyglycerol and fatty acids (E475) in the amount of 0.3–0.5%. The use of these two emulsifiers in the margarine formulation makes it possible to obtain uniform porosity in the baked products, to keep fat during baking, not to let it out, and there is also an increase in the volume of the finished products. The confectionery creams, cooked using such margarines, have a large volume and a high thermal stability. It should also be noted that in the production of margarines for such purposes, it is advisable to introduce 5–15% of coconut or palm kernel oil into the fat base.
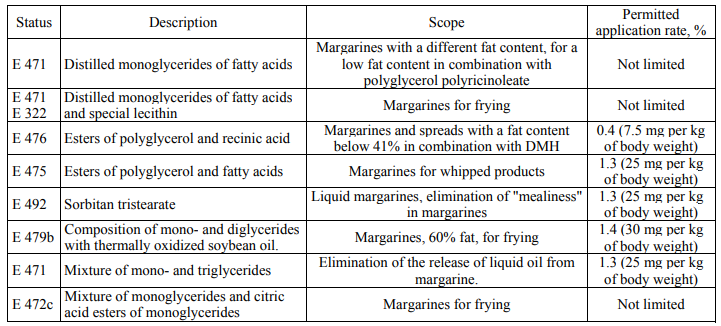
To prepare margarine for puff pastry to retain plasticity during dough making, without being torn and keeping the same thickness of the layer when being rolled (at the same time it should not melt and impregnate the dough layers), it is advisable to use complex emulsifiers that are a mixture of monoglycerides (E 471) and polyglycerol esters (E 475) to control margarine crystallization processes at all the stages of its production and use in the amount of 0.8–1.0% and to additionally apply 0.6–0.8% of lecithin [13]. Table 7 presents some variants of emulsifiers and their compositions for margarines and spreads.
In addition to its technological functions, an emulsifier can increase the nutritional value of the emulsion product being created. A complex food emulsifier may contain phospholipids that have good emulsifying properties and are also physiologically valuable ingredients with, among other things, antioxidant activity. When this emulsifier was added to the spread and margarine formulations, there was an increase in the oxidative stability of the products.
The type of lecithin used basically depends on the salt content in margarine. For household margarines and industrial types for baking and making puff pastry, both standard and modified lecithins are suitable. Since salt is the additional stabilizer in classic margarines, the system becomes less stable in its absence. Therefore, it is recommended to use fractionated lecithin for saltfree and low-salted margarines which is enriched with phosphatidylcholine to provide a further increase in the stability of a margarine emulsion. At present, there is a stable trend of transition from standard lecithins to enzymatically hydrolyzed ones. They are well suited for the emulsions with a relatively high water content. In addition, they are more convenient to use than standard lecithins.
The lecithins used in the margarine formulation for frying improve its characteristics, in particular, heat resistance. The addition of lecithin prevents margarine from darkening during reheating and prevents splashing during frying. The margarine components prone to sedimentation, for example, milk casein, are covered with a thin layer of lecithin, which prevents their burning. In addition, the margarines that contain lecithin do not foam under heat treatment.
Adding lecithin to low-fat margarines improves their taste. In this case, in combination with monodiglycerides, it forms a fine emulsion, which has a beneficial effect on the increase in shelf life.
The use of modified lecithins in margarine allows the product to resist heating to 280°C without splashing and darkening. The most optimal ratio of lecithin and mono- and diglycerides is 1 : 1; 1 : 2; 1 : 3 and 2 : 3.
Lecithin is a fat-soluble component, and therefore is added into the fat phase. There are a number of rules that should be followed when adding lecithin to oil (Table 8). The oil must be heated to a temperature of 55°C. Lecithin is added to oil gradually with constant stirring. When monoglycerides are used in the production of margarines, they must be dissolved in the oil phase together with lecithin.
It is very important that the mixture of emulsifiers is constantly mechanically treated over the whole technological process, the temperature should not be raised to more than 60°C.
Thus, based on the foregoing, it can be concluded that when selecting a particular emulsifier, all the features of the food emulsion it is intended for should be taken into account. Preference should be given to the food supplement a set of technological functions of which provides the maximum technological effect while preserving the nutritional value and organoleptic properties of the final product. In the final product, the emulsifiers should provide the stability of the water-inoil emulsion due to their effective impact on the process of fat dispersion in the plasma, promote the formation of a homogeneous, plastic consistency of the product resistant to freezing-thawing due to the formation of structural bonds in the product that are easily restored after destruction. In addition, the emulsifiers in the composition of margarines and spreads control the processes of fat crystallization, i.e. affect the modification of the polymorphic form, the size and growth rate of fat crystals thereby providing the improvement of the creamy taste and other properties. The correct choice of an emulsifier makes it possible to increase the hardness and thermal stability of the product by taking part in the formation of its structure and also to prevent the release of moisture and liquid fat in the product monolith during long-term storage [11].
The most effective to provide the stability of an emulsion are the synergistic systems that consist of several emulsifiers. Despite the fact that the development of completely new emulsifiers is laborintensive and is related to the need for long-term testing, the variety of factors that determine the composition and properties of emulsion products indicates that when creating new types of fat-and-oil products, the selection of optimal emulsifying systems, including those that consist of several emulsifiers, will be the subject of intensive scientific research and technological developments in future [14, 15].
СПИСОК ЛИТЕРАТУРЫ
- Berton C., Ropers M.-H., Viau M., and Genot C. Contribution of the interfacial layer to the protection of emulsified lipids against oxidation. Journal of Agricultural and Food Chemistry, 2011, vol. 59, no. 9, pp 5052-5061. DOI: 10.1021/jf200086n.
- Fuller G.T. Studies on the Shear Stability of Partially Crystalline Oil-in-Water Emulsions. Diss. Dr. of Philosophy in Food Technology. Palmerston North, New Zealand, 2015. 239 p.
- Hasenhuettl G.L. and Hartel R.W. Food Emulsifiers and Their Applications: Second Edition. New York: Springer New York, 2008. 426 p. DOI: 10.1007/978-0-387-75284-6.
- Helmenstine A.M. Emulsifier Definition - Emulsifying Agent. ThoughtCo. Available at: https://www.thoughtco.com/definition-of-emulsifier-or-emulsifying-agent-605085 (accessed 12 June 2017).
- Yu H., Huang Y., and Huang Q. Synthesis and characterization of novel antimicrobial emulsifiers from ε-Polylysine. Journal of Agricultural and Food Chemistry, 2010, vol. 58, no. 2, pp 1290-1295. DOI: 10.1021/jf903300m.
- Munk M.B., Larsen F.H., Van Den Berg F.W.J., Knudsen J.C., and Andersen M.L. Competitive Displacement of Sodium Caseinate by Low-Molecular-Weight Emulsifiers and the Effects on Emulsion Texture and Rheology. Langmuir, 2014, vol. 30, no. 29, pp. 8687-8696. DOI: 10.1021/la5011743.
- Munk M.B., Erichsen H.R., and Andersen M.L. The effects of low-molecular-weight emulsifiers in O/W-emulsions on microviscosity of non-solidified oil in fat globules and the mobility of emulsifiers at the globule surfaces. Journal of Colloid Interface Science, 2014, vol. 419, pp. 134-141. DOI: 10.1016/j.jcis.2013.12.036.
- Phan T.T., Le T.T., Van der Meeren P., and Dewettinck K. Comparison of emulsifying properties of milk fat globule membrane materials isolated from different dairy by-products. Journal of Dairy Science, 2014, vol. 97, no. 8, pp. 4799-4810. DOI: 10.3168/jds.2014-8030.
- Wu S., Wang G., Lu Z., et al. Effects of glycerol monostearate and Tween 80 on the physical properties and stability of recombined low-fat dairy cream. Dairy Science and Technology, 2016, vol. 96, no. 3, pp. 377-390. DOI: 10.1007/s13594-015-0274-x.
- Tran T., Green N.L., and Rousseau D. Spheroidal Fat Crystals: Structure Modification via Use of Emulsifiers. Crystal Growth and Design, 2015, vol. 15, no. 11, pp. 5406-5415. DOI: 10.1021/acs.cgd.5b01033.
- Vega C. and Roos Y.H. Invited review: Spray-dried dairy and dairy-like emulsions-Compositional considerations. Journal of Dairy Science, 2006, vol. 89, no. 2, pp. 383-401. DOI: 10.3168/jds.S0022-0302(06)72103-8.
- Wang Z., Song J, Zhang S., Xu X.-Q., and Wang Y. Formulating Polyethylene Glycol as Supramolecular Emulsifiers for One-Step Double Emulsions. Langmuir, vol. 33, no. 36, pp. 9160-9169. DOI: 10.1021/acs.langmuir.7b02326.
- Petrova M. Emulsifiers for confectionery and bakery products. Confectionery and Baking Industry, 2014, nos 11-12, p. 29. (In Russian).
- Starovoytova K.V. and Tereshchuk L.V. Teoriya i praktika primeneniya poverkhnostno-aktivnykh veshchestv v proizvodstve pishchevykh emul'siy [Theory and practice of application of surfactants in the production of food emulsions]. Kemerovo: KemIFST Publ., 2016. 152 p.
- Tereshchuk L.V. and Umanskiy M.S. Molochno-zhirovye kompozitsii: aspekty konstruirovaniya i ispol'zovaniya [Milk-fat compositions: aspects of the design and use]. Kemerovo: KemIFST Publ., 2006. 209 p.