Аннотация
Polyphenols are present as nutrient components in many functional food formulations. However, their bioavailability is quite low, and they tend to degrade under extreme technological conditions, e.g., heating, pH, etc. Moreover, polyphenols are known for their specific bitter taste. As a result, a large amount of polyphenols spoils the sensory properties of the finished product. Encapsulation seems a prospective solution to this problem. This article provides a comprehensive review of scientific publications on various methods of polyphenol encapsulation.The review covered publications registered in PubMed, Google Scholar, ResearchGate, Elsevier, eLIBRARY.RU, and Cyberleninka in 2002–2023 with a focus on original research articles published after 2012. The search involved such keywords as polyphenols, encapsulation, flavonoids, delivery systems, and functional products.
Encapsulating materials are made of organic or inorganic substances, as well as of their combinations. Mineral salts delay the contact between polyphenols and taste buds. However, they are not resistant enough to gastric juice. In this respect, organic matrices are more effective. Carbohydrates protect active molecules from degradation in the stomach. Liposomes increase the bioavailability of polyphenols. Milk or whey proteins also proved quite effective for a number of reasons. First, they mask the astringent taste, which makes it possible to include more polyphenols in functional food formulations. Second, the resulting product is fortified with valuable proteins and essential amino acids. Third, high concentrations of polyphenols possess enough antioxidant properties to increase the shelf-life.
Polyphenol encapsulation is an effective method of functional product design, especially in the sphere of foods made for dietary nutrition, sports, preventive diets, etc.
Ключевые слова
Polyphenols, biological activity, encapsulation, functional ingredientsВВЕДЕНИЕ
Recent decades have seen a surge of scientific interest in phenolic compounds of plant origin in the fields of physiology, medicine, and functional foods. Polyphenols possess antioxidant, anticarcinogenic, antimicrobial, anti-inflammatory, and organoprotective properties [1–7]. Foods fortified with primary elements, micronutrients, and biologically active substances contribute to treatment and prevention of major cardiovascular, endocrine, and oncological diseases.
Polyphenols with their beneficial properties are an important part of any healthy diet. The Federal Service for Surveillance on Consumer Rights Protection and Human Welfare was the first to specify the content of polyphenols and flavonoids in its Dietary Recommendations [8].
In nature, polyphenols occur in very small quantities. Moreover, they usually remain out of human consumption, being discarded as production waste, e.g., grape peels and seeds in wine production, citrus peels and membranes in juice production, olive pomace in olive oil production, etc. [9]. Given their low bioavailability, mere dietary adjustments cannot provide human body with polyphenols, which makes food fortification the only option available. Unfortunately, their bitter and astringent taste gives polyphenols a rather unfavorable sensory profile. Free polyphenols interact with salivary mucin and weaken its coating effect on taste buds, resulting in a specific taste. Encapsulation can camouflage the unpleasant taste of polyphenols while improving their resistance to some external factors, preserving biological activity, and even increasing bioavailability [9–14]. For instance, the pharmaceutical industry uses encapsulation to create new polyphenol-based drugs [15–17]. In the food industry, phenolic compounds with their antioxidant effect can extend the shelf-life of finished products [18, 19]. Therefore, the success of new polyphenol-containing functional foods depends on the correct choice of encapsulating material and technological conditions.
In our study, we focused on scientific publications on various methods of polyphenol encapsulation.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
This review covered publications registered in PubMed, Google Scholar, ResearchGate, Elsevier, eLIBRARY.RU, and Cyberleninka databases in 2002– 2023, with the focus on original studies published within the last 10 years in peer-reviewed sources. The search involved such keywords as polyphenols, encapsulation, flavonoids, delivery systems, and functional products.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
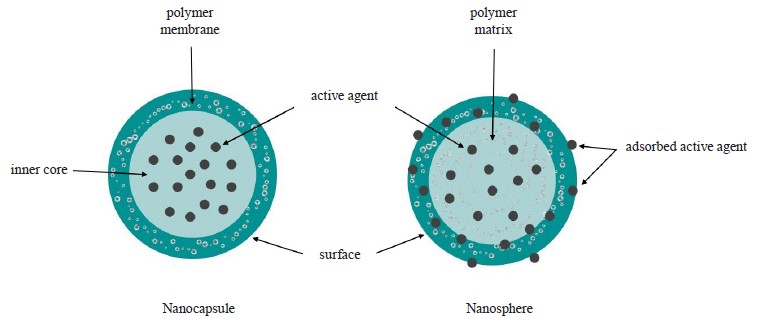
Encapsulation technologies. During encapsulation, a certain carrier substance develops particles, or capsules, that capture some other substance or a group of similar substances. Encapsulation isolates the biologically active substance from the environment. Various physicochemical interactions allow the carrier substance to include biologically active substances in the capsule wall or envelop them. Capsule particles can be of several types. Microspheres contain the active substance inside or as part of the wall. In microcapsules, the active substance is coated with the wall of some natural or synthetic carrier (Fig. 1) [17]. Particles vary in size from several micrometers to several millimeters.
Encapsulation is a well-tested reliable technology that makes it possible to modify the necessary properties and deliver all kinds of polyphenolic compounds. This method improves the bioavailability of polyphenols, masks the astringency, increases the shelf-life, and delivers molecules to specific parts of the intestine for metabolism or absorption.
Modern research publications feature such encapsulation technologies as spray (freeze) drying, spray cooling, extrusion coating, fluidized bed encapsulation, liposome capture, simple/complex coacervation, gelation, etc.
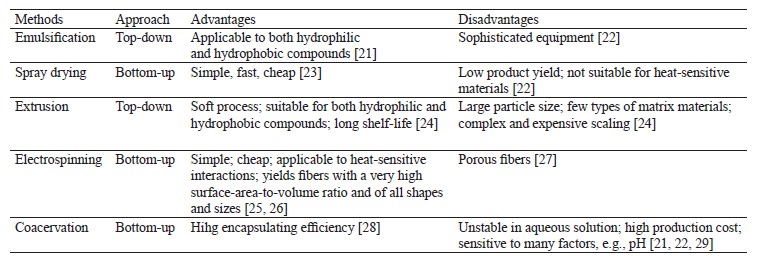
All encapsulation methods can be divided according to the strategy employed as top-down or bottom-up. The top-down strategy occurs in extrusion of emulsification. It breaks large solid or liquid structures into smaller fragments by external force, e.g., cutting, impact, or pressure. This method requires special equipment, e.g., homogenizers, which increases its operating and maintenance costs.
The bottom-up strategy uses self-organization potential to form larger molecules. Microcapsules appear when the components to be encapsulated and the carrier are mixed together in a solution. The process is sensitive to pH, temperature, ionic strength, etc., but it is energy efficient and provides microcapsules of preprogrammed shape and size. Coacervation, spray drying, electrospinning, bonding, and antisolvent precipitation are the most popular bottom-up strategy methods [20].Table 1 illustrates the advantages and disadvantages of each method [20].
In each specific case, the choice of encapsulation method depends on the chemical nature and properties of the molecules and the equipment available. Properly selected conditions make it possible to obtain capsules of the required structure, shape, and size.
The choice of optimal carrier medium is also of great importance because it delivers active molecules to certain parts of digestive tract, protects them from aggressive enzymes, ensures their bioavailability and thermal stability, improves sensory properties, etc. [30–34].
A wide range of mineral and organic substances can serve as carrier media during encapsulation.
Inorganic carriers. The bitterness of some polyphenols, e.g., green tea catechins, can be avoided by binding with inorganic salts, e.g., carbonates, phosphates, calcium and sodium chlorides, etc. Elabbadi et al. reported that this method masked the bitter taste without affecting the bioavailability of catechins, which did not degrade in the gastrointestinal tract [35]. They used the coprecipitation method by mixing a green tea extract with sodium carbonate and/or phosphate and adding calcium chloride. The resulting microcapsules proved resistant to washing, the maximal binding efficiency being as high as 65%. Such variables as flow rate, cations-to-anions ratio, and total ions also affected the degree of binding, particle sizes, and their tendency to agglomerate. The carbonate content was especially important. The optimal carbonate content was 75% since catechins appeared to be unstable in alkaline environment. When the proportion of carbonate was low, catechins were leached from the capsules during washing. Green tea extracts usually contain a mix of different catechins. Some catechins, e.g., catechin gallate, gallocatechin gallate, and gallocatechin methyl gallate, could bind more efficiently than the corresponding epicatechins. Gallates were better binders than similar molecules with no gallate radical.
More complex microcapsule compositions may include calcium chloride. For example, the bonds between calcium cations and carbonyl groups of pectinamide resulted in a network that stabilized the structure of the capsule [36]. When Wang et al. tried to encapsulate ferulic acid with zein, calcium chloride affected the structure of the resulting complexes [37]. The proportion of α-helices and β-sheets dropped whereas the proportion of disordered turns increased, resulting in smooth spherical particles. Calcium chloride was also reported as a hardening agent in combination with organic carriers [38].
Mineral carriers mask the bitter taste of polyphenols because capsules pass through the oral cavity very quickly to dissolve elsewhere in the digestive tract. The biggest disadvantage of mineral salts is that they cannot modify the breakdown and subsequent adsorption of polyphenols in the gastrointestinal tract. In this regard, bioorganic carriers seem to have better prospects. Carbohydrates, lipids, proteins, and their combinations are valuable nutrients that are able to protect polyphenols from aggressive hydrochloric acid and enzymes. They deliver polyphenols to certain parts of the gastrointestinal tract and/or increase their bioavailability.
Carbohydrates. The food industry knows a wide variety of carbohydrates, e.g., maltodextrin, β-cyclodextrin, pullulan, gelled glucan, curdlan, sodium alginate, pectin, etc. Their polysaccharides are stable at low pH; as a result, active components manage to reach the intestine intact to be absorbed there.
Dey et al. constructed a model of artificial digestion to encapsulate quercetin with gellan gum and registered the maximal release of quercetin at pH 7.4 [39]. Vallejo-Castillo et al. used a pectin-alginate composite to obtain enteric capsules with a papaya exocarp extract, which is rich in rutin and transferulic acid [40]. Guzmán-Díaz et al. included green tea extracts in double emulsions of chia seed mucilage [41]. After adding thixogam and carrageenan, they ensured the maximal release of catechins in the intestinal phase while preserving their antioxidant activity. Another study used chitosan, maltodextrin, and gum arabic to mask the astringent taste, increase the antioxidant activity, and prolong the shelf-life [42].
Although the encapsulation mechanisms are similar, each carrier has some specific properties. For example, alginate is stable in acidic environment while alkaline environment makes its capsules swell and disintegrate. Chitosan has favorable antibacterial and antioxidant properties, as well as effective mucoadhesion [43, 44]. Chitosan binds cholesterol and controls the release of active substances but dissolves only under acidic conditions. Guar gum is highly resistant to digestive enzymes and very stable in the upper gastrointestinal tract [45]. Between corn starch and quinoa-derived starch, quinoa starch appeared to form smaller quercetin capsules, which were more effective in protecting the active substance during storage [46]. Similar data were reported by Remanan and Zhu for rutin [47].
Polyphenols are known for their low hydrophilicity, which restricts the technological process and complicates absorption in the gastrointestinal tract. Cyclodextrin glucanotransferase triggers the development of cycloamylose. As it encapsulates resveratrol, its solubility in water increased by 6000 times, but the chemical stability, antioxidant effect, and anti-inflammatory properties remained the same [48].
The matrix material also affects the stability of the encapsulated active substance. Capsules based on a combination of alginate and guar gum were able to preserve anthocyanins during two weeks of storage [45]. Other research teams reported similar results [49]. In addition, alginate capsules proved very effective in preserving phenolic acids [45].
Polyphenol may both increase and decrease in content during storage as condensed molecules depolymerize and monomers bind to the capsule matrix. Composite matrices maintain higher antioxidant values of polyphenols, both in fresh capsules and after two or three weeks of storage, especially with guar gum.
Anthocyanins are the most common plant pigments, which makes them popular food colorants. The color intensity of encapsulated concentrates was reported to grow during storage, especially when a composite matrix was involved. Chitosan increased the intensity of the yellow color while alginate or its combination with guar gum intensified the red color [45]. Vergara et al. encapsulated extracts of purple potatoes, which owe their color to anthocyanins [50]. Purple potatoes have good prospects as a natural and safe food colorant. The scientists achieved 86% encapsulation efficiency by using maltodextrin. The resulting capsules provided higher stability of anthocyanins during storage. As much as 45% of the encapsulated anthocyanins remained after 138 days at 60°C while the control sample managed to maintain only 10% of the original amount after two days. A similar protective effect was reported for a gastrointestinal digestion model.
Costanzo & Angelico used encapsulation to preserve the active components of Silybum marianum extracts, improve their antioxidant and anti-inflammatory activity, and increase their bioavailability, thus raising the effectiveness of the final medicinal product [51]. Such extracts exhibit various important physiological effects and include a lot of biologically active substances, e.g., flavolignans, flavonoids, silibin A, silybin B, isosilybin A, isosilybin B, silicristin, isosilicristin, silydianin, taxifolin, etc. [52–55].
Sansone et al. used carboxymethylcellulose as coating and sodium lauryl sulfate as surfactant [56]. The liquid phase consisted of water, ethyl alcohol, and acetone in a ratio of 50:15:35. Spray drying made it possible to obtain the desired particle size and increase the bioavailability of plant components. If modified, this method can be used to encapsulate other natural biologically active substances.
Lachowicz et al. used maltodextrin and inulin to produce juneberry extracts, which they dried by vacuum or freeze drying to obtain powder [57]. Maltodextrin rendered the powders a higher content of polyphenols from berries and juices while inulin was more effective with berry peel samples. Freeze drying provided more active components and better antioxidant indicators than vacuum drying. Such powders can become an effective fortification means for functional foods with biologically active components.
Ćorković et al. performed a comparative analysis of pectin and alginate as encapsulating carriers for polyphenols and volatile substances in chokeberry juice [38]. Spectroscopy and high-performance liquid chromatography showed that alginate capsules had a higher concentration of polyphenols. Also, alginate capsules had better antioxidant results according to some other tests, e.g., FRAP (Ferric Reducing/Antioxidant Power), CUPRAC (CUPric Reducing Antioxidant Capacity), DPPH (2,2-diphenyl-1-picrylhydrazyl), and ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid).
Haładyn et al. used a combination of polysaccharides to encapsulate chokeberry concentrates and powder [45]. The researchers obtained the maximal content of active substances using a three-component carrier while the minimal one belonged to alginate capsules. Three-component matrices also demonstrated the greatest agreement between the polyphenolic profile of the capsules and the original concentrate and powder. Apparently, different polysaccharides differed in their abilities to bind fractions of phenolic compounds. Therefore, multicomponent carriers proved more effective in encapsulating natural extracts with complex chemical compositions.
To sum it up, carbohydrates solve a wide range of problems, from stabilizing the active substances, maintaining favorable physiological properties, and increasing bioavailability, to improving the sensory profile of the final product. By choosing the optimal carrier and technological conditions, food producers can obtain particles of a particular size with a lot of active substance.
Lipids. Emulsions, liposomes, nanoliposomes, and solid lipid particles are popular means of encapsulation. They deliver biologically active molecules in the food industry, pharmacy, and cosmetics [58–60]. Hydrophilic components are included in the aqueous fraction in the center of the liposome while fat-soluble components are distributed in the lipid layer. Amphiphilic phospholipids are natural components of cell membranes and reliable liposome agents. They are found in soy, sunflower, and egg lecithin. Phospholipids from rice bran were reported to produce nanoliposomes with quercetin [61]. Jahanfar et al. prepared encapsulated rosemary extract using phosphatidylcholine by freeze-drying at 70°C [62].
Lipid microparticles can be a mix of hydrogenated and interesterified vegetable oils. Cutrim et al. heated oils and tea extracts to 80°C to dry them in a cooled medium in a mini spray dryer [63]. As a result, they achieved an encapsulation efficiency of 83.5%.
Pan et al. applied the рН-driven encapsulation method to low-soluble polyphenols [64]. The method relied on the deprotonation of hydroxyl groups in a highly alkaline environment. As a result, negatively-charged molecules increased the solubility of the capsule. However, this method is quite limited because some polyphenols become unstable in alkaline environment. A greater number of hydroxyl groups increases the chance of oxidation. Therefore, quercetin is less stable than resveratrol and curcumin, which reduces its encapsulation efficiency.
Peng et al. reported that encapsulation depends on the concentration [65]. For example, curcumin exhibited its maximal solubility at a concentration of 0.36 mg/mL. By increasing the concentration of curcumin in the solution, the scientists achieved no increase in its uptake by liposomes. Resveratrol was reported to demonstrate a similar pattern. However, quercetin increased its encapsulating abilities at larger concentrations because it went unstable in alkaline environment. Therefore, this type of encapsulation depends on two factors, i.e., solubility in water and stability at high pH values.
Moghaddasi et al. combined black pepper oil with polysorbate 80 to obtain an emulsion [66]. They managed to optimize the solubility of curcumin and obtain microcapsules of 16 nm in size with a better antioxidant profile than in an aqueous curcumin suspension. Kumar et al. encapsulated resveratrol using the ultrasound method in a nanoemulsion with lecithin in combination with polysorbate 80 [67]. The particle size reached 20 nm, and the binding efficiency was as high as 99%.
Resveratrol emulsion turned out to be more resistant to ultraviolet irradiation than its aqueous or alcoholic solutions. Both liquid (medium chain fatty acids + polysorbate 80) and semi-solid resveratrol nanoemulsions (laurylpolyoxyl-32 glyceride + caprylcaproylpolyoxyl-8 glyceride) were more bioavailable as they were more efficient in penetrating the wall of the small intestinal [68]. Emulsion stability and encapsulation efficiency could be increased by adding magnesium salts and poloxamers, i.e., molecules containing central hydrophobic and lateral hydrophilic regions [69].
Liposomes make it possible to encapsulate not only isolated substances, but also their combinations. For instance, liposomes with curcumin and resveratrol were more stable during storage than liposomes containing only one type of polyphenol. Spectroscopic and fluorescence analyzes made it possible to evaluate the distribution of different molecules in a liposome. Thus, curcumin predominated in the hydrophobic region while resveratrol predominated in the hydrophilic region. The resulting synergistic effect of polyphenols on liposome stability provided an encapsulation efficiency of 80.42% and a better preservation of antioxidant properties [70].
Chen et al. succeeded in increasing antioxidant activity by encapsulating epigallocatechin gallate and quercetin in liposomes [71]. Conversely, polyphenols were reported to reduce lipid oxidation [72]. This property can be used to fortify products with valuable oils and essential fatty acids.
Exosomes are phospholipid-based membrane vesicles. In humans and animals, exosomes are produced by many tissues and are responsible for intercellular transport. Some technologies make it possible to extract vesicles from biological fluids, e.g., cow’s milk, and incorporate various biologically active or medicinal substances [73–76]. Vashisht et al. described the technology for producing exosomes from milk. In their study, exosomal curcurmin was more stable and bioavailable, compared to the free control [77]. Other authors reported similar data [78, 79]. Extracellular vesicles of plant origin are known to contain biologically active components, including polyphenols [80, 81]. Such vesicles have good prospects as a delivery system for nutrients and pharmacological substances meant for oral administration.
Thus, lipid encapsulation increases the stability and bioavailability of polyphenols while maintaining their physiological activity.
Proteins. Encapsulation of polyphenols in proteinbased nanoparticles is a popular research topic. Proteins seem an appropriate solution to the sensory characteristics’ problem that producers of fortified foods often have to face. However, proteins affect the color of the final product and may make the solution opaque [82].
Protein-polyphenol interaction is based on both covalent and non-covalent bonds, e.g., hydrogen, hydrophobic, or Van der Waals bonds [83]. Larger and more hydrophobic molecules attach to protein more effectively. Hydrophobic bonds develop the interaction between the aromatic rings of polyphenols and the pyrrolidine rings of proline residues. Hydrogen bonds are formed by hydrogen acceptors in the protein and hydroxyl groups of polyphenols. Catechins bind to β-lactoglobulin (β-Lg) as the number of hydroxyl groups increases in the series of epigallocatechin gallate > epigallocatechin > epicatechin > catechin [84].
Flavonols follow the same pattern when interacting with collagen. An increase in the number of hydroxyl groups in the kaempferol – quercetin – myricetin chain raises the melting point of the complex, which indicates its greater stability [85]. The amount of gallate radicals is another important factor. Spectroscopy and fluorescence tests showed that the bond between epigallocatechin gallate and β-Lg is stronger than that of epigallocatechin [86].
The mature of protein also affects the encapsulation process. Hydrophobic proteins attach better to polyphenols if the former are rich in proline and basic residues, as well as possess an open and mobile structure. In a study of whey proteins, the more hydrophobic β-lactoglobulin (β-Lg) had a greater affinity for polyphenols than α-lactalbumin (α-La) [87]. If preheated, proteins facilitate the formation of bonds with polyphenols. At ≥ 60°C, covalent bonds between epigallocatechin and β-Lg occurred fast, with lysine residues acting as a binding site [88]. Open-structured native globular proteins often demonstrate intramolecular interactions that promote protein aggregation [89].
These interactions depend on the molecular structure. For instance, a comparative analysis of galangin and genistein showed that galangin had a greater affinity for whey proteins and caseinate due to its flatter stereochemical structure and random B-ring rotation. Genistein, on the other hand, demonstrated localized isomerism and a twisted stereochemical structure [87, 90].
Jia et al. observed hydrogen and Van der Waals bonds during the formation of complexes of β-Lg with phenolic chlorogenic and ferrulic acids whereas hydrophobic interactions predominated in complexes with epigallocatechin gallate [91]. Temperature also proved an important variable. Under thermal treatment, chlorogenic and ferullic acids developed weaker bonds with β-Lg while epigallocatechin gallate bonds grew stronger [91].
The ability of proteins to bind to phenolic compounds are limited. For example, flexible gelatin molecules were reported to be able to bind to punicalagins (pomegranate polyphenols from the tannin group), whereas bovine serum albumin failed [92]. To select a protein carrier, functional food developers are to find out how a specific protein binds to a specific phenolic compound.
Scientific articles describe numerous methods for encapsulating polyphenols with proteins.
For example, Rodríguez-Félix et al. produced quercetin nanoparticles from zein by electrospraying [18]. They used Fourier transform infrared spectroscopy (FTIR) to establish the hydrogen bonds between quercetin and protein molecules. Such nanoparticles can increase the bioavailability of quercetin in medicine. Their antioxidant properties can prolong the shelf-life of functional foods. Bruni et al. also used zein to encapsulate leaf extracts of Paraguayan holly (yerba mate tea) by the method of electrospinning [93]. The resulting fibers retained antioxidant activity and increased the thermal stability of phenolic extracts, compared to the non-encapsulated samples.
When encapsulated by gelation of β-Lg matrix, epigallocatechin gallate could protect catechins in the stomach, thus preserving them intact to be absorbed in the intestine [94]. Another study involved thermally denatured β-Lg [95]. In the abovementioned studies, particle size and encapsulation efficiency depended on such variables as pH, temperature, molar ratio of β-Lg vs. epigallocatechin gallate, and β-Lg concentration.
Lestringant et al. encapsulated epigallocatechin gallate into a native β-Lg carrier, which they heated to 85°C to remove the solvent from the solution [96]. They tested the resulting native complex of β-Lg and epigallocatechin gallate for stability, physical properties, and bioactivity. The complexes showed comparable stability and binding efficiency to heated β-Lg nanoparticles at a molar ratio of 1:1 epigallocatechin gallate/β-Lg. The heated and desolvated β-Lg nanoparticles were of similar size; however, the desolvated samples possessed the highest binding affinity for epigallocatechin.
Cheng et al. reported that resveratrol, folic acid, and α-tocopherol bound with the central cavity of the outer surface of β-Lg [97]. The binding occurred near the amino acids of tryptophan (position 19) and arginine (position 124). Another binding area was the hydrophobic pocket in the space between α-helix and β-sheet structure of the protein. Therefore, β-Lg can simultaneously bind three biologically active substances to form protein-polyligand complexes. Xiang et al. described β-Lg as a transport molecule capable of binding low molecular weight lipophilic ligands, including polyphenols [98].
A pH-dependent encapsulation with casein can be applied to substances with low hydrophilicity, e.g., cur- cumin. The same method was once applied to nanoliposomes [99]. At pH 12, sodium caseinate dissociated and curcumin deprotonated. The subsequent neutralization triggered encapsulation of curcumin into self-assembling casein capsules, which were then spray dried. Ultraviolet and nuclear magnetic resonance spectroscopies revealed that sodium caseinate prevented curcumin from degradation, as a rule, in alkaline environment. This cheap technology enhances the biological properties of curcumin, in particular, its antiproliferative effect against colon and pancreatic cancer cells.
Xue et al. used a nanocomplex of glycosylated casein to encapsulate epigallocatechin gallate [100]. The resulting capsules did not aggregate during storage. An intestinal digestion model in vitro showed that the nanocomplex had a slow and prolonged release. Li et al. encapsulated naringenin with micellar casein and detected an increase in its solubility, as evidenced by its concentration in the aqueous phase [101]. Such capsules demonstrated a good potential as carriers of hydrophobic nutrients, e.g., flavonoids.
Polyphenols themselves can modify the biological and technological properties of proteins, increased whey proteins. Phenolic compounds are able to change the secondary structure of proteins, which is a scientifically proven fact.
Kanakis et al. used spectroscopic methods, i.e., circular dichroism and IR spectroscopy with Fourier transform [84]. Hydrophobic and hydrophilic bonds formed during the interaction between β-Lg and epigallocatechin gallate could increase the proportion of β-sheets and α-helices in the protein. The resulting protein structure proved reliably stable. In another study, the transformation of β-sheets into α-helices induced fewer twists and turns [91, 102]. The intensity depended on the specific features of individual molecules [103]. Flavonoids reduced the hydrophobicity of the protein surface and the number of sulfhydryl groups, which may denote increased protein aggregation [91, 103].
Thus, proteins can be used in functional food products. If combined with polyphenols, they can act as antioxidants, emulsifiers, foaming agents, and gelling agents [104–106]. However, the effect may be quite opposite. For example, baicalein, alone or with chrysin, was reported to enhance the foaming ability of β-Lg, while chrysin, on the contrary, weakened it [103].
Polyphenols were able to reduce the allergenic effect of milk protein complexes by weakening their binding to immunoglobulin epitopes in specialized foods for patients with food allergies to milk proteins and, in particular, β-Lg [102, 107].Multicomponent capsules are getting more and more scientific attention [108–110]. By combining different materials in the matrix, researchers obtain capsules with predesigned properties, e.g., particle size, binding efficiency, pH-related solubility, stability, release rate, absorption by enterocytes, nutraceutical value, etc.
Luo et al. used dextran-zein fibers obtained by electrospinning to encapsulate curcumin [111]. By adding zein to dextran, they achieved a nonlinear increase in the hydrophobicity of the resulting fibers: 30% of zein increased the flexibility, elasticity, and tensile fiber strength. However, low concentrations had quite the opposite effect. The ratio of the protein and polysaccharide components also affected the release rate of active molecules. The maximal rate of curcumin release was registered at the 15% zein content.
Gómez-Mascaraques et al. encapsulated a grape juice extract using a mix of gelatin and carrageenan [30]. The optimal ratio protein to carbohydrate was 85 to 15. This ratio provided the maximal absorption rate of biologically active substances. The release of the extract from the matrix turned out to be pH-dependent and increased in alkaline environment. Calcium chloride boosted the encapsulation efficiency but eliminated the pH-dependence of the release. As a result, the extract release did not run well in alkaline environment. The researchers, however, managed to solve the problem by changing the sequence of encapsulation: they extruded carrageenan and extract through gelatin in the presence of calcium chloride. In this way, they obtained capsules for intestinal release that were resistant to acidic gastric juice.
Wang et al. used nanoparticles made from chitosan in combination with bovine serum albumin to encapsulate polyphenols obtained from Pinus koraiensis pine cones [112]. The capsules showed high stability over a long storage period at room temperature. An artificial digestion model proved that the biologically active substances could reach the intestine.
Caballero et al. combined pea protein and high methoxyl pectin (1:1) at pH 4.0 to encapsulate hesperidin [113]. The resulting capsules were 10 times more soluble in water while exhibiting higher antioxidant activities and a better bioavailability in vitro than unencapsulated hesperidin. The researchers mentioned their good commercial prospects in food and drink fortification.
Viljanen et al. observed anthocyanins in a wheybased emulsion [114]. Anthocyanins obtained from black currant, raspberry, and lingonberry exhibited dose-dependent antioxidant properties in relation to both proteins and lipids. Such effect was brought about by the optimal ratio of delphinidin and cyanidin. Most anthocyanins were distributed in the aqueous fraction, exhibiting antioxidant activity against proteins. The presence of lipids reduced the amount of anthocyanin in the aqueous phase. About 20% of anthocyanins manifested their antioxidant properties right where they bound to proteins, i.e., on the border between lipid and water phases [115].
Aceituno-Medina et al. used the method of electrospinning to encapsulate quercetin in amaranth protein isolates in combination with pullulan ultrafine fibers [116]. Quercetin molecules distributed quite evenly throughout the smooth fibers. A digestion test in vitro showed that the encapsulated compounds had a better antioxidant capacity than the non-encapsulated ones.
Yadav et al. combined whey protein concentrate, maltodextrin, and gum arabic to encapsulate grape juice extract by the ultrasound method [117]. They achieved an encapsulation efficiency of 87.9–91.13% with the smallest particle size and maximal preservation of antioxidant properties. These results belonged to microcapsules coated with whey protein concentrate and maltodextrin or gum arabic at ratios of 4:1 and 3:2, with a core-to-coating ratio of 1:5.
Emulsion thermal gelation is another common encapsulation method. Betz & Kulozik encapsulated blueberry extracts in whey protein [118]. The average diameter of the resulting microcapsules was 0.5–2.5 mm. However, the particles were large (≥ 100 µm), which spoiled the sensory properties of the finished products. The particle size decreased as the stirring speed reached 1350 rpm. Further acceleration did not reduce the particle size. Emulsifiers reduced the surface tension, thus preventing the particles from aggregation and sticking. This measure made it possible to reduce the particle size to 70 μm. However, the emulsifier promoted the transition of anthocyanins from the protein solution to the oil phase. The pH value was another important factor that affected the particle size. When blueberry extract was added at ≥ 10% of total volume at pH 3.0, the resulting aggregates were irregular in shape an did not form spherical capsules. The scientists explained it by the effect of pH on the electrostatic interactions between protein and polyphenol molecules.
Ha et al. proposed an interesting technological solution by combining all three types of organic encapsulating agents, i.e., proteins, carbohydrates, and lipids [119]. They used chitosan bound to linoleic acid and combined with β-lactoglobulin (chitosan – linoleic acid/β-Lg) as a carrier to encapsulate quercetin. The nanoparticles were prepared by modified ionic gelation. They treated a mix of quercetin with chitosan-bound linoleic acid and β-Lg at 5, 10, 15, and 20°C, followed by tripolyphosphate. As the amount of linoleic acid increased and the temperature went down, the association efficiency of chitosan and β-Lg increased, as did the quercetin encapsulation efficiency. The researchers explained the effect by an increase in the hydrophobicity of β-Lg, which, in its turn, triggered an increase in hydrophobic interactions between molecules. Lower temperatures also reduced the particle size.
Guo et al. used a three-component matrix to encapsulate curcumin [120]. Pea protein isolate, high-methoxyl pectin, and rhamnolipid served as surfactants. When the ratio of protein to curcumin was 40 to 1, the encapsulation efficiency reached 93%. According to the Fourier transform infrared spectroscopy, the complexes appeared as a result of hydrogen bonds and electrostatic interaction. Curcumin was not crystalline. The capsules showed greater stability with respect to ultraviolet irradiation, thermal effects, and a gastrointestinal model.Polysaccharides, e.g., gum arabic, can act as emulsion stabilizers as evidenced by the size distribution of the resulting particles. For example, Zhang et al. registered two size groups of particles in an emulsion of whey proteins and sunflower oil [72]. The smaller particles (130 nm) corresponded to aggregated proteins, and the larger ones (550 nm) were formed by the emulsion. By adding gum arabic, the researchers reduced the proportion of the smaller particles in a dose-dependent manner. At 1% gum arabic and 1.6 mM calcium chloride, only one peak was observed, which meant that all particles were ≤ 2580 nm. The ζ-potential also became more negative as the concentration of gum arabic and calcium chloride continued to grow. This effect occurred because the electrostatic repulsion of the particles grew and the steric stabilization of the emulsion prevented its aggregation. The encapsulation of resveratrol occurred at the hydrophobic part, i.e., at the water and oil interface, so the presence of gum arabic and calcium chloride affected the efficiency of the process. Consequently, the resveratrol fluorescence increased together with the concentration of these matrix components.
Shao et al. used an emulsion of whey protein and medium chain triglycerides stabilized with gum arabic to reach a 50% resveratrol encapsulation efficiency [121].
Not only the capsule, but also its filling can be multicomponent, i.e., several biologically active substances can be encapsulated at once. For example, a combination of curcumin and β-carotene was reported to increase the bioavailability and stability of β-carotene [32].
Another study demonstrated a higher photostability of caffeic and folic acids when encapsulated together, compared to single encapsulation [122]. A combination of various polyphenols, e.g., low molecular weight genistein and high molecular weight icariin, increased the total flavonoid content in capsules, compared to isolated encapsulation [123].
Biological properties of encapsulated polyphenols. Polyphenol capsules must be safe to be used in the food and pharmaceutical industries. Scientist developed several methods to measure the release of active molecules and the preservation of the beneficial effects during technological processing.
Several in vitro studies on various models demonstrated the absence of cytotoxicity in polyphenol capsules [62, 124]. The increased bioavailability of polyphenols is also well documented. For example, consumption of resveratrol capsules made from casein or casein with β-cyclodextrin increased its concentration in the blood by 10 times, compared to free resveratrol tests [125, 126].
Kardum & Glibetic suggested that protein binding could stabilize or even increase antioxidant properties and reduce autoxidation brought about by the changes in pH or temperature during food processing [82].
Polyphenols encapsulated with chitosan, maltodextrin, gum arabic, gelatin, and whey proteins also resulted in good antioxidant properties, long shelf life, and appropriate sensory profile in regard with taste [42]. For example, Pedrozo et al., who used bovine serum albumin, recorded a twofold increase in antioxidant activity [127]. Quercetin nanoliposomes from rice bran phospholipids showed a thousandfold increase in antioxidant activity, compared to free quercetin [61]. By preserving the antioxidant activity of quercetin, capsules from chitosan and alginate were able to protect cells from oxidative stress [128]. However, encapsulation of blueberry extract using whey proteins had no significant effect on its antioxidant profile [129]. Some research teams even reported a decrease in antioxidant properties of quercetin, myricetin, and morin when they were bound to albumin, milk, and soy proteins [130].
This contradiction may be explained by different experimental conditions. In [88], the antioxidant properties of epigallocatechin gallate in combination with β-Lg did not change after heat treatment at 25–60°C. However, this indicator dropped when the temperature reached 85°C. The slower release of the active substance from microcapsules could be another explanation, but a slow release usually means a prolonged effect [131].
Capsules with curcumin made of zein and propylene glycol alginate preserved anticarcinogenic properties in vitro and demonstrated a 7.2-time increase in bioavailability in vivo, compared to crystalline curcumin [99].
Liposomes with rosemary extract retained their antiproliferative effect on cell lines and antimicrobial properties against Gram-positive bacteria [62]. Beconcini et al. studied the anti-inflammatory properties of encapsulated cherry extract, i.e., suppression of IL-6 and TNF-α and inflammatory cytokines in vitro [132]. They used chitosan derivatives and polylactic-glycolic acid. The abovementioned beneficial properties proved more pronounced in the capsuled cherry extract than in the pure one.
The anti-inflammatory effects of nanocurcumin were studied in COVID-19 patients. The mRNA expression and secretion of interleukins IL-1β, IL-6, TNF-α, and IL-18 were significantly lower in patients treated with nano-curcumin [133]. Piceatannol is a hydroxylated stilbene derivative with excellent anticarcinogenic properties. Encapsulated with bovine serum albumin, it demonstrated better anticarcinogenic and anti-inflammatory activity in laboratory mice [134].
In a human retinal cell line, epigallocatechin gallate liposomes had a more pronounced protective effect against oxidative stress compared to pure epigallocatechin gallate [69]. Haładyn et al. studied antidiabetic effects based on the inhibition of the glycolytic enzymes α-amylase and α-glucosidase. In their research, the encapsulated chokeberry concentrates and powders were more active than the non-encapsulated ones [45]. Capsules made of nanoporous silicone and β-cyclodextrin were able to preserve the antioxidant and antiangiogenic properties of polyphenols, in particular, of caffeic acid [135]. The organoprotective properties of curcumin in experimental gentamicin-induced nephropathy were stronger in liposomes than in crystalline forms [136].
Resveratrol liposomes also demonstrated greater stability than resveratrol nanoparticles. As a result, resveratrol liposomes had a better physiological effect on the 3T3-L1 fibroblast cell line. Nanoparticles, however, provided a longer release. Both encapsulation methods increased the solubility and bioavailability of resveratrol [137].
The longer storage stability is an important advantage of encapsulation. Quercetin nanoliposomes made from phospholipids retained their properties at 4°C for 6 months and at 27°C for 5 months [61]. Liposomes with chitosan or epigallocatechin gallate conjugates retained activity against Gram-positive and Gram-negative bacteria during storage for 28 days at 30°C [138]. β-Lactoglobulin-encapsulated quercetin, as well as ferulic and vanillic acids, maintained good photo- and thermal stability and anti-glycation properties [139]. Co-encapsulation of various polyphenols, such as epigallocatechin gallate and quercetin, made it possible to preserve their antioxidant properties [71].
Thus, encapsulation preserves the biological activity of polyphenols and even optimizes it because it protects active molecules from aggressive environment, increases solubility, and improves bioavailability.
ВЫВОДЫ
The review revealed a great scientific interest to various methods of encapsulating various biologically active substances, such as polyphenols. Some of them use isolated carriers while others develop complexes.
The efficiency of polyphenol encapsulation vary from 50 to 90% or more, depending on the method, encapsulation conditions, and the carrier. In most cases, encapsulated polyphenols retain their biological activity, e.g., anticancer, antioxidant, or anti-inflammatory properties.
To sum it up, micro- and nanocapsules are able to:
– mask the astringency typical of pure polyphenols and their extracts in functional foods;
– protect molecules from digestive juices, control their release, and deliver them to certain parts of the gastrointestinal tract;
– increase the bioavailability of polyphenols by improving their absorption by the walls of the digestive tract; as well as
– extend the shelf-life of the finished products.
Food producers may obtain polyphenols not only as pure substances or plant extracts: they can be harvested from by-products, e.g., pomace, cake, peels, etc. Since these discarded materials are rich in polyphenols, their recycling can open way to sustainable technologies in food production [140–145].
Polyphenol encapsulation technologies make it possible to create a wide range of fortified functional products to be used in healthy diets, preventive treatment, sports food, etc.
Вклад авторов
I.A. Evdokimov, G.S. Anisimov, and R.O. Budkevich developed the research concept. T.N. Bobrysheva, M.S. Zolotoreva, and A.K. Muravyev performed the research. T.N. Bobrysheva wrote the draft. G.S. Anisimov, M.S. Zolotoreva, and R.O. Budkevich edited and proofread the manuscript. G.S. Anisimov supervised the research.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declared no conflict of interests regarding the publication of this article.
БЛАГОДАРНОСТИ
The authors express their gratitude to M.E. Kosenko for linguistic support and V.S. Nabilkova for the visualization.
ФИНАНСИРОВАНИЕ
The research was supported by the Ministry of Science and Higher Education of the Russian Federation (Minobrnauki) as part of the high-tech production project on prebiotic lactulose and functional dairy ingredients for import substitution in medicine, veterinary medicine, and baby food, as well as therapeutic and prophylactic products for people and animals (Agreement No. 075-11-2022-021 April 7, 2022; Decree No. 218 of the Government of the Russian Federation of April 9, 2010).СПИСОК ЛИТЕРАТУРЫ
- Blando F, Calabriso N, Berland H, Maiorano G, Gerardi C, Carluccio M, et al. Radical scavenging and anti-inflammatory activities of representative anthocyanin groupings from pigment-rich fruits and vegetables. International Journal of Molecular Sciences. 2018;19(1). https://doi.org/10.3390/ijms19010169
- Tian L, Tan Y, Chen G, Wang G, Sun J, Ou S, et al. Metabolism of anthocyanins and consequent effects on the gut microbiota. Critical Reviews in Food Science and Nutrition. 2019;59(6):982–991. https://doi.org/10.1080/10408398.2018.1533517
- Tena N, Martín J, Asuero AG. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants. 2020;9(5). https://doi.org/10.3390/antiox9050451
- Bendokas V, Stanys V, Mažeikienė I, Trumbeckaite S, Baniene R, Liobikas J. Anthocyanins: From the field to the antioxidants in the body. Antioxidants. 2020;9(9). https://doi.org/10.3390/antiox9090819
- Fernández-Fernández AM, Dellacassa E, Nardin T, Larcher R, Ibañez C, Terán D, et al. Tannat grape skin: A feasible ingredient for the formulation of snacks with potential for reducing the risk of diabetes. Nutrients. 2022;14(3). https://doi.org/10.3390/nu14030419
- Łysiak G. Ornamental flowers grown in human surroundings as a source of anthocyanins with high anti-inflammatory properties. Foods. 2022;11(7). https://doi.org/10.3390/foods11070948
- Batçıoğlu K, Küçükbay F, Alagöz MA, Günal S, Yilmaztekin Y. Antioxidant and antithrombotic properties of fruit, leaf, and seed extracts of the Halhalı olive (Olea europaea L.) native to the Hatay region in Turkey. Foods and Raw Materials. 2023;11(1):84–93. https://doi.org/10.21603/2308-4057-2023-1-557
- Popova AYu, Tutelyan VA, Nikityuk DB. On the new (2021) norms of physiological requirements in energy and nutrients of various groups of the population of the Russian Federation. Problems of Nutrition. 2021;90(4):6–19. (In Russ.). https://doi.org/10.33029/0042-8833-2021-90-4-6-19
- Caballero S, Li YO, McClements DJ, Davidov-Pardo G. Encapsulation and delivery of bioactive citrus pomace polyphenols: a review. Critical Reviews in Food Science and Nutrition. 2022;62(29):8028–8044. https://doi.org/10.1080/10408398.2021.1922873
- Maqsoudlou A, Assadpour E, Mohebodini H, Jafari SM. Improving the efficiency of natural antioxidant compounds via different nanocarriers. Advances in Colloid and Interface Science. 2020;278. https://doi.org/10.1016/j.cis.2020.102122
- Maqsoudlou A, Assadpour E, Mohebodini H, Jafari SM. The influence of nanodelivery systems on the antioxidant activity of natural bioactive compounds. Critical Reviews in Food Science and Nutrition. 2022;62(12):3208–3231. https://doi.org/10.1080/10408398.2020.1863907
- Steiner BM, Shukla V, McClements DJ, Li YO, Sancho‐Madriz M, Davidov‐Pardo G. Encapsulation of lutein in nanoemulsions stabilized by resveratrol and Maillard conjugates. Journal of Food Science. 2019;84(9):2421–2431. https://doi.org/10.1111/1750-3841.14751
- Choi SJ, McClements DJ. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Science and Biotechnology. 2020;29(2):149–168. https://doi.org/10.1007/s10068-019-00731-4
- McClements DJ. Advances in edible nanoemulsions: Digestion, bioavailability, and potential toxicity. Progress in Lipid Research. 2021;81. https://doi.org/10.1016/j.plipres.2020.101081
- Jhaveri A, Deshpande P, Pattni B, Torchilin V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. Journal of Controlled Release. 2018;277:89–101. https://doi.org/10.1016/j.jconrel.2018.03.006
- Szulc-Musioł B, Sarecka-Hujar B. The use of micro- and nanocarriers for resveratrol delivery into and across the skin in different skin diseases – A literature review. Pharmaceutics. 2021;13(4). https://doi.org/10.3390/pharmaceutics13040451
- Trindade LR, da Silva DVT, Baião DS, Paschoalin VMF. Increasing the power of polyphenols through nanoencapsulation for adjuvant therapy against cardiovascular diseases. Molecules. 2021;26(15). https://doi.org/10.3390/molecules26154621
- Rodríguez‐Félix F, Del‐Toro‐Sánchez CL, Cinco‐Moroyoqui FJ, Juárez J, Ruiz‐Cruz S, López‐Ahumada GA, et al. Preparation and characterization of quercetin‐loaded zein nanoparticles by electrospraying and study of in vitro bioavailability. Journal of Food Science. 2019;84(10):2883–2897. https://doi.org/10.1111/1750-3841.14803
- Hosseini H, Jafari SM. Introducing nano/microencapsulated bioactive ingredients for extending the shelf-life of food products. Advances in Colloid and Interface Science. 2020;282. https://doi.org/10.1016/j.cis.2020.102210
- Jia Z, Dumont M-J, Orsat V. Encapsulation of phenolic compounds present in plants using protein matrices. Food Bioscience. 2016;15:87–104. httpss://doi.org/10.1016/j.fbio.2016.05.007
- Augustin MA, Hemar Y. Nano- and micro-structured assemblies for encapsulation of food ingredients. Chemical Society Reviews. 2009;38(4):902–912. https://doi.org/10.1039/b801739p
- Joye IJ, McClements DJ. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Current Opinion in Colloid and Interface Science. 2014;19(5):417–427. https://doi.org/10.1016/j.cocis.2014.07.002
- Nesterenko A, Alric I, Silvestre F, Durrieu V. Vegetable proteins in microencapsulation: A review of recent interventions and their effectiveness. Industrial Crops and Products. 2013;42:469–479. https://doi.org/10.1016/j.indcrop.2012.06.035
- Gouin S. Microencapsulation: Industrial appraisal of existing technologies and trends. Trends in Food Science and Technology. 2004;15(7–8):330–347. https://doi.org/10.1016/j.tifs.2003.10.005
- Munteanu BS, Vasile C. Encapsulation of natural bioactive compounds by electrospinning – Applications in food storage and safety. Polymers. 2021;13(21). https://doi.org/10.3390/polym13213771
- Wang YH, Zhao M, Barker SA, Belton PS, Craig DQM. A spectroscopic and thermal investigation into the relationship between composition, secondary structure and physical characteristics of electrospun zein nanofibers. Materials Science and Engineering: C. 2019;98:409–418. https://doi.org/10.1016/j.msec.2018.12.134
- Neo YP, Ray S, Jin J, Gizdavic-Nikolaidis M, Nieuwoudt MK, Liu D, et al. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein-gallic acid system. Food Chemistry. 2013;136(2):1013–1021. https://doi.org/10.1016/j.foodchem.2012.09.010
- Ezhilarasi PN, Karthik P, Chhanwal N, Anandharamakrishnan C. Nanoencapsulation techniques for food bioactive components: A review. Food and Bioprocess Technology. 2013;6:628–647. https://doi.org/10.1007/s11947-012-0944-0
- Munin A, Edwards-Lévy F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics. 2011;3(4):793–829. https://doi.org/10.3390/pharmaceutics3040793
- Gómez-Mascaraque LG, Llavata-Cabrero B, Martínez-Sanz M, Fabra MJ, López-Rubio A. Self-assembled gelatin-ι-carrageenan encapsulation structures for intestinal-targeted release applications. Journal of Colloid and Interface Science. 2018;517:113–123. https://doi.org/10.1016/j.jcis.2018.01.101
- Zhang H, Wang T, He F, Chen G. Fabrication of pea protein-curcumin nanocomplexes via microfluidization for improved solubility, nano-dispersibility and heat stability of curcumin: Insight on interaction mechanisms. International Journal of Biological Macromolecules. 2021;168:686–694. https://doi.org/10.1016/j.ijbiomac.2020.11.125
- Guo Q, Bayram I, Shu X, Su J, Liao W, Wang Y, et al. Improvement of stability and bioaccessibility of β-carotene by curcumin in pea protein isolate-based complexes-stabilized emulsions: Effect of protein complexation by pectin and small molecular surfactants. Food Chemistry. 2022;367. https://doi.org/10.1016/j.foodchem.2021.130726
- Molino S, Rufián Henares JÁ, Gómez-Mascaraque LG. Impact of gelatine coating on the performance of tannin-loaded pectin microbeads obtained through external gelation. Food Structure. 2022;32. https://doi.org/10.1016/j.foostr.2022.100256
- Molino S, Rufián Henares JÁ, Gómez-Mascaraque LG. Tannin-rich extracts improve the performance of amidated pectin as an alternative microencapsulation matrix to alginate. Current Research in Food Science. 2022;5:243–250. https://doi.org/10.1016/j.crfs.2022.01.014
- Elabbadi A, Jeckelmann N, Haefliger OP, Ouali L. Complexation/encapsulation of green tea polyphenols in mixed calcium carbonate and phosphate micro-particles. Journal of Microencapsulation. 2011;28(1):1–9. https://doi.org/10.3109/02652048.2010.520091
- Oidtmann J, Schantz M, Mäder K, Baum M, Berg S, Betz M, et al. Preparation and comparative release characteristics of three anthocyanin encapsulation systems. Journal of Agricultural and Food Chemistry. 2012;60(3):844–851. https://doi.org/10.1021/jf2047515
- Wang Q, Tang Y, Yang Y, Lei L, Lei X, Zhao J, et al. Interactions and structural properties of zein/ferulic acid: The effect of calcium chloride. Food Chemistry. 2022;373. https://doi.org/10.1016/j.foodchem.2021.131489
- Ćorković I, Pichler A, Ivić I, Šimunović J, Kopjar M. Microencapsulation of chokeberry polyphenols and volatiles: application of alginate and pectin as wall materials. Gels. 2021;7(4). https://doi.org/10.3390/gels7040231
- Dey M, Ghosh B, Giri TK. Enhanced intestinal stability and pH sensitive release of quercetin in GIT through gellan gum hydrogels. Colloids and Surfaces B: Biointerfaces. 2020;196. https://doi.org/10.1016/j.colsurfb.2020.111341
- Vallejo‐Castillo V, Rodríguez‐Stouvenel A, Martínez R, Bernal C. Development of alginate‐pectin microcapsules by the extrusion for encapsulation and controlled release of polyphenols from papaya (Carica papaya L.). Journal of Food Biochemistry. 2020;44(9). https://doi.org/10.1111/jfbc.13331
- Guzmán-Díaz DA, Treviño-Garza MZ, Rodríguez-Romero BA, Gallardo-Rivera CT, Amaya-Guerra CA, Báez-González JG. Development and characterization of gelled double emulsions based on chia (Salvia hispanica L.) mucilage mixed with different biopolymers and loaded with green tea extract (Camellia sinensis). Foods. 2019;8(12). https://doi.org/10.3390/foods8120677
- Massounga Bora AF, Ma S, Li X, Liu L. Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: Review and recent advances. Food Research International. 2018;105:241–249. https://doi.org/10.1016/j.foodres.2017.11.047
- Sánchez-Machado DI, López-Cervantes J, Correa-Murrieta MA, Sánchez-Duarte RG, Cruz-Flores P, de la Mora-López GS. Chitosan. In: Nabavi SM, Silva AS, editors. Nonvitamin and nonmineral nutritional supplements. Academic Press; 2019. pp. 485–493. https://doi.org/10.1016/b978-0-12-812491-8.00064-3
- Elbehairi SEI, Ismail LA, Alfaifi MY, Elshaarawy RFM, Hafez HS. Chitosan nano-vehicles as biocompatible delivering tools for a new Ag(I)curcuminoid-Gboxin analog complex in cancer and inflammation therapy. International Journal of Biological Macromolecules. 2020;165:2750–2764. https://doi.org/10.1016/j.ijbiomac.2020.10.153
- Haładyn K, Tkacz K, Wojdyło A, Nowicka P. The types of polysaccharide coatings and their mixtures as a factor affecting the stability of bioactive compounds and health-promoting properties expressed as the ability to Inhibit the α-amylase and α-glucosidase of chokeberry extracts in the microencapsulation process. Foods. 2021;10(9). https://doi.org/10.3390/foods10091994
- Jiang F, Du C, Zhao N, Jiang W, Yu X, Du S. Preparation and characterization of quinoa starch nanoparticles as quercetin carriers. Food Chemistry. 2022;369. https://doi.org/10.1016/j.foodchem.2021.130895
- Remanan MK, Zhu F. Encapsulation of rutin using quinoa and maize starch nanoparticles. Food Chemistry. 2021;353. https://doi.org/10.1016/j.foodchem.2020.128534
- Jeong H-M, Lee Y, Shin Y-J, Woo S-H, Kim J-S, Jeong D-W, et al. Development of an enzymatic encapsulation process for a cycloamylose inclusion complex with resveratrol. Food Chemistry. 2021;345. https://doi.org/10.1016/j.foodchem.2020.128777
- Pieczykolan E, Kurek MA. Use of guar gum, gum arabic, pectin, beta-glucan and inulin for microencapsulation of anthocyanins from chokeberry. International Journal of Biological Macromolecules. 2019;129:665–671. https://doi.org/10.1016/j.ijbiomac.2019.02.073
- Vergara C, Pino MT, Zamora O, Parada J, Pérez R, Uribe M, et al. Microencapsulation of anthocyanin extracted from purple flesh cultivated potatoes by spray drying and its effects on in vitro gastrointestinal digestion. Molecules. 2020;25(3). https://doi.org/10.3390/molecules25030722
- di Costanzo A, Angelico R. Formulation Strategies for enhancing the bioavailability of silymarin: The state of the art. Molecules. 2019;24(11). https://doi.org/10.3390/molecules24112155
- Tajmohammadi A, Razavi BM, Hosseinzadeh H. Silybum marianum (milk thistle) and its main constituent, silymarin, as a potential therapeutic plant in metabolic syndrome: A review. Phytotherapy Research. 2018;32(10):1933–1949. https://doi.org/10.1002/ptr.6153
- Fallah M, Davoodvandi A, Nikmanzar S, Aghili S, Mirazimi SMA, Aschner M, et al. Silymarin (milk thistle extract) as a therapeutic agent in gastrointestinal cancer. Biomedicine and Pharmacotherapy. 2021;142. https://doi.org/10.1016/j.biopha.2021.112024
- Hüttl M, Markova I, Miklankova D, Zapletalova I, Poruba M, Racova Z, et al. The beneficial additive effect of silymarin in metformin therapy of liver steatosis in a pre-diabetic model. Pharmaceutics. 2021;14(1). https://doi.org/10.3390/pharmaceutics14010045
- Amer ME, Amer MA, Othman AI, Elsayed DA, El-Missiry MA, Ammar OA. Silymarin inhibits the progression of Ehrlich solid tumor via targeting molecular pathways of cell death, proliferation, angiogenesis, and metastasis in female mice. Molecular Biology Reports. 2022;49:4659–4671. https://doi.org/10.1007/s11033-022-07315-2
- Sansone F, Esposito T, Lauro MR, Picerno P, Mencherini T, Gasparri F, et al. Application of spray drying particle engineering to a high-functionality/low-solubility milk thistle extract: Powders production and characterization. Molecules. 2018;23(7). https://doi.org/10.3390/molecules23071716
- Lachowicz S, Michalska-Ciechanowska A, Oszmiański J. The Impact of maltodextrin and inulin on the protection of natural antioxidants in powders made of saskatoon berry fruit, juice, and pomace as functional food ingredients. Molecules. 2020;25(8). https://doi.org/10.3390/molecules25081805
- Upputuri RTP, Mandal AKA. Sustained release of green tea polyphenols from liposomal nanoparticles; release kinetics and mathematical modelling. Iranian Journal of Biotechnology. 2017;15(4):277–283. https://doi.org/10.15171/ijb.1322
- Chimento A, de Amicis F, Sirianni R, Sinicropi MS, Puoci F, Casaburi I, et al. Progress to improve oral bioavailability and beneficial effects of resveratrol. International Journal of Molecular Sciences. 2019;20(6). https://doi.org/10.3390/ijms20061381
- Ozkan G, Kostka T, Esatbeyoglu T, Capanoglu E. Effects of lipid-based encapsulation on the bioaccessibility and bioavailability of phenolic compounds. Molecules. 2020;25(23). https://doi.org/10.3390/molecules25235545
- Rodriguez EB, Almeda RA, Vidallon MLP, Reyes CT. Enhanced bioactivity and efficient delivery of quercetin through nanoliposomal encapsulation using rice bran phospholipids. Journal of the Science of Food and Agriculture. 2019;99(4):1980–1989. https://doi.org/10.1002/jsfa.9396
- Jahanfar S, Gahavami M, Khosravi-Darani K, Jahadi M, Mozafari MR. Entrapment of rosemary extract by liposomes formulated by Mozafari method: Physicochemical characterization and optimization. Heliyon. 2021;7(12). https://doi.org/10.1016/j.heliyon.2021.e08632
- Cutrim CS, Alvim ID, Cortez MAS. Microencapsulation of green tea polyphenols by ionic gelation and spray chilling methods. Journal of Food Science and Technology. 2019;56(8):3561–3570. https://doi.org/10.1007/s13197-019-03908-1
- Pan K, Luo Y, Gan Y, Baek SJ, Zhong Q. pH-driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity. Soft Matter. 2014;10(35):6820–6830. https://doi.org/10.1039/c4sm00239c
- Peng S, Zou L, Zhou W, Liu W, Liu C, McClements DJ. Encapsulation of lipophilic polyphenols into nanoliposomes using pH-driven method: Advantages and disadvantages. Journal of Agricultural and Food Chemistry. 2019;67(26):7506–7511. https://doi.org/10.1021/acs.jafc.9b01602
- Moghaddasi F, Housaindokht MR, Darroudi M, Bozorgmehr MR, Sadeghi A. Synthesis of nano curcumin using black pepper oil by O/W Nanoemulsion Technique and investigation of their biological activities. LWT. 2018;92:92–100. https://doi.org/10.1016/j.lwt.2018.02.023
- Kumar R, Kaur K, Uppal S, Mehta SK. Ultrasound processed nanoemulsion: A comparative approach between resveratrol and resveratrol cyclodextrin inclusion complex to study its binding interactions, antioxidant activity and UV light stability. Ultrasonics Sonochemistry. 2017;37:478–489. https://doi.org/10.1016/j.ultsonch.2017.02.004
- Mamadou G, Charrueau C, Dairou J, Nzouzi NL, Eto B, Ponchel G. Increased intestinal permeation and modulation of presystemic metabolism of resveratrol formulated into self-emulsifying drug delivery systems. International Journal of Pharmaceutics. 2017;521(1–2):150–155. https://doi.org/10.1016/j.ijpharm.2017.02.036
- Minnelli C, Moretti P, Fulgenzi G, Mariani P, Laudadio E, Armeni T, et al. A Poloxamer-407 modified liposome encapsulating epigallocatechin-3-gallate in the presence of magnesium: Characterization and protective effect against oxidative damage. International Journal of Pharmaceutics. 2018;552(1–2):225–234. https://doi.org/10.1016/j.ijpharm.2018.10.004
- Huang M, Liang C, Tan C, Huang S, Ying R, Wang Y, et al. Liposome co-encapsulation as a strategy for the delivery of curcumin and resveratrol. Food and Function. 2019;10(10):6447–6458. https://doi.org/10.1039/c9fo01338e
- Chen W, Zou M, Ma X, Lv R, Ding T, Liu D. Co‐encapsulation of EGCG and quercetin in liposomes for optimum antioxidant activity. Journal of Food Science. 2018;84(1):111–120. https://doi.org/10.1111/1750-3841.14405
- Zhang H, Fan Q, Li D, Chen X, Liang L. Impact of gum Arabic on the partition and stability of resveratrol in sunflower oil emulsions stabilized by whey protein isolate. Colloids and surfaces B: Biointerfaces. 2019;181:749–755. https://doi.org/10.1016/j.colsurfb.2019.06.034
- Vázquez-Ríos AJ, Molina-Crespo Á, Bouzo BL, López-López R, Moreno-Bueno G, de la Fuente M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. Journal of Nanobiotechnology. 2019;17. https://doi.org/10.1186/s12951-019-0517-8
- Zhao L, Gu C, Gan Y, Shao L, Chen H, Zhu H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. Journal of Controlled Release. 2020;318:1–15. https://doi.org/10.1016/j.jconrel.2019.12.005
- Wan Z, Zhao L, Lu F, Gao X, Dong Y, Zhao Y, et al. Mononuclear phagocyte system blockade improves therapeutic exosome delivery to the myocardium. Theranostics. 2020;10(1):218–230. https://doi.org/10.7150/thno.38198
- Butreddy A, Kommineni N, Dudhipala N. Exosomes as naturally occurring vehicles for delivery of biopharmaceuticals: Insights from drug delivery to clinical perspectives. Nanomaterials. 2021;11(6). https://doi.org/10.3390/nano11061481
- Vashisht M, Rani P, Onteru SK, Singh D. Curcumin encapsulated in milk exosomes resists human digestion and possesses enhanced intestinal permeability in vitro. Applied Biochemistry and Biotechnology. 2017;183:993–1007. https://doi.org/10.1007/s12010-017-2478-4
- Oskouie MN, Moghaddam NSA, Butler AE, Zamani P, Sahebkar A. Therapeutic use of curcumin‐encapsulated and curcumin‐primed exosomes. Journal of Cellular Physiology. 2019;234(6):8182–8191. https://doi.org/10.1002/jcp.27615
- Feng X, Chen X, Zheng X, Zhu H, Qi Q, Liu S, et al. Latest trend of milk derived exosomes: Cargos, functions, and applications. Frontiers in Nutrition. 2021;8. https://doi.org/10.3389/fnut.2021.747294
- Ali NB, Razis AFA, Ooi DJ, Chan KW, Ismail N, Foo JB. Theragnostic applications of mammal and plant-derived extracellular vesicles: Latest findings, current technologies, and prospects. Molecules. 2022;27(12). https://doi.org/10.3390/molecules27123941
- Wang Y, Wang J, Ma J, Zhou Y, Lu R. Focusing on future applications and current challenges of plant derived extracellular vesicles. Pharmaceuticals. 2022;15(6). https://doi.org/10.3390/ph15060708
- Kardum N, Glibetic M. Polyphenols and their interactions with other dietary compounds: Implications for human health. Advances in Food and Nutrition Research. 2018;84:103–144. https://doi.org/10.1016/bs.afnr.2017.12.001
- Huang J, He Z, Cheng R, Cheng Z, Wang S, Wu X, et al. Assessment of binding interaction dihydromyricetin and myricetin with bovine lactoferrin and effects on antioxidant activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2020;243. https://doi.org/10.1016/j.saa.2020.118731
- Kanakis CD, Hasni I, Bourassa P, Tarantilis PA, Polissiou MG, Tajmir-Riahi H-A. Milk β-lactoglobulin complexes with tea polyphenols. Food Chemistry. 2011;127(3):1046–1055. https://doi.org/10.1016/j.foodchem.2011.01.079
- Yagolnik EA, Muzafarov EN, Kim YuA, Tarahovsky YuS. The interaction of flavonol quercetin with collagen. Izvestiya Tula State University. Natural Sciences. 2015;(2):121–132. (In Russ.). https://elibrary.ru/UJEGYL
- Zhang L, Wang Y, Xu M, Hu X. Galloyl moieties enhance the binding of (−)-epigallocatechin-3-gallate to β-lactoglobulin: A spectroscopic analysis. Food Chemistry. 2017;237:39–45. https://doi.org/10.1016/j.foodchem.2017.05.048
- Ma C-M, Zhao X-H. Depicting the non-covalent interaction of whey proteins with galangin or genistein using the multi-spectroscopic techniques and molecular docking. Foods. 2019;8(9). https://doi.org/10.3390/foods8090360
- Qie X, Chen Y, Quan W, Wang Z, Zeng M, Qin F, et al. Analysis of β-lactoglobulin–epigallocatechin gallate interactions: the antioxidant capacity and effects of polyphenols under different heating conditions in polyphenolic–protein interactions. Food and Function. 2020;11(5):3867–3878. https://doi.org/10.1039/d0fo00627k
- Nieuwland M, Geerdink P, Brier P, van den Eijnden P, Henket JTMM, Langelaan MLP, et al. Food-grade electrospinning of proteins. Innovative Food Science and Emerging Technologies. 2013;20:269–275. https://doi.org/10.1016/j.ifset.2013.09.004
- Ma C-M, Zhao X-H. The Non-covalent interactions and in vitro radical scavenging activities of the caseinate-galangin and caseinate-genistein complexes. Antioxidants. 2019;8(9). https://doi.org/10.3390/antiox8090354
- Jia J, Gao X, Hao M, Tang L. Comparison of binding interaction between β-lactoglobulin and three common polyphenols using multi-spectroscopy and modeling methods. Food Chemistry. 2017;228:143–151. https://doi.org/10.1016/j.foodchem.2017.01.131
- Li Z, Percival SS, Bonard S, Gu L. Fabrication of nanoparticles using partially purified pomegranate ellagitannins and gelatin and their apoptotic effects. Molecular Nutrition and Food Research. 2011;55(7):1096–1103. https://doi.org/10.1002/mnfr.201000528
- Bruni GP, Acunha TS, de Oliveira JP, Fonseca LM, da Silva FT, Guimarães VM, et al. Electrospun protein fibers loaded with yerba mate extract for bioactive release in food packaging. Journal of the Science of Food and Agriculture. 2020;100(8):3341–3350. https://doi.org/10.1002/jsfa.10366
- Shpigelman A, Cohen Y, Livney YD. Thermally-induced β-lactoglobulin–EGCG nanovehicles: Loading, stability, sensory and digestive-release study. Food Hydrocolloids. 2012;29(1):57–67. https://doi.org/10.1016/j.foodhyd.2012.01.016
- Li B, Du W, Jin J, Du Q. Preservation of (−)-epigallocatechin-3-gallate antioxidant properties loaded in heat treated β-lactoglobulin nanoparticles. Journal of Agricultural and Food Chemistry. 2012;60(13):3477–3484. https://doi.org/10.1021/jf300307t
- Lestringant P, Guri A, Gülseren İ, Relkin P, Corredig M. Effect of processing on physicochemical characteristics and bioefficacy of β-lactoglobulin–epigallocatechin-3-gallate complexes. Journal of Agricultural and Food Chemistry. 2014;62(33):8357–8364. https://doi.org/10.1021/jf5029834
- Cheng H, Ni Y, Bakry AM, Liang L. Encapsulation and protection of bioactive nutrients based on ligand- binding property of milk proteins. International Journal of Nutrition and Food Sciences. 2015;2(7).
- Xiang L-W, Melton LD, Leung IKH. Interactions of β-lactoglobulin with small molecules. In: Melton L, Shahidi F, Varelis P, editors. Encyclopedia of food chemistry. Elsevier; 2019. pp. 560–565. https://doi.org/10.1016/b978-0-08-100596-5.21488-1
- Li M, Liu Y, Liu Y, Zhang X, Han D, Gong J. pH-driven self-assembly of alcohol-free curcumin-loaded zein-propylene glycol alginate complex nanoparticles. International Journal of Biological Macromolecules. 2022;213:1057–1067. https://doi.org/10.1016/j.ijbiomac.2022.06.046
- Xue J, Tan C, Zhang X, Feng B, Xia S. Fabrication of epigallocatechin-3-gallate nanocarrier based on glycosylated casein: Stability and interaction mechanism. Journal of Agricultural and Food Chemistry. 2014;62(20):4677–4684. https://doi.org/10.1021/jf405157x
- Li M, Fokkink R, Ni Y, Kleijn JM. Bovine beta-casein micelles as delivery systems for hydrophobic flavonoids. Food Hydrocolloids. 2019;96:653–662. https://doi.org/10.1016/j.foodhyd.2019.06.005
- Xu J, Hao M, Sun Q, Tang L. Comparative studies of interaction of β-lactoglobulin with three polyphenols. International Journal of Biological Macromolecules. 2019;136:804–812. https://doi.org/10.1016/j.ijbiomac.2019.06.053
- Li A, Chen L, Zhou W, Pan J, Gong D, Zhang G. Effects of baicalein and chrysin on the structure and functional properties of β-lactoglobulin. Foods. 2022;11(2). https://doi.org/10.3390/foods11020165
- Baba WN, McClements DJ, Maqsood S. Whey protein–polyphenol conjugates and complexes: Production, characterization, and applications. Food Chemistry. 2021;365. https://doi.org/10.1016/j.foodchem.2021.130455
- Liu Q, Sun Y, Cheng J, Zhang X, Guo M. Changes in conformation and functionality of whey proteins induced by the interactions with soy isoflavones. LWT. 2022;163. https://doi.org/10.1016/j.lwt.2022.113555
- Huang G, Jin H, Liu G, Yang S, Jiang L, Zhang Y, et al. An insight into the changes in conformation and emulsifying properties of soy β-conglycinin and glycinin as affected by EGCG: Multi-spectral analysis. Food Chemistry. 2022;394. https://doi.org/10.1016/j.foodchem.2022.133484
- Wu X, Lu Y, Xu H, Lin D, He Z, Wu H, et al. Reducing the allergenic capacity of β-lactoglobulin by covalent conjugation with dietary polyphenols. Food Chemistry. 2018;256:427–434. https://doi.org/10.1016/j.foodchem.2018.02.158
- Devi N, Sarmah M, Khatun B, Maji TK. Encapsulation of active ingredients in polysaccharide–protein complex coacervates. Advances in Colloid and Interface Science. 2017;239:136–145. https://doi.org/10.1016/j.cis.2016.05.009
- Bušić A, Belščak-Cvitanović A, Vojvodić Cebin A, Karlović S, Kovač V, Špoljarić I, et al. Structuring new alginate network aimed for delivery of dandelion (Taraxacum officinale L.) polyphenols using ionic gelation and new filler materials. Food Research International. 2018;111:244–255. https://doi.org/10.1016/j.foodres.2018.05.034
- Silva MP, Fabi JP. Food biopolymers-derived nanogels for encapsulation and delivery of biologically active compounds: A perspective review. Food Hydrocolloids for Health. 2022;2. https://doi.org/10.1016/j.fhfh.2022.100079
- Luo S, Saadi A, Fu K, Taxipalati M, Deng L. Fabrication and characterization of dextran/zein hybrid electrospun fibers with tailored properties for controlled release of curcumin. Journal of the Science of Food and Agriculture. 2021;101(15):6355–6367. https://doi.org/10.1002/jsfa.11306
- Wang L, Li X, Wang H. Fabrication of BSA-Pinus koraiensis polyphenol-chitosan nanoparticles and their release characteristics under in vitro simulated gastrointestinal digestion. Food and Function. 2019;10(3):1295–1301. https://doi.org/10.1039/C8FO01965G
- Caballero S, Li YO, McClements DJ, Davidov‐Pardo G. Hesperetin (citrus peel flavonoid aglycone) encapsulation using pea protein–high methoxyl pectin electrostatic complexes: Complex optimization and biological activity. Journal of the Science of Food and Agriculture. 2022;102(12):5554–5560. https://doi.org/10.1002/jsfa.11874
- Viljanen K, Kylli P, Hubbermann E-M, Schwarz K, Heinonen M. Anthocyanin antioxidant activity and partition behavior in whey protein emulsion. Journal of Agricultural and Food Chemistry. 2005;53(6):2022–2027. https://doi.org/10.1021/jf047975d
- Viljanen K, Kylli P, Kivikari R, Heinonen M. Inhibition of protein and lipid oxidation in liposomes by berry phenolics. Journal of Agricultural and Food Chemistry. 2004;52(24):7419–7424. https://doi.org/10.1021/jf049198n
- Aceituno-Medina M, Mendoza S, Rodríguez BA, Lagaron JM, López-Rubio A. Improved antioxidant capacity of quercetin and ferulic acid during in-vitro digestion through encapsulation within food-grade electrospun fibers. Journal of Functional Foods. 2015;12:332–341. https://doi.org/10.1016/j.jff.2014.11.028
- Yadav K, Bajaj RK, Mandal S, Mann B. Encapsulation of grape seed extract phenolics using whey protein concentrate, maltodextrin and gum arabica blends. Journal of Food Science and Technology. 2020;57(2):426–434. https://doi.org/10.1007/s13197-019-04070-4
- Betz M, Kulozik U. Microencapsulation of bioactive bilberry anthocyanins by means of whey protein gels. Procedia Food Science. 2011;1:2047–2056. https://doi.org/10.1016/j.profoo.2011.10.006
- Ha H-K, Kim JW, Lee M-R, Lee W-J. Formation and characterization of quercetin-loaded chitosan oligosaccharide/β-lactoglobulin nanoparticle. Food Research International. 2013;52(1):82–90. https://doi.org/10.1016/j.foodres.2013.02.021
- Guo Q, Su J, Shu X, Yuan F, Mao L, Liu J, et al. Fabrication, structural characterization and functional attributes of polysaccharide-surfactant-protein ternary complexes for delivery of curcumin. Food Chemistry. 2021;337. https://doi.org/10.1016/j.foodchem.2020.128019
- Shao P, Feng J, Sun P, Ritzoulis C. Improved emulsion stability and resveratrol encapsulation by whey protein/gum Arabic interaction at oil-water interface. International Journal of Biological Macromolecules. 2019;133:466–472. https://doi.org/10.1016/j.ijbiomac.2019.04.126
- Wusigale, Wang T, Hu Q, Xue J, Khan MA, Liang L, et al. Partition and stability of folic acid and caffeic acid in hollow zein particles coated with chitosan. International Journal of Biological Macromolecules. 2021;183:2282–2292. https://doi.org/10.1016/j.ijbiomac.2021.05.216
- Song X, Gan K, Qin S, Chen L, Liu X, Chen T, et al. Preparation and characterization of general-purpose gelatin-based co-loading flavonoids nano-core structure. Scientific Reports. 2019;9. https://doi.org/10.1038/s41598-019-42909-0
- Heep G, Almeida A, Marcano R, Vieira D, Mainardes RM, Khalil NM, et al. Zein-casein-lysine multicomposite nanoparticles are effective in modulate the intestinal permeability of ferulic acid. International Journal of Biological Macromolecules. 2019;138:244–251. https://doi.org/10.1016/j.ijbiomac.2019.07.030
- Peñalva R, Morales J, González-Navarro CJ, Larrañeta E, Quincoces G, Peñuelas I, et al. Increased oral bioavailability of resveratrol by its encapsulation in casein nanoparticles. International Journal of Molecular Sciences. 2018;19(9). https://doi.org/10.3390/ijms19092816
- Peñalva R, Esparza I, Morales-Gracia J, González-Navarro CJ, Larrañeta E, Irache JM. Casein nanoparticles in combination with 2-hydroxypropyl-β-cyclodextrin improves the oral bioavailability of quercetin. International Journal of Pharmaceutics. 2019;570. https://doi.org/10.1016/j.ijpharm.2019.118652
- Pedrozo RC, Antônio E, Khalil NM, Mainardes RM. Bovine serum albumin-based nanoparticles containing the flavonoid rutin produced by nano spray drying. Brazilian Journal of Pharmaceutical Sciences. 2020;56. https://doi.org/10.1590/s2175-97902019000317692
- Aluani D, Tzankova V, Kondeva-Burdina M, Yordanov Y, Nikolova E, Odzhakov F, et al. Еvaluation of biocompatibility and antioxidant efficiency of chitosan-alginate nanoparticles loaded with quercetin. International Journal of Biological Macromolecules. 2017;103:771–782. https://doi.org/10.1016/j.ijbiomac.2017.05.062
- Baum M, Schantz M, Leick S, Berg S, Betz M, Frank K, et al. Is the antioxidative effectiveness of a bilberry extract influenced by encapsulation? Journal of the Science of Food and Agriculture. 2014;94(11):2301–2307. https://doi.org/10.1002/jsfa.6558
- Rawel HM, Czajka D, Rohn S, Kroll J. Interactions of different phenolic acids and flavonoids with soy proteins. International Journal of Biological Macromolecules. 2002;30(3–4):137–150. https://doi.org/10.1016/s0141-8130(02)00016-8
- Roy P, Parveen S, Ghosh P, Ghatak K, Dasgupta S. Flavonoid loaded nanoparticles as an effective measure to combat oxidative stress in Ribonuclease A. Biochimie. 2019;162:185–197. https://doi.org/10.1016/j.biochi.2019.04.023
- Beconcini D, Felice F, Zambito Y, Fabiano A, Piras AM, Macedo MH, et al. Anti-inflammatory effect of cherry extract loaded in polymeric nanoparticles: Relevance of particle internalization in endothelial cells. Pharmaceutics. 2019;11(10). https://doi.org/10.3390/pharmaceutics11100500
- Valizadeh H, Abdolmohammadi-vahid S, Danshina S, Ziya Gencer M, Ammari A, Sadeghi A, et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. International Immunopharmacology. 2020;89. https://doi.org/10.1016/j.intimp.2020.107088
- Aljabali AAA, Bakshi HA, Hakkim FL, Haggag YA, Al-Batanyeh MK, Al Zoubi MS, et al. Albumin nano-encapsulation of piceatannol enhances its anticancer potential in colon cancer via downregulation of nuclear p65 and HIF-1α. Cancers. 2020;12(1). https://doi.org/10.3390/cancers12010113
- Guzmán-Oyarzo D, Hernández-Montelongo J, Rosas C, Leal P, Weber H, Alvear M, et al. Controlled release of caffeic acid and pinocembrin by use of nPSi-βCD composites improves their antiangiogenic activity. Pharmaceutics. 2022;14(3). https://doi.org/10.3390/pharmaceutics14030484
- Bulboacă AE, Porfire A, Bolboacă SD, Nicula CA, Feștilă DG, Roman A, et al. Protective effects of liposomal curcumin on oxidative stress/antioxidant imbalance, metalloproteinases 2 and -9, histological changes and renal function in experimental nephrotoxicity induced by gentamicin. Antioxidants. 2021;10(2). https://doi.org/10.3390/antiox10020325
- Zu Y, Overby H, Ren G, Fan Z, Zhao L, Wang S. Resveratrol liposomes and lipid nanocarriers: Comparison of characteristics and inducing browning of white adipocytes. Colloids and Surfaces B: Biointerfaces. 2018;164:414–423. https://doi.org/10.1016/j.colsurfb.2017.12.044
- Mittal A, Singh A, Benjakul S. Preparation and characterisation of liposome loaded with chitosan-epigallocatechin gallate conjugate. Journal of Microencapsulation. 2021;38(7–8):533–545. https://doi.org/10.1080/02652048.2021.1990425
- Zhang S, Li X, Ai B, Zheng L, Zheng X, Yang Y, et al. Binding of β-lactoglobulin to three phenolics improves the stability of phenolics studied by multispectral analysis and molecular modeling. Food Chemistry: X. 2022;15. https://doi.org/10.1016/j.fochx.2022.100369
- Ferrentino G, Asaduzzaman Md, Scampicchio MM. Current technologies and new insights for the recovery of high valuable compounds from fruits by-products. Critical Reviews in Food Science and Nutrition. 2018;58(3):386–404. https://doi.org/10.1080/10408398.2016.1180589
- Dimou C, Karantonis HC, Skalkos D, Koutelidakis AE. Valorization of fruits by-products to unconventional sources of additives, oil, biomolecules and innovative functional foods. Current Pharmaceutical Biotechnology. 2019;20(10):776–786. https://doi.org/10.2174/1389201020666190405181537
- Valencia-Hernandez LJ, Wong-Paz JE, Ascacio-Valdés JA, Chávez-González ML, Contreras-Esquivel JC, Aguilar CN. Procyanidins: From agro-industrial waste to food as bioactive molecules. Foods. 2021;10(12). https://doi.org/10.3390/foods10123152
- Cano-Lamadrid M, Artés-Hernández F. By-products revalorization with non-thermal treatments to enhance phytochemical compounds of fruit and vegetables derived products: A review. Foods. 2021;11(1). https://doi.org/10.3390/foods11010059
- Chamorro F, Carpena M, Fraga-Corral M, Echave J, Riaz Rajoka MS, Barba FJ, et al. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: A circular economy model. Food Chemistry. 2022;370. https://doi.org/10.1016/j.foodchem.2021.131315
- Hafizov SG, Musina ON, Hafizov GK. Extracting hydrophilic components from pomegranate peel and pulp. Food Processing: Techniques and Technology. 2023;53(1):168–182. (In Russ.). https://doi.org/10.21603/2074-9414-2023-1-2425