Аннотация
Preventing food spoilage and prolonging its shelf life are of great importance to meet the increasing food demand. Dietary fibers in red pitahaya are known to help maintain food freshness. Lactic acid bacteria have probiotic properties and can be a good alternative to additives in food production. Therefore, we aimed to investigate the potential use of gum-based edible films containing red pitahaya extract and probiotic as a coating material in the food industry.Firstly, we determined the antimicrobial activity of red pitahaya peel and flesh extracts against pathogenic microorganisms and probiotic strains. Then, we employed the well diffusion method to determine the antimicrobial activity of the edible films containing red pitahaya extracts and Limosilactobacillus fermentum MA-7 used as a probiotic strain.
The largest inhibition zone diameters of peel and flesh extracts were 12.97 and 13.32 mm, respectively, against Candida albicans ATCC 10231. The inhibition of the growth of lactic acid bacteria was lower as the extract concentration decreased. The gum-based films with flesh extract and probiotic had the largest inhibition zone diameters of 21.63 and 21.52 mm, respectively, against Aeromonas hydrophila ATCC19570 and C. albicans ATCC 10231.
The edible films containing red pitahaya extract and L. fermentum MA-7 may have the potential to prevent spoilage caused by microorganisms in the food industry and to extend the shelf life of foods.
Ключевые слова
Hylocereus polyrhizus, pitahaya, lactic acid bacteria, guar gum, coating material, antimicrobial activity, plant extractsВВЕДЕНИЕ
Today, the growing world population is increasing the demand for high-quality food. Extending the shelf life of foods is one of the ways to meet this demand. There is a lot of current research into packaging systems that prevent food spoilage. One of them, active packaging, is a system that maintains the product’s quality and extends its shelf life through the interaction between packaging, the product, and the environment [1, 2]. This interaction has become of great importance recently due to the problem of hazardous waste and the environmental damage caused by non-biodegradable materials [3–5]. Coating processes are commonly used in various industries, such as food, agricultural, pharmaceutical, cosmetic, and textile industries. Products are generally coated for protective, decorative, or functional purposes [6, 7]. Edible film coatings, for example, have a number of advantages. They are biodegradable and therefore do not pollute the environment. They also serve as a nutritional supplement for consumers and as flavoring and dyeing agents for the product. Finally, edible film coatings exhibit antimicrobial and antioxidant properties due to essential oils, nisin, and plant extracts that they contain [8–11].
Edible film solutions can be prepared from gums of natural origin since they are inexpensive, biocompatible, non-toxic, and readily available [12]. Among natural biopolymers, guar gum is receiving a lot of attention in the field of food packaging due to its good film-forming and biological properties [13]. Guar gum is a hydrophilic nonionic macromolecule of polysaccharides with a high molecular weight. It is of low cost and has excellent biodegradability and biocompatibility [14]. Guar gum is one of the most essential thickeners and a flexible ingredient for a variety of food applications [15].
Pitahaya belongs to the genus Hylocereus of the Cactaceae family and is commonly known as the dragon fruit [16]. Fifteen years ago, the pitahaya fruit was unheard of, but today it has gained popularity in the European market and such countries as Colombia, Costa Rica, Vietnam, Mexico, the USA (Florida and California), and Nicaragua [17]. In Turkey, pitahaya is grown in the Mediterranean region, especially in Mersin, Antalya and partially in Adana [18]. Pitahaya is considered a promising fruit with antioxidant, anticancerous, and antimicrobial properties, as well as prebiotic effects [19]. Dietary fibers in red pitahaya are important for maintaining the fruit’s freshness. Therefore, red pitahaya can be potentially used to preserve food freshness [20].
Probiotics are non-pathogenic living microorganisms [21]. They can be found in various types of products such as foods, medicines, and dietary supplements [22]. Recently, probiotics have been increasingly used as a biocontrol agent in the food industry. In particular, lactic acid bacteria are excellent biocontrol agents due to their probiotic potential. Various methods have been developed to preserve the biological activities of probiotics during food processing and storage [23]. One of them is the use of edible films and coatings as potential carriers for probiotics [24]. The inclusion of probiotics in edible film solutions or coatings promotes the survival of these microorganisms [25]. This can also contribute to better food stability and safety due to the antimicrobial activity of probiotics against spoilage or pathogenic bacteria [26].
Unlike synthetic additives, new natural coating materials can inhibit the growth of pathogenic and food spoilage microorganisms without having any negative effects on health. In this regard, we aimed to study the potential use of gum-based edible films containing red pitahaya extracts and the probiotic candidate strain Limosilactobacillus fermentum MA-7 in the food industry. First, we investigated the antimicrobial activity of red pitahaya extracts against pathogenic test microorganisms. Then, the extracts were tested on the probiotic candidate strains. Finally, we determined the antagonistic effect of the film solutions prepared with red pitahaya extracts and L. fermentum MA-7 as natural biocontrol agents against pathogenic test microorganisms.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Preparation of red pitahaya methanol extracts. Red pitahaya fruits were obtained from Kumluca (Antalya, Turkey) in October 2021 (Fig. 1). Then, their flesh was separated from the peel, and they were left to dry. After grinding, the powder from red pitahaya peel and flesh (10 g) was extracted with 99.7% methanol (30 mL) in two repetitions for two days. For this, we sonicated the mixes on ice for 10 min every day using a sonication device (Hielscher, 30 kHz, 100% amplitude). The crude red pitahaya methanol extracts were stored at 4°C until used.
Microorganisms and growth conditions. Candida albicans ATCC 10231 was cultured at 30°C for 24 h in YPD (Yeast Peptone Dextrose). Aeromonas hydrophila ATCC 19570 and Listeria monocytogenes ATCC 7644 were cultured at 37°C for 24 h in NB (Nutrient Broth) and TSB (Tryptic Soy Broth). Yersinia ruckeri and Vibrio anguillarum A4 were cultured in TSB and TSB/NaCl medium at 25°C for 24 h. Limosilactobacillus fermentum MA-7, Lactobacillus gasseri MA-1, Limosilactobacillus vaginalis MA-10, and Lactobacillus delbrueckii MA-9 were cultured at 37°C in MRS (Man, Rogosa, and Sharpe) for 24 h. Streptococcus thermophilus MAS-1 was cultured at 37°C in M17 broth medium for 24 h.
Disc diffusion susceptibility test. The disc diffusion susceptibility test was used to determine the inhibitory effect of the red pitahaya peel and flesh methanol extracts against pathogenic test microorganisms and probiotic lactic acid bacteria. The prepared culture suspension (adjusted to 0.5 McFarland) was inoculated on an agar medium using the spread method and sterile discs (6 mm in diameter) were placed on the agar. The red pitahaya methanol extracts dissolved in dimethyl sulfoxide were dripped onto the sterile discs. Kanamycin (K; 30 µg/disc) and Ampicillin (AM; 10 µg/disc) antibiotic discs were used as controls for pathogenic microorganism strains, and Fluconazole (FCA; 25 µg/disc) was used for yeast. The culture dishes were incubated for 24 h at the suitable temperatures indicated previously. Then, the inhibition zone around the discs was measured using a caliper.
Micro-dilution assay. The micro-dilution assay was used to determine minimum inhibitory concentrations, as well as minimum fungicidal or bactericidal concentrations of the red pitahaya extracts. For this, the extracts were added to the growth medium and diluted by a two-fold serial dilution method to obtain a final concentration of 80–5 µg/µL. The culture suspension (0.5 McFarland) was added to each tube and then incubated under the conditions required for each microorganism as mentioned above. After incubation, the extract’s concentration in the tube without microbial growth was determined according to turbidity and the lowest concentration was recorded as a minimum inhibitory concentrations value. Minimum bactericidal or fungicidal concentrations values were determined by inoculating samples from the mixture onto an agar medium. The culture dishes were incubated at the appropriate temperature for 24 h. The lowest concentration without growth at the end of incubation was defined as minimum bactericidal or fungicidal concentrations values.
Microbial and physicochemical characterization of edible film solutions containing red pitahaya and L. fermentum MA-7. Preparation of edible film solutions. An edible film formulation was designed by modifying the methods of Kılınç et al. and Bambace et al. from commercially available guar gum, the pitahaya methanol extract, and the human milk-originated probiotic candidate strain L. fermentum MA-7 [27, 28]. This study included a control group and three different experimental test groups. The control group contained guar gum (1%, w/v) adjusted to the final volume with distilled water. The edible film formulation test groups included: guar gum (1%, w/v) with L. fermentum MA-7, guar gum (1%, w/v) with red pitahaya extract (10%, w/v), and guar gum (1%, w/v) with red pitahaya extract (10%, w/v) and L. fermentum MA-7. Glycerol (3%, w/v) was used as a plasticizer in all the groups. First, we determined the antimicrobial activity of the edible film solutions. Then, the solutions were dried in a Pasteur oven until they reached constant weight, to be used in characterization tests (Fig. 2).
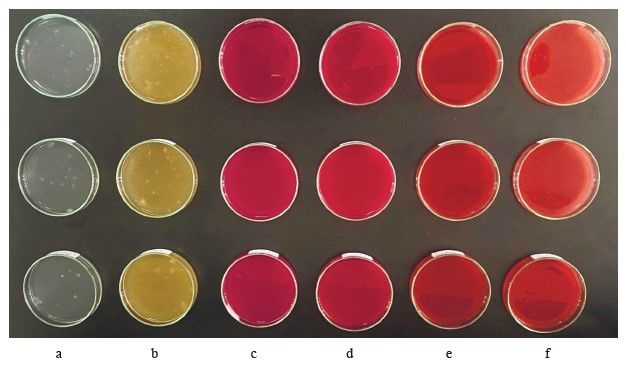
Antimicrobial activity of edible film solutions. The antifungal and antibacterial activities of the edible film formulation test groups were determined using the well diffusion assay. The test microorganisms included C. albicans ATCC 10231, A. hydrophila ATCC 19570, L. monocytogenes ATCC 7644, Y. ruckeri, and V. anguillarum A4. The culture suspensions (0.5 McFarland, 100 µL) were spread on the surface of the medium. Then, 100 µL of the mixture from the test groups and the control were added to each well (6 mm diameter, 0.1 cm3 volume). The experiment was carried out in triplicate. After incubation of the petri dishes for 24 h at appropriate temperatures, the inhibition zone diameters were obtained using a caliper.
Thickness and density of edible film solutions. The thickness of the films was determined with a digital micrometer. The density of the films was reported as the ratio of the cut mass of the film to its volume (Thickness × Surface Area) [29].
Moisture content of edible film solutions. The moisture content, %, was determined using the oven-drying method by differential weighing of a film sample before and after drying. Three different film samples from each group were oven-dried at 90°C to constant weight. The film content was calculated using Eq. (1):
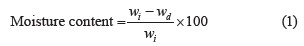
where wi is the initial weight of the film sample, g; wd is the weight of the oven-ried film sample, g. Each of the groups was tested in triplicate [30].
Transparency and light transmission of edible film solutions. The transparency andwlight transmission values were determined using a UV-VIS spectrophotometer (Beckman Coulter, USA) by reading the absorbance of the number of films at wavelengths in the range of 200–800 nm [30]. The film samples were cut into three strips (0.7×3 cm). Each of the strips was placed in a quartz cuvette and its absorbance was read against an empty cuvette. The relative transparency, A600/mm, of the film strip was measured at 600 nm and calculated using Eq. (2):
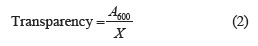
where A600 is the absorbance value at 600 nm and X is the film thickness, mm. Triplicate readings were made for each film formulation.
The light transmittance, %, of the film groups was recorded by making spectrophotometric readings at 50 nm intervals at 200–800 nm and calculated using the Lambert-Beer equation:
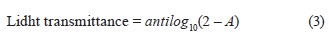
where A is the absorbance value of the film strip.
Water solubility of edible film solutions. The film samples were cut into square pieces in triplicate. The films were weighed in glass Petri dishes and then 30 mL of distilled water was added. After immersion at room temperature (~ 25°C) for 24 h, the residues were filtered and weighed to determine the degree of swelling or dried in an oven at 70°C to constant weight to determine their solubility [30]. The solubility in water, %, was calculated using Eq. (4):
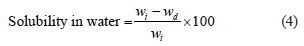
where wi is the initial weight of the film sample, g; wd is the weight of the oven-dried film sample, g. Triplicate readings were made for each of the film solution groups.
Statistical analysis. The analysis of variance (oneway ANOVA) was performed using the SPSS program (GNU) to determine significant differences between antimicrobial activity values. Tukey’s post-hoc test was used for multiple comparisons between different groups with 5% statistical significance (p < 0.05).
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
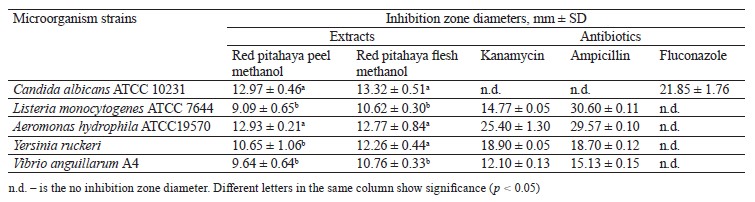
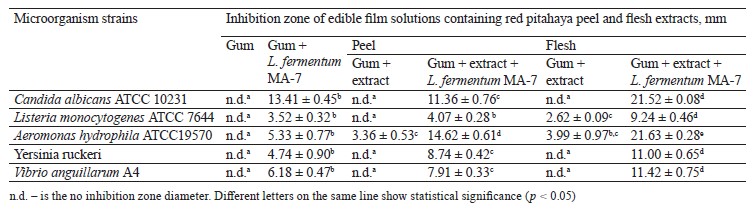
Increasing antibiotic resistance in the world has led researchers to look for plant-based natural alternatives to control pathogenic microorganisms instead of synthetic preservatives [31]. In this study, we determined the biological activity of red pitahaya extracts against food-borne, fish, and yeast microorganisms by using the disc diffusion susceptibility and micro-dilution methods (Table 1). The largest inhibition zone diameters in the peel and flesh extracts were determined as 12.97 and 13.32 mm, respectively, against Candida albicans ATCC 10231. The smallest inhibition zone diameters in the peel and flesh extracts were determined as 9.09 and 10.62 mm, respectively, against Listeria monocytogenes ATCC 7644. The difference in antimicrobial activity between the C. albicans ATCC 10231 and L. monocytogenes ATCC 7644 strains was statistically significant (p < 0.05) in both extracts.
In our previous study, where we investigated the biological activity of fruit and peel methanol extracts from white pitahaya, the inhibition zone diameters were determined against L. monocytogenes ATCC 7644 (6.30 and 6.35 mm, respectively) and against C. albicans ATCC 10231 (11.66 and 13.15 mm, respectively) [32]. The differences in the phenolic content, especially betalain, of fruits may cause different results in antimicrobial activity against the same test microorganisms [33, 34].
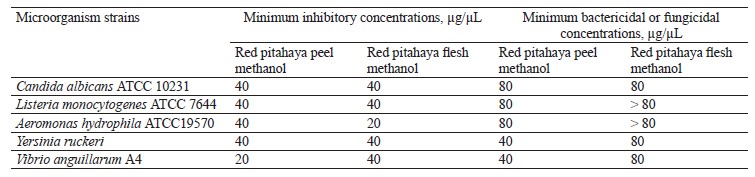
Antimicrobial agents may have a static or cidal effect. The static effect has the ability to prevent the growth or reproduction of microorganisms, while the cidal effect has the ability to kill microorganisms [35]. The disc diffusion assay alone is not sufficient to determine whether the antimicrobial activity is a static or a cidal effect [36]. For this reason, it is necessary to determine minimum inhibitory concentrations, as well as bactericidal or fungicidal concentrations of the extracts. The micro-dilution assay results for red pitahaya extracts are presented in Table 2. As can be seen, the minimum inhibitory concentrations value for the peel extract was determined as 40 µg/µL against all the test microorganisms, except V. anguillarum A4 (20 µg/µL). The flesh extract had a minimum inhibitory concentrations value of 40 µg/µL against all the test microorganisms, except A. hydrophila ATCC19570 (20 µg/µL). The minimum bactericidal concentrations values were determined in the range of 40– 80 µg/µL in the peel extract and 80 and higher µg/µL in the flesh extract. The minimum fungicidal concentrations value was 80 µg/µL for both extracts.
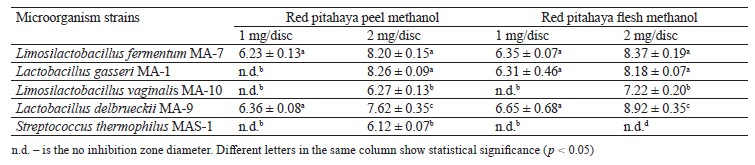
Lactic acid bacteria are an important group of probiotic microorganisms. One of them is Limosilactobacillus fermentum, a generally recognized as safe bacterium used for food fermentation [25–38]. Table 3 shows the inhibition zone diameters of red pitahaya extracts against the probiotic candidate lactic acid bacteria strains at 1 and 2 mg/disc concentrations. As can be seen, the inhibitory zone diameters of 6.23 and 6.36 mm were determined in 1 mg/disc peel extract against L. fermentum MA-7 and Lactobacillus delbrueckii MA-9, respectively. Similarly, low inhibition activities against L. fermentum MA-7 (6.35 mm), Lactobacillus gasseri MA-1 (6.31 mm), and L. delbrueckii MA-9 (6.65 mm) were observed for 1 mg/disc flesh extract. At a concentration of 1 mg/disc, both extracts had a statistically insignificant antibacterial effect against the tested lactic acid bacteria (p > 0.05). As the extract’s concentration decreased, its inhibitory activity against the lactic acid bacteria also decreased (Table 3).
A study by Siregar and Julianti showed no antibacterial activity of water, ethanol, and ethyl acetate extracts of red pitahaya peel against Lactobacillus acidophilus [39]. The differences in antimicrobial activity may be due to the environmental conditions in which pitahaya is grown, as well as the solvent, extraction method, and microorganism strains.
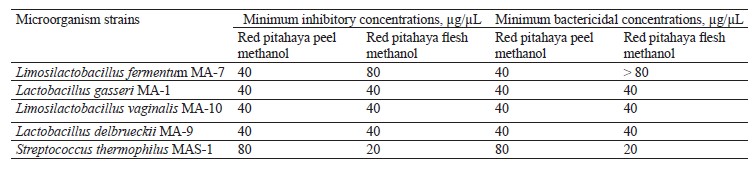
In our study, the minimum inhibitory and bactericidal concentrations values of the extracts against the tested lactic acid bacteria strains were determined in the range of 20 to > 80 µg/µL (Table 4). The highest minimum bactericidal concentrations value in the flesh extract was > 80 µg/µL against L. fermentum MA-7. The high minimum inhibitory and bactericidal concentrations values indicate that the red pitahaya extracts may have lower inhibitory activity against the lactic acid bacteria strains tested.
Edible film coatings with antimicrobial properties have been developed to provide consumers with foods that preserve high quality, do not spoil easily, keep microorganism growth under control, and have a long shelf life [40]. Literature has reported that L. fermentum strains produce various food-preservative antimicrobial peptides (fermenticins) and bacteriocins that can be used as an alternative to antibiotics [41]. These natural compounds are involved in antimicrobial activity in food bio-preservation and biomedicine [42]. For this reason, we used L. fermentum MA-7, which meets the criteria for being a good probiotic, to develop an edible film solution [43]. In addition, the tested red pitahaya extracts had relatively high minimum inhibitory and bactericidal concentrations values against L. fermentum MA-7.
Table 5 shows the biological activity of gum-based edible film solutions containing red pitahaya extracts and L. fermentum MA-7 (GEL) against the test microorganism. In most of the GEL groups, the antimicrobial activity was higher than in the other test groups, indicating a synergistic effect of pitahaya extracts with the probiotic. The antimicrobial activity of the GEL groups was statistically significant when compared to the gumbased film solutions without red pitahaya extract or L. fermentum MA-7 G (p < 0.05).
The GEL film solutions containing red pitahaya peel and flesh extracts showed inhibition zone diameters of 11.36 and 21.52 mm, respectively, against C. albicans ATCC 10231. Yeasts are important contaminants that enter the food chain during food processing, storage, and transportation [44]. Restricting yeast and fungi growth in food remains of high priority in the food and agricultural industries [45–47]. Edible films containing antimicrobials have the potential to prevent food spoilage caused by yeasts [48]. We found that our GEL film formulation has the potential to be used in preventing yeast-induced spoilage.
The GEL group showed statistically significant (p < 0.05) antimicrobial activity against A. hydrophila ATCC 19570, with inhibition zone diameters of 14.62 and 21.63 mm for the samples with the peel and flesh extracts, respectively. The use of antimicrobial film coatings in meat, fish, and seafood shows promising results for maintaining microbial stability during storage and ultimately increasing shelf life [49]. Our study indicated that the film formulations containing red pitahaya extract and L. fermentum MA-7 may had the potential to extend the shelf life of meat, fish, and seafood.
Qin et al. determined the antimicrobial activity of different film formulations obtained from red pitahaya peel extract against Staphylococcus aureus, L. monocytogenes, Escherichia coli, and Salmonella by the well diffusion method [50]. They found that the film solutions with large inhibition zones had the potential to be used not only as active packaging to extend the shelf life of foods, but also as smart packaging to preserve the freshness of protein-rich animal foods. Further, in a more recent study, edible films containing 0.5 and 1% concentrations of red pitahaya peel extract showed inhibition zone diameters of 1.24 and 1.69 mm, respectively, against S. aureus [51].
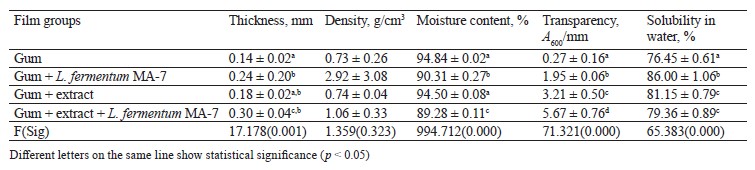
Table 6 shows the physicochemical characterization of the films with the red pitahaya peel extract. As can be seen, the thickness and density of the control group were the lowest compared to the films with L. fermentum MA-7 and the films with both the peel extract and the probiotic (p < 0.05). The difference between the control group and the group with the peel extract was not statistically significant (p > 0.05). The moisture contents of the control films and the ones with the extract were 94.84 and 94.50%, respectively. These values were higher compared to the films with L. fermentum MA-7 or the films with a combination of the red pitahaya peel extract and the probiotic. The addition of the extract and the probiotic changed the moisture content of the film by 5.56%, as well as decreased its transparency. The water solubility of the control group was 76.45%, while the group with L. fermentum MA-7 had the highest water solubility among the test groups (p < 0.05).
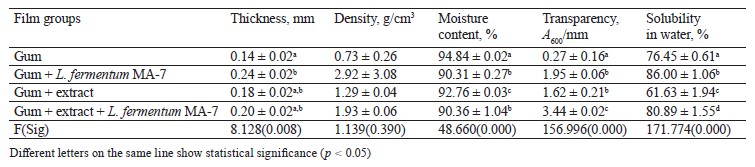
Table 7 presents the physicochemical characterization of the films containing the red pitahaya flesh extract. The highest thickness was detected in the films with L. fermentum MA-7. The films with both the flesh extract and the probiotic had a higher density (1.93 g/cm3) compared to the control group (0.73 g/cm3), whereas their moisture content was lower compared to the control group (p < 0.05). We found a statistically significant (p < 0.05) difference in transparency between the control films and the ones containing the flesh extract and L. fermentum Gum+extract + Lwferm entumr MAl7bility was determined as 61.63% in the . lms with the extract. The difference in water solubility among the film test groups was statistically significant (p < 0.05).
In a study by López-Díaz et al., the films with red pitahaya had thickness values between 0.037 and 0.060 mm, whereas their moisture content ranged between 21.3 and 32.4% [52]. In a study by Azlim et al., the films with red pitahaya peel extract had a moisture content between 0.24 and 0.28%, while their water solubility varied between 30.63 and 52.73% [53].
Thickness is one of the properties of edible films that affects the shelf life and biological structure of foods. The optimal thickness for edible films or coatings is ≤ 0.25 mm [54, 55]. In our study, the films with red pitahaya flesh and L. fermentum MA-7 were 0.20 mm thick, which was 0.05 mm thicker than the desired value stated in literature. High moisture content is a desirable criterion for coating foods. In our study, the moisture content of the films with red pitahaya extracts and L. fermentum MA-7 was found to be higher than in the study by Šuput et al. [56]. High-resolution films are materials that dissolve easily but do not have the ability to hold water [55]. High water resistance is preferable for coatings, since water sensitivity of some products may lead to a loss of quality. For this reason, edible films need high solubility and rapid dissolution in water [57]. In our study, the films with red pitahaya extracts and L. fermentum MA-7 had high solubility in accordance with literature.
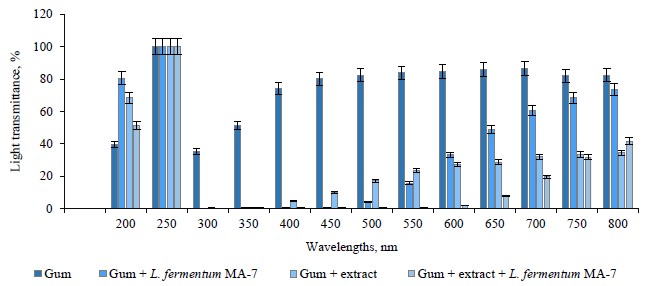
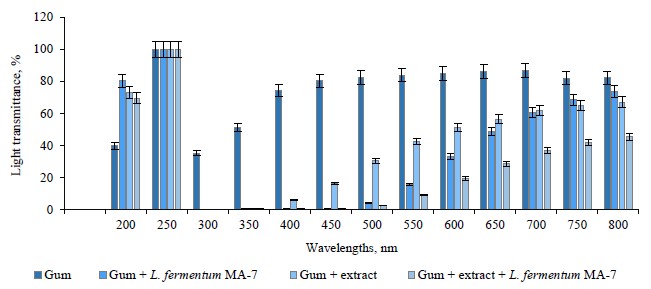
Figures 3 and 4 present the light transmittance of the films containing the red pitahaya peel and flesh extracts. As can be seen, the light transmittance of the control group (82.28–39.68%) was higher than in the test groups with the extract and/or L. fermentum MA-7 (51.31–41.57%).
The light transmittance of the extract-containing films ranged between 73.26 and 66.94%, while in the films with both the extract and L. fermentum MA-7, it varied between 69.34 and 45.47%. We found that light transmittance increased as the wavelength increased.
Socaciu et al. reported the light transmittance of films in the range of 0.01 to 70.65% [30]. The appearance of a product is important for presenting its quality and appeal to the consumer. Therefore, the transparency of films should not change the appearance, taste, or smell of the food [58]. The interaction of food with light depends on the relationship between packaging material and light. In this respect, it is important to know the optical properties of the packaging material. The interaction between the food material and light may cause unwanted photochemical reactions in the food depending on its composition [59]. In our study, the addition of red pitahaya extract and L. fermentum MA-7 to the films reduced their light transmittance.
ВЫВОДЫ
We investigated the use of extracts from red pitahaya grown in Turkey as a natural antimicrobial agent and the potential use of edible films prepared with these extracts as a coating material in the food industry. Consumers are concerned about the potential dangers of synthetic preservatives for human health. Therefore, there is an increasing tendency toward using natural antimicrobial agents. According to our results, the gum-based edible films containing red pitahaya extract and Limosilactobacillus fermentum MA-7 had a cidal/static effect against pathogenic microorganism strains. These film solutions had large inhibition zones against the bacteria and yeast. Thus, the use of edible film formulations with antimicrobial effects as a coating material can be an alternative solution to prevent the deterioration of foods and extend their shelf life. Our study proved that the gum-based film formulations with red pitahaya extract and L. fermentum MA-7 have high biological activity and may be used as a coating material in the food industry. Since literature offers limited studies on pitahaya, there is a need for more research and our results study can be used in further in vivo studies. However, since literature offers limited studies on pitahaya, there is a need for more research and our results study can be used in further in vivo studies.
Вклад авторов
All authors have contributed equally to this project.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare no conflict of interest regarding the authorship and publication of this article.
СПИСОК ЛИТЕРАТУРЫ
- Carina D, Sharma S, Jaiswal AK, Jaiswal S. Seaweeds polysaccharides in active food packaging: A review of recent progress. Trends in Food Science and Technology. 2021;110:559–572. https://doi.org/10.1016/j.tifs.2021.02.022
- Dominguez ET, Nguyen PH, Hunt HK, Mustapha A. Antimicrobial coatings for food contact surfaces: Legal framework, mechanical properties, and potential applications. Comprehensive Reviews in Food Science and Food Safety. 2019;18(6):1825–1858. https://doi.org/10.1111/1541-4337.12502
- Deshmukh RK, Gaikwad KK. Natural antimicrobial and antioxidant compounds for active food packaging applications. Biomass Conversion and Biorefinery. 2022;44:4419–4440. https://doi.org/10.1007/s13399-022-02623-w
- Kadam AA, Singh S, Gaikwad KK. Chitosan based antioxidant films incorporated with pine needles (Cedrus deodara) extract for active food packaging applications. Food Control. 2021;124:107877. https://doi.org/10.1016/j.foodcont.2021.107877
- Andrade R, Skurtys O, Osorio F. Drop impact behavior on food using spray coating: Fundamentals and applications. Food Research International. 2013;54(1):397–405. https://doi.org/10.1016/j.foodres.2013.07.042
- Falguera V, Quintero JP, Jiménez A, Muñoz JA, Ibarz A. Edible films and coatings: Structures, active functions, and trends in their use. Trends in Food Science and Technology. 2011;22(6):292–303. https://doi.org/10.1016/j.tifs.2011.02.004
- Lin D, Zhao Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Comprehensive Reviews in Food Science and Food Safety. 2007;6(3):60–75. https://doi.org/10.1111/j.1541-4337.2007.00018.x
- Nayik GA, Majid I, Kumar V. Developments in edible films and coatings for the extension of shelf life of fresh fruits. American Journal of Nutrition and Food Science. 2015;2(1):16–20. https://doi.org/10.12966/ajnfs.01.03.2015
- Aloui H, Khwaldia K. Natural antimicrobial edible coatings for microbial safety and food quality enhancement. Comprehensive Reviews in Food Science and Food Safety. 2016;15(6):1080–1103. https://doi.org/10.1111/1541-4337.12226
- Behbahani BA, Falah F, Vasiee A, Yazdi FT. Control of microbial growth and lipid oxidation in beef using a Lepidium perfoliatum seed mucilage edible coating incorporated with chicory essential oil. Food Science and Nutrition. 2021;9(5):2458–2467. https://doi.org/10.1002/fsn3.2186
- Jouki M, Yazdi FT, Mortazavi SA, Koocheki A. Quince seed mucilage films incorporated with oregano essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Food Hydrocolloids. 2014;36:9–19. https://doi.org/10.1016/j.foodhyd.2013.08.030
- Salehi F. Edible coating of fruits and vegetables using natural gums: A review. International Journal of Fruit Science. 2020;20(2):S570–S589. https://doi.org/10.1080/15538362.2020.1746730
- Sun J, Jiang H, Wu H, Tong C, Pang J, Wu C. Multifunctional bionanocomposite films based on konjac glucomannan/chitosan with nano-ZnO and mulberry anthocyanin extract for active food packaging. Food Hydrocolloids. 2020;107:105942. https://doi.org/10.1016/j.foodhyd.2020.105942
- Kumar P, Kumar L, Tanwar R, Singh S, Gaikwad KK. Active edible coating based on guar gum with mint extract and antibrowning agents for ber (Ziziphus mauritiana) fruits preservation. Journal of Food Measurement and Characterization. 2023;17:129–142. https://doi.org/10.1007/s11694-022-01609-6
- Vanaamudan A, Sadhu M, Pamidimukkala P. Chitosan-Guar gum blend silver nanoparticle bionanocomposite with potential for catalytic degradation of dyes and catalytic reduction of nitrophenol. Journal of Molecular Liquids. 2018;271:202–208. https://doi.org/10.1016/j.molliq.2018.08.136
- Zainoldin KH, Baba AS. The effect of Hylocereus polyrhizus and Hylocereus undatus on physicochemical, proteolysis, and antioxidant activity in yogurt. World Academy of Science, Engineering and Technology. 2009;3(12):884–889.
- Al-Alwani MAM, Mohamad AB, Kadhum AAH, Ludin NA. Effect of solvents on the extraction of natural pigments and adsorption onto TiO2 for dye-sensitized solar cell applications. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2015;138:130–137. https://doi.org/10.1016/j.saa.2014.11.018
- Seday Ü, Sanal D. Pitaya cultivation and production potential in Turkey. Agromedya. 2017;50–52. (In Turkish).
- Hsu C-T, Chang Y-H, Shiau S-Y. Color, antioxidation, and texture of dough and Chinese steamed bread enriched with pitaya peel powder. Cereal Chemistry. 2019;6:76–85. https://doi.org/10.1002/cche.10097
- Ai Y, Wang G, Fang F, Zhang F, Liao H. Development of real‐time intelligent films from red pitaya peel and its application in monitoring the freshness of pork. Journal of the Science of Food and Agriculture. 2022;102(12):5512–5522. https://doi.org/10.1002/jsfa.11906
- Guidelines for the evaluation of probiotics in food. Geneva: FAO and WHO; 2002.
- De S, Pramanik A, Das AKR, Paul S, Jana S, Pramanik P. Isolation and characterization of Lactobacillus spp. from curd and its pharmacological application in probiotic chocolate. Journal of Phytopharmacology. 2017;6(6):335–339. https://doi.org/10.31254/phyto.2017.6605
- Espitia PJP, Batista RA, Azeredo HMC, Otoni CG. Probiotics and their potential applications in active edible films and coatings. Food Research International. 2016;90:42–52. https://doi.org/10.1016/j.foodres.2016.10.026
- Pandhi S, Kumar A, Alam T. Probiotic edible films and coatings: Concerns, applications and future prospects. Journal of Packaging Technology and Research. 2019;3:261–268. https://doi.org/10.1007/s41783-019-00069-6
- Shahrampour D, Khomeiri M, Razavi SMA, Kashiri M. Development and characterization of alginate/pectin edible films containing Lactobacillus plantarum KMC 45. LWT. 2020;118:108758. https://doi.org/10.1016/j.lwt.2019.108758
- Pop OL, Pop CR, Dufrechou M, Vodnar DC, Socaci SA, Dulf FV, et al. Edible films and coatings functionalization by probiotic incorporation: A review. Polymers. 2019;12(1):12. https://doi.org/10.3390/polym12010012
- Kılınç B, Yalçın HT, Sürengil G. Determination of the efficiencies of fruit peels as antimicrobial agent and edible film for foods. The Black Sea Journal of Sciences. 2018;8(1):144–157. https://doi.org/10.31466/kfbd.409052
- Bambace MF, Alvarez MV, Moreira MR. Novel functional blueberries: Fructo-oligosaccharides and probiotic lactobacilli incorporated into alginate edible coatings. Food Research International. 2019;122:653–660. https://doi.org/10.1016/j.foodres.2019.01.040
- Ghiasi F, Golmakani MT. Innovative design of bio-functional Persian gum-based edible films by incorporating crocin and cinnamaldehyde: Free versus single and double emulsion fabrication techniques. Food Hydrocolloids. 2023;135:108164. https://doi.org/10.1016/j.foodhyd.2022.108164
- Socaciu M-I, Fogarasi M, Semeniuc CA, Socaci SA, Rotar MA, Mureşan V, et al. Formulation and characterization of antimicrobial edible films based on whey protein isolate and tarragon essential oil. Polymers. 2020;12(8):1748. https://doi.org/10.3390/polym12081748
- Gullon B, Pintado ME, Pérez-Álvarez JA, Viuda-Martos M. Assessment of polyphenolic profile and antibacterial activity of pomegranate peel (Punica granatum) flour obtained from co-product of juice extraction. Food Control. 2016;59:94–98. https://doi.org/10.1016/j.foodcont.2015.05.025
- Asan-Ozusaglam M, Celik I. White pitahaya as a natural additive: potential usage in cosmetic industry. Foods and Raw Materials. 2023;11(1):57–63. https://doi.org/10.21603/2308-4057-2023-1-552
- Al-Zoreky NS. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. International Journal of Food Microbiology. 2009;134(3):244–248. https://doi.org/10.1016/j.ijfoodmicro.2009.07.002
- Zakaria NNA, Mohamad AZ, Harith ZT, Rahman NA, Daud MF. Antioxidant and antibacterial activities of red (Hylocereus polyrhizus) and white (Hylocereus undatus) dragon fruits. Journal of Tropical Resources and Sustainable Science. 2022;10:9–14.
- Purssell E. Antimicrobials. In: Hood P, Khan E, editors. Understanding pharmacology in nursing practice. Cham: Springer; 2020. pp. 147–165. https://doi.org/10.1007/978-3-030-32004-1_6
- Altuner EM, Cetin B. Antimicrobial activity of Thuidium delicatulum (bryopsida) extracts. Institute of Natural and Applied Science Journal. 2009;2(2):85–92.
- Pimentel TC, da Costa WKA, Delfino TP, de Oliveira SML, Sivieri K, Magnani M. Foods and supplements as probiotic delivery vehicles. In: de Souza EL, de Brito Alves JL, Fusco V, editors. Probiotics for human nutrition in health and disease. Academic Press; 2022. pp. 115–142. https://doi.org/10.1016/B978-0-323-89908-6.00005-4
- Hossain TJ. Functional genomics of the lactic acid bacterium Limosilactobacillus fermentum LAB-1: metabolic, probiotic and biotechnological perspectives. Heliyon. 2022;8(11):e11412. https://doi.org/10.1016/j.heliyon.2022.e11412
- Siregar TW, Lubis Z, Julianti E. Effectiveness test of red dragon fruit skin (Hylocereus costaricensis jack) as natural preservation for nila fish (Oreochromis niloticus). IOP Conference Series: Earth and Environmental Science. 2020;515:012056. https://doi.org/10.1088/1755-1315/515/1/012056
- Kaba N, Duyar HA. Antimicrobial packaging. Journal of Fisheries and Aquatic Sciences. 2008;25:181–185.
- Naghmouchi K, Belguesmia Y, Bendali F, Spano G, Seal BS, Drider D. Lactobacillus fermentum: A bacterial species with potential for food preservation and biomedical applications. Critical Reviews in Food Science and Nutrition. 2020;60(20):3387–3399. https://doi.org/10.1080/10408398.2019.1688250
- Singh VP. Recent approaches in food bio-preservation – A review. Open Veterinary Journal. 2018;8(1):104–111. https://doi.org/10.4314/ovj.v8i1.16
- Asan-Ozusaglam M, Gunyakti A. Lactobacillus fermentum strains from human breast milk with probiotic properties and cholesterol-lowering effects. Food Science and Biotechnology. 2019;28(2):501–509. https://doi.org/10.1007/s10068-018-0494-y
- Liu Y, Yamdeu JHG, Gong YY, Orfila C. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Comprehensive Reviews in Food Science and Food Safety. 2020;19(4):1521–1560. https://doi.org/10.1111/1541-4337.12562
- Magan N, Hope R, Cairns V, Aldred D. Post-harvest fungal ecology: Impact of fungal growth and mycotoxin accumulation in stored grain. European Journal of Plant Pathology. 2003;109:723–730. https://doi.org/10.1023/A:1026082425177
- Magan N. Mycotoxin contamination of food in Europe: Early detection and prevention strategies. Mycopathologia. 2006;162:245–253. https://doi.org/10.1007/s11046-006-0057-2
- Garvey M. Food pollution: A comprehensive review of chemical and biological sources of food contamination and impact on human health. Nutrire. 2019;44:1. https://doi.org/10.1186/s41110-019-0096-3
- Mellinas C, Valdés A, Ramos M, Burgos N, Garrigos MC, Jiménez A. Active edible films: Current state and future trends. Journal of Applied Polymer Science. 2016;133(2):42631. https://doi.org/10.1002/app.42631
- Umaraw P, Munekata PES, Verma AK, Barba FJ, Singh VP, Kumar P, et al. Edible films/coating with tailored properties for active packaging of meat, fish, and derived products. Trends in Food Science and Technology. 2020;98:10–24. https://doi.org/10.1016/j.tifs.2020.01.032
- Qin Y, Liu Y, Zhang X, Liu J. Development of active and intelligent packaging by incorporating betalains from red pitaya (Hylocereus polyrhizus) peel into starch/polyvinyl alcohol films. Food Hydrocolloids. 2020;100:105410. https://doi.org/10.1016/j.foodhyd.2019.105410
- Sipayung K, Sinaga H, Suryanto D. Edible coating made of taro starch and red dragon fruit peel extract. IOP Conference Series: Earth and Environmental Science. 2021;782:032101. https://doi.org/10.1088/1755-1315/782/3/032101
- López-Díaz AS, Barriada-Bernal LG, Rodríguez-Ramírez J, Méndez-Lagunas LL. Characterization of pitahaya (Hylocereus undatus) mucilage-based films. Applied Food Research. 2023;3(1):100266. https://doi.org/10.1016/j.afres.2023.100266
- Azlim NA, Mohammadi Nafchi A, Oladzadabbasabadi N, Ariffin F, Ghalambor P, Jafarzadeh S, et al. Fabrication and characterization of a pH‐sensitive intelligent film incorporating dragon fruit skin extract. Food Science and Nutrition. 2022;10(2):597–608. https://doi.org/10.1002/fsn3.2680
- Skurtys O, Acevedo C, Pedreschi F, Enronoe J, Osorio F, Aguilera JM. Food hydrocolloid edible films and coatings. Nova Science Pub Inc; 2011. 66 p.
- Hatmi RU, Apriyati E, Cahyaningrum N. Edible coating quality with three types of starch and sorbitol plasticizer. Web of Conferences. 2020;142:02003. https://doi.org/10.1051/e3sconf/202014202003
- Šuput D, Lazić V, Popović S, Hromiš N, Bulut S. Biopolymer films synthesis and characterisation. Journal on Processing and Energy in Agriculture. 2017;21(1):9–12. https://doi.org/10.5937/JPEA1701009S
- Janjarasskul T, Krochta JM. Edible packaging materials. Annual Review of Food Science and Technology. 2010;1:415–448. https://doi.org/10.1146/annurev.food.080708.100836
- Pająk P, Przetaczek-Rożnowska I, Juszczak L. Development and physicochemical, thermal and mechanical properties of edible films based on pumpkin, lentil and quinoa starches. International Journal of Biological Macromolecules. 2019;138:441–449. https://doi.org/10.1016/j.ijbiomac.2019.07.074
- Isık H, Daghan S, Gokmen S. A research on edible coatings used in the food industry. Electronic Journal of Food Technologies. 2013;8(1):26–35. (In Turkish).