Аннотация
Synthesized in plants, polyphenols are powerful antioxidants and protect against stressful conditions. We aimed to identify different kinds of phytochemicals in fruits and provide detailed information on the roles they play in promoting good health in the human body. We also discussed the biological activities of phytochemicals found in several fruits.Google Scholar and PubMed databases were used to search for relevant information that could assist in answering our research questions. We selected and reviewed both research and review articles related to the purpose of our study.
Fruits contain numerous antioxidants which neutralize the negative impact of free radicals on the body. Free radicals are destructive species that can be produced during normal body metabolism or come from exogenous sources such as smoking or exposure to radiation. Due to their unstable nature, they can cause damage to cellular macromolecules, resulting in the development of degenerative diseases. Phytochemicals are diverse groups of bioactive compounds found in fruits that have potent antioxidant activity and exhibit several health-promoting properties in both in vivo and in vitro studies. There are two major groups of antioxidants: natural (or dietary) antioxidants and synthetic antioxidants. Natural antioxidants have gained much popularity in recent times because of the safety concerns surrounding the use of synthetic antioxidants.
The consumption of fruits plays a critical role in disease prevention, especially diseases resulting from oxidative damage to cells. The inclusion of fruits in one’s daily diet helps improve their overall wellbeing.
Ключевые слова
Fruits, antioxidants, bioavailability, polyphenols, carotenoids, biological activityВВЕДЕНИЕ
Fruits are important in the human diet because they provide the body with nutrients that promote growth and development, as well as maintain good health. Insufficient intake of fruits is one of the top ten risk factors responsible for death globally. Increasing fruit consumption can save the lives of about 2.7 million people each year [1]. Many individuals recognize the importance of diet in promoting good health and preventing disease. This is driving the search for new diet regimens with a positive impact on human health. Scientists are vigorously screening various food products, such as fruits, for potential disease-preventing properties. Fruits are referred to as “functional foods”, and studies have established the need to include them in the human diet [2].
Reactive oxygen species are free radicals involved in oxidation reactions in the body. They are harmful and can cause the breakdown of cell membranes, damage to membrane proteins, and DNA mutations. This can result in aging and many diseases such as arteriosclerosis, cancer, diabetes mellitus, liver injury, inflammation, skin damage, coronary heart disease, or arthritis. Antioxidants can inhibit the oxidation of molecules by breaking the chains of free radical reactions by donating their own electrons to free radicals [3]. Fruits are endowed with many bioactive compounds such as vitamin C, vitamin E, carotenoids, flavonoids, tannins, and other phenolic constituents, which act as potent antioxidants [4].
Although mostly consumed fresh, fruits can be processed into different kinds of food products such as canned foods, juices, pastes, etc. due to their free radical scavenging and health-promoting properties [2]. The consumption of bioactive compounds from fresh fruits or their processed products is linked with the prevention and decreased risk of many degenerative diseases [2]. Fruit-based products such as juices are widely available on the market, and they have been shown to contain a lot of compounds that exhibit antioxidant properties [5, 6]. Studies have found that polyphenols present in grape juice can protect individuals from heart disease, while juice from berries and apples can inhibit hypercholesterolemia [7]. Also, juice obtained from oranges, pineapples, and grapefruits contains good quantities of folic acid derivatives that are involved in the prevention of diseases of the nervous system and malformations such as spina bifida [8].
Studies are demonstrating that plant-based diets containing a lot of fruits and other nutrient-rich plant foods can lower the risk of diseases associated with oxidative stress [9]. Antioxidants from natural sources such as fruits are now gaining much popularity, especially in the food industry, due to their safety, unlike synthetic antioxidants, as well as their preventative and therapeutic effects on the body. Although synthetic antioxidants are used in food production to prevent lipid oxidation, butylated hydroxyanisole and hydroxytoluene are suspected to cause cancer and liver damage in animal studies [10, 11]. Diets containing adequate amounts of plant-based foods are highly important for maintaining good health. Food from plants such as fruits, vegetables, and whole grains contains enough antioxidants and minimizes the occurrence of chronic diseases [12]. Individuals who consume fruits in large quantities have a reduced risk of developing cancer and early death, especially from cancers involving epithelial cells, such as those in the cervix, lungs, stomach, esophagus, pancreas, and colon [12].
This review provides current information on phytochemicals present in fruits and shows them as potent antioxidants that promote good health by preventing diseases such as cancer, diabetes, etc. It also discusses some of the factors that can reduce fruit phytochemical bioavailability and thus decrease their health benefits for the human body.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
We reviewed relevant scientific literature found in Google Scholar and PubMed. Some of the search terms we used to retrieve articles included the effect of free radicals on the human body; phytochemicals and their fruit sources, biological activity, digestion, and bioavailability; and the importance of antioxidants for human health. The articles were selected based on the purpose of this review and our research questions.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
Generation of free radicals in the body and their role in cellular pathology. Free radicals are highly reactive molecules produced from either endogenous metabolic processes or external sources. They consist of reactive oxygen species and reactive nitrogen species. The endogenous sources include peroxisomal metabolism, mitochondrial respiration, phagocyte activity, inmation, arachidonate pathways, ischaemia, exercise, and reactions involving iron and other transition metals. Тhe external sources involve exposure to radiation, ozone, cigarette smoke, air pollutants, and industrial chemicals [13, 14]. Nitric oxide radical (NO•), superoxide anion radical (O2•–), perhydroxyl radical (HOO•), hydrogen peroxide (H2O2), hydroxyl radical (•OH), singletoxygen (1O2), hypochlorous acid (HOCl), peroxynitrite (ONOO–), hypochlorite radical (ClO–), and lipid peroxides (LOPs) are examples of free radicals capable of causing damage to biomolecules such as DNA, proteins, and lipids [15]. Polyunsaturated fatty acids can undergo peroxidation to produce compounds such as malondialdehyde, isoprostanes, 4-hydroxy-2-nonenal, etc. These compounds are known to cause diabetes, neurodegenerative diseases, and heart disease. Lipoproteins can be destroyed by peroxynitrites, resulting in the lipid peroxidation of cell membranes. The production of free radicals can interfere with protein synthesis and protein functions [15].
Oxidative stress is defined as an imbalance between the production of reactive oxygen species and the body’s antioxidant defense system [16]. Excessive production of free radicals may lead to a buildup of oxidative stress in cells and subsequent damage to proteins, DNA, lipids, and carbohydrate molecules. Many human diseases can be caused by oxidative stress, including brain dysfunction, heart disease, inflammatory diseases, diabetes, cardiovascular malfunctions, autoimmune diseases, and aging [2, 17]. Overproduction of reactive oxygen species may lead to overexpression of oncogenes, mutagen formation, and the initiation of atherogenic processes or inflammation. Maintaining redox homeostasis in the body is necessary for good health and disease prevention [11]. Since excessive production of free radicals is harmful, organisms possess natural antioxidant defense enzymes such as glutathione peroxidase, catalase, superoxide dismutase, and glutathione reductase to neutralize the effect of free radicals on the body [13]. However, certain conditions such as stress, illnesses, a high intake of processed foods, and environmental pollution can cause an imbalance in the body’s natural antioxidant defense processes [8].
Free radicals become a problem for the body when its antioxidant defense system is inadequate to neutralize or scavenge them [18]. Oxygen plays a crucial role in supporting human metabolic processes. However, in the process of certain metabolic events, oxygen can be transformed into highly reactive species that are destructive to cells in the body. Most of these reactive species are free radicals, possessing unpaired electrons. Since free radicals are unstable, they can either donate or accept electrons from other cellular molecules [14]. Increasing the intake of dietary antioxidants from fruit sources can reduce the stress imposed on the body by reactive oxygen species by protecting biomolecules (proteins, nucleic acids, and lipids) from oxidative damage, suppressing inflammatory responses, and controlling vascular homeostasis [19, 20].
Major phytochemicals in fruits. Fruits are important sources of phytochemicals for human consumption. About 200 000 phytochemicals have been identified, of which 20 000 can be found in vegetables, fruits, and cereals. Phytochemicals are bioactive compounds of plant origin that do not supply the body with energy but possess essential health benefits [21]. They are secondary metabolites present in plants. The main phytochemicals are phenolic compounds, carotenoids, and glucosinolates [12]. These chemicals are produced in plants to protect them from predators or diseases. They are referred to as “non-essential” nutrients because the human body can function without them [22]. Fruits contain different kinds of phytochemicals, such as phenolic acids, carotenoids, and flavonoids. These phytochemicals exert a wide range of biological activities and provide protection against chronic diseases. For instance, they may prevent the proliferation of cancer cells and regulate inflammatory and immune responses [23].
Carotenoids are lipid-soluble compounds synthesized in plants and microorganisms but not in animals. They are found in subcellular organelles such as chloroplasts and chromoplasts. Carotenoids are mainly conjugated with proteins in chloroplasts and serve as an additional pigment for photosynthesis. However, they exist in chromoplasts in crystalline form or as oil droplets. Carotenoids impart yellow, orange, and red colors to different kinds of plants and fruits. They are used in food processing as colorants and food supplements. During photosynthesis, they act as photosensitizers and protect plants from photodamage [12, 24]. Many carotenoids bind to chlorophylls, giving rise to xanthophyllchlorophyll and carotene-chlorophyll complexes (giving fruits a variety of colors). When fruits mature, their chlorophyll content is reduced, retaining only colored pigments [25]. Fruit color can indicate the type of carotenoids present; for example, yellow-orange fruits are high in β-carotene and α-carotene [26].
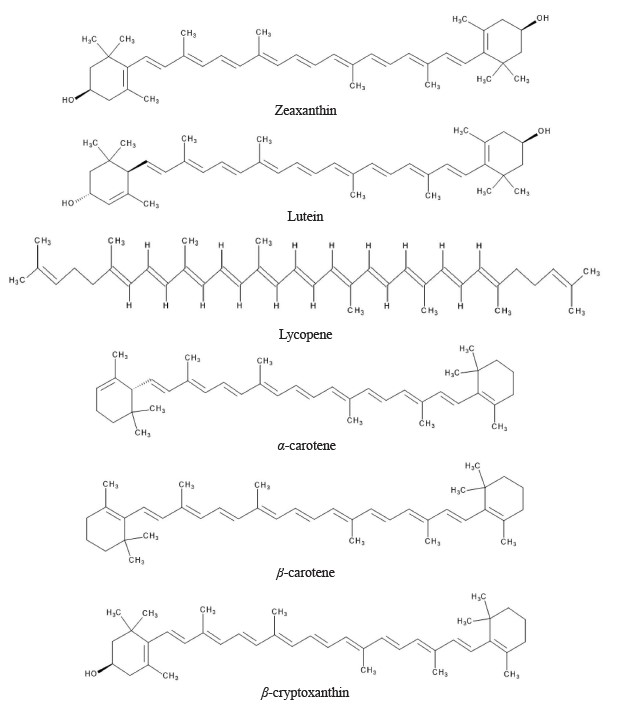
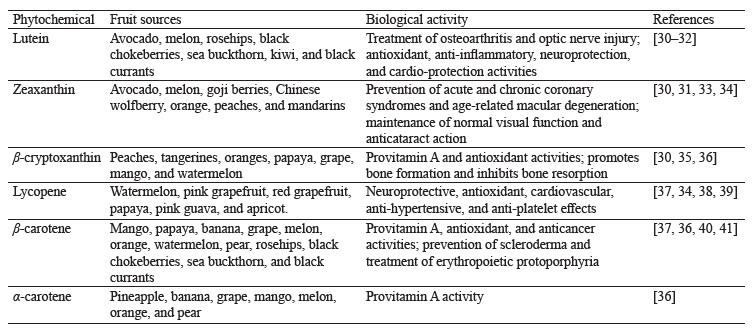
Typically, carotenoids contain the C40 skeleton, also known as tetraterpenoids. Humans can obtain approximately 50 carotenoids through diet. There are two classes of carotenoids, namely carotenes and xanthophylls. Carotenes include α-carotene, β-carotene, β,ψ-carotenes, and lycopene, whereas xanthophylls include β-cryptoxanthin, zeaxanthin, lutein, astaxanthin, fucoxanthin, and peridinin. These carotenoids contain oxygen in the form of hydroxy, aldehyde, carbonyl, carboxylic, furanoxide, and epoxide groups. Chemical structure of major carotenoids in fruits is shown in Fig. 1. There is a growing interest in screening plants and underutilized fruits for carotenoids. This is because fruits are identified as rich sources of carotenoids in the human diet [27–29]. There are a lot of health benefits associated with the intake of foods containing carotenoids. Table 1 shows some major carotenoids, their fruit sources, and their potential biological activities.
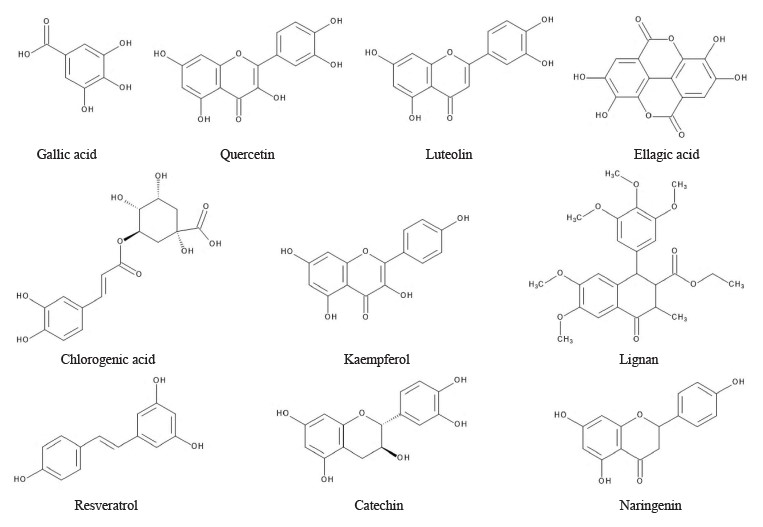
Polyphenols are among the most abundant groups of substances present in fruits, with about 8000 phenolic structures identified so far [15, 42]. They are mainly classified into flavonoids, phenolic acids, lignans, and stilbenes. Flavonoids are the most abundant group of phytochemicals [21]. They possess a minimum of one hydroxyl group linked to an aromatic ring. Dietary polyphenols are grouped into flavonoids and nonflavonoids. Flavonoids include flavones (luteolin and apigenin), flavonols (quercetin, kaempferol, and myricetin), flavonones (narigenin), isoflavonoids (daidzein and genistein), anthocyanidins (malvidin and cyanidin), and flavanols (epicatechin, catechin, epigallocatechin, epigallocatechin gallate, epicatechin gallate). Chemical structure of selected polyphenols in fruits is shown in Fig. 2. Nonflavonoids include stilbenes, phenolic acids, tannins, lignans, anthocyanidins, anthraquinones, and coumarins. They are characterized based on their carbon atom arrangements. Most polyphenols derived from plants, such as flavonoids and phenolic acids, are conjugated. They are bound to one or more sugar moieties or residues through their hydroxyl groups [37, 43]. They can also be conjugated to amines, organic acids, lipids, carboxylic acids, and other phenolic compounds [44].
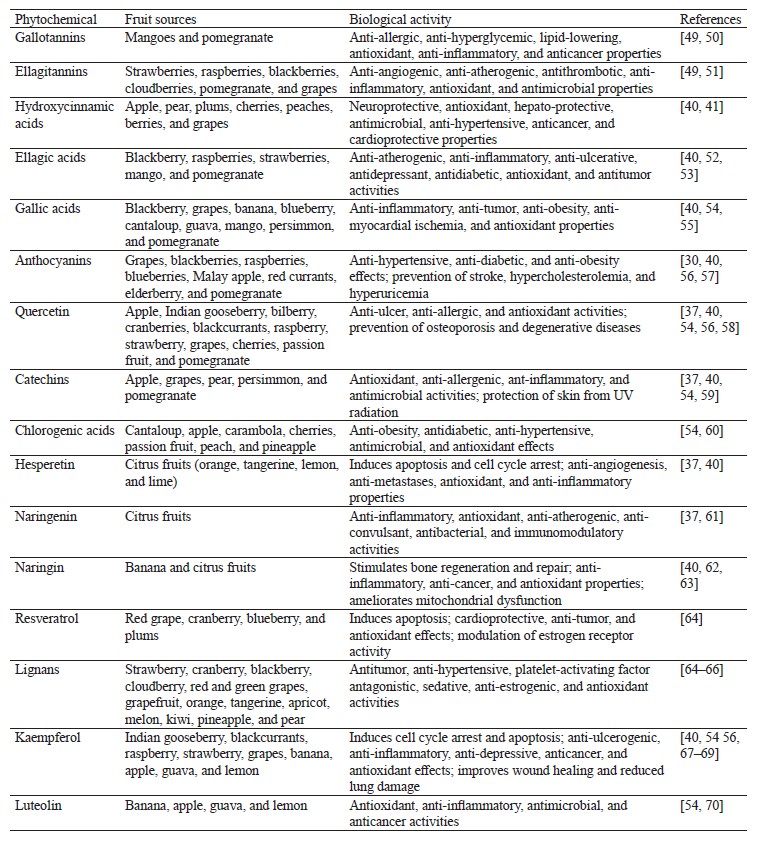
Polyphenols are synthesized in plants as a form of protection against stressful conditions such as exposure to UV, temperature fluctuations, and infection by pathogens [45]. They are powerful antioxidants with metal chelating properties that can reduce lipid peroxidation and trap nitrates to prevent the formation of mutagenic nitroso compounds [13]. Phenolic acids, flavonoids, and tannins constitute the most common polyphenols present in the human diet [46]. The composition of phenolic compounds found in fruits is determined by fruit ripeness, cultivar, physiological conditions, weather, and soil conditions. Postharvest treatments (e.g., processing) and storage can also influence the content of polyphenols in fruits [47]. Higher concentrations of polyphenols can be found in the outer parts of fruits such as apples, watermelons, oranges, etc. The concentration of polyphenols present in some plant food can be as high as 500 mg per 100 g of food [48]. Table 2 lists various polyphenolic compounds and the fruits that contain them.
Antioxidant and health-promoting properties of fruit phytochemicals. The role of phytochemicals in cancer prevention and management. Cancer is a disease characterized by the abnormal growth of cells able to invade and metastasize to other areas of the body. This disease develops due to the changes taking place within the genes that control normal body functions [71]. It is one of the major health problems affecting people globally, both in developed and developing countries. About 18.1 million new cases of cancer were recorded in 2018 worldwide, and this number is expected to increase to about 23.6 million by 2030 [72].
The current remedies for cancer treatment include surgical removal and treatment of cancer cells with radiation accompanied by chemotherapy [73]. Chemoprevention of cancer makes use of natural and/or artificial mediators to interrupt carcinogenesis by inhibiting specific molecular signaling pathways. The chemotherapeutic mediators involved in cancer treatment may be grouped as blocking and suppressing agents [74, 75]. Chemotherapy has certain disadvantages, such as drug resistance, cancer recurrence, and harmful effects on non-targeted tissues. These are some of the challenges arising from the use of anticancer drugs for cancer treatment. To avoid the side effects emerging from the use of chemotherapeutic agents, scientists are investigating new anticancer agents with better effectiveness and fewer or no side effects [76]. Several epidemiological studies have found that eating fruits on a regular basis can significantly reduce the risk of developing cancer. Polyphenols offer protection to healthy body cells, as well as kill, or are toxic to, premalignant and malignant cells [74].
Screening plants for potent phytochemicals is a better alternative for improving cancer treatment due to their lower side effects. Phytochemicals are biologically active compounds that possess potent anti-tumor properties [76]. They participate in slowing or preventing carcinogenesis by mobbing free radicals, suppressing the survival and spreading of malignant cells, and reducing the invasiveness and angiogenesis of tumors [77–79]. Phytochemicals prevent cancer progression by acting on several molecular targets and signal transduction pathways, such as membrane receptors, downstream tumor-activator or suppressor proteins, kinases, transcription factors, caspases, microRNAs, and cyclins [76].
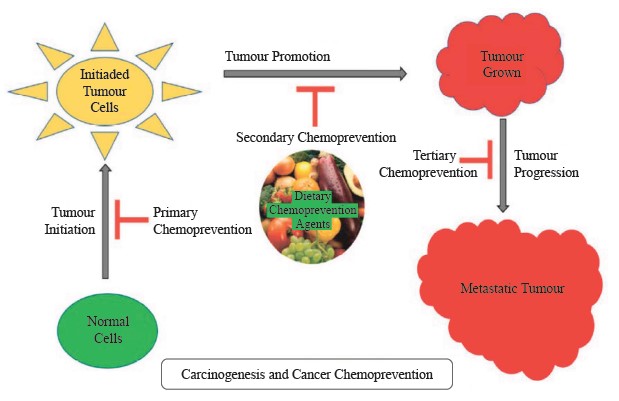
Apigenin, a flavonoid found in fruits, possesses potent anticancer properties [80]. According to the research, аpigenin in the amount of 5 mg/kg suppresses tumor growth while also reducing Ki67 expression and stimulating apoptosis in an athymic nude mouse xenograft with human chondrosarcoma Sw1353 cells. Apigenin can stimulate the expression of Bcl-2 family proteins and activate the caspase cascade to induce G2/M phase arrest and apoptosis [79]. The proliferation of tumor cells such as those in the lung, breast, colon, prostate, and liver can be suppressed by resveratrol (found in fruits such as grapes and berries) [81]. It has been shown to suppress the growth and metastasis of tumors in the lungs of mice with metastatic Lewis lung carcinoma tumors [82]. The antimetastatic and antitumor properties of resveratrol could be due to its ability to inhibit DNA synthesis, angiogenesis, and neovascularization [81]. Several signaling pathways can be altered by resveratrol to reduce the growth and proliferation of cancer cells, initiate programmed cell death, reduce inflammation and angiogenesis, as well as prevent tumor metastases [76]. Diets rich in lycopene content (e.g., fruits) are also shown to reduce the risk of prostate cancer diagnosis in men [83]. Polyphenolic compounds play a crucial role in cancer treatment and prevention by interrupting the initiation, promotion, and progression of cancer cells via the modification of several signal transduction pathways [84]. The role of phytochemicals in cancer prevention at various stages is demonstrated in Fig. 3 [74].
The role of phytochemicals in diabetes management. Diabetes mellitus is one of the main causes of death worldwide, with a prevalence of 8.8% in adults aged 20–79 years [52]. It is generally classified into three major groups, namely type 1 diabetes, type 2 diabetes, and gestational diabetes [85]. A lot of factors are responsible for the development of diabetes in individuals, with innate immunity regarded as the main factor in its pathophysiology [86]. Type 1 diabetes is caused by the immune system’s inflammatory response to pancreatic islet cells, which causes β-cells to lose function. In type 2 diabetes, low-grade systemic inflammation is a common mediator for the initiation and development of micro- and macro-vascular problems [87]. It is distinguished by insulin resistance and a loss of β-cell function, resulting in an insufficient supply of insulin to meet the body’s metabolic needs. People who suffer from type 2 diabetes are unable to control their blood sugar, which results in the excess accumulation of glucose in their blood and urine. Type 2 diabetes is an important public health concern because it accounts for 90% of all diabetes cases worldwide [88].
In recent times, phytochemicals have been under intense consideration for their use as drug candidates to prevent and treat several metabolic disorders such as hyperglycemia and dyslipidemia [52]. Phytochemicals induce important hypoglycemic effects and contribute to the prevention of diabetes-related vascular complications [89, 90]. Certain groups of flavonoids exhibit potent hypoglycemic properties by enhancing glucose and oxidative metabolism in diabetic conditions. The hypoglycemic property of phytochemicals has been established by studies in human and animal models of type 2 diabetes [91].According to Keshari et al., flavonoids such as naringenin, apigenin, and quercetin isolated from Fiscus racemose stems (these compounds are also present in fruits) demonstrated a hypoglycemic effect by reducing the levels of glucose from 300 to 185 mg/dL after one week of oral administration (100 mg/kg) in vivo [92]. Also, the administration of flavonoids to rats improves the glycogen content in the liver when compared with that of untreated diabetic rats. Flavonoids exert their antidiabetic activity by binding to glucose transporter 1 receptors and peroxisome proliferator-activated receptor gamma [92]. This promotes glucose uptake, lipid metabolism, improves insulin activity, and enhanced glucose tolerance in diabetic animals and humans [93]. Flavonoids act through different mechanisms, such as increasing the levels of superoxide dismutase, catalase, and glutathione in the pancreas, normalizing the levels of aspartate transaminase and alanine transaminase in plasma, and enhancing glucose uptake by the cells [94].
Anthocyanins, polyphenols present in fruits such as berries, can prevent type 2 diabetes and obesity. Anthocyanins influence glucose absorption, insulin secretion, level, and action, as well as lipid metabolism in both in vitro and in vivo studies. Several in vitro studies suggested that anthocyanins could decrease glucose absorption from the intestine by delaying the release of glucose during digestion [95]. Some phytochemicals with known anti-diabetic properties include ellagic acid (found in berries, dried fruits, and pomegranate), epigallocatechin gallate (cranberries, strawberries, cherries, pears, kiwi, peaches, black berries, apples, and avocados), naringenin (citrus fruits, cherries, and grapefruit), hesperetin (orange and lemon), chrysin (passion fruit), kaempferol (grapes, raspberry, strawberries, peach, cowberries, and apples), apigenin (grape fruit and orange), quercetin (apples and berries), and resveratrol (grapes) [52].
Anti-inflammatory properties of phytochemicals. Inflammation is a biological response to tissue damage which may be caused by harmful agents produced from biological, physical, or chemical sources. Inflammation can activate several signaling pathways, including phosphatidylinositol-3-kinase (PI3K), Janus-activated kinase (JAK), and mitogen-activated protein kinase (MAPK). Chronic inflammation activates chemokines, cytokines (IL-4, IL-5), inducible nitric oxide synthase (iNOS), signal transducer and activator of transcription 3 (STAT3), and cyclooxygenase enzyme (COX) [96]. Prolonged inflammation interrupts metabolism and induces stress in cells. As a result, it can cause inflammatory diseases such as asthma, autoimmune disorders, allergies, arthritis, and inflammatory bowel disease [96, 97]. Inflammatory diseases can be prevented or suppressed by blocking the pathways that produce inflammatory mediators, particularly proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukins (IL-1β, IL-6) [98].
Phytochemicals mediate inflammation through kinases such as mitogen-activated protein kinase and protein kinase C. Phytochemicals inhibit the activity of these enzymes by changing the DNA-binding potential of transcription factors such as nuclear factor kappa-B (NF-κB), one of the main effector molecules that mediates inflammation [99, 100]. Flavonoids such as quercetin inhibit the activity of enzymes such as cyclooxygenase and lipoxygenase in arachidonic acid metabolism to reduce the production of prostaglandins and leukotrienes [101, 102]. Apigenin inhibits the production of prostaglandin E2 (PGE2) and the activity of cyclooxygenase (COX-2), as well as NF-κB-dependent pathways [64]. Hesperidin is another phytochemical that inhibits the synthesis of pro-inflammatory mediators such as arachidonic derivatives, thromboxane A2, and prostaglandins E2 and F2 [103]. Nitric oxide is one of the main mediators of inflammation, and phytochemicals that can prevent its production without damaging endothelial or neuronal nitric oxide synthase (NOS) may be considered potent compounds for treating inflammation [104].
Antioxidant activity of phytochemicals. Antioxidants refer to molecules with the potential to react with free radicals by neutralizing or terminating chain reactions initiated by free radicals to prevent them from destroying other molecules [14]. Antioxidants can protect cells by converting reactive oxygen species to non-radical species, breaking auto-oxidative chain reactions, or reducing the concentration of localized oxygen [105]. Evidence of oxidative stress is implicated in several diseases, such as cancer, cardiovascular, neurological, and pulmonary diseases, rheumatoid arthritis, nephropathy, ocular diseases, and the induction of pre-eclampsia during pregnancy. These conditions develop when free radicals begin to alter cell membranes, proteins, lipoproteins, and DNA; stimulate the overexpression of specific genes; activate various kinases and transcription factors such as AP-1 and NF-kappa B; and contribute to the production of toxic peptides (β-amyloid) [106].
Natural antioxidants are primarily obtained from plants, as well as fruits, in the form of vitamins, carotenoids, flavonoids, tannins, alkaloids, terpenoids, isothiocyanates, lectins, polypeptides, and other phenolic compounds [107–110]. Phenolic compounds exhibit potent antioxidant activity, which is shown through their ability to neutralize the deleterious effects of free radicals and serve as antitumor, cardioprotective, and antimutagenic agents [111]. Studies have shown that the intake of dietary antioxidants, especially from fruits with a high total phenolic content, increases serum antioxidant scavenging capacity and provides a boost for the body’s antioxidant defense system [112, 113]. Flavonoids such as quercetin, combined with other bioactive compounds, exhibit strong antioxidant activities and are responsible for the disease-preventing properties of fruits. Quercetin is one of the most potent antioxidant compounds that protects the body against oxidative stress induced by amyloid deposits [64]. Rutin (quercetin 3-O-rhamnoglucoside), which is found in fruits, has a similar level of antioxidant activity. It exertsits antioxidant activity by donating electrons to free radicals to convert them into more stable and less reactive species. Enzymes that are involved in the production of reactive oxygen species can be inhibited by rutin to lower oxidative stress.
This can prevent diseases caused by oxidative stress, e.g.,neurodegenerative diseases [64, 114].
Carotenoids such as zeaxanthin, astaxanthin, and lutein are powerful lipid-soluble antioxidants that are involved in mobbing free radicals mainly in lipidsoluble environments. High intakes of carotenoids can prevent lipid oxidation and oxidative stress caused by free radicals [30]. Some carotenoids serve as precursors for vitamin A, while others possess effective antioxidant properties that act to scavenge reactive oxygen species. Carotenoids such as α-carotene, β-carotene, and cryptoxanthin are shown to have provitamin A activity, while lutein and lycopene have strong antioxidant activity [115]. Photo-oxidative damage, which occurs because of UV irradiation of the skin, affects macromolecules such as proteins, lipids, and DNA. This may lead to conditions such as premature skin aging, erythema, photodermatoses, and skin cancer. Intake of carotenoids from diets has been shown to improve skin texture, color, strength, and elasticity. Because of their antioxidant activity, carotenoids offer protection to the skin from the sun and harmful ultraviolet radiation [116]. Several studies have found that diets rich in phytochemicals with high antioxidant activity can help prevent a variety of chronic degenerative diseases caused by oxidative stress. Regular consumption of fruits is important for maintaining good health and disease prevention [117].
Bioavailability of phytochemicals and factors that hinder their bioavailability in the body. Digestion and absorption of phytochemicals in fruits. The absorption of phytochemicals from the food matrix into the body depends on their solubility, structure, degree of glycosylation or acylation, molecular size, the individual’s microbiome, and the presence of complementary compounds [118]. Most of phytochemicals exist as esters, glycosides, or polymers in food, while others covalently bind to cell wall components in food matrices, preventing their release for absorption in the gastrointestinal tract. Apart from isoflavonols, all flavonoids exist in their glycosylated form. However, aglycones and some glucosides, such as resveratrol and quercetin, are easily absorbed from the small intestine [22, 119, 120].
The absorption of phytochemicals from food (e.g., fruits) begins with chewing and digestion in the mouth. Chewing and the action of digestive enzymes, particularly α-amylase, aid in the reduction of food particle size and the release of bioactive compounds (polyphenols or carotenoids) from the food matrix [121]. The digest is delivered to the stomach, where digestive enzymes, an acidic pH, and further mixing disintegrate the food matrix to release more polyphenols or carotenoids. The acidic chyme from the stomach is released into an alkaline environment in the duodenum. Enzymes released by the liver and pancreas and the presence of bile salts at this stage also help release additional bioactive compounds and completely break down the food matrix into simple, absorbable macronutrient units. Polyphenols are then released into the bloodstream and delivered to target tissues. Those phenolic compounds which are not digested are metabolized by the microbiota in the colon to produce metabolites, which are also absorbed into the bloodstream [122]. Carotenoids are lipophilic compounds that are insoluble in aqueous solutions. In the stomach, they are packaged into micelles (which help transport fat-soluble materials) to make them more accessible to the intestinal epithelium for absorption into the bloodstream [34, 36].
Factors that may hinder the bioavailability of phytochemicals in fruits. Bohn defines bioavailability as the amount or concentration of a nutrient or non-nutrient substance absorbed into the human body to stimulate physiological activities or for storage [48]. Bioaccessibility, on the other hand, is defined as the amount of nutrients released from a food matrix during digestion, making the nutrient available or easily accessible for absorption in the gastrointestinal tract [22]. The ability of phytochemicals to exert health-promoting effects on the body depends primarily on their bioaccessibility and bioavailability [123]. Phytochemicals such as polyphenols exist in foods as polymers or in glycosylated form, which may hinder their absorption. To improve the bioavailability of phytochemicals from fruits, they must undergo hydrolysis by colonic microbiota and intestinal enzymes for easy absorption [123, 124]. The involvement of colonic microflora reduces the efficiency of polyphenols absorbed due to the degradation of aglycones to produce several simple aromatic acids during the process [49].
Some phytochemicals, such as tannins, can interact with proteins to form insoluble complexes, and this can limit or prevent their absorption [125, 126]. β-casein can also bind to (+)-catechins and (–)-epicatechin through its proline residues [127]. Phytochemicals can undergo different kinds of changes during gastrointestinal digestion, and those that are able to pass through the intestinal walls can either be metabolized or excreted, which reduces the level (low bioavailability) delivered to target organs or tissues for therapeutic effect [128].
Phytochemicals (such as flavonoids) can bind to components in the digestive system secretions such as saliva, pancreatic, and gastric juices. This may also reduce the amount absorbed into systemic circulation for physiological activities. Research has shown that phenolic compounds have a good affinity for proline-rich proteins and histatins present in human saliva. They either form a covalent or non-covalent bond to increase the size of the phenolic compound to prevent passage through absorptive cells of the small intestine [129]. Enzymes, such as pancreatic α-amylase and trypsin, have been shown to lose activity in the presence of phenolic compounds [130]. In addition, polyphenols can also bind to enterocyte brush border enzymes, and this prevents their release for absorption into the body [129].
ВЫВОДЫ
Fruits are rich sources of phytochemicals which perform several biological activities by acting as potent antioxidant, anticancer, antidiabetic, and anti-inflammatory agents. The modes of action of these bioactive compounds include scavenging free radicals or inhibiting the activity of enzymes and transcription factors that play a critical role in signaling pathways involved in disease initiation and progression. The excess accumulation of free radicals in the body and the inability of the body’s antioxidant defenses to effectively destroy free radicals cause oxidative stress and may lead to diseases such as cancer, diabetes, etc. It is therefore imperative that fruits rich in phytochemicals be regularly consumed to reduce an individual’s risk of suffering from several chronic disease conditions.
Вклад авторов
E.K. Danyo designed the research concept, wrote the original draft, and edited the manuscript. M.N. Ivantsova wrote, reviewed, and edited the manuscript. Both of the authors read and approved the final version of the manuscript.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare that there is no conflict of interest.
БЛАГОДАРНОСТИ
We gratefully acknowledge the research funding from the Ministry of Science and Higher Education of the Russian Federation (Ural Federal University Program of Development within the Priority-2030 Program).
СПИСОК ЛИТЕРАТУРЫ
- Khoo H-E, Nagendra Prasad K, Kong K-W, Jiang Y, Ismail A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules. 2011;16(2):1710–1738. https://doi.org/10.3390/molecules16021710
- Toydemir G, Gultekin Subasi B, Hall RD, Beekwilder J, Boyacioglu D, Capanoglu E. Effect of food processing on antioxidants, their bioavailability and potential relevance to human health. Food Chemistry: X. 2022;14. https://doi.org/10.1016/j.fochx.2022.100334
- Dontha S. A review on antioxidant methods. Asian Journal of Pharmaceutical and Clinical Research. 2016;9(2):14–32. https://doi.org/10.22159/ajpcr.2016.v9s2.13092
- Sas K, Robotka H, Toldi J, Vécsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. Journal of the Neurological Sciences. 2007;257(1–2):221–239. https://doi.org/10.1016/j.jns.2007.01.033
- Martí N, Mena P, Cánovas JA, Micol V, Saura D. Vitamin C and the role of citrus juices as functional food. Natural Product Communications. 2009;4(5):677–700. https://doi.org/10.1177/1934578X0900400506
- Jacob K, Periago MJ, Böhm V, Berruezo GR. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. British Journal of Nutrition. 2007;99(1):137–146. https://doi.org/10.1017/S0007114507791894
- Habanova M, Saraiva JA, Holovicova M, Moreira SA, Fidalgo LG, Haban M, et al. Effect of berries/apple mixed juice consumption on the positive modulation of human lipid profile. Journal of Functional Foods. 2019;60. https://doi.org/10.1016/j.jff.2019.103417
- Starowicz M, Achrem-Achremowicz B, Piskuła MK, Zieliński H. Phenolic compounds from apples: Reviewing their occurrence, absorption, bioavailability, processing, and antioxidant activity – A review. Polish Journal of Food and Nutrition Sciences. 2020;70(4):321–336. https://doi.org/10.31883/pjfns/127635
- Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Washington: AICR; 2007. 14 p.
- Saad B, Sing YY, Nawi MA, Hashim NH, Mohamed Ali AS, Saleh MI, et al. Determination of synthetic phenolic antioxidants in food items using reversed-phase HPLC. Food Chemistry. 2007;105(1):389–394. https://doi.org/10.1016/j.foodchem.2006.12.025
- Szymanska R, Pospíšil P, Kruk J. Plant-derived antioxidants in disease prevention 2018. Oxidative Medicine and Cellular Longevity. 2018;2018. https://doi.org/10.1155/2018/2068370
- Li H, Tsao R, Deng Z. Factors affecting the antioxidant potential and health benefits of plant foods. Canadian Journal of Plant Science. 2012;92:1101–1111. https://doi.org/10.4141/cjps2011-239
- Fernandez-Panchon MS, Villano D, Troncoso AM, Garcia-Parrilla MC. Antioxidant activity of phenolic compounds: From in vitro results to in vivo evidence. Critical Reviews in Food Science and Nutrition. 2008;48(7):649–671. https://doi.org/10.1080/10408390701761845
- Hajhashemi V, Vaseghi G, Pourfarzam M, Abdollahi A. Are antioxidants helpful for disease prevention? Research in Pharmaceutical Sciences. 2010;5(1):1–8.
- Rudrapal M, Khairnar SJ, Khan J, Dukhyil AB, Ansari MA, Alomary MN, et al. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Frontiers in Pharmacology. 2022;13. https://doi.org/10.3389/fphar.2022.806470
- Djenidi H, Khennouf S, Bouaziz A. Antioxidant activity and phenolic content of commonly consumed fruits and vegetables in Algeria. Progress in Nutrition. 2020;22(1):224–235.
- Law BMH, Waye MMY, So WKW, Chair SY. Hypotheses on the potential of rice bran intake to prevent gastrointestinal cancer through the modulation of oxidative stress. International Journal of Molecular Sciences. 2017;18(7). https://doi.org/10.3390/ijms18071352
- Wang S, Melnyk JP, Tsao R, Marcone MF. How natural dietary antioxidants in fruits, vegetables and legumes promote vascular health. Food Research International. 2011;44(1):14–22. https://doi.org/10.1016/j.foodres.2010.09.028
- Law MR, Morris JK. By how much does fruit and vegetable consumption reduce the risk of ischaemic heart disease? European Journal of Clinical Nutrition. 1998;52:549–556. https://doi.org/10.1038/sj.ejcn.1600603
- Ness AR, Powles JW. Fruit and vegetables, and cardiovascular disease: A review. International Journal of Epidemiology. 1997;26(1):1–13. https://doi.org/10.1093/ije/26.1.1
- Ramadan S, Ibrahim AAA. Fruits and vegetables as sources of functional phytochemicals for the prevention and management of obesity, diabetes, and cancer. In: Egbuna C, Hassan S, editors. Dietary phytochemicals. A source of novel bioactive compounds for the treatment of obesity, cancer and diabetes. Cham: Springer; 2021. pp. 147–167. https://doi.org/10.1007/978-3-030-72999-8_8
- Langston FMA, Nash GR, Bows JR. The retention and bioavailability of phytochemicals in the manufacturing of baked snacks. Critical Reviews in Food Science and Nutrition. 2023;63(14):2141–2177. https://doi.org/10.1080/10408398.2021.1971944
- Boyer J, Liu RH. Apple phytochemicals and their health benefits. Nutrition Journal. 2004;3. https://doi.org/10.1186/1475-2891-3-5
- Bartley GE, Scolnik PA. Plant carotenoids: Pigments for photoprotection, visual attraction, and human health. The Plant Cell. 19985;7(7):1027–1038. https://doi.org/10.1105/tpc.7.7.1027
- Marín A, Ferreres F, Tomás-Barberán FA, Gil MI. Characterization and quantitation of antioxidant constituents of sweet pepper (Capsicum annuum L.). Journal of Agricultural and Food Chemistry. 2004;52(12):3861–3869. https://doi.org/10.1021/jf0497915
- Saini RK, Nile SH, Park SW. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Research International. 2015;76(3):735–750. https://doi.org/10.1016/j.foodres.2015.07.047
- Meléndez-Martínez AJ, Mapelli-Brahm P, Benítez-González A, Stinco CM. A comprehensive review on the colorless carotenoids phytoene and phytofluene. Archives of Biochemistry and Biophysics. 2015;572:188–200. https://doi.org/10.1016/j.abb.2015.01.003
- Meléndez-Martínez AJ, Mandić AI, Bantis F, Böhm V, Borge GIA, Brnčić M, et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Critical Reviews in Food Science and Nutrition. 2022;62(8):1999–2049. https://doi.org/10.1080/10408398.2020.1867959
- Maoka T. Carotenoids as natural functional pigments. Journal of Natural Medicines. 2020;74:1–16. https://doi.org/10.1007/s11418-019-01364-x
- Chandra S, Sah K, Bagewadi A, Keluskar V, Shetty A, Ammanagi R, et al. Additive and synergistic effect of phytochemicals in prevention of oral cancer. European Journal of General Dentistry. 2012;1(3):142–147. https://doi.org/10.4103/2278-9626.105354
- Olsson ME, Gustavsson K-E, Andersson S, Nilsson Å, Duan R-D. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidant levels. Journal of Agricultural and Food Chemistry. 2004;52(24):7264–7271. https://doi.org/10.1021/jf030479p
- Nurul Fuad NI, Sekar M, Gan SH, Lum PT, Vaijanathappa J, Ravi S. Lutein: A comprehensive review on its chemical, biological activities and therapeutic potentials. Pharmacognosy Journal. 2020;12(6s):1769–1778. https://doi.org/10.5530/pj.2020.12.239
- Sathasivam R, Ki J-S. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Marine Drugs. 2018;16(1). https://doi.org/10.3390/md16010026
- Rodriguez-Concepcion M, Avalos J, Bonet ML, Boronat A, Gomez-Gomez L, Hornero-Mendez D, et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Progress in Lipid Research. 2018;70:62–93. https://doi.org/10.1016/j.plipres.2018.04.004
- Burri BJ, la Frano MR, Zhu C. Absorption, metabolism, and functions of β-cryptoxanthin. Nutrition Reviews. 2016;74(2):69–82. https://doi.org/10.1093/nutrit/nuv064
- Fernández-García E, Carvajal-Lérida I, Jarén-Galán M, Garrido-Fernández J, Pérez-Gálvez A, Hornero-Méndez D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Research International. 2012;46(2):438–450. https://doi.org/10.1016/j.foodres.2011.06.007
- Liu RH. Health-promoting components of fruits and vegetables in the diet. Advances in Nutrition. 2013;4(3):384S–392S. https://doi.org/10.3945/an.112.003517
- Khan J, Deb PK, Priya S, Medina KD, Devi R, Walode SG, et al. Dietary flavonoids: Cardioprotective potential with antioxidant effects and their pharmacokinetic, toxicological and therapeutic concerns. Molecules. 2021;26(13). https://doi.org/10.3390/molecules26134021
- Khan UM, Sevindik M, Zarrabi A, Nami M, Ozdemir B, Kaplan DN, et al. Lycopene: Food sources, biological activities, and human health benefits. Oxidative Medicine and Cellular Longevity. 2021;2021. https://doi.org/10.1155/2021/2713511
- Shahidi F, Ambigaipalan P, Chandrasekara A. Recent advances in phytochemicals in fruits and vegetables. In: Yahia EM, editor. Fruit and Vegetable Phytochemicals: Chemistry and human health, 2nd ed. Chichester: John Wiley & Sons; 2017. pp. 1323–1356. https://doi.org/10.1002/9781119158042.ch71
- Sova M, Saso L. Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients. 2020;12(8). https://doi.org/10.3390/nu12082190
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55(6):481–504. https://doi.org/10.1016/S0031-9422(00)00235-1
- Rudrapal M, Chetia D. Plant flavonoids as potential source of future antimalarial leads. Systematic Reviews in Pharmacy. 2017;8(1):13–18.
- Kondratyuk TP, Pezzuto JM. Natural product polyphenols of relevance to human health. Pharmaceutical Biology. 2004;42(1):46–63. https://doi.org/10.3109/13880200490893519
- Faller ALK, Fialho E. Polyphenol content and antioxidant capacity in organic and conventional plant foods. Journal of Food Composition and Analysis. 2010;23(6):561–568. https://doi.org/10.1016/j.jfca.2010.01.003
- King A, Young G. Characteristics and occurrence of phenolic phytochemicals. Journal of the American Dietetic Association. 1999;99(2):213–218. https://doi.org/10.1016/S0002-8223(99)00051-6
- Preti R, Tarola AM. Study of polyphenols, antioxidant capacity and minerals for the valorisation of ancient apple cultivars from Northeast Italy. European Food Research and Technology. 2021;247:273–283. https://doi.org/10.1007/s00217-020-03624-7
- Bohn T. Dietary factors affecting polyphenol bioavailability. Nutrition Reviews. 2014;72(7):429–452. https://doi.org/10.1111/nure.12114
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: Food sources and bioavailability. The American Journal of Clinical Nutrition. 2004;79(5):727–747. https://doi.org/10.1093/ajcn/79.5.727
- Patel SS, Goyal RK. Biotransformation of gallotannins from fresh fruit juice of Emblica officinalis in in-vitro system. Research Journal of Phytochemistry. 2013;7(1):18–23. https://doi.org/10.3923/rjphyto.2013.18.23
- Lipińska L, Klewicka E, Sójka M. The structure, occurrence and biological activity of ellagitannins: A general review. Acta Scientiarum Polonorum Technologia Alimentaria. 2014;13(3):289–299. https://doi.org/10.17306/J.AFS.2014.3.7
- Kong M, Xie K, Lv M, Li J, Yao J, Yan K, et al. Anti-inflammatory phytochemicals for the treatment of diabetes and its complications: Lessons learned and future promise. Biomedicine and Pharmacotherapy. 2021;133. https://doi.org/10.1016/j.biopha.2020.110975
- Debnath B, Singh WS, Das M, Goswami S, Manna K. Biodynamic activities of ellagic acid: A dietary polyphenol. Journal of Nature and Science of Medicine. 2020;3(2). https://doi.org/10.4103/JNSM.JNSM_32_19
- Fu L, Xu B-T, Xu X-R, Gan R-Y, Zhang Y, Xia E-Q, et al. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chemistry. 2011;129(2):345–350. https://doi.org/10.1016/j.foodchem.2011.04.079
- Bai J, Zhang Y, Tang C, Hou Y, Ai X, Chen X, et al. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomedicine and Pharmacotherapy. 2021;133. https://doi.org/10.1016/j.biopha.2020.110985
- Khoo HE, Azlan A, Kong KW, Ismail A. Phytochemicals and medicinal properties of indigenous tropical fruits with potential for commercial development. Evidence-Based Complementary and Alternative Medicine. 2016;2016. https://doi.org/10.1155/2016/7591951
- Liu J, Zhou H, Song L, Yang Z, Qiu M, Wang J, et al. Anthocyanins: Promising natural products with diverse pharmacological activities. Molecules. 2021;26(13). https://doi.org/10.3390/molecules26133807
- Anand David AV, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacognosy Reviews. 2016;10(20):84–89. https://doi.org/10.4103/0973-7847.194044
- Bae J, Kim N, Shin Y, Kim S-Y, Kim Y-J. Activity of catechins and their applications. Biomedical Dermatology. 2020;4. https://doi.org/10.1186/s41702-020-0057-8
- Naveed M, Hejazi V, Abbas M, Ali Kamboh A, Khan GJ, Shumzaid M, et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomedicine and Pharmacotherapy. 2018;97:67–74. https://doi.org/10.1016/j.biopha.2017.10.064
- Salehi B, Tsouh Fokou PV, Sharifi-Rad M, Zucca P, Pezzani R, Martins N, et al. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals. 2019;12(1). https://doi.org/10.3390/ph12010011
- Chen R, Qi Q-L, Wang M-T, Li Q-Y. Therapeutic potential of naringin: An overview. Pharmaceutical Biology. 2016;54(12):3203–3210. https://doi.org/10.1080/13880209.2016.1216131
- Kanazawa K, Sakakibara H. High content of dopamine, a strong antioxidant, in Cavendish banana. Journal of Agricultural and Food Chemistry. 2000;48(3):844–848. https://doi.org/10.1021/jf9909860
- Majdan M, Bobrowska-Korczak B. Active compounds in fruits and inflammation in the body. Nutrients. 2022;14(12). https://doi.org/10.3390/nu14122496
- Rodríguez-García C, Sánchez-Quesada C, Toledo E, Delgado-Rodríguez M, Gaforio JJ. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules. 2019;24(5). https://doi.org/10.3390/molecules24050917
- Zhang J, Chen J, Liang Z, Zhao C. New lignans and their biological activities. Chemistry and Biodiversity. 2014;11(1):1–54. https://doi.org/10.1002/cbdv.201100433
- Campos-Vidal Y, Herrera-Ruiz M, Trejo-Tapia G, Gonzalez-Cortazar M, Aparicio AJ, Zamilpa A. Gastroprotective activity of kaempferol glycosides from Malvaviscus arboreus Cav. Journal of Ethnopharmacology. 2021;268. https://doi.org/10.1016/j.jep.2020.113633
- Kim JK, Park SU. Recent studies on kaempferol and its biological and pharmacological activities. EXCLI Journal. 2020;19:627–634.
- Kim TW, Lee SY, Kim M, Cheon C, Ko S-G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death and Disease. 2018;9. https://doi.org/10.1038/s41419-018-0930-1
- Lopez-Lazaro M. Distribution and biological activities of the flavonoid luteolin. Mini-Reviews in Medicinal Chemistry. 2009;9(1):31–59. https://doi.org/10.2174/138955709787001712
- Ranjan A, Ramachandran S, Gupta N, Kaushik I, Wright S, Srivastava S, et al. Role of phytochemicals in cancer prevention. International Journal of Molecular Sciences. 2019;20(20). https://doi.org/10.3390/ijms20204981
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492
- Nussbaumer S, Bonnabry P, Veuthey J-L, Fleury-Souverain S. Analysis of anticancer drugs: A review. Talanta. 2011;85(5):2265–2289. https://doi.org/10.1016/j.talanta.2011.08.034
- George BP, Chandran R, Abrahamse H. Role of phytochemicals in cancer chemoprevention: Insights. Antioxidants. 2021;10(9). https://doi.org/10.3390/antiox10091455
- Landis-Piwowar KR, Iyer NR. Cancer chemoprevention: Current state of the art. Cancer Growth and Metastasis. 2014;7:19–25. https://doi.org/10.4137/CGM.S11288
- Choudhari AS, Mandave PC, Deshpande M, Ranjekar P, Prakash O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Frontiers in Pharmacology. 2020;10.
- Lee W-L, Huang J-Y, Shyur L-F. Phytoagents for cancer management: Regulation of nucleic acid oxidation, ROS, and related mechanisms. Oxidative Medicine and Cellular Longevity. 2013;2013. https://doi.org/10.1155/2013/925804
- Lu L, Zhao Z, Liu L, Gong W, Dong J. Combination of baicalein and docetaxel additively inhibits the growth of non-small cell lung cancer in vivo. Traditional Medicine and Modern Medicine. 2018;01(03):213–218. https://doi.org/10.1142/S2575900018500131
- Yan X-B, Xie T, Wang SD, Wang Z, Li H-Y, Ye Z-M. Apigenin inhibits proliferation of human chondrosarcoma cells via cell cycle arrest and mitochondrial apoptosis induced by ROS generation-an in vitro and in vivo study. International Journal of Clinical and Experimental Medicine. 2018;11(3):1615–1631.
- Madunić J, Madunić IV, Gajski G, Popić J, Garaj-Vrhovac V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Letters. 2018;413:11–22. https://doi.org/10.1016/j.canlet.2017.10.041
- Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12- dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Research. 2002;62:4945–4954.
- Kimura Y, Okuda H. Resveratrol isolated from Polygonum cuspidatum root prevents tumor growth and metastasis to lung and tumor-induced neovascularization in Lewis lung carcinoma-bearing mice. The Journal of Nutrition. 2001;131(6):1844–1849. https://doi.org/10.1093/jn/131.6.1844
- Chen J, Song Y, Zhang L. Lycopene/tomato consumption and the risk of prostate cancer: A systematic review and meta-analysis of prospective studies. Journal of Nutritional Science and Vitaminology. 2013;59(3):213–223. https://doi.org/10.3177/jnsv.59.213
- Ramos S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Molecular Nutrition and Food Research. 2008;52(5):507–526. https://doi.org/10.1002/mnfr.200700326
- Improving care and promoting health in populations: Standards of medical care in diabetes – 2019. Diabetes Care. 2019;42:S7–S12. https://doi.org/10.2337/dc19-S001
- Pollack RM, Donath MY, LeRoith D, Leibowitz G. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes Care. 2016;39:S244–S252. https://doi.org/10.2337/dcS15-3015
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. https://doi.org/10.1038/nature05485
- Zhao C, Yang C, Wai STC, Zhang Y, Portillo MP, Paoli P, et al. Regulation of glucose metabolism by bioactive phytochemicals for the management of type 2 diabetes mellitus. Critical Reviews in Food Science and Nutrition. 2019;59(6):830–847. https://doi.org/10.1080/10408398.2018.1501658
- He L, Wang H, Gu C, He X, Zhao L, Tong X. Administration of traditional Chinese blood circulation activating drugs for microvascular complications in patients with type 2 diabetes mellitus. Journal of Diabetes Research. 2016;2016. https://doi.org/10.1155/2016/1081657
- Xie W, Du L. Diabetes is an inflammatory disease: Evidence from traditional Chinese medicines. Diabetes, Obesity and Metabolism. 2010;13(4):289–301. https://doi.org/10.1111/j.1463-1326.2010.01336.x
- Bailey CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care. 1989;12(8):553–564. https://doi.org/10.2337/diacare.12.8.553
- Keshari AK, Kumar G, Kushwaha PS, Bhardwaj M, Kumar P, Rawat A, et al. Isolated flavonoids from Ficus racemosa stem bark possess antidiabetic, hypolipidemic and protective effects in albino Wistar rats. Journal of Ethnopharmacology. 2016;181:252–262. https://doi.org/10.1016/j.jep.2016.02.004
- Rebhun JF, Glynn KM, Missler SR. Identification of glabridin as a bioactive compound in licorice (Glycyrrhiza glabra L.) extract that activates human peroxisome proliferator-activated receptor gamma (PPARγ). Fitoterapia. 2015;106:55–61. https://doi.org/10.1016/j.fitote.2015.08.004
- Teoh SL, Das S. Phytochemicals and their effective role in the treatment of diabetes mellitus: A short review. Phytochemistry Reviews. 2018;17:1111–1128. https://doi.org/10.1007/s11101-018-9575-z
- Gaikwad SB, Krishna Mohan G, Rani MS. Phytochemicals for diabetes management. Pharmaceutical Crops. 2014;5:11–28. https://doi.org/10.2174/2210290601405010011
- Kim JG, Lim BO. Anti-inflammatory activity of fruit such as berries on the body. Japanese Journal of Gastroenterology and Hepatology. 2019;2.
- Tan WSD, Liao W, Zhou S, Wong WSF. Is there a future for andrographolide to be an anti-inflammatory drug? Deciphering its major mechanisms of action. Biochemical Pharmacology. 2017;139:71–81. https://doi.org/10.1016/j.bcp.2017.03.024
- Kim K-M, Kwon Y-G, Chung H-T, Yun Y-G, Pae H-O, Han J-A, et al. Methanol extract of Cordyceps pruinosa inhibits in vitro and in vivo inflammatory mediators by suppressing NF-κB activation. Toxicology and Applied Pharmacology. 2003;190(1):1–8. https://doi.org/10.1016/S0041-008X(03)00152-2
- Maleki SJ, Crespo JF, Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chemistry. 2019;299. https://doi.org/10.1016/j.foodchem.2019.125124
- Zhu F, Du B, Xu B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Critical Reviews in Food Science and Nutrition. 2018;58(8):1260–1270. https://doi.org/10.1080/10408398.2016.1251390
- Deng S, Palu AK, West BJ, Su CX, Zhou B-N, Jensen JC. Lipoxygenase inhibitory constituents of the fruits of noni (Morinda citrifolia) collected in Tahiti. Journal of Natural Products. 2007;70(5):859–862. https://doi.org/10.1021/np0605539
- Xiao X, Shi D, Liu L, Wang J, Xie X, Kang T, et al. Quercetin suppresses cyclooxygenase-2 expression and angiogenesis through inactivation of P300 signaling. PLoS ONE. 2011;6(8). https://doi.org/10.1371/journal.pone.0022934
- Benavente-García O, Castillo J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. Journal of Agricultural and Food Chemistry. 2008;56(15):6185–6205. https://doi.org/10.1021/jf8006568
- Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. Journal of Pharmacological Sciences. 2004;96(3):229–245. https://doi.org/10.1254/jphs.CRJ04003X
- Oroian M, Escriche I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Research International. 2015;74:10–36. https://doi.org/10.1016/j.foodres.2015.04.018
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. International Journal of Biomedical Science. 2008;4(2):89–96. https://doi.org/10.59566/IJBS.2008.4089
- Ali SS, Kasoju N, Luthra A, Singh A, Sharanabasava H, Sahu A, et al. Indian medicinal herbs as sources of antioxidants. Food Research International. 2008;41(1):1–15. https://doi.org/10.1016/j.foodres.2007.10.001
- Negi PS. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. International Journal of Food Microbiology. 2012;156(1):7–17. https://doi.org/10.1016/j.ijfoodmicro.2012.03.006
- Oz AT, Kafkas E. Phytochemicals in fruits and vegetables. In: Waisundara VY, Shiomi N, editors. Superfood and functional food – An overview of their processing and utilization. IntechOpen; 2017. https://doi.org/10.5772/66987
- Sikora E, Cieślik E, Topolska K. The sources of natural antioxidants. Acta Scientiarum Polonorum, Technologia Alimentaria. 2008;7(1):5–17.
- Tekos F, Makri S, Skaperda Z-V, Patouna A, Terizi K, Kyriazis ID, et al. Assessment of antioxidant and antimutagenic properties of red and white wine extracts in vitro. Metabolites. 2021;11(7). https://doi.org/10.3390/metabo11070436
- Prakash D, Upadhyay G, Pushpangadan P, Gupta C. Antioxidant and free radical scavenging activities of some fruits. Journal of Complementary and Integrative Medicine. 2011;8(1). https://doi.org/10.2202/1553-3840.1513
- Zhang Y-J, Gan R-Y, Li S, Zhou Y, Li A-N, Xu D-P, et al. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20(12):21138–21156. https://doi.org/10.3390/molecules201219753
- Park S-E, Sapkota K, Choi J-H, Kim M-K, Kim YH, Kim KM, et al. Rutin from Dendropanax morbifera Leveille protects human dopaminergic cells against rotenone induced cell injury through inhibiting JNK and p38 MAPK signaling. Neurochemical Research. 2014;39:707–718. https://doi.org/10.1007/s11064-014-1259-5
- Sidhu JS, Zafar TA. Bioactive compounds in banana fruits and their health benefits. Food Quality and Safety. 2018;2(4):183–188. https://doi.org/10.1093/fqsafe/fyy019
- Walia A, Gupta AK, Sharma V. Role of bioactive compounds in human health. Acta Scientific Medical Sciences. 2019;3(9):25–33.
- Ferreira Gomes CC, de Siqueira Oliveira L, Rodrigues DC, Ribeiro PRV, Canuto KM, Duarte ASG, et al. Evidence for antioxidant and anti‐inflammatory potential of mango (Mangifera indica L.) in naproxen‐induced gastric lesions in rat. Journal of Food Biochemistry. 2021;46(3). https://doi.org/10.1111/jfbc.13880
- Epriliati I, Irine R. Bioavailability of phytochemicals. In: Rao AV, editor. Phytochemicals – A global perspective of their role in nutrition and health. IntechOpen; 2012. https://doi.org/10.5772/26702
- Felgines C, Talavéra S, Texier O, Lamaison J-L, Gonthier M-P, Scalbert A, et al. Strawberry anthocyanins are recovered in urine as glucuro- and sulfoconjugates in humans. The Journal of Nutrition. 2003;133(5):1296–1301. https://doi.org/10.1093/jn/133.5.1296
- Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. The American Journal of Clinical Nutrition. 2005;81(1):243S–255S. https://doi.org/10.1093/ajcn/81.1.243S
- Lewandowska U, Szewczyk K, Hrabec E, Janecka A, Gorlach S. Overview of metabolism and bioavailability enhancement of polyphenols. Journal of Agricultural and Food Chemistry. 2013;61(50):12183–12199. https://doi.org/10.1021/jf404439b
- Shi M, Gu J, Wu H, Rauf A, Emran TB, Khan Z, et al. Phytochemicals, nutrition, metabolism, bioavailability, and health benefits in lettuce – A comprehensive review. Antioxidants. 2022;11(6). https://doi.org/10.3390/antiox11061158
- Polia F, Pastor-Belda M, Martínez-Blázquez A, Horcajada M-N, Tomás-Barberán FA, García-Villalba R. Technological and biotechnological processes to enhance the bioavailability of dietary (poly)phenols in humans. Journal of Agricultural and Food Chemistry. 2022;70(7):2092–2107. https://doi.org/10.1021/acs.jafc.1c07198
- Selma MV, Espín JC, Tomás-Barberán FA. Interaction between phenolics and gut microbiota: Role in human health. Journal of Agricultural and Food Chemistry. 2009;57(15):6485–6501. https://doi.org/10.1021/jf902107d
- Lu Y, Bennick A. Interaction of tannin with human salivary proline-rich proteins. Archives of Oral Biology. 1998;43(9):717–728. https://doi.org/10.1016/S0003-9969(98)00040-5
- Wróblewski K, Muhandiram R, Chakrabartty A, Bennick A. The molecular interaction of human salivary histatins with polyphenolic compounds. European Journal of Biochemistry. 2001;268(16):4384–4397. https://doi.org/10.1046/j.1432-1327.2001.02350.x
- Arts MJTJ, Haenen GRMM, Wilms LC, Beetstra SAJN, Heijnen CGM, Voss H-P, et al. Interactions between flavonoids and proteins: Effect on the total antioxidant capacity. Journal of Agricultural and Food Chemistry. 2002;50(5):1184–1187. https://doi.org/10.1021/jf010855a
- Velderrain-Rodríguez GR, Palafox-Carlos H, Wall-Medrano A, Ayala-Zavala JF, Chen C-YO, Robles-Sánchez M, et al. Phenolic compounds: Their journey after intake. Food and Function. 2014;5:189–197. https://doi.org/10.1039/C3FO60361J
- Laurent C, Besançon P, Caporiccio B. Flavonoids from a grape seed extract interact with digestive secretions and intestinal cells as assessed in an in vitro digestion/Caco-2 cell culture model. Food Chemistry. 2007;100(4):1704–1712. https://doi.org/10.1016/j.foodchem.2005.10.016
- Rohn S, Rawel HM, Kroll J. Inhibitory effects of plant phenols on the activity of selected enzymes. Journal of Agricultural and Food Chemistry. 2002;50(12):3566–3571. https://doi.org/10.1021/jf011714b