Аннотация
The main objective of the present study was to improve the oxidative stability of sunflower oil (SFO) and soybean oil (SBO). The aqueous ethanol extracts (80% ethanol) of pomegranate and Baladi orange peels were used as natural antioxidants at concentrations of 800 and 1,200 ppm in SFO and SBO in comparison to butylated hydroxytoluene (BHT). Their antioxidant activities were estimated via the Rancimat method and over 24 days of storage at 65°C. The effect of extracts on the stability of sunflower and soybean oils during the storage period was studied by measuring the peroxide value (PV), conjugated dienes (CD) at 232 nm, conjugated trienes (CT) at 270 nm, free fatty acids (FFAs), iodine value (IV), and the refractive index (RI). A great difference in PVs was observed between the control sample and the oil samples containing natural extracts which slowed the rate of peroxide formation. Generally, the results showed that during the storage period at 65°C, the conjugated diene formation followed a similar pattern relative to PV accumulation. The PV, CD, CT, FFA, and RI values of SFO and SBO containing a pomegranate peel extract (PPE) and Baladi orange peel extract (BOPE) at concentrations of 800 and 1,200 ppm were lower than those of SFO and SBO containing 200 ppm BHT, and this trend became apparent during the storage period. The rate of reduction of IV in the control was higher than that in SFO and SBO containing both synthetic and natural antioxidants. These findings confirmed that the natural antioxidants under investigation could be used as alternatives to synthetic antioxidants to improve the oxidative stability of edible oils in the food industry.Ключевые слова
Pomegranate peel extract, baladi orange peel extract, sunflower oil, soybean oil, oxidative stabilityВВЕДЕНИЕ
Lipid peroxidation that results from the reaction between unsaturated fatty acids and molecular oxygen is a severe problem for the oil and fat industry [1]. This process not only deteriorates the quality of fats and fatty foods and results in chemical spoilage, but also produces free radicals, such as peroxyl and hydroxyl radicals, and reactive oxygen species (ROS) that are reportedly related to carcinogenesis, mutagenesis, inflammation, ageing, and cardiovascular diseases [2].
Antioxidants are the substances that can prevent or inhibit oxidation processes in the human body and deterioration in food products [3]. Synthetic antioxidants are widely used as food additives to prevent rancidification due to their high performance, low cost, and wide availability [4]. Hence, synthetic antioxidants, such as butylated hydroxytoluene (BHT), tertiary butyl hydroquinone (TBHQ) and butylated hydroxyanisole (BHA), are used in edible vegetable oils. These antioxidants used as preservatives in the food industry may be responsible for liver damage and carcinogenesis; for this reason, an interest in the use of natural antioxidants has increased [5].
Plant extracts offer a unique range of applications for holistic health and wellness [6]. Secondary plant metabolites, such as phenolic compounds from plant sources, are highly valuable for their therapeutic attributes as antioxidants [2]. The sources of these metabolites include different plant organs, such as seeds, leaves, and peels.
Pomegranate (Punica granatum L.) peels and Baladi orange (Citrus sinensis) peels have been studied as potential antioxidant sources due to their high antioxidant activities. The processing of pomegranate fruits produces 46% peels, 14% seeds, and 40% juice, with by-products representing more than 50% of the total weight [7]. Pomegranate peels contain 249.4 mg/g of phenolic compounds in comparison to only 24.4 mg/g of phenolic compounds in the pulp of pomegranates [8]. Citrus peel that represents approximately half of the fruit mass contains the highest concentrations of flavonoids [9].
These by-products have high antioxidant activities, few studies have dealt with the use of food wastes to stabilize edible oils. Thus, the aim of the present study was to evaluate the antioxidant efficiency of PPE and BOPE as natural antioxidants for improving the oxidative stability of refined bleached deodorized (RBD) sunflower and soybean oils because these peels are the richest sources of phenolic compounds with antioxidant activities.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Materials. Freshly refined, bleached and deodorized sunflower and soybean oil samples without any added antioxidants were obtained from Arma Company for Food Industries in the 10th of Ramadan City, Sharkia Governorate, Egypt. The oil samples were immediately stored at -18°C until further analyses. Pomegranate fruits (Punica granatum L.) and Baladi orange fruits (Citrus sinensis) were obtained from the local markets in Zagazig City, Sharkia Governorate, Egypt. All the chemicals and reagents used in the analytical methods (analytical grade) were obtained from Sigma Chemical Company (St. Louis, MO, U.S.A.) and El-Gamhouria Trading Chemicals and Drugs Company, Zagazig, Egypt.
Methods. Preparation of extracts. The plant materials were washed with distilled water and then peeled. The fresh peels were cut into small pieces with a sharp knife for easy drying and directly dried in a hot-air oven at 40°C until completely dried. The dried samples were ground to fine powder using a laboratory blender. The extracts were prepared according to [1] with slight modifications. The material passed through an 80-mesh sieve was used for extraction purposes. The powdered material (10 g) from each sample was extracted with 100 mL of aqueous (80%) ethanol using a magnetic stirrer at room temperature overnight. The extracts were separated from the residues by filtering through a Whatman No. 1 filter paper. The residues were re-extracted twice with the same solvent under the same conditions, and the extracts were pooled. All the combined extracts were concentrated by a rotary evaporator under a vacuum (BÜCHI Rotavapor, Model R-124, Germany) at 45°C to obtain crude extracts.
Incorporation of natural extracts with the oil samples. Fresh RBD sunflower and soybean oil samples without any additives and those containing 1,200 and 800 ppm PPE and BOPE were prepared separately. The crude concentrated extracts of pomegranate and Baladi orange peels were individually added to preheated (50°C) RBD sunflower and soybean oils at concentrations of 800 and 1,200 ppm (w/w). The extracts were weighed and dissolved in 5 mL of 2- propanol to facilitate their dispersion in sunflower and soybean oils [2]. The mixtures were stirred for 30 min at 50°C for uniform dispersion [10]. A synthetic antioxidant, BHT, was used at its legal limit of 200 ppm to compare the efficiency of the natural antioxidants. A control sample (without any added antioxidant extract) and a sample containing BHT were also prepared for both sunflower and soybean oils under the same conditions. Each 120 g of the prepared sample was placed in an airtight dark brown glass bottle. Subsequently, the prepared oil samples were stored in an oven at a temperature of 65°C for 24 days, whereby each day of storage was equivalent to one month of storage at ambient temperature [11]. The oil samples were periodically analyzed at different intervals.
Determination of oxidative stability by Rancimat. The oxidative stability of the oil samples was estimated as an induction period (hr) according to the method described by [12] using a Rancimat Metrohm Instrument (Ud.CH-9100 Herisau, Switzerland, Model 679) at 100°C with the air pumped at a flow rate of 20 L/h. This assay was conducted at the Oils & Fats Research Dept., Food Technology Research Institute, ARC, Egypt.
Measurement of chemical and physical properties of oils. The peroxide value (PV), free fatty acids, iodine value (measured according to the Hannus method), and the refractive index using a refractometer (INYRL-3- Poland) at 20°C were determined in different oil samples according to the method described by [13].
The UV absorbencies of the oil samples at 232 and 270 nm (K232 and K270) were determined according to the European Official Methods of Analysis [14]. Each oil sample (0.1 g) was dissolved in 10 mL of cyclohexane. The absorption was then read at 232 nm (conjugated dienes) and at 270 nm (conjugated trienes) in a quartz cell with a 1 cm long path using a 1% solution of oil against cyclohexane as a blank in a Pye-Unicam double-beam recording spectrophotometer, model L.
Statistical analyses. The experiments were conducted in triplicate, and the resulting data were expressed as the mean ± SD. The statistical analyses of the data were performed using SPSS software (17.0 for Windows) and analyzed by one-way ANOVA. Significant differences between the means were determined using Duncan's multiple range tests (Duncan, 1955). The significance level was P ≤ 0.05.≤ 0.05.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
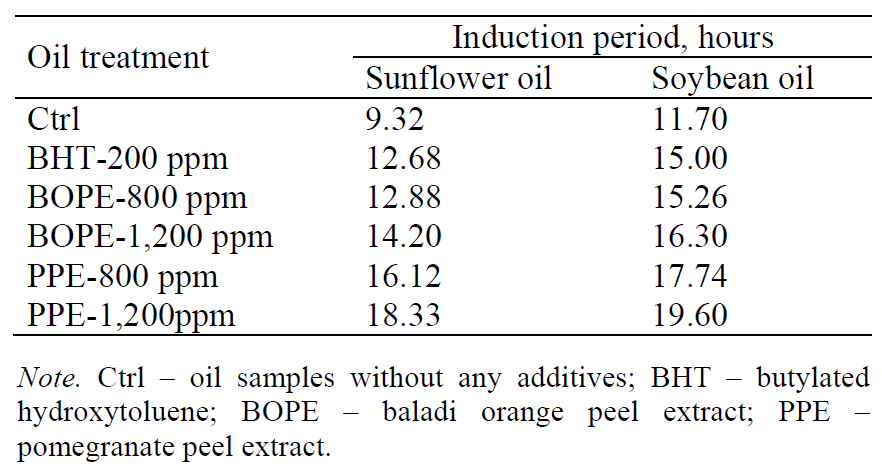
Oxidative stability. The Rancimat method is commonly used to estimate the antioxidative potency of various antioxidants [15]. As shown in Table 1, the addition of the natural antioxidants extracted from pomegranate and Baladi orange peels to sunflower and soybean oil samples resulted in an increase in the oxidative stability of the oils. This increase depended on the concentration of antioxidants as well as the type of oil. The stability of sunflower oil increased from 9.32 hours in the control oil sample (without any additives) to 12.68, 12.88, 14.20, 16.12 and 18.33 hours in the sunflower oil treated with BHT, BOPE (800 ppm), BOPE (1,200 ppm), PPE (800 ppm) and PPE (1,200 ppm), respectively. In addition, the stability of soybean oil increased from 11.70 hours in the control oil sample (without any additives) to 15.00, 15.26, 16.30, 17.74 and 19.60 hours in the soybean oil treated with BHT, BOPE (800 ppm), BOPE (1,200 ppm), PPE (800 ppm) and PPE (1,200 ppm), respectively. In addition, the results revealed that the PPE showed the highest value for the induction period followed by BOPE. These natural antioxidants are considered beneficial to human health because they inhibit lipid peroxidation and scavenge free radicals. Generally, PPEs exhibit good antioxidant capacities and act as the effective scavengers of several ROS due to their high levels of polyphenolic compounds [16]. Various phenolic compounds present in orange peel extracts prolonged the oxidative stability of the oil samples according to the Rancimat method [17]. The obtained results are close to those reported by [18] and [19].
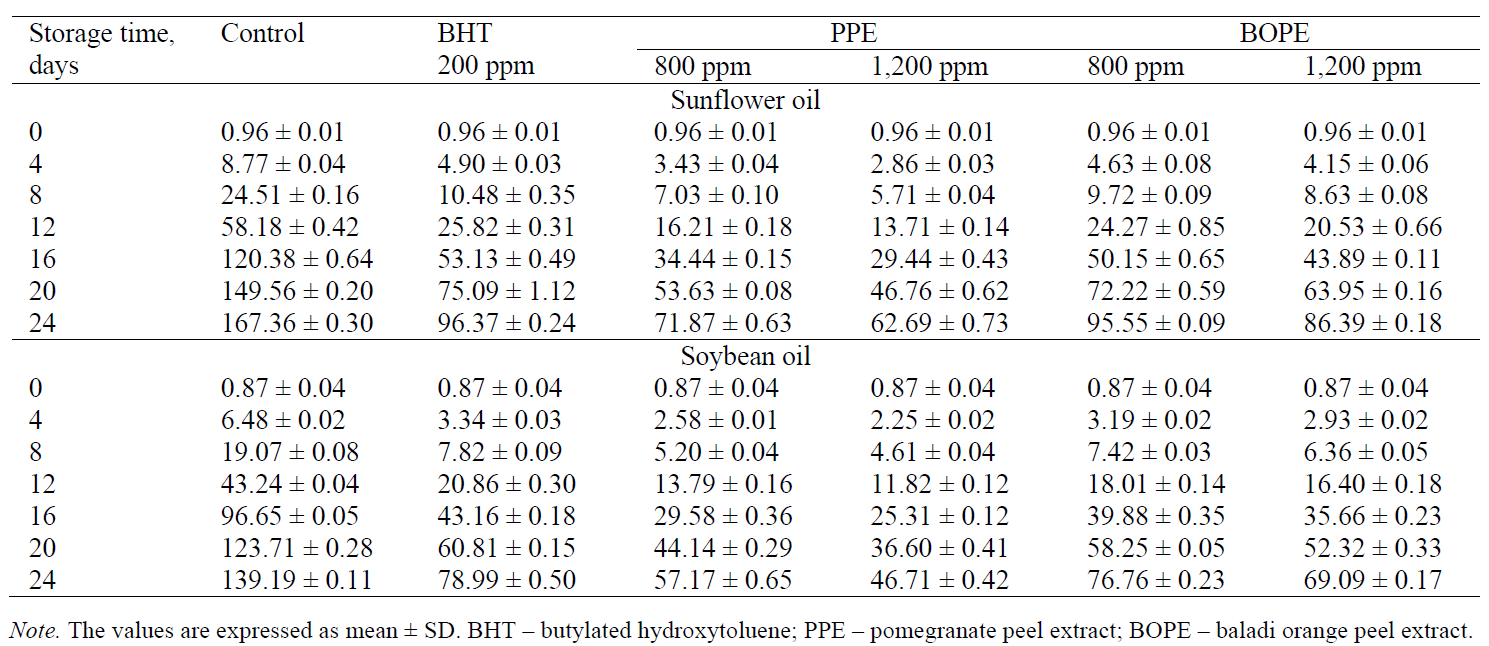
Peroxide value. PV measures the concentration of peroxides and hydroperoxides formed during the initial stages of lipid oxidation. PV is a good indicator of the primary oxidation products of oils [20]. A saturated iodine solution added to the oil sample reacts with the resulting hydroperoxide from the lipid oxidation and releases free iodine as a product. The liberated iodine is then titrated against sodium thiosulphate. The titration value can be calculated and reported as PV in milliequivalents of oxygen per 1kg of oil [21]. A continuous increase in PV with the increased storage period was observed in all the samples. This increase in the PV is attributed to the formation of hydroperoxides. The results in Table 2 show the extent of the changes in the PV of the sunflower and soybean oils supplemented with PPE and BOPEs at different concentrations compared to the control and sunflower and soybean oil samples containing synthetic antioxidant over the storage period. The PV ranged from 0.96 to 71.87 meqO2/kg oil for sunflower oil supplemented with PPE and from 0.96 to 95.55 meqO2/kg oil for sunflower oil supplemented with BOPE. The sunflower oil sample without any antioxidants (control) reached the maximum PV of 167.36 meqO2/kg oil after 24 days of storage. A great difference in PV was observed between the control and sunflower oil samples containing natural extracts, which slowed the rate of peroxide formation. The PV of sunflower oil containing 1,200 ppm of PPE and BOPE as well as BHT at a concentration of 200 ppm was found to be 62.69, 86.39 and 96.37 meqO2/kg oil after 24 days of storage, respectively. Regarding the effect of the natural antioxidants extracted from pomegranate and Baladi orange peels on the PV of soybean oil during the storage period, the obtained results showed that PV ranged from 0.87 to 57.17 meqO2/kg oil for the soybean oil supplemented with PPE, and from 0.87 to 76.76 meqO2/kg oil for the soybean oil supplemented with BOPE. The soybean oil without any antioxidants (control) reached the maximum PV of 139.19 meqO2/kg oil after 24 days of storage. There was also a great difference in PV between the control and soybean oil samples containing natural extracts. The PV of soybean oil containing 1,200 ppm of PPE and BOPE as well as BHT at a concentration of 200 ppm was observed to be 46.71, 69.09, and 78.99 meqO2/kg oil after 24 days of storage, respectively. Based on PV, the studied antioxidants can be arranged in the following descending order according to their efficiency in maintaining the quality of the studied oils: PPE (1,200 ppm), PPE (800 ppm), BOPE (1,200 ppm), BOPE (800 ppm), and BHT (200 ppm). The inhibition of oil oxidation was highly dependent on concentration; therefore, all the extracts were effective at 800 ppm as compared to BHT (200 ppm). These data suggest the superior antioxidant activity of pomegranate and orange peel extracts compared to a synthetic antioxidant. These results confirm the findings of [22], [23], and [6].
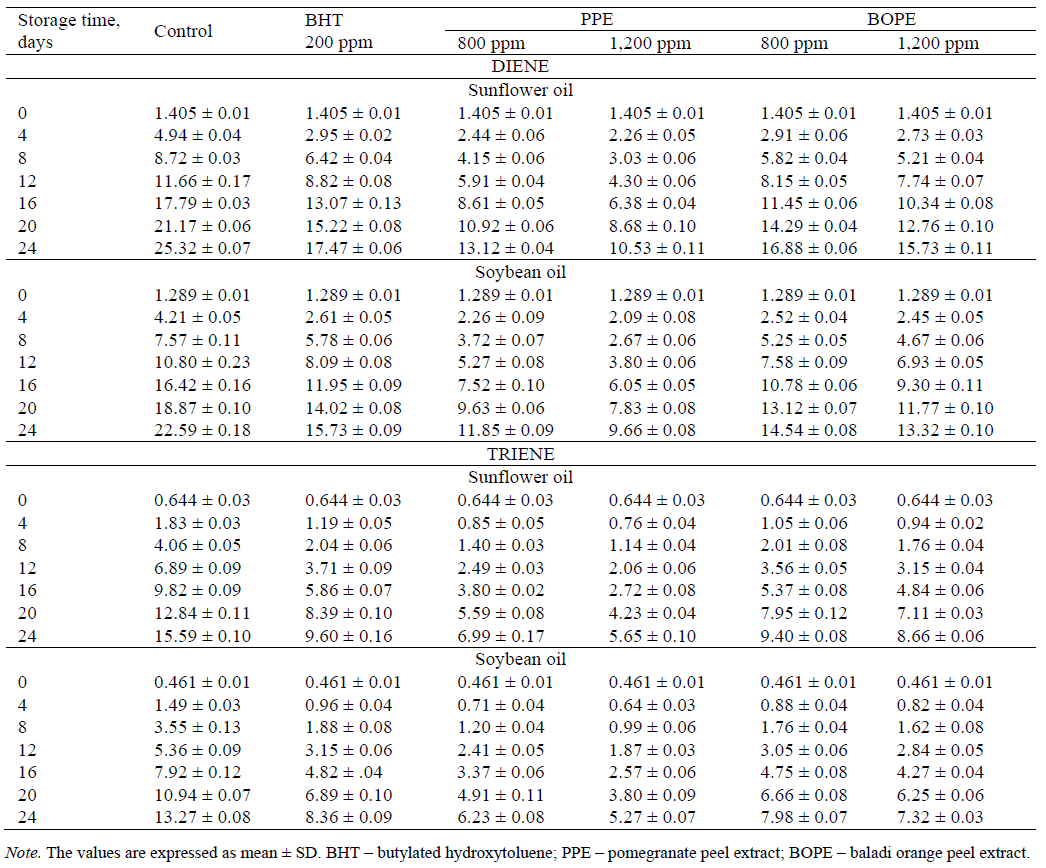
Conjugated dienes. The estimation of the conjugated dienes (CD) at 232 nm is a good measure of the oxidation state of oils [20]. CDs are hydroperoxides formed with the shift in the position of the double bond(s) during the oxidation of polyunsaturated fatty acids [24]. The results in Table 3 show that the UV absorbance at 232 nm for the sunflower and soybean oils without any additives at the beginning of the storage period were 1.405 and 1.289, respectively. Subsequently, they increased at the end of the storage period to 25.32 and 22.59 for the sunflower and soybean oils, respectively. The content of CDs (in the case of the natural antioxidants extracted from pomegranate peel and Baladi orange peel) was increased at the end of the storage period to reach 10.53 and 9.66 for PPE at a concentration of 1,200 ppm and 15.73 and 13.32 for BOPE at a concentration of 1,200 ppm for the sunflower and soybean oils, respectively. The UV absorbance values at 232 nm for the sunflower and soybean oils treated with BHT at a concentration of 200 ppm at the end of the storage period were 17.47 and 15.73, respectively. Generally, the results showed that during the storage period at 65°C, the conjugated diene formation followed a similar pattern relative to PV accumulation. Both of these indices measure the primary products of lipid oxidation. It was found in [6] that the conjugated diene content of sunflower oil without any additives (control) was 25.55 ± 0.38, whereas the contents in the sunflower oil containing BHA at a concentration of 200 ppm, kenaf seed extract (KSE), roselle seed extract (RSE), and roselle extract (RE) at a concentration of 1,500 ppm were 18.26±0.59, 17.53 ± 0.41, 13.55 ± 0.33 and 15.13 ± 0.46, respectively, after 24 days of storage at 65°C. The obtained results are consistent with the findings of [10] who reported that the conjugated diene and triene values of sunflower oil with and/or without the different extracts were higher than those of soybean oil with and/or without different extracts after 15 days of storage at 65°C.
Conjugated trienes. The estimation of conjugated trienes (CT) is a good parameter for measuring the oxidative deterioration of oils: it indicates the effectiveness of antioxidants in oils [21]. CT may be produced by the dehydration of conjugated diene hydroperoxides [25]. The effects of BHT, PPE, and BOPE additions to sunflower oil on ultraviolet absorbance at 270 nm are also shown in Table 3. There were regular increases in CT for all the samples over the storage period. The increase in CT can be arranged in descending order as follows: SFO-Ctrl > SFO-BHT > SFO-BOPE (800 ppm) > SFO-BOPE (1,200 ppm) > SFO-PPE (800 ppm) > SFO-PPE (1,200 ppm). The results showed that the PPE and BOPE controlled the CT values appreciably, and their CT values were lower than those of BHT at every interval revealing the antioxidants efficiency in improving the stability of sunflower oil by delaying the formation of secondary oxidation products. [26] described the antioxidant activity of garlic extracts in sunflower oil as assessed under accelerated conditions when using CT as an indicator of oxidative degradation. With regard to the effect of the natural antioxidants extracted from pomegranate and Baladi orange peels on ultraviolet absorbance at 270 nm in the soybean oil, Table 3 shows a relative increase in the CT contents of soybean oil under accelerated storage. The CT values continued to increase with the increased storage time. The highest CT content was observed for SBO-Ctrl showing a higher intensity of oxidation, followed by SBO-BHT, SBO-BOPE (800 ppm), SBO-BOPE (1,200 ppm), SBO-PPE (800 ppm) and SBO-PPE (1,200 ppm). The CT contents of PPE and BOPE also increased but at a slower rate than that in the control. This finding demonstrated the antioxidant potential of PPE and BOPE in the stabilizing oils. Notably, BHT showed relatively low antioxidant properties in preventing lipid oxidation compared to PPE and BOPE. The similar results were found by [22] who reported that the control exhibited the highest CD and CT contents followed by BHT and PPE. [1] reported that the corn oil without any additives exhibited the highest CD and CT contents followed by BHT, citrus peel extract, and pomegranate peel extract.
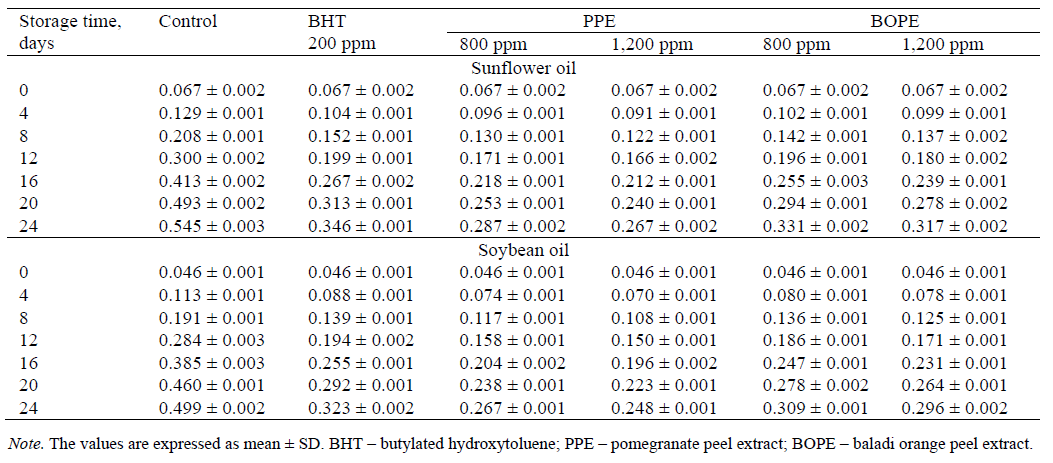
Free fatty acids. The free fatty acid content (FFA % as oleic acid) is used as an indicator of oil hydrolysis. FFA are formed due to the hydrolysis of triglycerides in oil and are considered one of the important indicators of oil rancidity [27]. Table 4 depicts the extent of changes in the FFAs in the sunflower and soybean oils stored with PPE, BOPE, and BHT. The SFO samples containing PPE and BOPE at concentrations of 800 and 1,200 ppm had a lower FFA content than the SFO sample containing 200 ppm BHT over the storage period. The FFA values of SFO containing PPE and BOPE were lower than those of SFO without any additives (control). Table 4 also shows the effect of the storage period on FFA content in the soybean oil treated with different antioxidants. The FFA content of the SBO sample without any additives was higher than that of the SBO samples that contained PPE and BOPE. The FFA values of SBO containing PPE and BOPE at concentrations of 800 and 1,200 ppm were lower than those of SBO containing 200 ppm BHT, and this trend became apparent during the storage period. These results confirmed the findings of [23] who reported that the soybean oil treated with an ethanolic extract of orange peel at concentrations of 800, 1,200 and 1,600 ppm showed lower FFA contents than the soybean oil treated with synthetic antioxidants (BHT and BHA, 200 ppm) at the end of the storage period. [28] reported that after 7 days of storage at 80°C, the FFA contents of the sunflower oil supplemented with pomegranate peel extract and sour orange peel extract at a concentration of 800 μg/mL were lower than those of the sunflower oil supplemented with BHT (200 μg/mL).
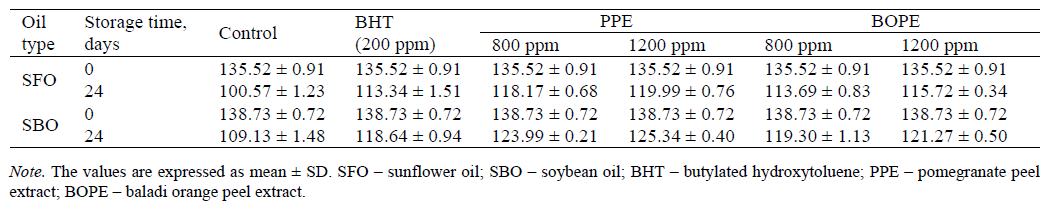
Iodine value. Sunflower and soybean oils contain a great deal of polyunsaturated fatty acids (PUFAs). These PUFAs are highly prone to lipid oxidation [29]. During storage, the double bonds of these PUFAs are attacked by free radicals, which results in the formation of conjugated bonds [30]. Hence, the measurement of the amount of the unsaturated fatty acids present in sunflower and soybean oils can be used as a reference to determine the freshness of oil [31]. The freshness of sunflower and soybean oils can be determined quantitatively by measuring the iodine value (IV) [27]. Unsaturated fatty acids react with iodine monobromide and release free iodine. Free iodine can then react with sodium thiosulphate. The results are then calculated and expressed in g I2/100 g. [32] demonstrated that IV highly diminished with increasing the storage time. The double bonds of unsaturated fatty acids in the oil are broken during storage, and IV decreased [33]. The variations in IV for the supplemented oil samples and the control after an accelerated storage period at 65°C for 24 days are shown in Table 5. IV was 135.52 g I2/100 g for all the sunflower oil samples before storage. After 24 days of storage, IV were 100.57, 113.34, 119.99, 118.17, 115.72, and 113.69 g I2/100 g for SFO-Ctrl, SFO- BHT, SFO-PPE (1,200 ppm), SFO-PPE (800 ppm), SFO- BOPE (1,200 ppm) and SFO-BOPE (800 ppm), respectively. However, IV was 138.73 g I2/100 g for all the soybean oil samples before storage. After 24 days of storage, IV were 109.13, 118.64, 125.34, 123.99, 121.27 and 119.30 g I2/100 g for SBO-Ctrl, SBO-BHT, SBO- PPE (1,200 ppm), SBO-PPE (800 ppm), SBO-BOPE (1,200 ppm) and SBO-BOPE (800 ppm), respectively. IV was shown to decrease with the increased storage time for all the samples. The rate of reduction of IV in the control was higher than that in the sunflower and soybean oils containing both synthetic and natural antioxidants. A decreasing in IV in oils is generally attributed to a breakdown in the double bonds of the fatty acids caused by the oxidation process. IV at the beginning and end of the storage period was determined according to [34]. These results are in accordance with those reported by [6] and [35] who reported that after 24 days of storage at 65°C, the rate of reduction in IV of sunflower oil without any extracts was higher than that in the sunflower oil supplemented with natural extracts.
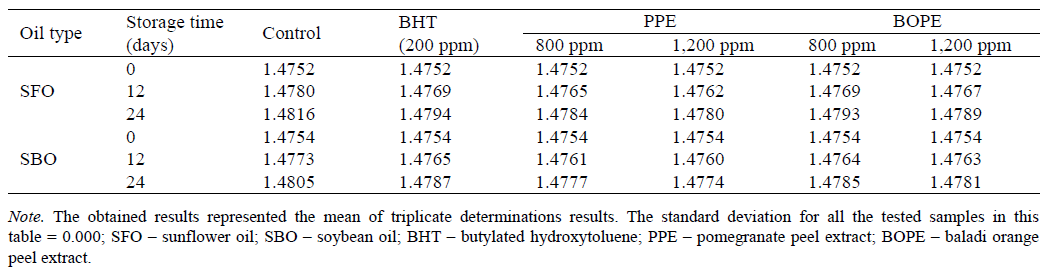
Refractive index (RI). The data in Table 6 show the effect of PPE and BOPE on the refractive index (RI) of sunflower and soybean oils during the accelerated storage at 65°C for 24 days. The RI values of the supplemented sunflower and soybean oil samples and the control demonstrated a gradual increase with the duration of the storage period. After 24 days of storage, the untreated sunflower and soybean oil samples (control) demonstrated higher RT values than the sunflower and soybean oil samples containing natural extracts and BHT. The RI values of the supplemented sunflower oil samples and control were 1.4752 before storage, and this value became 1.4816, 1.4794, 1.4780, 1.4784, 1.4789, and 1.4793 at the end of the storage period for SFO-Ctrl, SFO-BHT, SFO-PPE (1,200 ppm), SFO-PPE (800 ppm), SFO-BOPE (1,200 ppm) and SFO-BOPE (800 ppm), respectively. With regard to the extent of the changes in RI of the soybean oil supplemented with PPE and BOPEs when compared to the control and the soybean oil samples containing synthetic antioxidant after 24 days of storage, the results showed that the RI values of the supplemented soybean oil samples and control were 1.4754 before storage, and this value reaching 1.4805, 1.4787, 1.4774, 1.4777, 1.4781, and 1.4785 at the end of the storage period for SBO-Ctrl, SBO-BHT, SBO-PPE (1,200 ppm), SBO- PPE (800 ppm), SBO-BOPE (1,200 ppm) and SBO- BOPE (800 ppm), respectively. These results are consistent with [20] who reported that oxidation may result in an increase in RI of edible oils due to oxidative deterioration and increased conjugation.
ВЫВОДЫ
The present study demonstrates that pomegranate and orange peel extracts have strong protective effects against the oxidation of sunflower and soybean oils. In addition, the study showed a positive correlation between the results obtained using the Rancimat method and the oven test (during storage at 65°C for 24 days) in the sunflower and soybean oils treated with the natural antioxidants extracted from pomegranate and orange peels at concentrations of 800 and 1,200 ppm. These findings have confirmed that the natural antioxidants under investigation could be used as alternatives to synthetic antioxidants to improve the oxidative stability of edible oils in the food industry.
СПИСОК ЛИТЕРАТУРЫ
- Sultana B., Anwar F., Asi M.R., and Chatha S.A.S. Antioxidant potential of extracts from different agro wastes: Stabilization of corn oil. Grasas y Aceites, 2008, vol. 59, no. 3, pp. 205–217. DOI: https://doi.org/10.3989/gya.2008.v59.i3.510.
- Sikwese F. and Duodu K.G. Antioxidant effect of a crude phenolic extract from sorghum bran in sunflower oil in the presence of ferric ions. Food Chemistry, 2007, vol. 104, no. 1, pp. 324–331. DOI: https://doi.org/10.1016/j.foodchem.2006.11.042.
- Babbar N., Oberoi H.S., Sandhu S.K., and Bhargav V.K. Influence of different solvents in extraction of phenolic compounds from vegetable residues and their evaluation as natural sources of antioxidants. Journal of Food Science Technology, 2014, vol. 51, no. 10, pp. 2568–2575. DOI: https://doi.org/10.1007/s13197-012-0754-4.
- Valantina R.S. and Neelamegam P. Antioxidant potential in vegetable oil. Research Journal Chemistry Environment, 2012, vol. 16, no. 2, pp. 87–94.
- Kim J., Kim D.N., Lee S.H., Yoo S.-H., and Lee S. Correlation of fatty acid composition of vegetable oils with rheological behaviour and oil uptake. Food Chemisty, 2010, vol. 118, no. 2, pp. 398–402. DOI: https://doi.org//10.1016/j.foodchem.2009.05.011.
- Nyam K., Wong M., Long K., and Tan C. Oxidative stability of sunflower oils supplemented with kenaf seeds extract, roselle seeds extract and roselle extract, respectively under accelerated storage. International Food Research Journal, 2013, vol. 20, no. 2, pp. 695–701.
- Suryawanshi P.C., Kirtane R.D., Chaudhari A.B., and Kothari R.M. Conservation and recycling of pomegranate seeds and shells for value addition. Journal of Renewable and Sustainable Energy, 2009, vol. 1, no. 1. Available at: https://aip.scitation.org/doi/10.1063/1.3078510. (accessed 27 Februaru 2018).
- Rudra S.G., Nishad J., Jakhar N., and Kaur C. Food industry waste: mine of nutraceuticals. International Journal of Science, Environment, 2015, vol. 4, no. 1, pp. 205–229.
- Manthey J.A. and Grohmann K. Phenols in citrus peel byproducts. Concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. Journal of Agricultural and Food Chemistry, 2001, vol. 49, no. 7, pp. 3268–3273. DOI: https://doi.org/10.1021/jf010011r.
- Anwar F., Jamil A., Iqbal S., and Sheikh M.A. Antioxidant activity of various plant extracts under ambient and accelerated storage of sunflower oil. Grasas y Aceites, 2006, vol. 57, no. 2, pp. 189–197. DOI: https://doi.org/10.3989/gya.2006.v57.i2.36.
- Evans C.D., List G.R., Moser H.A., and Cowan J.C. Long term storage of soybean and cottonseed salad oils. Journal of the American Oil Chemists Society, 1973, vol. 50, no. 6, pp. 218–222.
- Tsaknis J., Lalas S., Gergis V., Dourtoglou V., and Spiliotis V. Characterization of Moringa oleifera variety Mbololo seed oil of Kenya. Journal of Agricultural and Food Chemistry, 1999, vol. 47, no. 11, pp. 4495–4499. DOI: https://doi.org/10.1021/jf9904214.
- Official Methods of Analysis of the Association of the Official Analytical Chemists. 19th end. Arlington. Virginia, USA. 2012.
- Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Official Journal of the European Communities, 1991, L248, pp. 1–82.
- Chen C.-W. and Ho C.-T. Antioxidant properties of polyphenols extracted from green and black teas. Journal of Food Lipids, 1995, vol. 2, no. 1, pp. 35–46. DOI: https://doi.org/10.1111/j.1745-4522.1995.tb00028.x.
- Kulkarni A.P. and Aradhya S.M. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chemistry, 2005, vol. 93, no. 2, pp. 319–324. DOI: https://doi.org/10.1016/j.foodchem.2004.09.029.
- Yassari S. and Yasari E. Effects of extracts of Thompson orange peels on the stability of canola oil. Journal of Agriculture and Crop Sciences, 2013, vol. 5, no.4, pp. 410–420.
- Warner K., Frankel E., and Mounts T. Flavor and oxidative stability of soybean, sunflower and low erucic acid rapeseed oils. Journal of the American Oil Chemists' Society, 1989, vol. 66, no. 4, pp. 558–564. DOI: https://doi.org/10.1007/BF02885448.
- Ibrahium M.I. Efficiency of pomegranate peel extract as antimicrobial, antioxidant and protective agents. World Journal of Agricultural Sciences, 2010, vol. 6, no. 4, pp. 338–344.
- McGinely L. Analysis and quality control for processing and processed fats. In:Rossel J.B. and Pritchard J.L.R. (eds) Analysis of oilseeds, fats and fatty foods. New York: Elseiver Applied Science, 1991. pp. 460–470.
- Shadidi F. and Wanasundara U. Measurements of Lipid Oxidation and Evaluation of Antioxidant Activity. Natural Antioxidants: Chemistry, Health Effects and Applications, 1997, p. 379.
- Iqbal S., Haleem S., Akhtar M., Zia-ul-Haq M., and Akbar J. Efficiency of pomegranate peel extracts in stabilization of sunflower oil under accelerated conditions. Food Research International, 2008, vol. 41, no. 2, pp. 194–200. DOI: https://doi.org/10.1016/j.foodres.2007.11.005/.
- Abd El-aal H.A., Halaweish F.T. Food preservative activity of phenolic compounds in orange peel extracts (Citrus sinensis L.). Lucrări Ştiinţifice, 2010, vol. 53, pp. 457–464.
- White P. Conjugated diene, anisidine value, and carbonyl value analyses. In: Warner K. and Eskin N.A.M. (eds) Methods to assess quality and stability of oils and fat-containing foods. Champaing: AOCS Press, 1995. pp. 159–178.
- Fishwick M.J. and Swoboda P.A. Measurement of oxidation of polyunsaturated fatty acids by spectrophotometric assay of conjugated derivatives. Journal of the Science of Food and Agriculture, 1977, vol. 28, no. 4, pp. 387–393. DOI: https://doi.org/10.1002/jsfa.2740280412.
- Iqbal S. and Bhanger M. Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chemistry, 2007, vol. 100, no. 1, pp. 246–254. DOI: https://doi.org/10.1016/j.foodchem.2005.09.049.
- O’Keefe S.F. and Pike O.A. Fat characterization. In: Nielsen S.S. (ed) Food analysis. New York: Springer, 2010. pp. 239–260.
- Lutfullah G., Tila H., Khattak S.U., et al. Antioxidant properties of agro-industrial waste and their use as natural preservative for sunflower oil. Journal of Applied Environmental and Biological Sciences, 2015, vol. 5, no. 11, pp. 10–16.
- Zhang Y., Yang L., Zu Y., et al. Oxidative stability of sunflower oil supplemented with carnosic acid compared with synthetic antioxidants during accelerated storage. Food Chemistry, 2010, vol. 118, no. 3, pp. 656–662. DOI: https://doi.org/10.1016/j.foodchem.2009.05.038.
- Kanner J., Gorelik S., Roman S., and Kohen R. Protection by polyphenols of postprandial human plasma and low-density lipoprotein modification: the stomach as a bioreactor. Journal of Agricultural and Food Chemistry, 2012, vol. 60, no. 36, pp. 8790–8796. DOI: https://doi.org/10.1021/jf300193g.
- Mei W.S.C., Ismail A., Esa N.M., et al. The effectiveness of rambutan (Nephelium lappaceum L.) extract in stabilization of sunflower oil under accelerated conditions. Antioxidants, 2014, vol. 3, no. 2, pp. 371–386. DOI: https://doi.org/10.3390/antiox3020371.
- Nor F.M., Mohamed S., Idris N.A., and Ismail R. Antioxidative properties of Pandanus amaryllifolius leaf extracts in accelerated oxidation and deep frying studies. Food chemistry, 2008, vol. 110, no. 2, pp. 319–327. DOI: https://doi.org/10.1016/j.foodchem.2008.02.004.
- Alireza S., Tan C., Hamed M., and Che Man Y. Effect of frying process on fatty acid composition and iodine value of selected vegetable oils and their blends. International Food Research Journal, 2010, vol. 17, no. 2, pp. 295–302.
- Yalcin H., Karaman S., and Ozturk I. Evaluation of antioxidant efficiency of potato and orange peel and apple pomace extract in sunflower oil. Italian Journal of Food Science, 2011, vol. 23, no. 1, pp. 55–61.
- Ling S.S.C., Chang S. K., Sia W.C.M., and Yim H.S. Antioxidant effcacy of unripe banana (musa acuminata Colla) peel extracts in sunflower oil during accelerated storage. Acta Scientiarum Polonorum, Technologia Alimentaria, 2015, vol. 14, no. 4, pp. 343–356. DOI: https://doi.org/10.17306/J.AFS.2015.4.34.