Аннотация
Neurodegenerative disorder leads to a progressive memory loss that has only limited known medications. The use of ashwagandha, probiotics, or their combination may improve cholinergic activity, consequently providing therapeutic potency against amnesia and neuroplasticity disorders. We aimed to explore the modulatory benefits of ashwagandha extract and probiotics against induced behavioral and neurochemical retardations.Acid curd (Karish) cheese samples were supplemented with ashwagandha extract and/or probiotics and subjected to chemical, microbiological, rheological, sensorial, and biological investigations by standard techniques.
The supplementation of Karish cheese with ashwagandha never deteriorated its chemical composition or rheological parameters. On the contrary, it exerted high antioxidant and phenolic potentials. Also, ashwagandha extract performed antimicrobial action against the tested pathogenic bacteria and showed better prebiotic effects with Lactobacillus plantarum. The biological study revealed that treating dementia-modeled rats with Karish cheese supplemented with ashwagandha and/or probiotics resulted in a detectable improvement in the behavioral and neurochemical measurements. However, the cheese supplemented with a formula of ashwagandha and probiotics had the greatest regenerating effect.
The supplementation of Karish cheese with ashwagandha and/or probiotics exhibited a modulatory efficiency against experimentally induced behavioral and neurochemical disorders.
Ключевые слова
Ashwagandha, Karish cheese, Lactobacillus plantarum, probiotic, therapeutic effect, dementia, ratsВВЕДЕНИЕ
White soft cheese is a rudimentary product at the breakfast table and a meal, or a basic sandwich, for school children at the beginning of the day [1]. Acid curd, or Karish, cheese is an excellent choice of white soft cheeses mixed with many natural additives such as herbs, spices, or vegetables. Made from defatted milk, acid curd cheese can also be added to edible oils, with olive oil being the most famous. So, this cheese can be used with many additives that increase its health and nutritional value [2]. Several studies have shown that fermented dairy products improve memory functions. In addition, individuals who consume low-fat dairy products, including acid curd (Karish) cheese and yogurt (at least once a week), have a higher level of awareness than others. A close link has also been found between the consumption of fermented dairy products and a lower risk of dementia [3–6].
The physiological effects of fermented dairy products are due to the presence of fatty acids and bioactive peptides. They are naturally released during the fermentation process that occurs due to the presence of lactic acid bacteria in milk and its products [7].
Alzheimer’s disease has serious effects on the patient, their family, and society. It is linked with severe cognitive damage and some metabolic defects. Prevention is the best way to fight this disease since its treatment can neither delay nor stop its progress [8]. Studies indicate links between lower pathogens, lifestyle changes, lower injury rates, and better prevention [9]. Accordingly, following a healthy diet and exercising are some of the most successful ways to avoid many diseases to date [10]. Using herbs and medicinal plants in food for health benefits has been a trend over the past few years. Since milk and dairy products are commonly preferred by different segments of society, they are among the most important carriers of phytochemicals present in herbs (mainly polyphenols) for health benefits [1, 11].
Ashwagandha (Withania somnifera L.), Indian ginseng or winter cherry, has many health and medical benefits, both therapeutic and protective [12]. It can efficiently prevent thyroid dysfunction, reduce its complications for the nervous system, and treat hypertension [13, 14]. Ashwagandha is also considered an adaptogen, a memory enhancer, and a cardiovascular protector. It is known for its antioxidant, anxiolytic, antiparkinsonian, antivenom, anti-inflammatory, antitumorous, immunomodulatory, hypolipidemic, and antibacterial properties [15]. Ashwagandha extract was reported to possess antioxidant and anti-inflammatory effects against aluminum neurotoxicity, reduce cholinergic activity by maintaining normal acetylcholine esterase activity, and enhance memory [16]. It is added to many formulations to increase energy, improve overall health and longevity, as well as prevent various diseases. The dried roots of the plant are used to treat nervous and sexual disorders [17].
Probiotics have a positive effect on psychological well-being through enhancing human mood and sleep quality, etc. [18–20]. Different studies on experimental animals indicated a close relation between probiotic consumption and cognitive function. For example, a 12-week probiotic consumption had a positive effect on the cognitive function and some metabolic conditions in Alzheimer’s patients [21]. Many previous studies confirmed anti-Alzheimer’s properties of Lactobacillus plantarum [22]. In particular, this probiotic enhances the production of acetylcholine neurotransmitter, prevents memory deficit, and improves learning ability [23]. Combining probiotics, mainly L. plantarum, with ashwagandha extract as a supplement has proven to have a positive effect against aluminum chloride-induced neurotoxicity. Recently, Mustafa et al. confirmed the amelioration effect of ashwagandha extract combined with a probiotic strain in bio-yogurt against AlCl3 neurotoxicity in rats [24]. In this regard, we aimed to evaluate the effect of ashwagandha ethanolic extract (AEE) on the properties of acid curd (Karish) cheese as a milk model system. For this, AEE and/or a probiotic were added to UF-retentate to deliver health benefits. The supplemented cheese was subjected to chemical, physical, textural, and microbiological determination, as well as a biological evaluation for cognitive or learning difficulties in rats.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Our materials were obtained from the following sources:
– skimmed UF-retentate: from the Animal Production Research Institute, the Agriculture Research Center (Giza, Egypt);
– ashwagandha (Withania somnifera L., NCBI: txid126910) roots: from Imtenan Co. (Giza, Egypt);
– aluminum chloride (AlCl3, 99%, BN AC196): from Alpha-Chemika (India);
– Bacillus cereus (ATCC133018), Salmonella typhimuum 9027, and Staphaurius (ATCC 25175): from the stock cultures of the Agricultural Research Centre (Giza, Egypt);
– Escherichia coli O157:H7 (ATCC 6933), Listeria monocytogenes V7, and Yersenia enterocolitica (ATCC9610TM): from Liofilchem S.r.l. (Rosetodegli Abruzzi, TE, Italy);
– Streptococcus thermophiles, Lactobacillus delbrueckii spp. bulgaricus, and Lactobacillus casei: from the stock cultures of the Dairy Microbiology Lab., the National Research Centre (Giza, Egypt);
– Lactobacillus rhamnosus (Tistr 541), Lactobacillus plantarum (Dsaz 0174), and Lactobacillus reuteri (B-14171): from the Cairo Microbiology Resources Center, the Faculty of Agriculture at Ain Shams University;
– Bifidobacterium bifidum (Bb-12) and Lactobacillus acidophilus (N4495): from Chr. Hansen’s Lab. A/S (Copenhagen, Denmark).
Based on the microbiological results, L. plantarum was selected for this study.
Ashwagandha ethanolic extract (AEE) was prepared as previously described by Mustafa et al. [24]. In brief, ashwagandha roots were milled into fine powder, soaked in ethanol (70%), and stirred overnight. The mixture was then filtered (Whatman No. 1) and the solvent was evaporated using a rotary evaporator (under reduced pressure at 40°C), whereas the water residuals were freeze-dried. Finally, the dry extract was stored at –20°C until further in vitro and in vivo assessments.
The total phenolic content of ashwagandha ethanolic extract was analyzed spectrophotometrically by the modified Folin-Ciocalteu colorimetry and expressed as catechin equivalents using the catechin standard curve [24].
Radical scavenging activity. The capacity of ash- wagandha antioxidants to quench the DPPH radical was determined as described by Salama et al. [25]. The radical scavenging activity (RSA, %) of the extract was calculated according to the following Eq. (1):
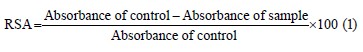
Preparation of acid curd (Karish) cheese. Karish cheese was made by using skimmed UF-retentate heat-treated at 90°C/5 min and cooled to 42°C. For the experiments, 200 mg of ashwagandha ethanolic extract (AEE) was added to one litter of UF-retentate, then inoculated with a 2% active starter-mixture and/or a 2% probiotic (L. plantarum). The cheese treatments were divided as follows: 1) the control (C) contained the active starter-mixture (without AEE); 2) the first treatment (T1) contained the active starter-mixture and the probiotic (L. plantarum); 3) the second treatment (T2) contained the active starter-mixture and AEE; 4) the third treatment (T3) contained the active starter-mixture, the probiotic (L. plantarum), and AEE. The different treatments were packed into plastic cubs (100 mL) and left at 42°C until complete coagulation. After coagulation, the treated cheeses were cooled and stored at 5°C until sensory, chemical, and microbiological analyses before and during storage [2].
Chemical analysis. The cheese samples were analyzed for the content of moisture, fat, total solids, total nitrogen, soluble nitrogen/total nitrogen ratio, and ash according to the Association of Official Analytical Chemists [26]. The pH value was measured using a digital laboratory pH-meter. Diacetyl and acetaldehyde levels were determined spectrophotometrically.
Antioxidant activity and total phenolic compounds. The antioxidant activity and total phenolic content were determined in both the pure ashwagandha ethanolic extract and the supplemented cheese samples according to Salama et al. [25].
The texture profile analysis of all the cheese samples was carried out according to the standards of the International Dairy Federation [27].
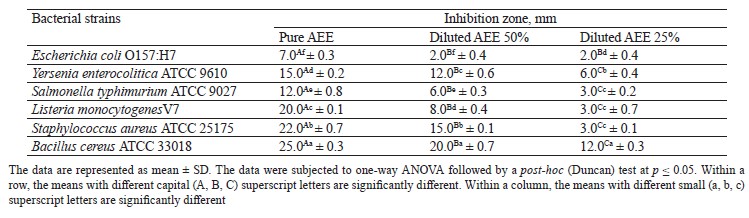
Screening for anti-microbial activity. The anti-bacterial activity of ashwagandha ethanolic extract (AEE) against pathogenic strains was evaluated according to Tepe et al. [28]. For this, the crude dry extract was considered as level 100%; AEE was diluted with distilled water to obtain levels 50 and 25%.
Evaluation of prebiotic activity. The prebiotic activity of ashwagandha ethanolic extract was based on the growth of the given probiotic strains according to the methods of Lourence & Viljoen [29].
Microbiological analysis. The total aerobic colony count, as well as mold, yeast, and coliform counts of the cheese samples were microbiologically examined as previously described by El-Shenawy et al. during different storage periods [30].
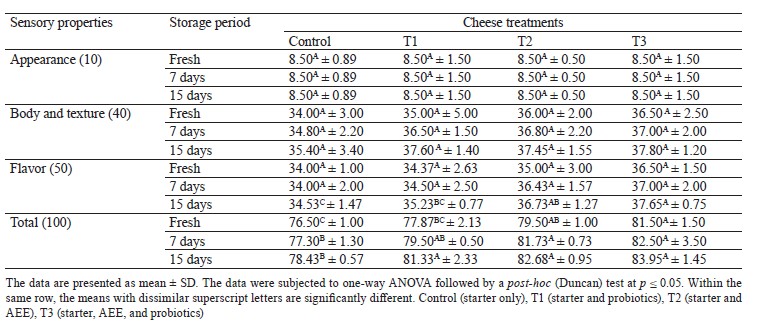
Sensory evaluation. The sensory properties of the cheese samples during different storage periods were evaluated according to Salama [1]. Ten (4 women (35– 55 years) and 6 men (25–60 years)) expert panelists from the staff of the Dairy Science Department at the National Research Center (Egypt) were previously trained with commercial samples of cheese according to Cost-95. They evaluated appearance (10), body & texture (40), and flavor (50) of the cheese samples. The cheese samples were cut into cubes (1.5×1.5×1.5 cm), covered with plastic wrap to prevent dehydration, coded with three-digit random numbers, and held for at least 1 h at 20°C to equilibrate. Each panelist was given three cheese cubes to score each sample on the hedonic scale. The evaluation took place in a tasting room equipped as specified in Standard IS0 8589 (1988).
Animals and experimental study. Thirty adult female Wistar albino rats (150–200 g) were obtained from the Animal Colony of the Research Institute of Ophthalmology, Egypt. They were housed in suitable plastic cages and maintained under controlled conditions (temperature 25 ± 2°C, humidity 50 ± 5%, and 12 h lightdark cycles). The rats had free access to a commercial ration used as a basal diet and water ad libitum. After seven days (acclimatization period), they were randomly assigned into five groups (6 animals each) as follows. Group I included rats that orally received 2 mL/kg/day of starter-emulsified cheese for one month and served as the control. Group II included rats orally intoxicated with AlCl3 (300 mg/kg/day) for one month to induce dementia [31]. Group III included rats that orally received 2 mL/kg/day (equivalent to 109 CFU/kg/day) of probiotic/starter-emulsified cheese in addition to intoxication with AlCl3 for one month [32]. Group IV included rats that orally received 200 mg/kg/day of ashwagandha ethanolic extract-emulsified cheese alongside their intoxication with AlCl3 for a similar period [24]. Group V included rats that orally received 2 mL/kg/day of starter/ probiotic/AEE-emulsified cheese, as well as being AlCl3- intoxicated for a month. All the animals were gavaged once a day, with the doses adjusted on a weekly basis according to their body weights. At the end of the experiment, behavioral observations and biochemical measurements were conducted.
Behavioral profile. Open field test. In this test, the rats were gently placed into a corner of a cleaned and sterilized planed arena and observed for 3 min. Both exploratory behaviors (ambulation and crossing of squares as well as rearing) and non-exploratory measures (grooming) were scored as absolute counts [33].
Modified elevated plus maze test. Depending on the aversion of rats to the open space, their spatial longterm memory was measured as described by Hliňák & Krejčı́ [34].
The novel object recognition test (hippocampusdependent memory impairment) was performed via an automated tracking of rats with a video tracking system (Anymaze 4.20, Stoelting, USA). All exploratory actions were measured automatically and manually as explained by Gümüş [35].
Y-maze test. The short-term memory and locomotor activity were measured according to Wright [36]. The rats were video-tracked for 5 min using a video tracking system (Anymaze 4.20, Stoelting, USA) that recorded the number of arm entries and the distance travelled by each animal. The alternation percent was calculated according to the Eq. (2):

Brain tissue sampling. After the last administration and behavioral tests, the animals were fasted overnight and euthanized by sudden decapitation. The brains of some animals (in each group) were dissected out and anatomized into two similar halves. The first half was homogenized in phosphate buffer (0.1 M, pH 7.4) to determine oxidative stress markers. The second half was homogenized in 0.1 M perchloric acid containing 3, 4-dihydroxybenzylamine at a final concentration of 25 ng/mL to assess acetylcholinesterase activity and the level of biogenic amines. The brains of the rest of the animals (in each group) were dissected out and immersed in formaldehyde-saline (10 %, v/v) buffer for histopathological examination.
Acetylcholinesterase activity, dopamine, and serotonin. Acetylcholinesterase activity was determined according to the modified method of Ellman [37]. Dopamine and serotonin levels were measured by high-performance liquid chromatography (Waters, Milford, USA) following the method of Kim [38].
Oxidative stress status. Oxidative stress markers (glutathione, nitric oxide, malondialdehyde, and superoxide dismutase) were determined in brain phosphate buffer homogenates using reagent kits obtained from Biodiagnostic (Giza, Egypt).
Histopathological examination. Formaldehyde-saline (10 %, v/v) buffer-immersed brains were hydrated in ascending grades of ethanol, cleared in xylene, and embedded in paraffin. Then, 5-μm-thick sections were cut and stained with hematoxylin and eosin for pathohistological examination using light microscopy.
Statistical analysis. Comparisons between means were carried out using one-way ANOVA, followed by a post-hock (Duncan) multiple comparisons test at p ≤ 0.05, using the statistical analysis system (SAS) program software (SAS Institute Inc., Cary, NC, USA).
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
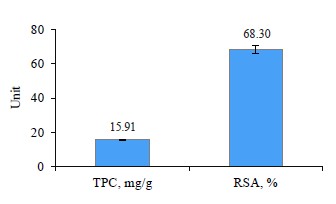
The total antioxidant capacity and total phenolic content of ashwagandha ethanolic extract (AEE) are presented in Fig. 1 . As can be seen, AEE can be considered an excellent source of antioxidants and phenolic compounds, as previously reported by Munir et al. [39]. In addition, the probiotic (Lactobacillus plantarum) and starter used in our study performed a detectable antioxidant activity resulting from metabolic and milk protein hydrolyses via the spontaneous action of the starter and beneficial bacterial strains, as previously reported by Ali et al. and Wang et al. [40, 41].
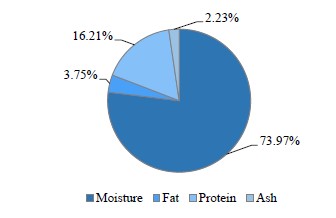
Figure 2 illustrates the main chemical analysis of different acid curd (Karish) cheese samples supplemented with AEE and/or probiotic bacteria. As we can see, neither AEE nor the probiotic bacteria made any changes in the chemical composition of manufactured Karish cheese, as previously reported [1, 22].
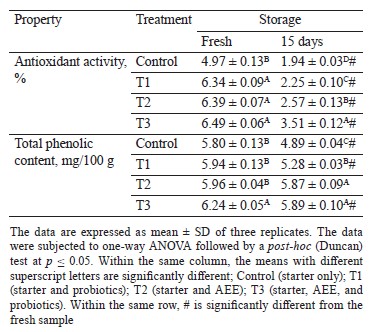
Antioxidant activity and total phenolic content. Interestingly, our study evidenced that adding ashwagandha ethanolic extract (AEE) and probiotic bacteria to Karish cheese improves its antioxidant activity and total phenolic content (Table 2). Among the fresh cheeses, the highest antioxidant activity and total phenolic content (6.49% and 6.24 mg/100 g, respectively) were found in the AEE/probiotic-supplemented sample (T3), followed by the AEE-treated cheese without probiotic bacteria (T2) and the probiotic-supplemented sample without AEE (T1). The lowest values were found in the control. However, 15 days of cold storage significantly decreased the antioxidant activity and total phenolic content of all the cheese treatments. The values were still quite high though, with the lowest found in the control (1.94% and 4.89 mg/100 g, respectively). The decrease in antioxidants and total phenols during storage agrees with the previous studies [24, 25].
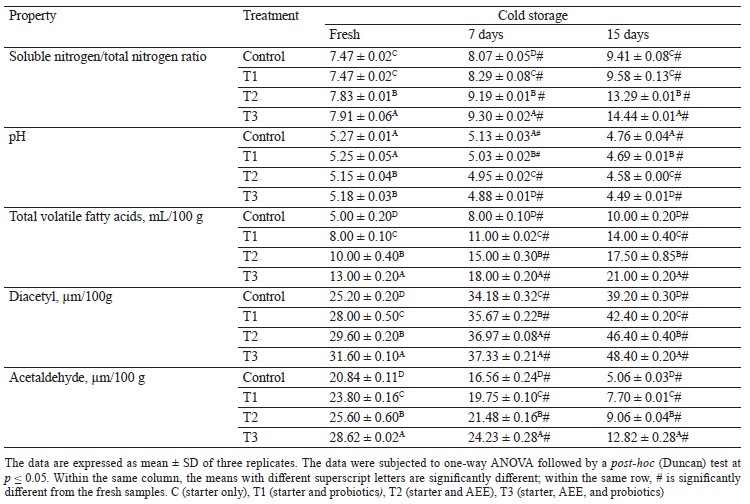
Physicochemical properties of acid curd (Karish) cheese. Table 3 shows the physicochemical analysis of different treatments of Karish cheeses before and after 15 days of cold storage. In the fresh samples, the soluble nitrogen/total nitrogen ratio significantly increased in the cheese treated with ashwagandha ethanolic extract (AEE) and probiotic bacteria, while being equal in the control and the probiotic-supplemented cheese (T1). During cold storage (7 and 15 days), the soluble nitrogen/total nitrogen ratio also significantly increased. This is attributed to the addition of AEE, which markedly enhanced the effect of starter and probiotic bacteria as a prebiotic improving the growth and fermentation. This finding clarifies the upsurge of the soluble nitrogen/total nitrogen ratio in the fresh samples or during storage [47, 48].
The pH values decreased slightly between the fresh samples as AEE was added alone or in combination with probiotic bacteria. However, they decreased significantly throughout storage up to 15 days. As mentioned before, the decrease in pH spontaneously resulted from the action of the starter and probiotic bacteria, which was markedly enhanced by the presence of AEE.
Total volatile fatty acids and diacetyl values increased significantly among the treatments, as well as during storage, with the highest values noted in the cheeses treated with AEE and/or probiotics.
Acetaldehyde values significantly elevated in the fresh treatments and markedly decreased during storage, with the lowest values recorded at the end of storage. The samples supplemented with AEE and/or probiotics had higher acetaldehyde levels compared to the control.
These physicochemical changes were consistent with several previous studies [25, 49, 50].
Texture analysis is an important test that reflects the product’s acceptability, varying with the type of cheese. Figure 3 presents the texture profile of acid curd (Karish) cheeses supplemented with ashwagandha ethanolic extract (AEE) and/or probiotic bacteria. While no significant differences were observed in the examined texture profile parameters among the treatments, there was a slight difference when comparing them with the control. This difference might be due to the addition of AEE, probiotics, and/or starter culture. Alternatively, it could result from the changes in pH and soluble nitrogen/total nitrogen ratios that affected the texture profile during storage. It was previously reported that the texture profile correlated with the physicochemical changes and the chemical composition of Karish cheese [51]. Generally, the addition of AEE and probiotic bacteria does not have a significant effect on the texture properties compared with the control.
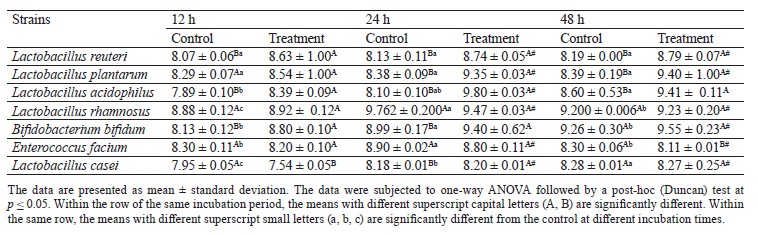
Prebiotic activity. Ashwagandha ethanolic extract (AEE) exhibits excellent prebiotic properties due to its high contents of phytochemicals, polyphenols, and other bioactive compounds. Therefore, we evaluated the prebiotic activity of various concentrations of AEE against foodborne microorganisms to choose the best strain with high prebiotic activity (best growth) for the manufacture of acid curd (Karish) cheese. Table 5 presents the selected probiotic strains in culture media containing AEE in comparison with the control. We found no significant changes in bacterial counts during the first 12 h. After that period, particularly after 24 h, the growth significantly increased. When the bacterial population reached its maximal growth, it remained constant during 48 h. L. plantarum showed significant growth after 24 h to reach a count of 9.35 CFU/mL, 1.0 log higher than that of the control group (8.38 log CFU/mL). As a result, we selected L. plantarum as a good carrier of AEE and probiotic bacteria to use in manufacturing Karish cheese. We also found increasing growth rates of the other probiotic strains at all the interval times. This is because AEE is a rich source of carbohydrates with high prebiotic potential [55]. Generally, herbs contain a good amount of carbohydrates, protein, minerals, and some vitamins. They serve as prebiotics and are a source of carbon and nitrogen enhancing the growth of different probiotic strains [56]. On the other hand, ashwagandha contains steroidal lactones and flavonoids, while its roots have suitable amounts of sominie, somniferin, somniferinine, withanine, and withanonine that supply the pH and titratable acidity [57].
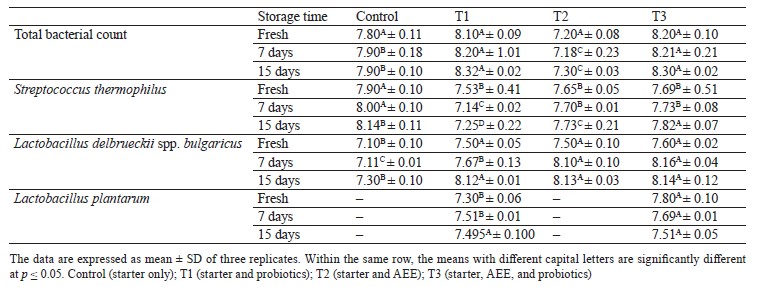
Viability of L. plantarum and starter culture in Karish cheese. The viability of probiotics, or the number of viable counts, in the final product until the end of its storage is an important quality indicator. A functional product should have a viable count of at least 10–9– 10–10 CFU/mL. Therefore, all fermented products are tested for viable counts until consumption. Table 6 shows the viability of L. plantarum and starter culture in Karish cheeses supplemented with аshwagandha ethanolic extract (AEE). As can be seen, the presence of AEE enhanced the growth of L. plantarum in fresh Karish cheese and during storage at 4°C, compared to the control, increasing the production of lactic acid. The viable counts of L. plantarum reached 7.51 log CFU/mL after 15 days of cold storage.
According to the Codex Alimentarius, a commercial probiotic beverage should possess a minimum viable count of 106 CFU/mL at the time of consumption [58]. The growth rates recorded in our study showed that ashwagandha is a good medium for probiotic growth and it can be considered a prebiotic. Also, the starter cultures (Streptococcus thermophilus and Lactobacillus delbrueckii spp. bulgaricus) showed greater growth in the AEE-treated cheese (T2) compared to the sample free from the extract, although the growth of S. thermophiles was higher in the control. However, the starter cultures were still above the minimum viable counts recommended by the Codex Alimentarius. These results are in contrast with those reported by Momin and Prajapati, who indicated that the increase in the log viable count was lower in the fermented milk supplemented with ashwagandha [47]. Nevertheless, our results are consistent with those of Khatoon and Gupta, who reported a suitable growth of starter culture in various combinations with AEE [54]. At the end of the storage period (15 days), the total bacterial count in all the Karish cheese treatments ranged from 7.8 (Control) to 8.37 (T3) log CFU/g. Molds, yeasts, or coliform bacteria were not detected in any of the samples throughout cold storage. This indicates the microbial quality of the final product until the end of storage, as well as good hygiene during preparations, manufacture, and storage.
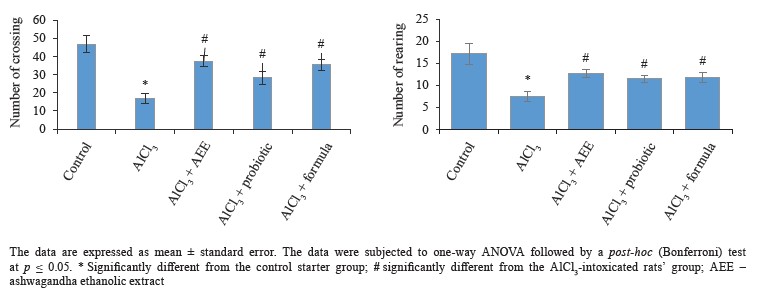
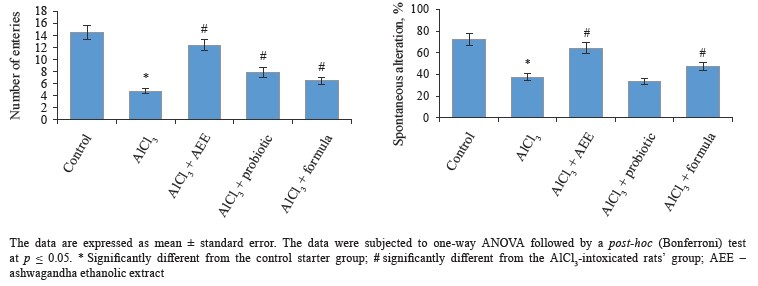
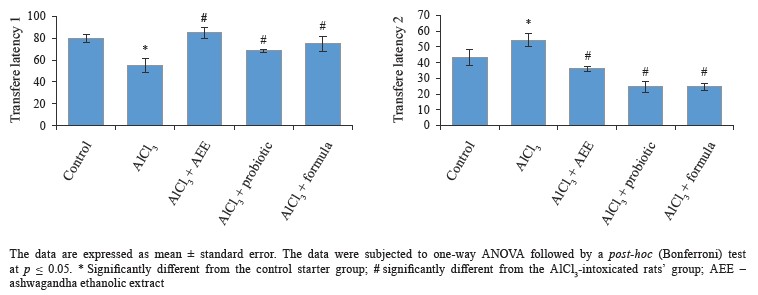
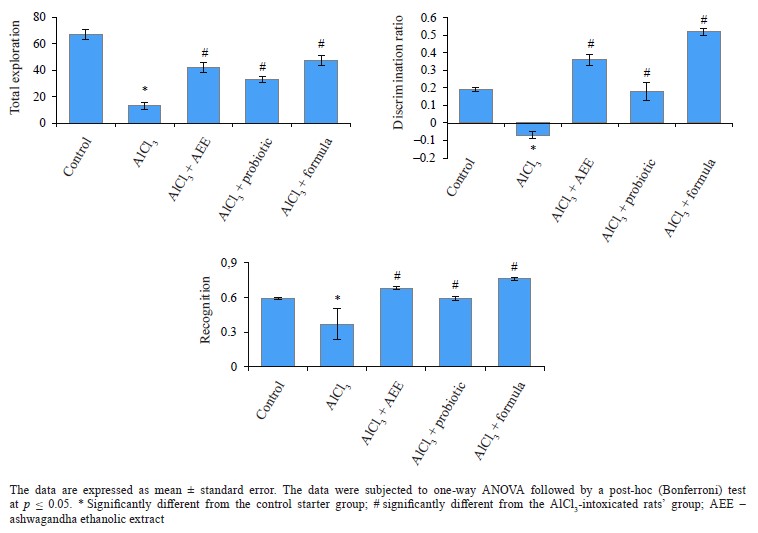
Biological study. Regarding behavioral measurements (Figs. 4–7), we found that the co-administration of rats with cheese supplemented with аshwagandha ethanolic extract (AEE), probiotics, or their combination significantly restored the behavioral deteriorations resulting from AlCl3 intoxication. This was evidenced by the marked improvement in locomotion deficits (vertical and horizontal activities) in the open field test.In particular, AEE significantly increased the number of arms in the maze, while the formula (AEE plus probiotics) increased the spontaneous alternation percentage, compared to the AlCl3-intoxicated rats’ group. In the modified elevated plus maze, the probiotics, AEE, and their formula significantly decreased the transfer latency. Further, the AEE-treated rats spent significantly more time exploring in the novel object recognition test, compared to the probiotic-treated rats, whereas the formula-treated rats showed a high discrimination ratio, as well a high recognition index, compared to the other groups.
The open field test was used to assess the rats’ locomotion and emotionality [59]. We found that AEE was able to alleviate some motor deficits and anxiety caused by AlCl3, which might be due to its chemical constituents with antioxidant properties. Thus, AEE can reverse the AlCl3-induced cognitive defects. These findings are consistent with some previous experiments [60]. The effect of AEE on the rats’ motor activity was confirmed by the number of arms entered in the Y-maze, which also increased significantly in the AEE-treated group when compared with the other groups. In the Y-maze test, the formula was found better able to treat short-term memory deficits in the rats, compared to AEE or probiotics.
The modified elevated plus maze test is used to measure the long-term spatial memory of rats [61]. In our study, both AEE and probiotics, as well as their formula, markedly decreased the time to transfer to both closed arms. This indicated their ability to restore the long-term memory in the treated rats compared to the untreated-AlCl3 rats.The novel object recognition test is used to assess the ability of rats to recognize an object or stimulus seen in the previous 24 h [62]. The test requires intact dorsal hippocampus and cortex [63]. Our data revealed that the administration of AEE- or formula-supplemented cheese remarkably restored the cognitive deficits and recognitive memory disruptions that accompanied AlCl3-intoxication. This was indicated by the time spent exploring a novel object, a recognition index, and a discrimination ratio. In animal model investigations, strong associations have been reported between gastrointestinal microbiota and stress behavior. Particularly, the disruption or absence of gut bacteria was shown to increase the neuroendocrine stress response and behaviors associated with anxiety and stressinduced memory dysfunction [64, 65]. It was reported that probiotics reduced anxiety behavior in rodents, effectively reversing the effects of stress and improving memory and learning performance in terms of object recognition [66–68].
AlCl3 is known to induce behavioral, biochemical, and histopathological effects that are linked with cognitive impairments [69–72]. In our study, the supplementation of Karish cheese with AEE and/or probiotics appreciably ameliorated the neuronal, biochemical, and behavioral aberrations in the AlCl3-challenged demented rats. This indicated their curative and neuroprotective actions against dementia complications.
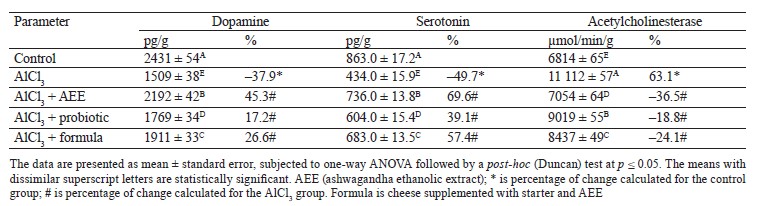
With respect to the neuro-and-biochemical investigations, we found that AlCl3 significantly decreased brain monoamines (dopamine and serotonin) and raised acetylcholinesterase activity, as previously observed [72–74]. The levels of monoamines were similar to those in patients with Alzheimer’s disease, suggesting a prominent role of AlCl3 in aging and development of neurodegenerative diseases [75]. Interestingly, the supplementation with AEE and/or probiotics was found to significantly alleviate the effect of AlCl3 on the biogenic monoamines, producing a therapeutic effect against neurodegenerative disorders. In addition, AEE and probiotics possessed significant antioxidant properties, which may be related to their contents of polyphenols, flavonoids, and other compounds. Compared with the control group, AlCl3-intoxication resulted in a significant decrease in dopamine and serotonin levels (–37.9 and –49.7%, respectively). However, a significant elevation (63.1%) was shown in acetylcholinesterase activity. Interestingly, the treatment of rats with Karish cheese supplemented with AEE, probiotics, or their combination (formula), in line with AlCl3-intoxication, markedly restored (at different degrees) the AlCl3-deteriorated neurochemical measurements. This was evidenced by a significant reduction in acetylcholinesterase activity (–28.4, –18.8, and –24.1%, respectively), as well as a notable increase in dopamine (45.3, 17.2, and 26.6%, respectively) and serotonin (69.6, 39.1, and 57.4%, respectively), compared with the AlCl3-intoxicated group. The highest improvement was performed by the AshEE-supplemented cheese (Table 7).
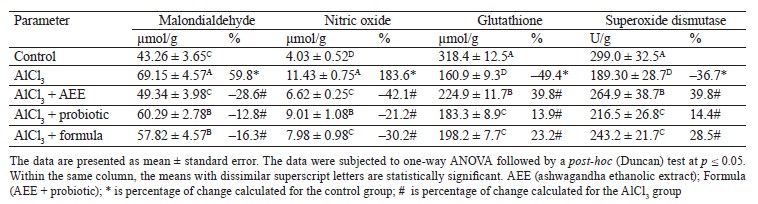
Our data showed a significant increase in the levels of malondialdehyde (59.6%) and nitric oxide (183.6%) in the brains of AlCl3-intoxicated rats. We also found a significant drop in the anti-oxidative battery resulting from a marked decrease in glutathione (–49.4%) and superoxide dismutase activity (–36.6%), compared to the control group. Noteworthily, the treatment of AlCl3-intoxicated rats with the Karish cheese supplemented with AshEE, probiotics, or their combination (formula) alleviated some AlCl3-induced oxidative deteriorations. This was evidenced by a marked decrease in the levels of malondialdehyde (–28.6, –12.8, and –16.3%, respectively) and nitric oxide (–42.1, –21.2, and –30.2%, respectively), as well as a remarkable rise in glutathione (39.8, 13.9, and 23.2%, respectively) and superoxide dismutase activity (39.8, 14.4, and 28.5%, respectively), compared to the AlCl3-intoxicated rats (Table 8).
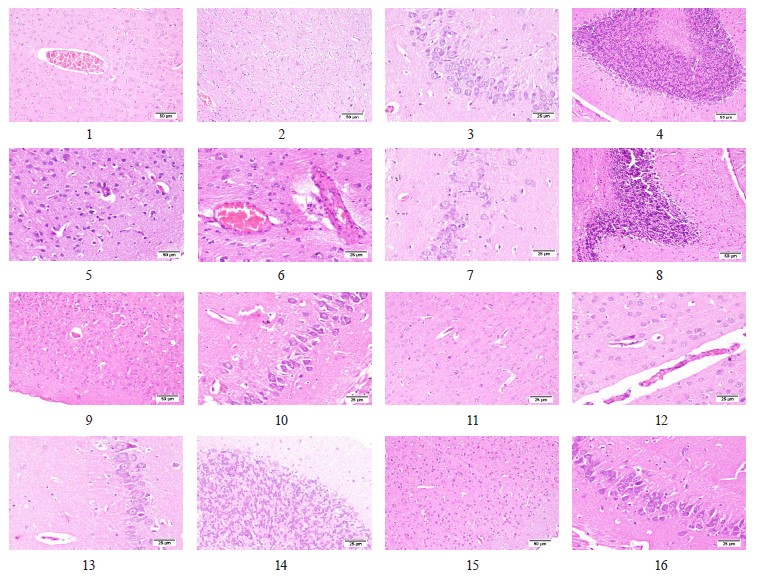
According to our results, the treatment of AlCl3-intoxicated rats with AshEE-supplemented Karish cheese resulted in a moderate protection against brain injury. In particular, the cerebral cortex appeared normal (Fig. 8, plate 9) and free from angiopathies; the hippocampus also appeared histologically normal (Fig. 8, plate 10). Similarly, the treatment of the AlCl3-intoxicated animals with probiotic-supplemented Karish cheese resulted in a mild alleviative action against AlCl3-induced brain damage. However, the cerebral cortex appeared normal, except for a few degenerating neurons with focal glial infiltrations (Fig. 8, plate 11). Cerebral angiopathy was also noticed with mild perivascular lymphocytic cuffing (Fig. 8, plate 12). A few degenerating neurons were observed in the hippocampus and the cerebellum (Fig. 8, plates 13 & 14). The treatment of the animals with the formula (AEE and probiotics)-supplemented Karish cheese exerted the best neuroprotective action. The cerebral cortex appeared normal (Fig. 8, plate 15), although both the hippocampus and the cerebellum showed a few necrotic cells (Fig. 8, plate 16).
The AlCl3-induced significant elevation in malondialdehyde and reduction in enzymatic (superoxide dismutase and CAT) and non-enzymatic (glutathione) antioxidant efficiency, as well as the extensive neuronal damage in the hippocampus were not consistent with the reports of Igbokwe et al. and Makhdoomi et al. [76, 77]. Since Al3+ and Fe3+ have similar ionic radii, Al3+ can bind to the Fe3+-binding protein transferrin and pass Al3+ by transferrin receptors to deliver Al3+ to the brain and initiate oxidative damage [78]. Moreover, Al3+ alters Ca2+ flux and causes abnormal augmentation of intracellular Ca2+, which can increase the production of reactive oxygen species via mitochondrial dysfunction [79]. This can result in oxidative damage, neuronal degeneration, neurochemical changes, and cognitive impairments. Since both inflammation and oxidative stress are interrelated, the latter induces inflammatory cytokine genes [80]. Thus, exposure to metals such as aluminum can increase the levels of proinflammatory cytokines in the brain [81]. Moreover, elevated nitric oxide inhibits microglia proliferation and stimulates glutamate release from astrocytes, leading to excitotoxicity of neurons and glia. Our study revealed that AEE- and/or probiotic-fortified cheese efficiently alleviated the AlCl3-associated pathophysiological deteriorations. It has been reported that AEE exhibited neuroprotection against oxidative stress by activating the Nrf2 pathway and upregulating cytoprotective genes, as well as Keap-Nrf2ARE signaling [82, 83]. Ashwagandha appears to increase the expression and translocation of Nrf2 that binds on ARE and causes the induction of several phase I and phase II metabolizing enzymes, phase III detoxifying proteins, and antioxidant proteins. The up-regulation of detoxification enzymes enhances cell survival and protection due to an improved redox state which prevents glutathionylated protein accumulation. Also, AEE-mediated neuroprotection was reported showing that Withanolide-A (the main constituent of AEE) increased glutathione synthesis in neuronal cells [84].
ВЫВОДЫ
Acid curd (Karish) cheese as a dairy product model can consider an excellent delivery system for ashwagandha and probiotics, mainly Lactobacillus plantarum, to provide maximum health benefits. The addition of ashwagandha extract and/or L. plantarum enhances the nutri-pharmaceutical value of Karish cheese without affecting its properties. The combination of ashwagandha extract and L. plantarum performed a modulatory effectiveness against induced neurotoxicity, especially related Alzheimer’s disease and learning difficulties. This effect was achieved through a remarkable improvement in the behavioral, neurochemical, and oxidative statusВклад авторов
L.K. Hassan and H.H. Salama were involved in the original research and manuscript writing. K.G. Abdel-Wahhab and H.M.A. Khalil conducted the biological study. S.M. Abdelhamid performed the: microbiological assessment. All the authors were involved in editing, reviewing, and proofreading the manuscript. All the authors read and approved the final version of the manuscript.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare that they have no conflict of interest which could hinder the publication of this article.
БЛАГОДАРНОСТИ
The authors are thankful to the National Research Centre for supporting this research.СПИСОК ЛИТЕРАТУРЫ
- Salama HH, Kholif AMM, Fouad MT, Koç GÇ. Properties of novel ultra-filtrated soft cheese supplemented with sumac extract. Egyptian Journal of Chemistry. 2022;65(6):219–231. https://doi.org/10.21608/ejchem.2021.99475.4627
- Al-Moghazy M, El-sayed HS, Salama HH, Nada AA. Edible packaging coating of encapsulated thyme essential oil in liposomal chitosan emulsions to improve the shelf life of Karish cheese. Food Bioscience. 2021;43. https://doi.org/10.1016/j.fbio.2021.101230
- Saper Al Garory NH, Abdul-Abbas SJ, Al-Hashimi AG. The role of fermented dairy products in human health. Revis Bionatura. 2023;8(2). https://doi.org/10.21931/RB/CSS/2023.08.02.66
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396(10248):413–446. https://doi.org/10.1016/S0140-6736(20)30367-6
- Kim H, Osuka Y, Kojima N, Sasai H, Nakamura K, Oba C, et al. Inverse association between cheese consumption and lower cognitive function in japanese community-dwelling older adults based on a cross-sectional study. Nutrients. 2023;15(14). https://doi.org/10.3390/nu15143181
- Ni J, Nishi SK, Babio N, Martínez‐González MA, Corella D, Castañer O, et al. Dairy product consumption and changes in cognitive performance: two‐year analysis of the PREDIMED‐plus cohort. Molecular Nutrition and Food Research. 2022;66(14). https://doi.org/10.1002/mnfr.202101058
- McDade E, Llibre-Guerra JJ, Holtzman DM, Morris JC, Bateman RJ. The informed road map to prevention of Alzheimer disease: A call to arms. Molecular Neurodegeneration. 2021;16. https://doi.org/10.1186/s13024-021-00467-y
- O’Donnell H. A review of primary, secondary, and tertiary prevention strategies for Alzheimer’s Disease. Undergraduate Journal of Public Health. 2023;7. https://doi.org/10.3998/ujph.3946
- Frisoni GB, Altomare D, Ribaldi F, Villain N, Brayne C, Mukadam N, et al. Dementia prevention in memory clinics: Recommendations from the European task force for brain health services. The Lancet Regional Health – Europe. 2023;26. https://doi.org/10.1016/j.lanepe.2022.100576
- Khalil HMA, Salama HH, Al-Mokaddem AK, Aljuaydi SH, Edris AE. Edible dairy formula fortified with coconut oil for neuroprotection against aluminium chloride-induced Alzheimer’s disease in rats. Journal of Functional Foods. 2020;75. https://doi.org/10.1016/j.jff.2020.104296
- El-Sayed HS, Salama HH, Edris AE. Survival of Lactobacillus helveticus CNRZ32 in spray dried functional yogurt powder during processing and storage. Journal of the Saudi Society of Agricultural Sciences. 2020;19(7):461–467. https://doi.org/10.1016/j.jssas.2020.08.003
- Mikulska P, Malinowska M, Ignacyk M, Szustowski P, Nowak J, Pesta K, et al. Ashwagandha (Withania somnifera) – Current research on the health-promoting activities: A narrative review. Pharmaceutics. 2023;15(4). https://doi.org/10.3390/pharmaceutics15041057
- Hosny EN, El-Gizawy MM, Sawie HG, Abdel-Wahhab KG, Khadrawy YA. Neuroprotective effect of ashwagandha extract against the neurochemical changes induced in rat model of hypothyroidism. Journal of Dietary Supplements. 2021;18(1):72–91. https://doi.org/10.1080/19390211.2020.1713959
- Polumackanycz M, Petropoulos SA, Śledziński T, Goyke E, Konopacka A, Plenis A, et al. Withania somnifera L.: Phenolic compounds composition and biological activity of commercial samples and its aqueous and hydromethanolic extracts. Antioxidants. 2023;12(3). https://doi.org/10.3390/antiox12030550
- Verma N, Gupta SK, Tiwari S, Mishra AK. Safety of ashwagandha root extract: A randomized, placebo-controlled, study in healthy volunteers. Complementary Therapies in Medicine. 2021;57. https://doi.org/10.1016/j.ctim.2020.102642
- Elhadidy ME, Sawie HG, Meguid NA, Khadrawy YA. Protective effect of ashwagandha (Withania somnifera) against neurotoxicity induced by aluminum chloride in rats. Asian Pacific Journal of Tropical Biomedicine. 2018;8(1):59–66. https://doi.org/10.4103/2221-1691.221139
- Ajgaonkar A, Jain M, Debnath K. Efficacy and safety of ashwagandha (Withania somnifera) root extract for improvement of sexual health in healthy women: A prospective, randomized, placebo-controlled study. Cureus. 2022;14(10). https://doi.org/10.7759/cureus.30787
- Marotta A, Sarno E, Del Casale A, Pane M, Mogna L, Amoruso A, et al. Effects of probiotics on cognitive reactivity, mood, and sleep quality. Frontiers in Psychiatry. 2019;10. https://doi.org/10.3389/fpsyt.2019.00164
- Asan-Ozusaglam M, Celik I. White pitahaya as a natural additive: potential usage in cosmetic industry. Foods and Raw Materials. 2023;11(1):57–63. https://doi.org/10.21603/2308-4057-2023-1-552
- Irokanulo EO, Yadung Q-EM, Orotayo DE, Nwonuma CO, Alonge OS. In vitro probiotic evaluation of yeasts from coconut and raffia juices. Food Processing: Techniques and Technology. 2023;53(4):672–679. https://doi.org/10.21603/2074-9414-2023-4-2467
- Akhgarjand C, Vahabi Z, Shab-Bidar S, Etesam F, Djafarian K. Effects of probiotic supplements on cognition, anxiety, and physical activity in subjects with mild and moderate Alzheimer’s disease: A randomized, double-blind, and placebo-controlled study. Frontiers in Aging Neuroscience. 2022;14. https://doi.org/10.3389/fnagi.2022.1032494
- Liu N, Yang D, Sun J, Li Y. Probiotic supplements are effective in people with cognitive impairment: A meta-analysis of randomized controlled trials. Nutrition Reviews. 2023;81(9):1091–1104. https://doi.org/10.1093/nutrit/nuac113
- Huang H-J, Chen J-L, Liao J-F, Chen Y-H, Chieu M-W, Ke Y-Y, et al. Lactobacillus plantarum PS128 prevents cognitive dysfunction in Alzheimer’s disease mice by modulating propionic acid levels, glycogen synthase kinase 3 beta activity, and gliosis. BMC Complementary Medicine and Therapies. 2021;21. https://doi.org/10.1186/s12906-021-03426-8
- Mustafa MA, Ashry M, Salama HH, Abdelhamid SM, Hassan LK, Abdel-Wahhab KG. Ameliorative role of ashwagandha/probiotics fortified yogurt against AlCl3 toxicity in rats. International Journal of Dairy Science. 2020;15(4):169–181. https://doi.org/10.3923/ijds.2020.169.181
- Salama HH, El-Said MM, Abdelhamid SM, Abozed SS, Mounier MM. Effect of fortification with sage loaded liposome on the chemical, physical, microbiological properties and cytotoxicity of yoghurt. Egyptian Journal of Chemistry. 2020;63(10):3879–3890. https://doi.org/10.21608/EJCHEM.2020.27321.2572
- Official methods of analysis of AOAC International. 19th ed. Gaithersburg: AOAC International; 2012.
- Guide for the care and use of agricultural animals in research and teaching. Champaign: Federation of Animal Science Societies; 2010.
- Tepe B, Donmez E, Unlu M, Candan F, Daferera D, Vardar-Unlu G, et al. Antimicrobial and antioxidative activities of the essential oils and methanol extracts of Salvia cryptantha (Montbret et Aucher ex Benth.) and Salvia multicaulis (Vahl). Food Chemistry. 2004;84(4):519–525. https://doi.org/10.1016/S0308-8146(03)00267-X
- Lourens-Hattingh A, Viljoen BC. Yogurt as probiotic carrier food. International Dairy Journal. 2001;11(1–2):1–17. https://doi.org/10.1016/S0958-6946(01)00036-X
- El-Shenawy M, Fouad MT, Hassan LK, Seleet FL, El-Aziz MA. A probiotic beverage made from tiger-nut extract and milk permeate. Pakistan Journal of Biological Sciences. 2019;22(4):180–187. https://doi.org/10.3923/pjbs.2019.180.187
- Morgese MG, Trabace L. Monoaminergic system modulation in depression and Alzheimer’s disease: A new standpoint? Frontiers in Pharmacology. 2019;10. https://doi.org/10.3389/fphar.2019.00483
- Chien C-Y, Chien Y-J, Lin Y-H, Lin Y-H, Chan S-T, Hu W-C, et al. Supplementation of Lactobacillus plantarum (TCI227) prevented potassium-oxonate-induced hyperuricemia in rats. Nutrients. 2022;14(22). https://doi.org/10.3390/nu14224832
- Gould TD, Dao DT, Kovacsics CE. The open field test. In: Gould TD, editor. Mood and anxiety related phenotypes in mice: Characterization using behavioral tests. Totowa: Humana Press; 2009. pp. 1–20. https://doi.org/10.1007/978-1-60761-303-9_1
- Hliňák Z, Krejčı́ I. Oxiracetam prevents the MK-801 induced amnesia for the elevated plus-maze in mice. Behavioural Brain Research. 2000;117(1–2):147–151. https://doi.org/10.1016/S0166-4328(00)00298-9
- Gümüş HG, Agyemang AA, Romantsik O, Sandgren R, Karlsson H, Gram M, et al. Behavioral testing and litter effects in the rabbit. Behavioural Brain Research. 2018;353:236–241. https://doi.org/10.1016/j.bbr.2018.02.032
- Wright RL, Lightner EN, Harman JS, Meijer OC, Conrad CD. Attenuating corticosterone levels on the day of memory assessment prevents chronic stress-induced impairments in spatial memory. European Journal of Neuroscience. 2006;24(2):595–605. https://doi.org/10.1111/j.1460-9568.2006.04948.x
- Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology. 1961;7(2):88–90. https://doi.org/10.1016/0006-2952(61)90145-9
- Kim C, Speisky MB, Kharouba SN. Rapid and sensitive method for measuring norepinephrine, dopamine, 5-hydroxytryptamine and their major metabolites in rat brain by high-performance liquid chromatography: Differential effect of probenecid, haloperidol and yohimbine on the concentrations. Journal of Chromatography. 1987;386:25–35. https://doi.org/10.1016/S0021-9673(01)94581-9
- Munir N, Mahmood Z, Shahid M, Afzal MN, Jahangir M, Ali Shah SM, et al. Withania somnifera chemical constituents’ in vitro antioxidant potential and their response on spermatozoa parameters. Dose-Response. 2022;20(1). https://doi.org/10.1177/15593258221074936
- Ali MA, Kamal MM, Rahman MH, Siddiqui MN, Haque MA, Saha KK, et al. Functional dairy products as a source of bioactive peptides and probiotics: current trends and future prospectives. Journal of Food Science and Technology. 2022;59(4):1263–1279. https://doi.org/10.1007/s13197-021-05091-8
- Wang Y, Wu J, Lv M, Shao Z, Hungwe M, Wang J, et al. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Frontiers in Bioengineering and Biotechnology. 2021;9. https://doi.org/10.3389/fbioe.2021.612285
- Balajirao KN, Akhre T, Hingane LD. Ashwagandha as antibacterial. International Journal of Innovative Research in Technology. 2021;8(7):350–355.
- Khanchandani N, Shah P, Kalwani T, Ardeshna A, Dharajiya D. Antibacterial and antifungal activity of ashwagandha (Withania somnifera L.): A review. Journal of Drug Delivery and Therapeutics. 2019;9(5-s):154–161. https://doi.org/10.22270/jddt.v9i5-s.3573
- Kumar A, Kumar SPJ, Chintagunta AD, Agarwal DK, Pal G, Singh AN, et al. Biocontrol potential of Pseudomonas stutzeri endophyte from Withania somnifera (ashwagandha) seed extract against pathogenic Fusarium oxysporum and Rhizoctonia solani. Archives of Phytopathology and Plant Protection. 2022;55(1):1–18. https://doi.org/10.1080/03235408.2021.1983384
- Reddy YM, Kumar SPJ, Saritha K V, Gopal P, Reddy TM, Simal-Gandara J. Phytochemical profiling of methanolic fruit extract of Gardenia latifolia Ait. by LC-MS/MS analysis and evaluation of its antioxidant and antimicrobial activity. Plants. 2021;10(3). https://doi.org/10.3390/plants10030545
- Turrini F, Donno D, Beccaro GL, Pittaluga A, Grilli M, Zunin P, et al. Bud-derivatives, a novel source of polyphenols and how different extraction processes affect their composition. Foods. 2020;9(10). https://doi.org/10.3390/foods9101343
- Momin JK, Prajapati JB. Effect of selected medicinal herbs on viability and acid production of lactic dairy starters. Emergent Life Sciences Research. 2019;5(2):35–42. https://doi.org/10.31783/elsr.2019.523542
- Peterson CT. Dysfunction of the microbiota-gut-brain axis in neurodegenerative disease: The promise of therapeutic modulation with prebiotics, medicinal herbs, probiotics, and synbiotics. Journal of Evidence-Based Integrative Medicine. 2020;25:1–19. https://doi.org/10.1177/2515690X20957225
- Zahran HA, Mabrouk AMM, Salama HH. Evaluation of yoghurt fortified with encapsulated echium oil rich in stearidonic acid as a low-fat dairy food. Egyptian Journal of Chemistry. 2022;65(4):29–41. https://doi.org/10.21608/ejchem.2021.99859.4642
- Mehanna NM, Elwahsh NAA, El-Deeb AM, Nasser AA. Impact of using eggshells powder as a natural source of calcium on composition and quality of bio-karish cheese. Current Science International. 2020;9(4):607–616. https://doi.org/10.36632/csi/2020.9.4.53
- Delgado FJ, Rodríguez-Pinilla J, Márquez G, Roa I, Ramírez R. Physicochemical, proteolysis and texture changes during the storage of a mature soft cheese treated by high-pressure hydrostatic. European Food Research and Technology. 2015;240:1167–1176. https://doi.org/10.1007/s00217-015-2420-3
- Alamdar Husain S, David J, Ibrahim M, Nayeem Ali M, Chandra D, Srivastava P. Studies on chemical properties and nutritive value of dairy dessert (sandesh) incorporated with ashwagandha (Withania somnifera) and tulsi (Ocimum sanctum). Journal of Pharma Research. 2015;4(8):281–283.
- Carvajal MA, Alaniz AJ, Gutierrez-Gomez C, Vergara PM, Sejian V, Bozinovic F. Increasing importance of heat stress for cattle farming under future global climate scenarios. Science of The Total Environment. 2021;801. https://doi.org/10.1016/j.scitotenv.2021.149661
- Khatoon N, Gupta RK. Probiotics beverages of sweet lime and sugarcane juices and its physiochemical, microbiological & shelf-life studies. Journal of Pharmacognosy and Phytochemistry. 2015;4(3):25–34.
- Mirzaei-Alamouti H, Moradi S, Shahalizadeh Z, Razavian M, Amanlou H, Harkinezhad T, et al. Both monensin and plant extract alter ruminal fermentation in sheep but only monensin affects the expression of genes involved in acid-base transport of the ruminal epithelium. Animal Feed Science and Technology. 2016;219:132–143. https://doi.org/10.1016/j.anifeedsci.2016.06.009
- Bakirci I. The effects of some herbs on the activities of thermophilic dairy cultures. Nahrung. 1999;43(5):333–335.
- Verma KC. Ashwagandha (Withania somnifera Dunal): Wonder medicinal plant. Agricultural Reviews. 2010;31(4):292–297.
- Callaway TR, Wright ADG, Brikis O, Edrington TS, Nisbet DJ. Evaluation of bacterial diversity in the rumen and feces of cattle. In: Nelson KE, editor. Encyclopedia of metagenomics. New York: Springer-Verlag; 2014. pp. 171–176.
- Ruan J, Yao Y. Behavioral tests in rodent models of stroke. Brain Hemorrhages. 2020;1(4):171–184. https://doi.org/10.1016/j.hest.2020.09.001
- Chen X, Zhang M, Ahmed M, Surapaneni KM, Veeraraghavan VP, Arulselvan P. Neuroprotective effects of ononin against the aluminium chloride-induced Alzheimer’s disease in rats. Saudi Journal of Biological Sciences. 2021;28(8):4232–4239. https://doi.org/10.1016/j.sjbs.2021.06.031
- Reddy DS, Kulkarni SK. Possible role of nitric oxide in the nootropic and antiamnesic effects of neurosteroids on aging- and dizocilpine-induced learning impairment. Brain Research. 1998;799(2):215–229. https://doi.org/10.1016/S0006-8993(98)00419-3
- Zhang S-Y, Chen S-Q, Zhang J-Y, Chen C-H, Xiang X-J, Cai H-R, et al. The effects of bilateral prostriata lesions on spatial learning and memory in the rat. Frontiers in Behavioral Neuroscience. 2022;16. https://doi.org/10.3389/fnbeh.2022.1010321
- Cinalli DA, Cohen SJ, Guthrie K, Stackman RW. Object recognition memory: Distinct yet complementary roles of the mouse CA1 and perirhinal cortex. Frontiers in Molecular Neuroscience. 2020;13. https://doi.org/10.3389/fnmol.2020.527543
- Halverson T, Alagiakrishnan K. Gut microbes in neurocognitive and mental health disorders. Annals of Medicine. 2020;52(8):423–443. https://doi.org/10.1080/07853890.2020.1808239
- Socała K, Doboszewska U, Szopa A, Serefko A, Włodarczyk M, Zielińska A, et al. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacological Research. 2021;172. https://doi.org/10.1016/j.phrs.2021.105840
- Mindus C, Ellis J, van Staaveren N, Harlander-Matauschek A. Lactobacillus-based probiotics reduce the adverse effects of stress in rodents: A meta-analysis. Frontiers in Behavioral Neuroscience. 2021;15. https://doi.org/10.3389/fnbeh.2021.642757
- Xiao J, Wang T, Xu Y, Gu X, Li D, Niu K, et al. Long-term probiotic intervention mitigates memory dysfunction through a novel H3K27me3-based mechanism in lead-exposed rats. Translational Psychiatry. 2020;10. https://doi.org/10.1038/s41398-020-0719-8
- Webberley TS, Masetti G, Bevan RJ, Kerry-Smith J, Jack AA, Michael DR, et al. The impact of probiotic supplementation on cognitive, pathological and metabolic markers in a transgenic mouse model of Alzheimer’s disease. Frontiers in Neuroscience. 2022;16. https://doi.org/10.3389/fnins.2022.843105
- Hassan HM, Elnagar MR, Abdelrazik E, Mahdi MR, Hamza E, Elattar EM, et al. Neuroprotective effect of naringin against cerebellar changes in Alzheimer’s disease through modulation of autophagy, oxidative stress and tau expression: An experimental study. Frontiers in Neuroanatomy. 2022;16. https://doi.org/10.3389/fnana.2022.1012422
- Skalny AV, Aschner M, Jiang Y, Gluhcheva YG, Tizabi Y, Lobinski R, et al. Molecular mechanisms of aluminum neurotoxicity: Update on adverse effects and therapeutic strategies. Advances in Neurotoxicology. 2021;5:1–34. https://doi.org/10.1016/bs.ant.2020.12.001
- Mold MJ, O’Farrell A, Morris B, Exley C. Aluminum and tau in neurofibrillary tangles in familial Alzheimer’s disease. Journal of Alzheimer’s Disease Reports. 2021;5(1).
- Abbas F, Eladl MA, El-Sherbiny M, Abozied N, Nabil A, Mahmoud SM, et al. Celastrol and thymoquinone alleviate aluminum chloride-induced neurotoxicity: Behavioral psychomotor performance, neurotransmitter level, oxidative-inflammatory markers, and BDNF expression in rat brain. Biomedicine and Pharmacotherapy. 2022;151. https://doi.org/10.1016/j.biopha.2022.113072
- Elmorsy E, Elsharkawy E, Alhumaydhi FA, Salama M. The protective effect of Indian Catechu methanolic extract against aluminum chloride-induced neurotoxicity, A rodent model of Alzheimer’s disease. Heliyon. 2021;7(2). https://doi.org/10.1016/j.heliyon.2021.e06269
- Elshamy S, Abdel Motaal A, Abdel-Halim M, Medhat D, Handoussa H. Potential neuroprotective activity of Mentha longifolia L. in aluminum chloride-induced rat model of Alzheimer’s disease. Journal of Food Biochemistry. 2021;45(4). https://doi.org/10.1111/jfbc.13644
- Kasem NRA, Mannaa FA, Abdel-Wahhab KG, Mourad HH, Gomaa HF. Preventive efficiency of Chelidonium majus ethanolic extract against aflatoxin B1 induced neurochemical deteriorations in rats. Pakistan Journal of Biological Sciences. 2022;25(3):234–244. https://doi.org/10.3923/pjbs.2022.234.244
- Igbokwe IO, Igwenagu E, Igbokwe NA. Aluminium toxicosis: a review of toxic actions and effects. Interdisciplinary Toxicology. 2019;12(2):45–70. https://doi.org/10.2478/intox-2019-0007
- Makhdoomi S, Mahboobian MM, Haddadi R, Komaki A, Mohammadi M. Silibinin-loaded nanostructured lipid carriers (NLCs) ameliorated cognitive deficits and oxidative damages in aluminum chloride-induced neurotoxicity in male mice. Toxicology. 2022;477. https://doi.org/10.1016/j.tox.2022.153260
- Ott DB, Hartwig A, Stillman MJ. Competition between Al3+ and Fe3+ binding to human transferrin and toxicological implications: structural investigations using ultra-high resolution ESI MS and CD spectroscopy. Metallomics. 2019;11(5):968–981. https://doi.org/10.1039/C8MT00308D
- De Nicolo B, Cataldi-Stagetti E, Diquigiovanni C, Bonora E. Calcium and reactive oxygen species signaling interplays in cardiac physiology and pathologies. Antioxidants. 2023;12(2). https://doi.org/10.3390/antiox12020353
- Ramos-González EJ, Bitzer-Quintero OK, Ortiz G, Hernández-Cruz JJ, Ramírez-Jirano LJ. Relationship between inflammation and oxidative stress and its effect on multiple sclerosis. Neurología. 2021. https://doi.org/10.1016/j.nrl.2021.10.003
- Antoniadou F, Papamitsou T, Kavvadas D, Kapoukranidou D, Sioga A, Papaliagkas V. Toxic environmental factors and their association with the development of dementia: A mini review on heavy metals and ambient particulate matter. Materia Socio-Medica. 2020;32(4):299–306. https://doi.org/10.5455/msm.2020.32.299-306
- Hybertson BM, Gao B, McCord JM. Effects of the phytochemical combination PB123 on Nrf2 activation, gene expression, and the cholesterol pathway in HepG2 cells. OBM Integrative and Complimentary Medicine. 2022;7(1). https://doi.org/10.21926/obm.icm.2201002
- Bashir A, Nabi M, Tabassum N, Afzal S, Ayoub M. An updated review on phytochemistry and molecular targets of Withania somnifera (L.) Dunal (Ashwagandha). Frontiers in Pharmacology. 2023;14. https://doi.org/10.3389/fphar.2023.1049334
- Wongtrakul J, Thongtan T, Kumrapich B, Saisawang C, Ketterman AJ. Neuroprotective effects of Withania somnifera in the SH-SY5Y Parkinson cell model. Heliyon. 2021;7(10). https://doi.org/10.1016/j.heliyon.2021.e08172