Аннотация
Coacervation is a low-energy method that is ideal for encapsulating heat-sensitive materials, e.g., limonene, citral, linalool, and isoamyl acetate.This research used a simple coacervation method to prepare flavoring beads with alginate and Tween 80. The methods of scanning electron microscopy (SEM) and fourier transform infrared (FTIR) spectroscopy made it possible to study the morphology and structure of the flavoring beads. After the extraction, the flavor retention and structure were described using the method of gas chromatography with mass spectrometry (GC-MS).
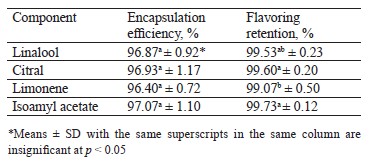
The microcapsules demonstrated a retention rate of 99.07–99.73% while the encapsulation efficiency remained as high as 96.40–97.07%. The microcapsules had a mononuclear structure and ranged from spherical to elongated ellipsoids; they were sealed without agglomeration. The particle size was below 1000 µm. The GC-MS chromatograms detected neither structural changes nor any new compounds. The FTIR spectra were similar to the control but demonstrated slight shifts, which suggested fundamental structural changes caused by the coacervation. We also fortified sponge cake and jelly with flavoring beads. The sensory analysis of the sponge cake samples revealed no significant differences compared to the control. All the fortified jelly samples had higher scores for smell, taste, texture, and overall preference than the control.
The coacervation method proved to be an excellent solution for the problem of heat-sensitive flavorings that often lose quality or sensory attributes in food products that undergo extensive thermal treatment.
Ключевые слова
Sodium alginate, flavorings, coacervation, GC/MS, FTIR, sensory propertiesВВЕДЕНИЕ
The market for flavorings is expected to increase from 14.66 billion $ in 2021 to 20.12 billion $ by 2028, with a Compound Annual Growth Rate of 4.64% [1, 2]. Flavorings affect consumption, acceptance, and food palatability. However, they are sensitive to environmental conditions and industrial processing. In free form, the flavor loss may reach 90% because flavoring substances are extremely volatile and react easily with other components [3]. Microencapsulation is an important method of improving flavor stability. During microencapsulation, solids or liquids are enclosed in polymeric matrices, e.g., maltodextrin, starches, alginates, etc. In addition, microencapsulation extends storage life by preventing unfavorable chemical or sensory changes, as well as increases the solubility of hydrophobic flavorings. The method also makes it possible to control the release of active constituents and create new applications [4].
Microparticles can be obtained by a variety of processes and methods, including fluidized beds, extrusion, centrifugal extrusion, coating, spray-drying, spray-chilling, coacervation, liposomes, and inclusion complexation. Industrial microencapsulation must be simple, reproducible, and quick, with low dependency on the solubility properties of the core substance and the coating polymer. Microencapsulation by coacervation has a few advantages over other methods. For instance, it provides effective control of the properties and quality of the final product. Microencapsulation by coacervation can be applied to heat-sensitive products and allows for continuous, cheap, and simple production. The obtained particles are highly soluble and stable while being uniform in size [5, 6].
The encapsulation of oils has received particular attention in scientific literature. Most publications feature citronella oil, sunflower oil, fish oil, linseed oil, orange oil, canola oil, olive oil, and thyme oil [7–14]. As for the application range, oil capsules are popular in medicine and pharmacy, where they provide controlled medication release and serve as antibacterial or nutraceutical agents. They can be used in cosmetics, molecular cuisine, food industry, animal feed, etc.
However, microencapsulation of flavorings by coacervation, especially with the help of alginate, has received very little scientific attention so far. The current study aimed at encapsulating limonene, citral, linalool, and isoamyl acetate using the coacervation method with alginate for coating. The flavorings belong to different chemical classes and are part of many commercial food formulations. In nature, they occur in oranges, bananas, lemons, etc.
This research focused on the physical and chemical properties of the microencapsulated flavorings. In addition, the fortified sponge cake and jelly samples underwent a sensory assessment to reveal the effect of processing conditions on the quality of the final product. The simple and reliable microencapsulation method could be applied to different flavorings in food products with extended shelf-life in order to control the flavor release and bring the flavor through severe processing conditions.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Ingredients and chemicals. The wheat flour, butter, fresh eggs, sugar, fresh skimmed milk, salt, baking powder, and gelatin were obtained from a local market in Giza, Egypt. The linalool, citral, limonene, isoamyl acetate, and Tween 80 were purchased from Sigma-Aldrich, USA. The sodium alginate with a medium viscosity of ≥ 2000 cp was isolated from brown algae with a molecular weight of 80–120 kDa and a mannuronic to guluronic acid ratio of 1.56. It was purchased from Sigma-Aldrich, USA, while the calcium chloride was provided by Rankem, India.
Preparing beads by coacervation. The encapsulation method with the simple coacervation technique described by Müller et al. was used to prepare flavoring beads [11]. To prepare sodium alginate solution, we dissolved 2.5% (w/v) of sodium alginate in distilled water at 1000 rpm for 30 min using a magnetic stirrer. Then, we added 1% (w/v) of the concentrated liquid flavoring to the sodium alginate solution. To prepare the alginate emulsion gel, we added 1% (w/v) Tween 80 as an emulsifying agent and stirred the resulting mix for 1 h. The mix of aroma compounds and alginate solution underwent stirring at 500 rpm for 1 h. After being transferred to a separating funnel, it went into a wide-mouth beaker with a 3% (w/v) calcium chloride solution. The procedure involved an 18-gauge needle of 38 mm in length and 1.27 mm in diameter, as well as a 100-mL glass syringe. For additional hardening, the flavoring beads were stirred at 10 rpm for 1 h. After that, we filtered the beads through cheesecloth and washed them under distilled water. After rinsing, the beads were stored in airtight containers for analysis. The control sample contained neither sodium alginate nor calcium chloride.
Determining encapsulation efficiency. The coacervated beads with limonene, citral, and linalool were evaluated after 24 h of hydrodistillation. The method involved dissolving 5 g of beads in 150 mL of distilled water for 3 h using a Clevenger-type apparatus. We added 2 mL of ethyl ether to extract the volatiles from the aqueous phase. The extract was concentrated in a rotary evaporator, followed by nitrogen gas to vaporize any remaining solvent until constant total weight [15].
As for isoamyl acetate microcapsules, we dissolved 5 g of coacervated beads in 30 mL of distilled water in a screw-cap vial and then mixed it using a vortex mixer. Then, we added 40 mL of diethyl ether solution to extract the flavor from the water phase, as described by Moawad et al. [16]. After collecting the flavoring, the ethyl ether was allowed to evaporate by rotary evaporation at 40°C, followed by nitrogen gas to vaporize any remaining solvent until constant total weight. Finally, the retention percentage was calculated using the Eq. (1) below:
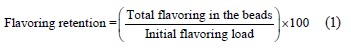
Shahidi Noghabi & Molaveisi assessed the encapsulation efficiency by a modified method that allowed them to determine the surface oil or flavoring [17]. They added 30 mL of hexane to 5 g of beads, followed by stirring at 300 rpm for 10 min. After filtration and washing with hexane, the solvent was vaporized under vacuum at 50°C. All residual solvent was evaporated with nitrogen until constant weight. Finally, they calculated the encapsulation efficiency, %, by using the Eq. (2) developed by Rubén et al. [18]:
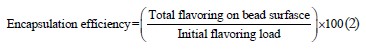
Scanning electron microscopy analysis (SEM). The scanning electron microscopy revealed the surface morphology of the flavoring microcapsules. We used a Quanta FEG 250 field emission electron microscope (Czech Republic) to examine the samples at an accelerating voltage of 10 kV. The gold-sputtered samples were mounted on aluminum stubs with double-sided adhesive tape and coated with gold using an Edwards sputter coater S150 A (Crawley, England). The magnification range was 50–15 000×.
Determining flavoring retention and structure by gas chromatography with mass spectrometry (GC-MS).
The analysis involved a Hewlett-Packard 5890 gas chromatograph and a Hewlett-Packard 5970 mass spectrometer. The volatiles were separated in a J&W DB-5 MS column, USA (30 m, 0.25 mm, 0.25 µm). The oven temperature remained 50°C for 5 min before climbing to 250°C at a rate of 4°C per 1 min. Helium served as a carrier gas, with a flow rate of 1.1 mL per 1 min. The sample size was 2 µL; the split ratio was 1:10; the injector temperature was 220°C. The mass spectra were acquired in the electron impact mode at 70 eV and a scan m/z range of 29–400 amu. The retention indices of the isolated volatile compounds were computed using the retention time of a series of n-alkanes (C6-C22), which were analyzed under identical conditions. The isolated peaks were identified according to the repository of mass spectra established by the National Institute of Standards and Technology (NIST) [19].
Structural analysis by Fourier transform infrared (FTIR) Spectroscopy. This test involved a transmittance mode iS50 Thermo Nicolet Nexus 670 FT-IR (Thermo Scientific, USA) with a built-in diamond crystal to capture the FTIR spectra of alginate and beads [20]. With 32 scans at a 4 cm–1 resolution, the analysis covered a spectral range of 400–4000 cm–1.
Preparing sponge cake and jelly. The sponge cake samples were prepared as recommended by Pasukamonset et al. [21]. The concentrations of flavoring beads followed the results of the encapsulation efficiency test and the allowable amounts reported for the creaming stage in the Fenaroli’s Handbook of Flavor Ingredients [22]. We scaled the dough into two aluminum molds (10×20 cm) to be baked in an electric oven (Universal, Egypt) at 175°C for 25 min. After that, it stayed there to cool at ambient temperature for 30 min. The resulting products were stored in airtight polyethylene pouches at 4°C for further analysis. The jelly samples were prepared with modifications as proposed by Cano-Lamadrid et al., who added 100 g of sugar to 200 mL of water and boiled it with 20 g of gelatin for 2 min [23]. The flavorings were added separately. Unflavored sponge cake and jelly served as control samples.
Sensory evaluation of sponge cake and jelly. The sensory evaluation involved 26 trained panelists from the Department for Food Technology and Nutrition, National Research Centre, Cairo, Egypt. They assessed the coacervated flavoring, sponge cake, and jelly samples using the nine-point hedonic scale, from 9 points for like extremely to 5 for neither like nor dislike and 1 for dislike extremely. Color, smell, taste, softness, and acceptability were the primary sensory attributes [24]. Each panelist was given a tray with core samples of cakes and jelly, a glass of water, and an evaluation sheet. The samples were randomly coded using a three-digit number. The panelists were instructed to rinse their palates between the samples. They had enough room to handle the samples and the questionnaire; the evaluation time was not limited.
Statistical analysis. The obtained data were evaluated using the analysis of variance (ANOVA), the Duncan’s Multiple Range test (DMRT), and the SPSS 22 Statistical Package for Social Sciences. The results were presented as mean ± SD with significant differences at p < 0.05.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
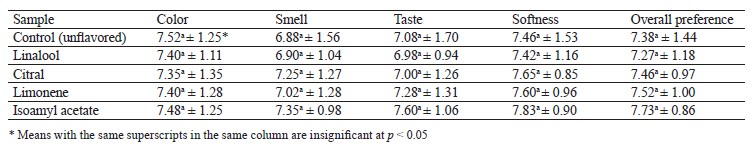
Flavoring retention and encapsulation efficiency. The total retention percentage of the encapsulated flavoring ranged from 99.07 to 99.73% (Table 1). The high retention percentage indicated that the loss of the coacervation-encapsulated volatiles was extremely low. The percentages recorded for the different flavorings were very close despite the differences in volatility, vapor pressure, and molecular weight. The significant difference in the total retention between limonene and the rest of the samples could be correlated with the polarity between the core material and the environment. The method is known to be suitable for core materials. In addition, the procedure presupposes no higher-energy steps for homogenization or encapsulation, which prevents the active constituents from going loose.
We recorded no significant differences (p ≤ 0.05) in encapsulation efficiency, which stayed between 96.40 and 97.07%. Table 1 demonstrates the excellent encapsulation of the core content despite the difference in some physicochemical properties. The non-significant differences were connected with the total retention content, where the highest efficiency percent (97.07%) belonged to isoamyl acetate, which also showed the highest retention percentage. The lowest efficiency percent (96.40%) was observed in the limonene sample, which also had the poorest retention properties.
Our findings agreed with those published by Müller et al., who coacervated orange oil with alginate and found the encapsulation efficiency as 99.51% [11]. Benavides et al., who studied coacervated thyme essential oil, established an inverse relationship between the content of encapsulated oil and the encapsulation efficiency percentage [14]. Similarly, Baranauskaite et al. reported that increasing oregano essential oil content decreased the encapsulation efficiency percentage of microspheres [25]. The authors explained it by the limited capacity of the capsule to contain oil. When they raised the oil content, a significant amount of it approached the microsphere surface. When the microsphere dried, this oil was lost by volatilization, thus reducing the encapsulation efficiency percentage [14].
Encapsulation efficiency percentage depends on some other variables. For example, a faster stirring rate causes a higher degree of dispersion. As a result, small clusters of oil appear in the microsphere. They are finely distributed between the alginate chains. Emulsifying agent is another important variable. In our case, Tween 80 improved the oil retention and flavoring [26]. The wall vs. core ratio is an important factor in enhancing encapsulation efficiency percentage, which is known to go down when the amount of essential oil increases. A larger amount of wall material or crosslinking agent makes the wall more compact, which prevents the release of oil or flavoring from the microcapsules [27].
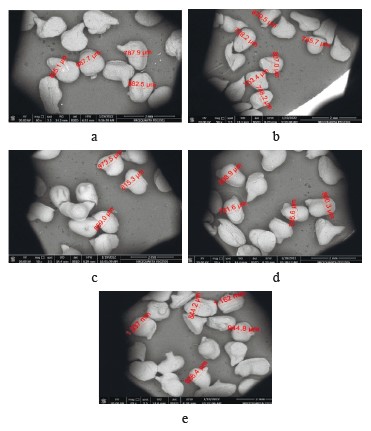
Morphological analysis of flavoring beads. Figure 1 demonstrates the results of the scanning electron microscopy, which we used to study the morphology of flavoring microcapsules. They showed spherical or elongated ellipsoids with few or no dents, cracks, or holes. These complete and sealed capsules protected the flavoring and the core material. Some irregular morphology was observed, but the structure was mononuclear, which means that the core material was sealed without agglomeration. The particle size was not homogeneous for different microcapsules. The average particle size was below 1000 µm.
Our findings confirmed those reported by Müller et al., who obtained coacervated orange oil microparticles with a mean volume diameter of 908.63 μm and a particle size of 346.37–1867.31 μm [11]. Piornos et al. studied the microscopic outer and inner structures of optimized linseed oil beads of approximately 1.80 mm [10]. They reported various external morphologies, i.e., rough and smooth surfaces, as a consequence of drying and subsequent shrinking. Some beads had small depressions on the surface. However, the authors discarded the possibility of deeper pores by zooming on the problematic zones. The microencapsulation of limonene essential oil using simple coacervation by chitosan showed that microcapsules had a mean size of 10 μm. They were rough and not spherical in shape, which resembles our findings. The shrinking was caused by the loss of encapsulated oils, as seen by the pores in microcapsules [3]. Gawad & Fellner compared the encapsulation of glycerol using a simple coacervation with alginate and a complex coacervation with alginate and chitosan [20]. The size and appearance they obtained were comparable with our data. Therefore, other studies also revealed the ability of simple coacervation to present an efficient and excellent encapsulation.
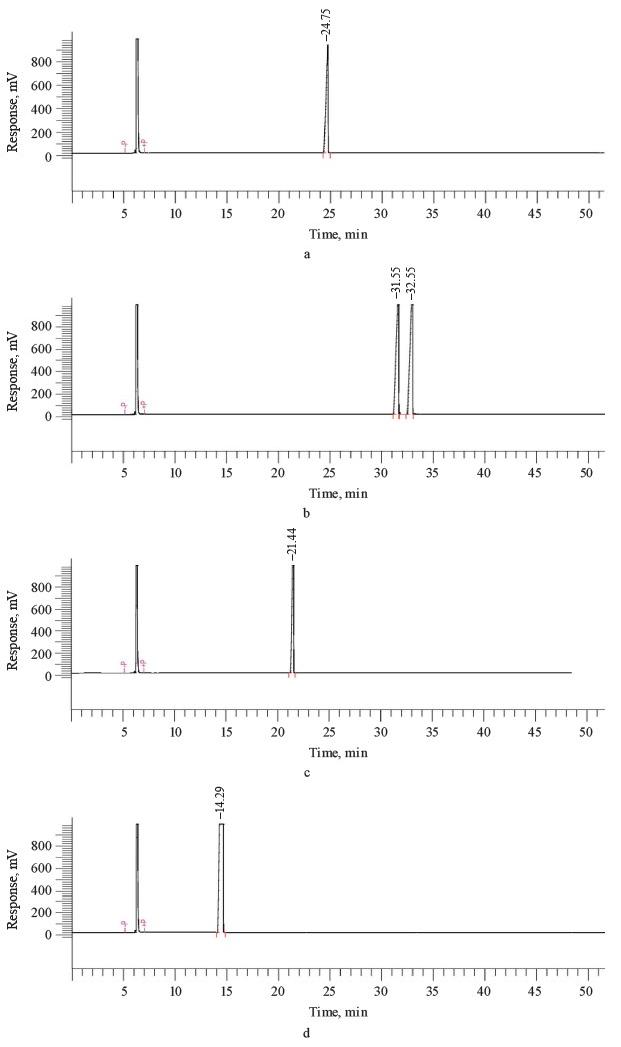
Studying the effect of coacervation on flavoring structure by gas chromatography with mass spectrometry (GC-MS). Figure 2 illustrates the retention potential of coacervated flavorings based on their different physical and chemical properties. Coacervation is a low or no-energy method, compared to other intensive-energy protocols, e.g., spray-drying. Therefore, coacervated flavorings are expected to be more stable than spray-dried ones. The chromatograms revealed neither changes nor any new compounds.
These results correspond with those reported by Li et al. [28]. The authors encapsulated citral essential oil by simple coacervation and found that the process did not degrade the two main compounds of the essential oil, namely α-citral and β-citral. The microcapsules they obtained were highly efficient in preserving the qualities of peaches during storage. Similarly, encapsulation did not affect the antifungal or antibacterial activities of citral essential oil.
Besharati et al. found that encapsulating flaxseed oil with chitosan minimizes the ruminal biohydrogenation process of unsaturated fatty acids: the microcapsules had excellent flavor retention and stability [29]. D-limonene, the major component of orange oil, was well retained (60–85%) in microcapsules prepared by Baiocco & Zhang based on shear stress [30].
Coacervation is a good method for heat-sensitive materials, e.g., essential oils or flavorings, because it does not involve thermal treatment. Microparticles obtained by coacervation have higher thermal stability than those obtained by intensive-energy methods, e.g., spray-drying. As a result, coacervated microparticles may be used in foods that undergo intense thermal treatment. Consequently, the microencapsulation method may be selected based on the final product, equipment availability, and the relation between production cost and sales price of the microencapsulated flavorings [11].
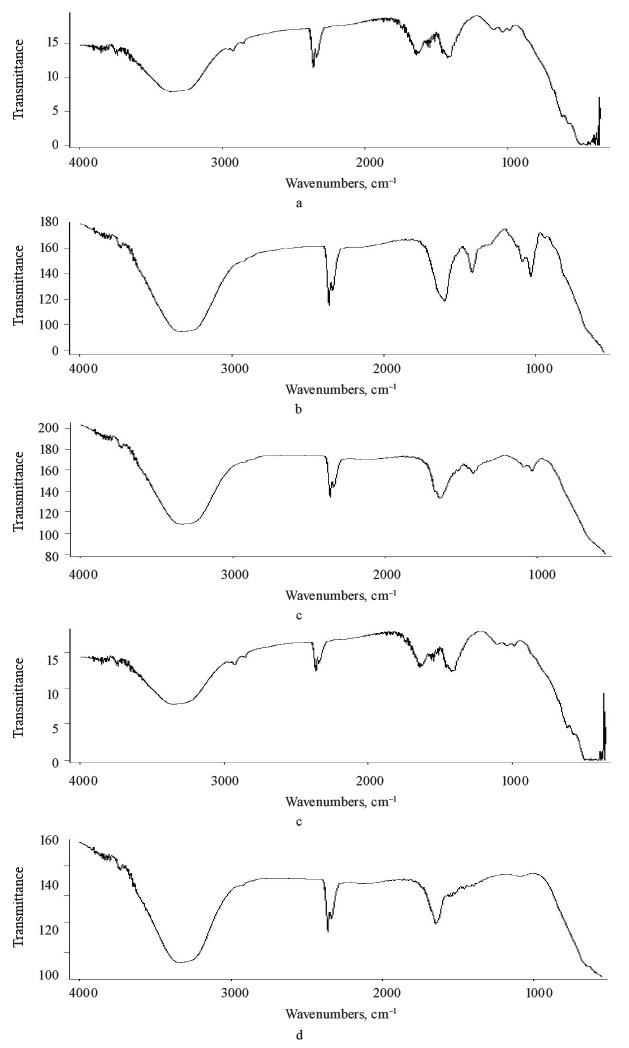
Fourier transformed infrared spectroscopy (FTIR) analysis. Figure 3 shows the FTIR spectra of flavorings encapsulated by coacervation and alginate (control). The alginate spectrum (control) had an OH-related broadband at 3.200–3.400 cm–1 stretching with strong hydgen bonding. The peak at 2.924 cm–1 could be ascribed to the overlapping symmetrical and asymmetrical C-H stretching vibration of aliphatic chains (-CH2-, -CH3). The asymmetric and symmetric vibrational modes of carboxylate ions (O-C-O) were recorded at 1.633 and 1.414 cm–1, respectively. The vibrational mode at 1.093 cm–1 was attributable to the C–O stretching vibration of a pyranose ring. Due to its polysaccharide structure, the stretching vibration of sodium alginate (C-O-C) manifested at 1.035 cm–1.
A C-H stretching was also identified for uronic acid (982 cm–1) and mannuronic acid (878 cm–1). These findings confirmed those reported by Helmiyati & Aprilliza [31]. The spectra of microencapsulated flavorings were related strongly to the control but with slight shifts suggesting fundamental structure changes caused by coacervation. For example, linalool, citral, and isoamyl acetate had narrower and more intense bands at 3.200– 3.400 cm–1 that reflected new hydrogen bonds between alginate and flavorings. However, nothing of similar kind was recorded for limonene due to the nature of this flavoring. The O-C-O signal was more intense in the alginate sample (control) than that in the microencapsulated samples. Again, an exception was the limonene beads, which revealed weaker ionic bonds between flavorings and carboxyl groups due to the absence of efficient functional groups in the flavoring. Generally, ionic bonds are essential evidence for the interaction during complex coacervation, e.g., between chitosan and alginate, as well as the broadening of some bands or the increase in the intensity of others [20].
Sensory evaluation of food products fortified with microencapsulated beads. Table 2 sums up the results of the sensory evaluation, which included color, smell, taste, texture, and overall preference of the control sponge cake and the cakes fortified with coacervated flavorings. No significant differences were obtained for color scores. The samples with coacervated citral, limonene, or isoamyl acetate got the best scores for smell, taste, softness, and overall preference. Again, no significant differences were observed between these experimental samples and the control. Choosing sponge cake as an application for the encapsulated flavorings was critical for assessing the success of the whole process and the limits of potential use for such formulated raw materials in different food products. Additionally, the application opens prospects for flavorings in many food products that need intensive cooking conditions without affecting the quality or sensory attributes.
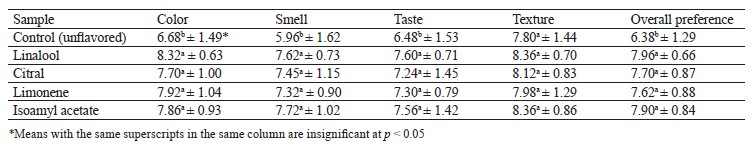
The jelly samples fortified with coacervated flavorings received higher mean scores than the control, and this time the differences were statistically significant (Table 3). Texture was the only variable with no significant differences. In contrast, all the experimental samples showed significant differences in smell, taste, and overall preference. The lower molecular weight and vapor pressure of aroma compounds were responsible for the highest sensory attributes. Immersing in water solutions increased the aroma release in the gas phase because sucrose had the so-called salting out effect, which constituted more than 50% of the jelly formulation. Vatankhah Lotfabadi et al. used time-intensity analysis and HS-GC/MS spectrometry to study D-limonene flavor in rock candy crystal sticks and reported similar results [32]. Dubova et al., who studied the natural flavor distillates obtained from melon and cucumber, also reported sensory characteristics that were closer to natural raw materials than the existing industrial analogs [33].
ВЫВОДЫ
In this research, the method of coacervation proved quite efficient in encapsulating such popular industrial flavorings as limonene, citral, linalool, and isoamyl acetate. The experimental samples demonstrated high retention percentage and excellent encapsulation efficiency. The capsules were complete and sealed, which means they provided good protection for the core material. The coacervated flavorings showed no physicochemical changes; no new compounds were detected. The experimental samples of sponge cake and jelly received better scores for all sensory attributes. Therefore, microencapsulation by coacervation could be an excellent solution for heat-sensitive food ingredients.
Вклад авторов
Amr Farouk, Mamdouh H. El-Kalyoubi, and Mohamed F. Khallaf developed the research concept, supervised the project, and performed the practical part of the study. Shimaa Moawad and Ramadan A. Gawad designed the methodology, as well as performed the statistical analysis, formal analysis, and data curation. Shimaa Moawad and Badr Saed prepared the samples and performed the extraction. Amr Farouk wrote the original draft, reviewed scientific articles, and edited the text. All the authors have read and agreed to the published version of the manuscript.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare that there is no conflict of interests related to the publication of this article.
БЛАГОДАРНОСТИ
The authors express their gratitude to the National Research Center, Cairo, Egypt, for supporting this study.
СПИСОК ЛИТЕРАТУРЫ
- Kazantseva EG, Lyamkin II. Micro-ingredient markets and their impact on the sustainability of food systems. Food Processing: Techniques and Technology. 2023;53(1):202–216. (In Russ.). https://doi.org/10.21603/2074-9414-2023-1-2424
- Food flavorings market size, share & COVID-19 Impact analysis by type (natural and synthetic), by application (bakery, beverage, confectionery, dairy, convenience foods, snacks, and others), and regional forecast, 2021–2028. Market Research Report. 2022.
- Cristina Ferrer Carneiro H, Hoster K, Reineccius G, Silvia Prata A. Flavoring properties that affect the retention of volatile components during encapsulation process. Food Chemistry: X. 2022;13. https://doi.org/10.1016/j.fochx.2022.100230
- Moawad S, El-Kalyoubi M, Khallaf M, Abd El Mageed MA, Ali H, Farouk A. Influence of carriers on the functional properties of spray-dried flavorings during storage. Egyptian Journal of Food Science. 2021;49(1):1–8.
- Choudhury N, Meghwal M, Das K. Microencapsulation: An overview on concepts, methods, properties and applications in foods. Food Frontiers. 2021;2(4):426–442. https://doi.org/10.1002/fft2.94
- Abd El-Aziz M, Salama HH, Sayed RS. Plant extracts and essential oils in the dairy industry: A review. Foods and Raw Materials. 2023;11(2):321–337. https://doi.org/10.21603/2308-4057-2023-2-579
- Agrawal M, Goel A. Microencapsulation of citronella oil for fragrant cotton fabrics. International Journal of Novel Research and Development. 2022;7(3):205–210.
- Morales E, Rubilar M, Burgos-Dıaz C, Acevedo F, Penning M, Shene C. Alginate/Shellac beads developed by external gelation as a highly efficient model system for oil encapsulation with intestinal delivery. Food Hydrocolloids. 2017;70:321–328. https://doi.org/10.1016/j.foodhyd.2017.04.012
- Wu Q, Zhang T, Xue Y, Xue C, Wang Y. Preparation of alginate core–shell beads with different M/G ratios to improve the stability of fish oil. LWT. 2017;80:304–310. https://doi.org/10.1016/j.lwt.2017.01.056
- Piornos JA, Burgos-Dıaz C, Morales E, Rubilar M, Acevedo F. Highly efficient encapsulation of linseed oil into alginate/ lupin protein beads: Optimization of the emulsion formulation. Food Hydrocolloids. 2017;63:139–148. https://doi.org/10.1016/j.foodhyd.2016.08.031
- Müller PS, Perussello C, Zawadzki SF, Scheer ADP. Encapsulation efficiency and thermal stability of orange essential oil microencapsulated by spray drying and by coacervation. B. CEPPA. Curitiba. 2016;34(1):133–150.
- Loyeau PA, Spotti MJ, Vinderola G, Carrara CR. Encapsulation of potential probiotic and canola oil through emulsification and ionotropic gelation, using protein/polysaccharides Maillard conjugates as emulsifiers. LWT. 2021;150. https://doi.org/10.1016/j.lwt.2021.111980
- Chaabane D, Yakdhane A, Vatai G, Koris A, Nath, A. Microencapsulation of olive oil: A comprehensive review. Periodica Polytechnica Chemical Engineering. 2022;66(3):354–366. https://doi.org/10.3311/PPch.19587
- Benavides S, Cortes P, Parada J, Franco W. Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chemistry. 2016;204:77–83. https://doi.org/10.1016/j.foodchem.2016.02.104
- Farouk A, El-Kalyoubi M, Ali H, Abd El Mageed M, Khallaf M, Moawad S. Effect of different carriers and their blends on flavor microencapsulation via spray drying. Pakistan Journal of Biological Sciences. 2020;23(3):257–263. https://doi.org/10.3923/pjbs.2020.257.263
- Moawad S, El-Kalyobi M, Khallaf M, Mohammed DM, Mahmoud KF, Farouk A. Effect of spray-drying on the physical, sensory, and in-vivo parameters of orange peel oil and limonene. Egyptian Journal of Chemistry. 2022;65(SI:13B):353–368.
- Shahidi Noghabi M, Molaveisi M. Microencapsulation optimization of cinnamon essential oil in the matrices of gum Arabic, maltodextrin, and inulin by spray-drying using mixture design. Journal of Food Process Engineering. 2020;43(2). https://doi.org/10.1111/jfpe.13341
- Bustos RO, Alberti FV, Matiacevich SB. Edible antimicrobial films based on microencapsulated lemongrass oil. Journal of Food Science and Technology. 2016;53(1):832–839. https://doi.org/10.1007/s13197-015-2027-5
- Augustini ALRM, Sielemann S, Telgheder U. Strategy for the identification of flavor compounds in e-liquids by correlating the analysis of GCxIMS and GC-MS. Talanta. 2021;230. https://doi.org/10.1016/j.talanta.2021.122318
- Gawad G, Fellner V. Evaluation of glycerol encapsulated with alginate and alginate-chitosan polymers in gut environment and its resistance to rumen microbial degradation. Asia-Australasian Journal of Animal Science. 2019;32(1):72–81. https://doi.org/10.5713/ajas.18.0110
- Pasukamonset P, Pumalee T, Sanguansuk N, Chumyen C, Wongvasu P, Adisakwattana S, et al. Physicochemical, antioxidant and sensory characteristics of sponge cakes fortified with Clitoria ternatea extract. Journal of Food Science and Technology. 2018;55(8):2881–2889. https://doi.org/10.1007/s13197-018-3204-0
- Burdock GA. Fenaroli's handbook of flavor ingredients. 6th ed. Boca Raton: CRC Press; 2009. 2159 p.
- Cano-Lamadrid M, Calín-Sánchez Á, Clemente-Villalba J, Hernández F, Carbonell-Barrachina ÁA, Sendra E, et al. Quality parameters and consumer acceptance of jelly candies based on pomegranate juice “Mollar de Elche”. Foods. 2020;9(4). https://doi.org/10.3390/foods9040516
- Krupa-Kozak U, Drabińska N, Rosell CM, Piłat B, Starowicz M, Jeliński T, et al. High-quality gluten-free sponge cakes without sucrose: Inulin-type fructans as sugar alternatives. Foods. 2020;9(12). https://doi.org/10.3390/foods9121735
- Baranauskaite J, Ockun MA, Uner B, Tas C, Ivanauskas L. Effect of the amount of polysorbate 80 and oregano essential oil on the emulsion stability and characterization properties of sodium alginate microcapsules. Molecules. 2021;26(20). https://doi.org/10.3390/molecules26206304
- Nawaz T, Iqbal M, Khan BA, Nawaz A, Hussain T, Hosny KM, et al. Development and optimization of acriflavine-loaded polycaprolactone nanoparticles using Box–Behnken design for burn wound healing applications. Polymers. 2022;14(1). https://doi.org/10.3390/polym14010101
- Enascuta CE, Stepan E, Oprescu EE, Radu A, Alexandrescu E, Stoica R, et al. Microencapsulation of essential oils. Revista De Chimie. 2018;69(7):1612–1615. https://doi.org/10.37358/RC.18.7.6381
- Li Z, Zheng M, He P, Gong W, Liu Z, Xu C, et al. Citral essential oil-loaded microcapsules by simple coacervation and its application on peach preservation. ACS Omega. 2022;7(46):42181–42190. https://doi.org/10.1021/acsomega.2c04928
- Besharati M, Giannenas I, Palangi V, Ayasan T, Noorian F, Maggiolino A, et al. Chitosan/calcium–alginate encapsulated flaxseed oil on dairy cattle diet: In vitro fermentation and fatty acid biohydrogenation. Animals. 2022;12(11). https://doi.org/10.3390/ani12111400
- Baiocco D, Zhang Z. Microplastic-Free microcapsules to encapsulate health-promoting limonene oil. Molecules. 2022;27(21). https://doi.org/10.3390/molecules27217215
- Helmiyati, Aprilliza M. Characterization and properties of sodium alginate from brown algae used as an ecofriendly superabsorbent. IOP Conference Series: Materials Science and Engineering. 2016;188. https://doi.org/10.1088/1757-899X/188/1/012019
- Vatankhah Lotfabadi S, Mortazavi SA, Yeganehzad S. Study on the release and sensory perception of encapsulated d-limonene flavor in crystal rock candy using the time–intensity analysis and HS-GC/MS spectrometry. Food Science and Nutrition. 2020;8(2):933–941. https://doi.org/10.1002/fsn3.1372
- Dubova HE, Sukmanov VA, Marynin AI, Zakharevych VB, Voskobojnyk VI. Studies of some aspects in the process of aroma restoration. Foods and Raw Materials. 2016;4(1):19–26. http://doi.org/10.21179/2308-4057-2016-1-19-26