Аннотация
Molecular genetic research methods make it possible to evaluate the genetic diversity of bovine leukemia virus (BLV) and are the most informative approaches to its genetic identification. Molecular genetic research methods work well for the phylogenetic analysis of sequenced nucleotide DNA sequences of the provirus, as well as for the polymerase chain reaction-restriction fragment length polymorphism analysis (PCR-RFLP) according to the phylogenetic classification of the pathogen. The purpose of the research was to study the scientific and methodological approaches to the genetic identification of bovine leukemia virus, integrated into the molecular monitoring of infection of cattle with BLV genotypes. The authors used PCR-RFLP-genotyping and comparative phylogenetic analysis of aligned nucleotide sequences of the env gene fragment of the BLV provirus isolates to detect the genotypic affiliation of the cattle from twenty-one livestock farms of the Republic of Tatarstan. As a result, isolates of four out of ten BLV genotypes were found in the Tatarstani cattle, namely genotypes 1, 4, 7, and 8. The research involved a comparative analysis of 505 nucleotide sequences of a fragment of the BLV env gene, including those deposited in GenBank NCBI. The analysis confirms the inconsistency of several earlier PCR-RFLP typing strategies with the current approach in assessing the genotypic diversity by phylogenetic analysis. The improved strategy of PCR-RFLP genotyping of BLV corresponds with its modern phylogenetic classification. The strategy makes it possible to identify all the known genotypes of the viral pathogen. Its validity has been proved by in silico modelling of restrictogrammes and a phylogenetic analysis of the env gene fragment of 57 reference isolates of ten BLV genotypes that generate 57 genotype-associated combinations of diagnostically significant PCR-RFLP profiles.Ключевые слова
Bovine leukaemia virus, BLV, cattle, gene, genotype, genetic identification, PCR, RFLP, sequencingВВЕДЕНИЕ
Enzootic Bovine Leukosis (EBL) is a chronic infectious disease of a tumorous nature. It causes significant economic damage to the dairy and beef cattle industry due to poor production, low quality, cattle mortality, and expensive epidemic prevention measures [1, 2].
Foodstuffs of infected animals can be dangerous to humans due to the harmful metabolites it contains. The causative agent affects all kinds of raw material (milk,meat, by-products) and products, remaining a potential source of human infection [3–5].
Pasteurization of milk inactivates the virus but does not degrade its genome. The genetic material of the provirus maintains its integrity in canned meat [6]. Moreover, there are dairy products with partial pasteurization regime, e.g. classic cheeses, granulated cottage cheese, powdered milk with a low heating temperature, etc. The temperature processing parameters used in accordance with the regulatory and technical documentation cannot destroy harmful metabolites and, in some cases, do not kill the virus [7]. According to some researches, DNA of BLV provirus was found in epithelial cells of human mammary glands, including those of breast cancer patients. The hypothesis states that BLV may destabilize the host genome, thus leading to cancerous degeneration of cells [8–12].
Obtaining high-quality raw materials of animal origin is the most important challenge for meat and dairy industry. The challenge includes the development of functional and gerodietic foods [13–15].
According to the requirements of the Technical Regulations of the Customs Union “On safety of milk and dairy products” (TR CU 033/2013), BLV preventive measures and eradication activities are extremely important, given the significant prevalence of this incurable disease in the Russian Federation [16, 17].
An early genetic diagnosing of the pathogen is part of the system of anti-epizootic measures, followed by the removal of infected animals from the herd. Molecular genetic research methods make it possible to assess the genetic diversity of BLV [18]. This is the most informative approach to the gene identification of the virus. Molecular genetic research methods work well for the phylogenetic analysis of nucleotide DNA sequences of the provirus, as well as for PCR-RFLP analysis according to the phylogenetic classification of the pathogen [19].
The current phylogenetic classification of BLV includes ten genotypes. The first seven genotypes were described by Argentinean scientists in 2009 [20], while genotype 8 was described by researchers from Russia [21–23], Croatia [24], and a European team of scientists [25] in 2011–2013. Genotype 9 was investigated by a team of Argentinean, Chilean, and Japanese scientists in 2016 [26]. Genotype 10 was described by a team of researchers from Thailand and South Korea [27] in 2016.
The objective of the current research was to study the scientific and methodological approaches to the genetic identification of BLV integrated into the molecular monitoring of infection of cattle herds with BLV genotypes. The following tasks were set:
- to establish the genotypes of BLV isolates in Tearstain cattle;
- to define the types of BLV isolates with deciphered nucleotide sequences of the env gene fragment, depending on the chosen gene identification strategy;
- to improve the strategy of PCR-RFLP-genotyping of BLV and make it consistent with the modern phylogenetic BLV classification.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
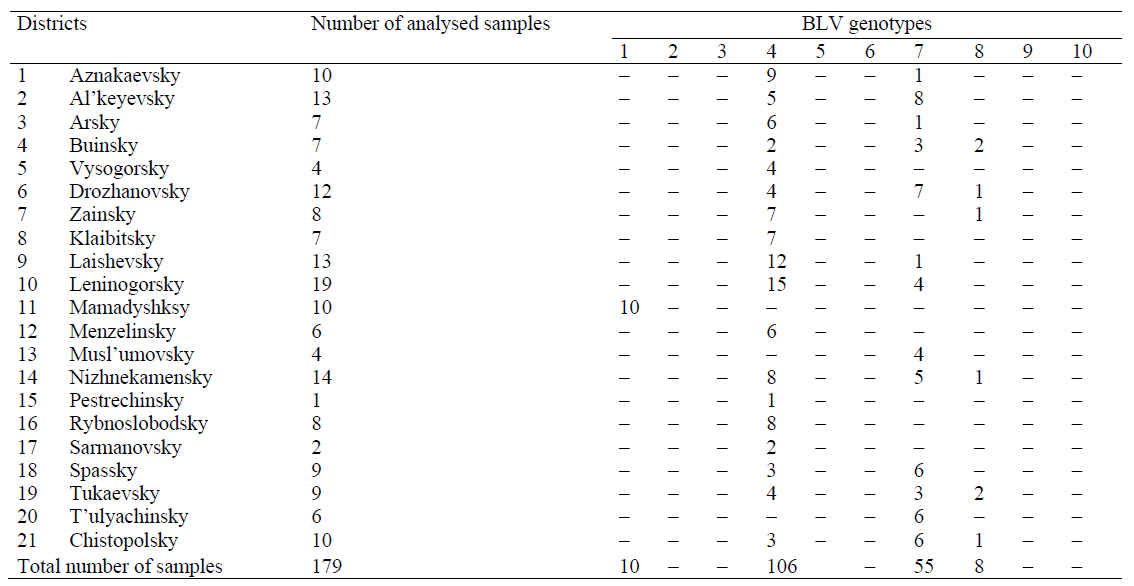
The research involved a total of 179 blood samples from AGID-positive cows. The samples were provided by agricultural enterprises from 21 districts of the Republic of Tatarstan. The samples were genetically examined for BLV. The examination included a phylogenetic analysis of sequenced env gene fragment of the pathogen and a PCR-RFLP-genotyping consistent with the phylogenetic classification of the infectious agent.
To extract DNA from the whole conserved blood, we used a commercial PCR diagnostic kit, ‘DNA-Sorb B’, produced by the Central Research Institute of Epidemiology of the Federal Supervisory Service for Consumer Rights and Human Welfare, Ministry of Health of the Russian Federation.
Nested PCR with extracted samples of BLV proviral DNA was performed with external (env5032 and env5608) and internal (env5099 and env5521) primers initiating the generation of env gene 444 bp fragment of causative agent at the final stage of reaction [28].
Restriction endonucleases used in PCR-RFLP- genotyping of BLV were consistent with its phylogenetic classification: BstYI (isoshizomer BstX2I), HphI (isoshizomer AsuHPI), HaeIII, PvuII, SspI. The NEBcutter v.2.0 web resource was used for PCR-RFLP modelling.
For the detection of the obtained results of PCR and PCR-RFLP analysis, 2.5% agarose gel horizontal electrophoresis was applied with a TBE buffer (pH 8.0) containing ethidium bromide. The electrophoregrammes were examined in a UV-transilluminator (λ = 310 nm). The sizes of the generated DNA fragments were compared with standard DNA molecular weight markers (SibEnzyme Ltd, Russia).
Sequencing of the PCR amplification products of the env gene fragment of detected BLV provirus isolates was performed on ABI PRISM 3100 Genetic Analyser (Applied Biosystems, USA) in the laboratory of Scientific and Technical Complex Sintol (Russia). Internal oligonucleotide primers env5099 and env5521 were used as sequencing. The sequenced fragments of the env gene of BLV provirus isolates were aligned with the corresponding nucleotide sequences of the reference BLV isolates from GenBank with the help of BLAST and MEGA-4 programmes. The last stage included a phylogenetic analysis.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
The study featured a PCR-RFLP-genotyping and a comparative phylogenetic analysis of the aligned sequences of the env gene fragment of BLV provirus isolates from 21 districts of the Republic of Tatarstan.
As a result, out of 179 identified isolates, ten isolates belonged to genotype 1; 106 isolates belonged to the cluster of genotype 4; 55 were characterized as genotype 7, and the remaining eight provirus isolates belonged to genotype 8 (Table 1).
According to the results obtained by PCR-RFLP- genotyping and phylogenetic analysis of sequenced env gene fragment, there are four out of ten currently known BLV genotypes in Tatarstan: 1, 4, 7, and 8.
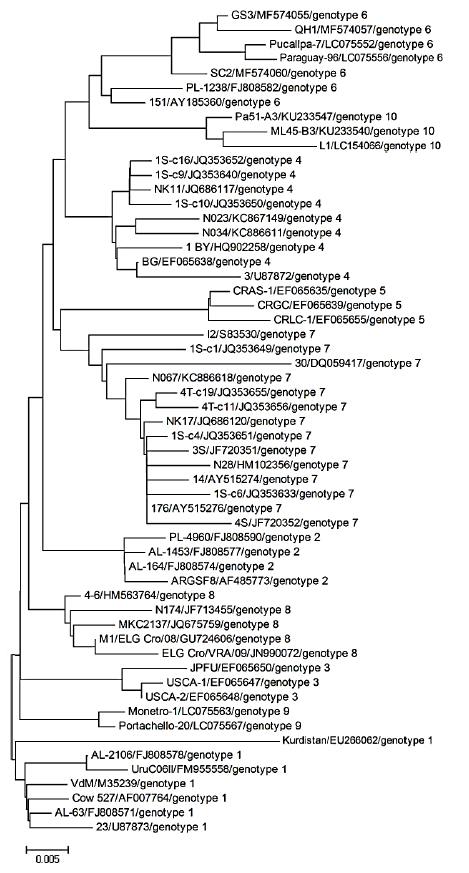
Fig. 1 shows the genotypes of BLV isolated with the help of phylogenetic analysis of nucleotide sequences of env gene fragment.
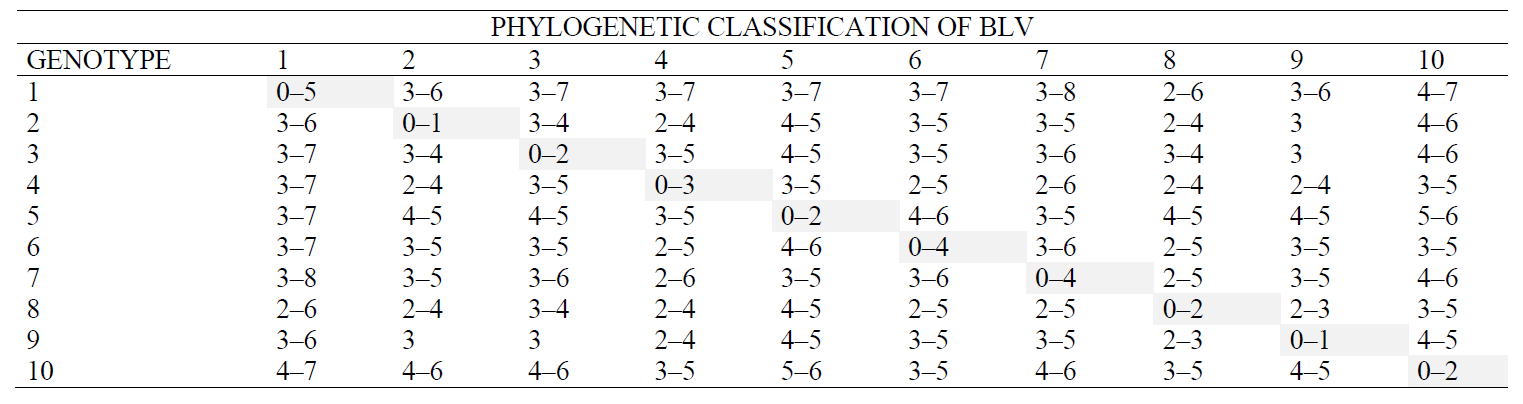
An additional assessment of the heterogeneity of the reference BLV representatives for the env gene included an analysis of the intra- and intergenotypic heterogeneity of genotypes. The data in Table 2 indicate that it is impossible to use the ‘heterogeneous’ criterion for assessing the genetic diversity of BLV.
As part of the next task, BLV isolates with the decoded nucleotide sequences of the env gene fragment were identified according to the chosen genetic identification strategy. The degree of consistency of genotypic approaches was assessed by comparing the data of the in silico PCR-RFLP and the phylogenetic analyses.
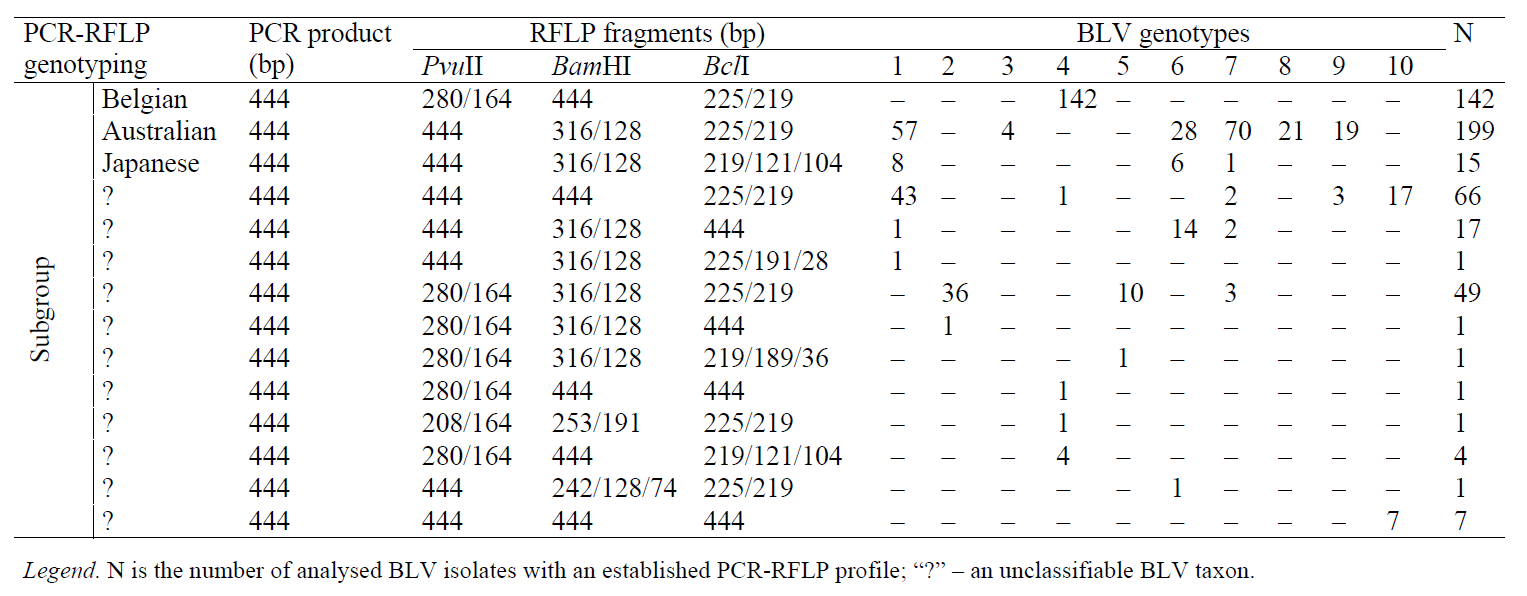
A comparative analysis of 505 nucleotide sequences of the BLV env gene locus, including those deposited with GenBank NCBI, confirms the inconsistency of a number of earlier PCR-RFLP typing strategies [28–30] with the current approach in assessing the genotypic diversity by means of phylogenetic analysis.
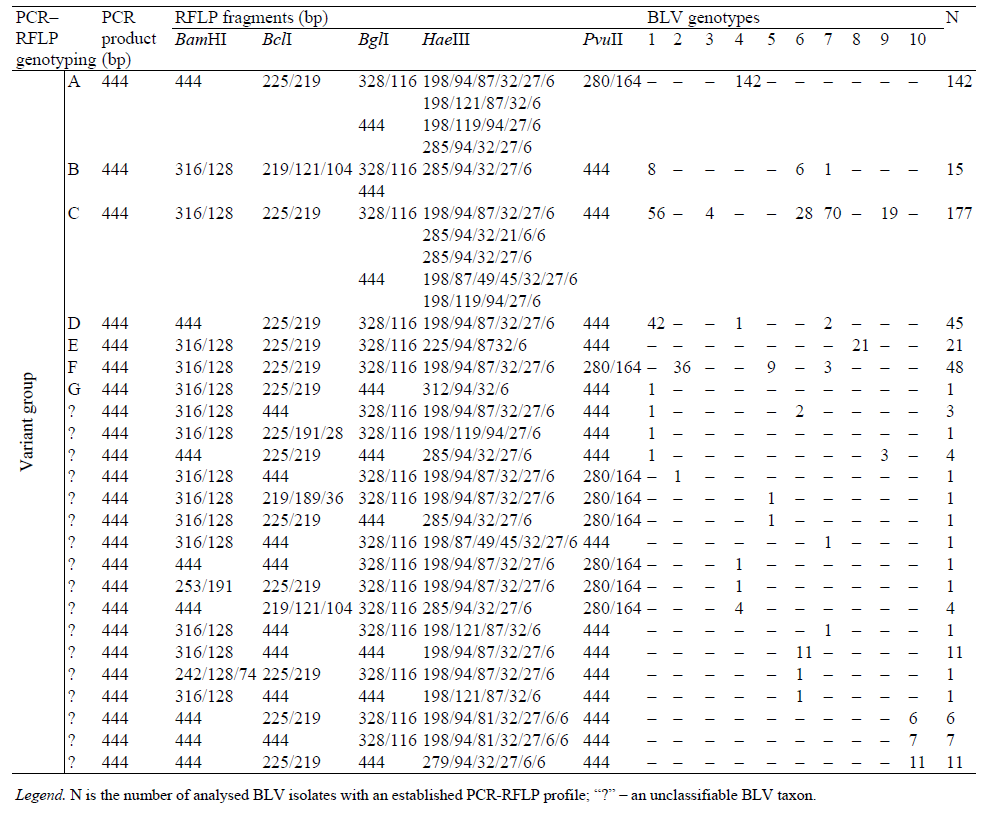
Thus, the BLV isolates that belong to the Belgian subgroup according to D. Beier et al. (2001) [28], belong to genotype 4 according to the phylogenetic classification; Australian subgroup can be referred to genotypes 1, 3, 6, 8, or 9; Japanese subgroup – to genotypes 1, 6, or 7. In addition, the genotyping strategy [28] includes 11 additional unique combinations of PCR-RFLP profiles, conditionally identical to 11 unclassifiable BLV subgroups (Table 3).
Besides, BLV isolates that belong to genotype 1 and 7 according to the phylogenetic analysis, can be referred to Australian and Japanese subgroups, as well as to three unclassifiable subgroups, according to the strategy of D. Beier et al. (2001) [28]; genotypes 2, 5, and 10 belong to two unclassifiable subgroups; genotypes 3 and 8 – to Australian subgroup; genotype 4 – to the Belgian subgroup and four unclassifiable subgroups; the genotype 5 – to two unclassifiable subgroups; genotype 6 – to the Australian, Japanese and two unclassifiable subgroups; genotype 9 – to the Australian and one unclassifiable subset (Table 3).
BLV isolates that were genotyped according to M. Licursi et al. (2002) [29] as genotype 1 may belong to genotypes 1, 4, 6, or 7 according to the phylogenetic classification; genotype 3 – to genotypes 1, 6, or 7; genotype 5 – to genotypes 1, 3, 6, 7, or 9; genotype 6 – to genotypes 2, 4, 5, or 7 (Table 4).
For the genotyping strategy described in [29], there are 19 unique combinations of PCR-RFLP profiles that are conditionally identical to 19 unclassifiable BLV genotypes (Table 4).
Besides, BLV isolates that are genotyped according to phylogenetic analysis as genotype 1 may refer to 1, 3, 5, and three unclassifiable BLV genotypes according to the strategy of M. Licursi et al. (2002) [29]; genotype 2 belongs to genotype 6 and two unclassifiable genotypes; the genotype 3 – to genotype 5 and one unclassifiable genotype; genotype 4 – to genotypes 1 and 6 and five unclassifiable genotypes; genotype 5 – to genotype 6 and two unclassifiable genotypes; genotype 6 – to genotypes 1, 3, and 5 and three unclassifiable genotypes; genotype 7 – to genotypes 1, 3, and 6 and four unclassifiable genotypes; genotype 8 – to one unclassifiable genotype; genotype 9 – to genotype 5; genotype 10 – to three unclassifiable genotypes (Table 4).
It should be mentioned that, when analyzing in silico PCR-RFLP data from 505 BLV representatives, we found not a single nucleotide sequence of the env gene fragment that would belong to genotypes 2 and 4 according to M. Licursi et al. (2002) (Table 4). This fact did not make it possible to prove the actual existence of PCR-RFLP profiles indicated for these two BLV genotypes.
Table 5 compares the in silico data of PCR-RFLP according to the strategy by H. Fechner et al. (1997) [30] and the phylogenetic analysis of a fragment of the BLV env gene.
Thus, the BLV isolates identified according to H. Fechner et al. (1997) [30] as variant group A belong to genotype 4 according to the phylogenetic classification; variant group B – to genotypes 1, 6, or 7; variant group C – to genotypes 1, 3, 6, 7, or 9; variant group D – to genotypes 1, 4, or 7; variant group F – to genotypes 2, 5, or 7; variant group G – to genotype 1 (Table 5).
For this typing strategy [30], there are 17 unique combinations of PCR-RFLP profiles that are conditionally identical to 17 unclassifiable variant BLV groups (Table 5). Besides, BLV isolates genotyped by phylogenetic analysis as genotype 1 are characterized as variant groups B, C, D, G and three unclassifiable variant groups of BLV according to the strategy of H. Fechner et al. (1997) [30]; the genotype 2 belongs to variant group F and one unclassifiable variant group; genotype 3 – to variant group C; genotype 4 – to variant groups A, D and three unclassifiable variant groups; genotype 5 – to variant group F and two unclassifiable variant groups; genotype 6 – to variant groups B and C and four unclassifiable variant groups; genotype 7 – to variant groups B, C, D, F and two unclassifiable variant groups; genotype 8 – to variant group E; genotype 9 – to variant group C and one unclassifiable variant group; genotype 10 – to three unclassifiable variant groups of BLV (Table 5).
The priority task of the research was to improve the strategy of PCR-RFLP genotyping of BLV by making it consistent with the phylogenetic classification and taking into account the update information on the genetic diversity of the ten known BLV genotypes.
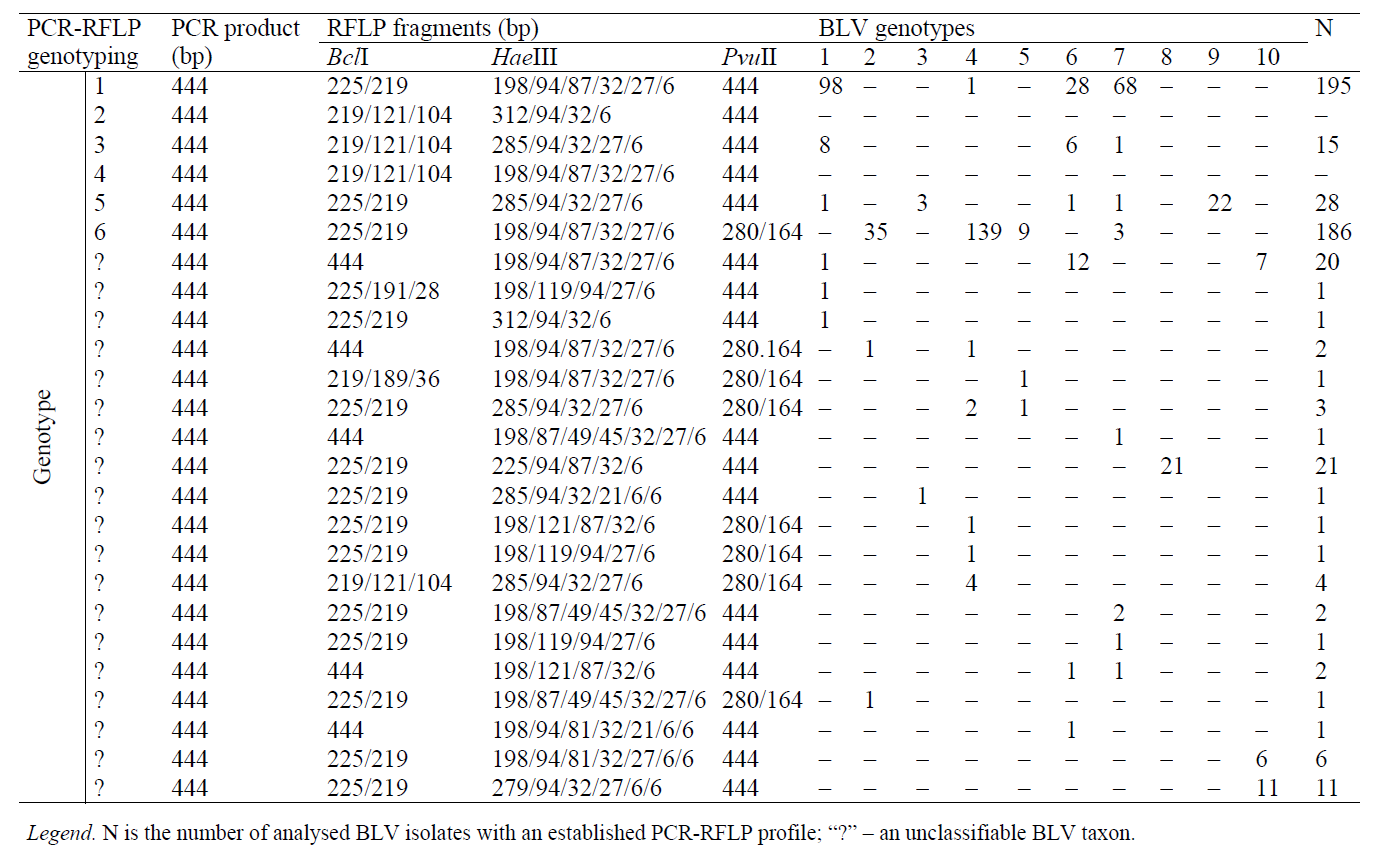
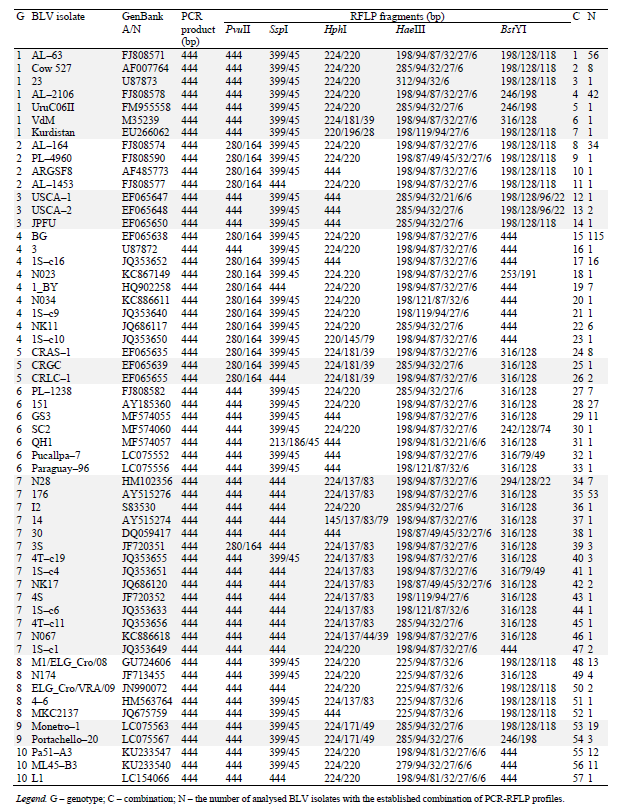
505 BLV isolates were generated during the analysis of restriction mappings of the env gene locus according to 5 restriction enzymes. The interpretation of their env-PCR-RFLP profiles actually reflects the strategy of PCR-RFLP genotyping of BLV in accordance with its phylogenetic classification. The data are represented in Table 6.
The PCR-RFLP-genotyping strategy of BLV, which we have improved, is consistent with its phylogenetic classification. The new classification makes it possible to identify all the ten currently known genotypes of the viral pathogen (Table 6).
It should be noted that genotype 1 is characterized by seven combinations of env-PCR-RFLP profiles (C 1-7), genotype 2 – by four combinations (C 8-11); genotype 3 – by three combinations (C 12-14); genotype 4 – by nine combinations (C15-23); genotype 5 – by three combinations (C 24-26); genotype 6 – by seven combinations (C27-33), genotype 7 – by fourteen combinations (C 34-47); genotype 8 – by five combinations (C 48-52); genotype 9 – by two combinations (C 53-54); genotype 10 – by 3 three combinations (C 55-57) (Table 6).
It should be emphasized that genotypes 8 and 9 can be easily identified even with the use of one restrictase, HaeIII, generating RFLP fragments (225/94/87/32/6 bp) that are characteristic of genotype 8; HphI – generating RFLP fragments (224/171/49 bp) that are characteristic of genotype 9. Representatives of genotypes 2 (BstYI and PvuII), 3 (HaeIII and HphI), and 5 (HphI and PvuII) can be identified with two restriction enzymes (Table 6).
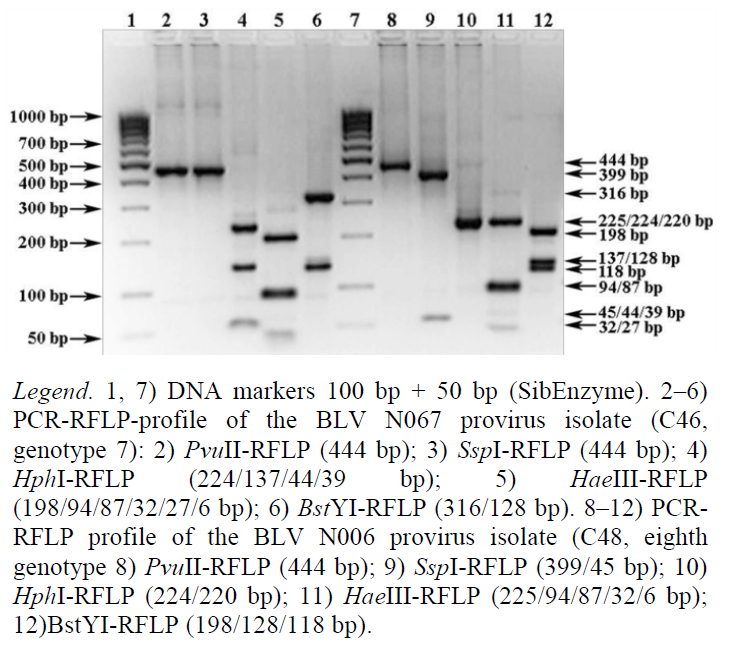
Figs. 2–4 show illustrative examples of the implementation of the strategy of BLV PCR-RFLP- genotyping in accordance with its phylogenetic classification.
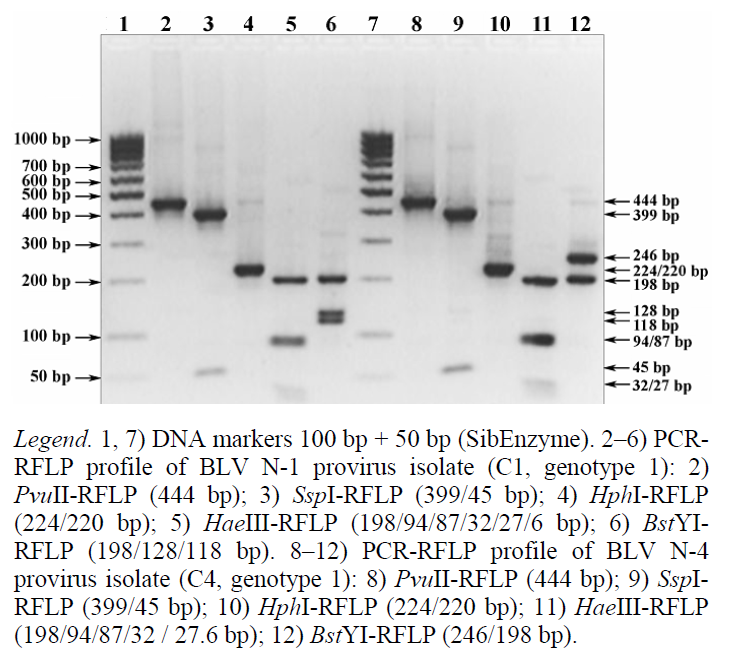
As one can see from the electrophoregramme in Fig. 2 (tracks 2–6), the PCR-RFLP profile of the BLV N-1 provirus isolate is identified as combination 1 (C1) of the env-PCR-RFLP profile of genotype 1, which includes at least 56 isolates deposited in the GenBank NCBI (Table 6).
The PCR-RFLP profile of the BLV N-4 provirus isolate (Fig. 2, tracks 8-12) characterizes combination 4 (C4) of the env-PCR-RFLP profile of genotype 1, with at least 42 identified representatives (Table 6).
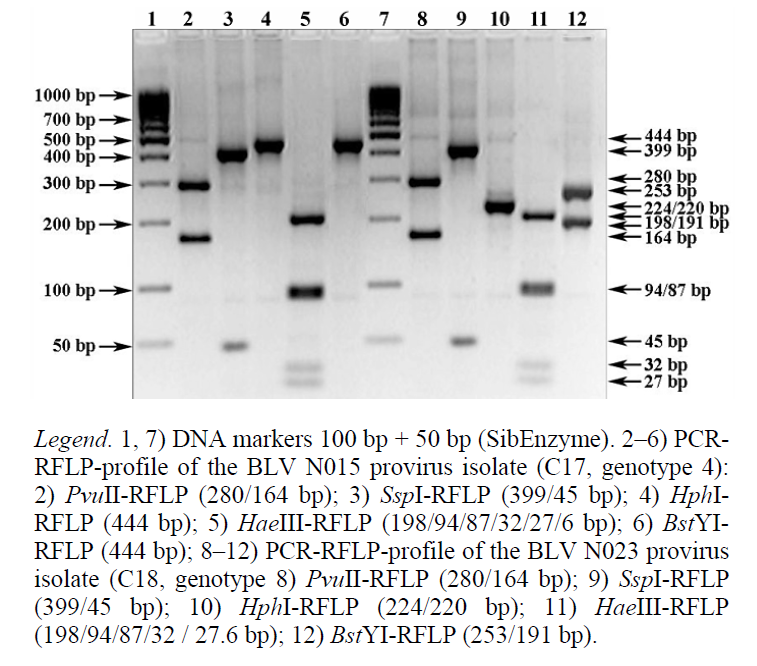
The PCR-RFLP profile of the BLV N015 provirus isolate (Fig. 3, tracks 2–6) is identified as combination 17 (C17) of the env-PCR-RFLP profile of genotype 4. It includes at least 16 representatives (Table 6), two of which affect cattle in Tatarstan. According to GenBank NCBI, these nucleotide sequences of the env gene fragment are isolate N015 (GenBank A/N: KC867143) and isolate N062 (GenBank A/N: KC886615).
The PCR-RFLP profile of the BLV N023 provirus isolate (Fig. 3, tracks 8-12) is identified as combination 18 (C18) of the env-PCR-RFLP profile of genotype 4 (Table 6). Its nucleotide sequence of the env gene fragment from the GenBank NCBI is the only variant for the given combination (isolate N023, GenBank A/N: KC867149).
The PCR-RFLP profile of the BLV N067 provirus isolate (Fig. 4, tracks 2–6) characterizes itself as combination 46 (C46) of the env-PCR-RFLP profile of genotype 7 (Table 6). Its nucleotide sequence of the env gene fragment from GenBank NCBI is the only one for this combination (isolate N067, GenBank A/N: KC886618).
The PCR-RFLP profile of the BLV N006 provirus isolate (Fig. 4, tracks 8–12) belongs to combination 48 (C48) of the env-PCR-RFLP profile of genotype 8. It includes at least 13 representatives (Table 6), three of which affect cattle populations in Tatarstan. According to GenBank NCBI, their nucleotide sequences of the env gene fragment are isolate N063 (GenBank A/N: KC886616), isolate N006 (GenBank A/N: KC867140), and isolate N089 (GenBank A/N: KC886624).
The improved strategy of PCR-RFLP-genotyping corresponds with the modern phylogenetic classification of BLV and makes it possible to identify all its known genotypes. Its accuracy is based upon in silico modelling of restrictogrammes and the phylogenetic analysis of the env gene fragment of 57 reference isolates of the ten known BLV genes (Fig. 5). They produce 57 diagnostically significant genotype- associated combinations of PCR-RFLP profiles.
ВЫВОДЫ
To determine the genotypes of BLV isolates obtained from Tatarstani cattle, we performed a phylogenetic analysis of the env gene fragment sequences and a PCR-RFLP analysis that corresponded with the phylogenetic classification of the infectious agent. The genotypic composition of 179 identified BLV isolates detected in cattle from livestock farms of 21 districts of the Republic of Tatarstan was represented by genotypes 1 (10 isolates), 4 (106 isolates), 7 (55 isolates), and 8 (8 isolates). Thus, we state the fact that four out of ten currently known BLV genotypes circulate on the territory of the Republic of Tatarstan, namely genotypes 1, 4, 7, and 8.
After that, we classified the BLV isolates with decoded nucleotide sequences of the env gene locus according to the chosen genetic identification strategy. Subsequently, we assessed the degree of consistency of genotypic approaches by comparing in silico PCR- RFLP data and the results of the phylogenetic analysis. We used 505 corresponding sequences, including those deposited in GenBank NCBI. As a result, we managed to prove that a number of previously used PCR-RFLP typing strategies were inconsistent with the current approach in assessing the genotypic diversity of BLV with the help of the phylogenetic analysis. The inconsistency of the three PCR-RFLP strategies for BLV typing with the modern phylogenetic classification is associated, among other things, with the on-going knowledge acquisition in the sphere of the genetic diversity of the ten known BLV genotypes.
During the final stage of the research, we improved the strategy of PCR-RFLP-genotyping of BLV to make it consistent with the phylogenetic classification. The new version takes into account the new data about the genetic diversity of BLV. It also includes an interpretation of the PCR-RFLP profiles of 505 BLV isolates. The interpretation resulted from a restriction mapping of the env gene fragment according to 5 restriction endonucleases. The improved strategy of PCR-RFLP-genotyping allows one to identify all the currently known BLV genotypes. The improved strategy owes its accuracy to in silico modelling of restrictogrammes and the phylogenetic analysis of the env gene of 57 reference isolates of ten BLV genes that generate 57 diagnostically significant genotype- associated combinations of PCR-RFLP profiles
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare that there are no conflict of interest related to this article.
ФИНАНСИРОВАНИЕ
The research was supported by the section of storage and processing of agricultural products of the Department of Agricultural Sciences (Russian Academy of Sciences) and V.M. Gorbatov Federal Research Center for Food Systems.
СПИСОК ЛИТЕРАТУРЫ
- Mishchenko V.A., Petrova O.N., Karaulov A.K., and Mishchenko A.V. Problema leykoza krupnogo rogatogo skota [The issues of bovine leukaemia]. Vladimir: FGBI ARRIAH Publ., 2018. 38 p. (In Russ.).
- Donnik I.M., Petropavlovsky M.V., Krivonogova A.S., et al. Revisiting the issue of the molecular–genetic structure of the causative agent of the bovine leukemia virus in the Russian Federation. Indian Journal of Science and Technology, 2016, vol. 9, no. 42. DOI: https://doi.org/10.17485/ijst/2016/v9i42/104253.
- Olaya–Galán N.N., Corredor–Figueroa A.P., Guzmán–Garzón T.C., et al. Bovine leukaemia virus DNA in fresh milk and raw beef for human consumption. Epidemiology & Infection, 2017, vol. 145, no. 15, pp. 3125–3130. DOI: https://doi.org/10.1017/S0950268817002229.
- Villalobos–Cortes A. Enzootic bovine leukosis and the risk to human health. African Journal of Biotechnology, 2017, vol. 16, no. 15, pp. 763–770. DOI: https://doi.org/10.5897/AJB2016.15736.
- Alabbody H.H.K. Bovine leukosis and the possibility to cause cancer in humans: A scientific review. Iraqi Journal of Veterinary Medicine, 2018, vol. 42, no. 1, pp. 52–60.
- Reichert M., Grundboeck–Jusko J., Rulka J., Stec J., and Kozaczynski W. Influence of selected technological treatments on the BLV provirus DNA occurring in the tissues of leukaemic cattle. Bulletin of the Veterinary Institute in Pulawy, 1994, vol. 38, no. 2, pp. 52–60.
- Sviridenko G.M. and Semova E.G. Leucose in the cattle and safety of milk products. Dairy industry, 2003, no. 7, pp. 8–10. (In Russ.).
- Mesa G., Ulloa J.C., Uribe A.M., and Gutierrez M.F. Bovine Leukemia Virus Gene Segment Detected in Human Breast Tissue. Open Journal of Medical Microbiology, 2013, vol. 3, no. 1, pp. 84–90. DOI: https://doi.org/10.4236/ojmm.2013.31013.
- Buehring G.C., Shen H.M., Jensen H.M., et al. Bovine Leukemia Virus DNA in Human Breast Tissue. Emerging Infectious Diseases, 2014, vol. 20, no. 5, pp. 772–782. DOI: https://doi.org/10.3201/eid2005.131298.
- Buehring G.C., Shen H.M., Jensen H.M., et al. Exposure to Bovine Leukemia Virus Is Associated with Breast Cancer: A Case–Control Study. PLoS One, 2015, vol. 10, no. 9. DOI: https://doi.org/10.1371/journal.pone.0134304.
- Buehring G.C., Shen H., Schwartz D.A., and Lawson J.S. Bovine leukemia virus linked to breast cancer in Australian women and identified before breast cancer development. PLoS One, 2017, vol. 12, no. 6. DOI: https://doi.org/10.1371/journal.pone.0179367.
- Khazipov N.Z., Vafin R.R., Shaeva A.Yu., and Zaynullin L.I. Transformation of cells under the influence of bovine leukemia virus is a real risk of developing the oncological diseases in humans. Modern problems of science and education, 2013, no. 6. Available at: http://www.science–education.ru/ru/article/view?id=11792. (accessed 27 July 2018). (In Russ.).
- Petrov A.N., Galstyan A.G., Radaeva I.A., et al. Indicators of quality of canned milk: Russian and international priorities. Foods and Raw Materials, 2017, vol. 5, no. 2, pp. 151–161. DOI: https://doi.org/10.21179/2308–4057–2017–2–151–161.
- Petrov A.N., Khanferyan R.A., and Galstyan A.G. Current aspects of counteraction of foodstuff's falsification. Problems of Nutrition, 2016, vol. 85, no. 5, pp. 86–92. (In Russ.).
- Galstyan A.G., Radaeva I.A., Chervetsov V.V., et al. Improvement of the canned milk products quality due to the application of the pasteurized raw milk. Dairy industry, 2015, no. 5, pp. 42–44. (In Russ.).
- Kuznetsova T.V., Kuznetsov A.A., and Kirillova S.V. Tekhnicheskiy reglamennt TS «O bezopasnosti moloka i molochnoy produktsii» i ehkonomicheskie aspekty ego realizatsii molochnymi tovaroproizvoditelyami Rossii [On safety of milk and dairy products’ and the economic aspects of its implementation by Russian dairy producers]. Agro food policy in Russia, 2015, vol. 48, no. 12, pp. 35–38. (In Russ.).
- Lysov A., Petropavlovskiy M., Donnik I., and Krivonogova A. The system of individual veterinary and zootechnie activities for healthy improvement from BLV of cattle on the example of Tyumen region. Issues of Legal Regulation in Veterinary Medicine, 2017, no. 3, pp. 40–43. (In Russ.).
- Vinogradova I.V., Gladyrʹ E.A., Kovalyuk N.V., et al. Izuchenie genotipicheskogo raznoobraziya, molekulyarno–geneticheskoy struktury i filogeneticheskiy analiz vozbuditelya leykoza krupnogo rogatogo skota, tsirkuliruyushchego v populyatsii zhivotnykh iz raznykh regionov Rossii [A study of the genotypic diversity, molecular–genetic structure and a phylogenetic analysis of the causative agent of bovine leukaemia in cattle from different regions of Russia]. Ekaterinburg: Ural State Agrarian University Publ., 2014. pp. 3–18. (In Russ.).
- Vafin R.R., Khazipov N.Z., Shaeva A.Y., et al. Genotypic identification of the bovine leukemia virus. Molecular Genetics, Microbiology and Virology, 2014, vol. 29, no. 4, pp. 195–203. DOI: https://doi.org/10.3103/S0891416814040120.
- Rodriguez S.M., Golemba M.D., Campos R.H., Trono K., and Jones L.R. Bovine leukemia virus can be classified into seven genotypes: evidence for the existence of two novel clades. Journal of General Virology, 2009, vol. 90, no. 11, pp. 2788–2797. DOI: https://doi.org/10.1099/vir.0.011791–0.
- Shaeva A.Y., Vafin R.R., Khazipov N.Z., et al. Genotypic identification of BLV isolates detected in livestock farms of the republic of Tatarstan. Uchenye zapiski Kazanskoy gosudarstvennoy akademii veterinarnoy meditsiny im. N.EH. Baumana [Scientific notes of the Kazan State Academy of Veterinary Medicine. N.E. Bauman], 2011, vol. 208, pp. 330–337. (In Russ.).
- Shaeva A.Y., Garaeva Z.R., Vafin R.R., Khazipov N.Z., and Alimov A.M. Identification of a new BLV genotype. Uchenye zapiski Kazanskoy gosudarstvennoy akademii veterinarnoy meditsiny im. N.EH. Baumana [Scientific notes of the Kazan State Academy of Veterinary Medicine. N.E. Bauman], 2012, vol. 211, pp. 192–197. (In Russ.).
- Vafin R.R., Khazipov N.Z., Shaeva A.Y., et al. Finding a new (8th) genotype of BLV in various regions of the world. Fundamental research, 2013, no. 10, part 7, pp. 1467–1471. (In Russ.).
- Balić D., Lojkić I., Periškić M., et al. Identification of a new genotype of bovine leukemia virus. Archives of Virology, 2012, vol. 157, no. 7, pp. 1281–1290. DOI: https://doi.org/10.1007/s00705–012–1300–4.
- Rola–Łuszczak M., Pluta A., Olech M., et al. The molecular characterization of bovine leukaemia virus isolates from Eastern Europe and Siberia and its impact on phylogeny. PLoS One, 2013, vol. 8, no. 3. DOI: https://doi.org/10.1371/journal.pone.0058705.
- Polat M., Takeshima S.N., Hosomichi K., et al. A new genotype of bovine leukemia virus in South America identified by NGS–based whole genome sequencing and molecular evolutionary genetic analysis. Retrovirology. 2016, vol. 13, no. 4. DOI: https://doi.org/10.1186/s12977–016–0239–z.
- Lee E., Kim E.J., Ratthanophart J., et al. Molecular epidemiological and serological studies of bovine leukemia virus (BLV) infection in Thailand cattle. Infection, Genetics and Evolution, 2016, vol. 41, no. 245–254. DOI: https://doi.org/10.1016/j.meegid.2016.04.010.
- Beier D., Blankenstein P., Marquardt O., and Kuzmak J. Identification of different BLV provirus isolates by PCR, RFLPA and DNA sequencing. Berliner und Munchener Tierarztliche Wochenschrift, 2001, vol. 114, no. 7–8, pp. 252–256.
- Licursi M., Inoshima Y., Wu D., et al. Genetic heterogeneity among bovine leukemia virus genotypes and its relation to humoral responses in hosts. Virus Research, 2002, vol. 86, no. 1–2, pp. 101–110.
- Fechner H., Blankenstein P., Looman A.C., et al. Provirus Variants of The Bovine Leukemia Virus and Their Relation to the Serological Status of Naturally Infected Cattle. Virology, 1997, vol. 237, no. 2, pp. 261–269. DOI: https://doi.org/10.1006/viro.1997.8784.