Аннотация
Oral non-tobacco nicotine products have gained enormous popularity in recent years. The countries of the Eurasian Economic Union also produce and sell this type of innovative but poorly studied goods. As a result, the safety profile and quality of such products as nicotine poaches require urgent comprehensive research. This study featured the changes in quality of nicotine poaches during storage, i.e. nicotine content, water activity, and microbiological index.The research featured nicotine poaches of several popular brands. The authors used standard research methods; the experiments were performed in the laboratory for chemistry and quality control, Institute of Tobacco, Makhorka, and Tobacco Products, Krasnodar, and at the Department of Bioorganic Chemistry and Technical Microbiology, Kuban State Technological University, Krasnodar.
The water activity was 0.8911–0.9502 Aw at the initial stage and remained stable in most samples even after six months of storage. Velo Freeze was the only brand to show significant variations in water activity. The nicotine content was 10.115–12.950 mg/g at the initial stage. Only four samples maintained the initial values after six months of storage. The fluctuations of nicotine content were also mentioned by the manufacturer. The microbiological profile remained stable during the six months of storage and met the requirements for similar products, i.e., chewing gum and unglazed caramel.
The project needs further research because the qualitative characteristics of nicotine poaches provided rather unambiguous results. Our study will help develop state standards for oral nicotine products. The results obtained will be used to formulate proposals to the organizations responsible for the future Technical Regulations of the Eurasian Economic Union for nicotine products.
Ключевые слова
Oral non-tobacco nicotine products, smokeless tobacco, water activity, nicotine, snus, safetyВВЕДЕНИЕ
Oral tobacco-free nicotine products have gained global popularity in recent years, and the Eurasian Economic Union is no exception.
The government of Sweden subjected the production and use of snus to the Food Act. A special technical specification controls its quality and safety. Sweden developed a special standard for oral non-tobacco nicotine products. In the late 1990s, this standard was used to develop a voluntary quality standard for Swedish snus called GothiaTek®. It is GothiaTek® that was adopted by the trade organization of the European Smokeless Tobacco Council (ESTOC). Eventually, it became the industry standard for all smokeless tobacco products in Europe [1].Sweden, Russia offers domestic consumers both tobacco and non-tobacco oral nicotine products.
Currently, Armenia’s Ministry of Economy is developing Technical Regulations for nicotine products for all Eurasian Economic Union counties. The draft regulations establish safety requirements for heated tobacco products, electronic nicotine delivery liquids, and oral non-tobacco nicotine products, e.g., nicotine poaches.
All such products, except nicotine poaches, are consumed as aerosol, which appears when the substance is heated. Nicotine poaches, however, are consumed orally, which means that the safety of this type of nicotine-containing products should be strictly controlled.
Oral non-tobacco nicotine products have an obvious advantage over conventional smoking: its consumption is individual and produces no tobacco smoke with its harmful effect on the so-called passive smokers.
In Russia, technical regulations for tobacco products can be found in Federal Law No. 268-FZ (December 22, 2008). However, the law standardizes neither nicotine content nor microbiological indicators, and such approach poses certain health risks for consumers.
Although the demand for these products keeps growing, consumers receive very little information about the properties, quality profile, and quantitative characteristics of oral nicotine products, not to mention their potential adverse impact on human health. In the absence of state standard, these products remain beyond the scope of any regulatory documents.
However, some domestic studies have been going on in this sphere; for example, Duruncha et al. developed a method for determining the mass fraction of nicotine in oral non-tobacco nicotine products [2].
What is even more important, the early years of nicotine poaches on the Russian market saw some comprehensive studies of nicotine content. Pankov et al. commented upon the absence of standards for nicotine poaches that were visioned to replace traditional cigarettes [3]. The authors reported that the nicotine content in nicotine poaches varied from 29.22 to 62.83 mg/g, and high nicotine content could harm consumer’s health.
Other early publications indicated that the lacking governmental regulation led to illegal homemade production of oral nicotine products [4]. This research again mentioned the major fluctuations in nicotine content in these products, which, in turn, could pose potential harm to consumers.
As a result, the qualitative characteristics and safety indicators of oral nicotine products are a prospective and relevant research area. Our study featured the quality profile of nicotine poaches during storage: nicotine content, water activity, and microbiological indicators. Our conclusions could be of great use to the future quality and safety standard of oral nicotine products.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Samples of nicotine poaches were provided by British American Tobacco, Belarus (Figs. 1–4). Other experimental materials of four brands came from BAT Pécsi Dohánygyár (2–8 Dohany St., Pecs, 7622, Hungary) (Table 1).
The research was conducted in the laboratory of chemistry and quality control, the All-Russian Research Institute of Tobacco, Makhorka, and Tobacco Products, Krasnodar, and included the following methods:
– the mass fraction of nicotine in oral non-tobacco nicotine products was measured using the method of gas chromatography, certificate No. 025-01.00281-2013-2020;
– another method of nicotine determination followed CORESTA CRM 62: Determination of Nicotine in Tobacco and Tobacco Products by Gas Chromatographic Analysis;
– the water activity was studied based on CORESTA CRM 88: Determination of Water Activity of Tobacco and Tobacco Products using an Aqualab TDL2 device with a tunable diode laser;
– the moisture content test relied on a 3-h standard method with a drying oven, where the samples were dried at 95°C for 3 h, as in State Standard 3935-2000.
The microbial tests were conducted at the Department of Bioorganic Chemistry and Technical Microbiology, Kuban State Technical University, Krasnodar, and involved the following guidelines: State Standard 10 444.15-94, State Standard 31747-2012, and State Standard 10 444.12-2013.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
We needed three stages to study the safety factors of oral nicotine products.
The first stage involved such variables as sample weight, filling volume, and type (encased/not encased), as well as their effect on water activity. These experiments included an Aqualab TDL2 device.
The sample weight and filling volume did not affect the water activity, provided that the sample stayed below the internal upper mark of the Aqualab bowl.
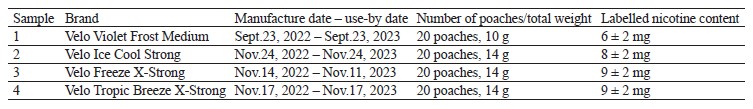
The second stage featured environmental variables, i.e., natural conditions, air-conditioning, and refrigeration chamber, as well as their impact on the water activity and nicotine content in four different samples of nicotine poaches, i.e., Velo Violet Frost, Velo Ice Cool, Velo Freeze, and Velo Tropic Breeze, after three and six months of storage (Table 2).
Table 2 shows that seven samples out of twelve (No. 1, 2, 4, 8, 9, 10, and 12) maintained stable water activity after three months of storage. Under different environmental conditions, these samples demonstrated insignificant fluctuations (≤ 0.012) in water activity indicator.
Seven samples (No. 1, 2, 4, 5, 8, 9, and 12) maintained stable water activity after six months of storage: even under different environmental conditions, fluctuations did not exceed 0.012 Aw.
The Velo Freeze sample (No. 3, 7, and 11) demonstrated a gradual increase in water activity under all storage conditions. As a result, these samples received special attention in the further studies because growing water activity may indicate an early change in the microbiological status of the product. Such products needed further monitoring after nine and twelve months of storage.
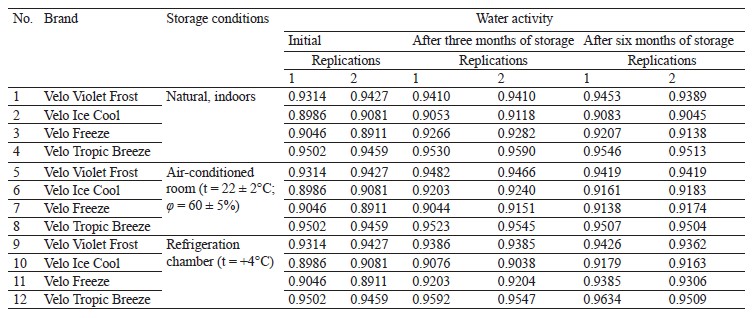
As for the nicotine content (Table 3), five samples (No. 1, 3, 4, 5, and 6) yielded stable values after three months of storage. Under different storage conditions, nicotine fluctuations were insignificant and stayed below 0.7 mg/g.
Four samples (No. 2, 5, 7, and 11) maintained the same nicotine content after six months of storage. Under different storage conditions, nicotine fluctuations were insignificant and stayed below 0.7 mg/g. Further monitoring is necessary after nine and twelve months of storage.
The third stage featured the microbiological parameters. This part of our research involved direct methods to test the samples for the presence or absence of microbiological contamination. The samples spent six months under various storage conditions.
The domestic food industry knows no microbiological standards for nicotine poaches. However, these products are similar in consumption to unglazed caramel and chewing gum, so we adopted the permissible levels of microorganisms from the microbiological safety standards stipulated by Technical Regulations of Customs Union TR CU 021/2011 (Clause 1.4: Sugar and Confectionery Products, Appendix 2: Microbiological Safety Standards) (Table 4).
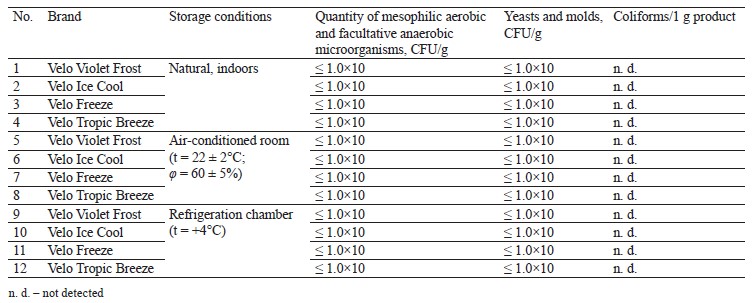
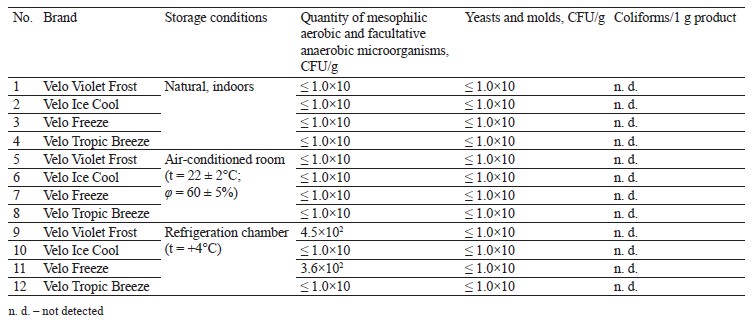
Tables 5 and 6 illustrate the results of the microbiological experiments.
Table 5 shows that all samples, regardless of storage conditions, revealed no microbiological changes after three months of storage. Table 6 shows that almost all samples, regardless of storage conditions, revealed no changes in microbiological status after six months of storage. However, we registered a slight increase in mesophilic aerobic and optionally anaerobic microorganisms after six months of refrigerated storage in the Velo Violet Frost and Velo Freeze samples.
These samples had rather high humidity (44–50%), which could potentially trigger the development of unwanted microflora. However, the microbiological indicators remained below the permissible content level of 500 CFU/g because the product formulation included preservatives.
Our results suggest that, even if water activity exceeds 0.7, oral nicotine products do not have to be banned from sale because they still comply with the permissible microbiological safety standards given in Table 4.
The All-Russian Research Institute of Tobacco, Mak- horka, and Tobacco Products submitted a proposal to standardize the water activity indicator for nicotine poaches as part of the future Technical Regulations of the Eurasian Economic Union for nicotine-containing products.
The technical regulation draft also establishes that the nicotine mass fraction in a nicotine poach is not to exceed 11 mg per product.
Microbiological monitoring should be repeated after nine and twelve months of storage, taking into account the expiry date and water activity dynamics.
ВЫВОДЫ
This research obtained important experimental data on the effect of various storage conditions on nicotine poaches after three and six months of storage, e.g., their water activity indicator, nicotine content, and microbiological parameters.
Samples of Velo Violet Frost and Velo Freeze revealed slight microbiological contamination with mesophilic aerobic and facultative anaerobic microorganisms after refrigerated storage at t = +4°C, but this indicator stayed well within the permissible microbiological safety standards of 500 CFU/g.
Since the poaches were refrigerated in original sealed packaging, the increase in mesophilic aerobic and facultative anaerobic microorganisms may indicate contamination at the production stage.
Some samples demonstrated a slight increase in water activity. Minor fluctuations in nicotine content did not exceed the permissible error. However, Velo Tropic Breeze poaches stored in a refrigerator at t = +4°C showed a nicotine content of 9.550–16.236 mg/g after three months of storage and 10.287–20.818 mg/g after six months. Such fluctuations were most likely caused by mixing errors during production.
The ambiguous quality characteristics means that the research needs to be continued. Eventually, these studies will help to develop a state standard for oral nicotine tobacco-free products. Now we are preparing a number of proposals to be included in the future Technical Regulations of the Eurasian Economic Union for nicotine products.
Вклад авторов
N.A. Pankov, E.V. Gnuchikh, and V.G. Lobanov supervised the project. N.A. Pankov, A.Yu. Lushnikova, and T.V. Vanitskaya provided the research data. N.A. Pankov, E.V. Gnuchikh, and T.A. Perezhogin collected the data and wrote the manuscript.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare no conflict of interests regarding the publication of this article.
СПИСОК ЛИТЕРАТУРЫ
- Rutqvist LE, Curvall M, Hassler T, Ringberger T, Wahlberg I. Swedish snus and the GothiaTek® standard. Harm Reduction Journal. 2011;8:11 https://doi.org/10.1186/1477-7517-8-11
- Duruncha NA, Perezhogina TA, Ostapchenko IM, Kokorina LV. Elaboration method for determination mass fraction of nicotine in non tobacco nicotine containing product of oral type of consuminf. Natural and Technical Sciences. 2021;154(3):24–28. (In Russ.). https://doi.org/10.25633/ETN.2021.03.01; https://elibrary.ru/UQZVRZ
- Pankov NA, Lushnikova AYu, Gnuchikh EV. Oral non-tobacco nicotine products: nicotine content and pH. Scientific support for technological development and competitiveness in the food and processing industry: Proceedings of the III International Scientific and Practical Conference; 2023; Krasnodar. Krasnodar: V.M. Gorbatov Federal Research Center for Food Systems of RAS; 2023. p. 380–384. (In Russ.). https://elibrary.ru/FLWSIU
- Don TA, Kalashnikov SV, Mirgorodskaya AG. Research of non-smoking products for oral consumption. New Technologies. 2020;15(4):53–59. (In Russ).