Аннотация
Seaweed has a unique chemical composition with an abundance of bioactive substances. In Russia, brown seaweed grows in the coastal areas of the Pacific Ocean (Far East) and the seas of the Arctic Ocean.This review focuses on the therapeutic and nutritional potential of functional components of brown seaweed. It was based on a systematic analysis of research and review articles published from 2010 to 2023 and indexed in Scopus, Web of Science, and eLIBRARY.RU. Our particular interest was in seaweed’s bioactive components such as polysaccharides, phenolic compounds, vitamins, lipids and fatty acids, proteins, peptides, and amino acids.
Compounds extracted from brown seaweed exhibit antioxidant, antiglycemic, antitumoral, neuroprotective, anti-inflammatory, anticoagulant, antibacterial, and immunostimulating properties. Brown seaweed and its derivatives are used as structural modifiers, antioxidants, preservatives, moisture-retaining agents, and sources of vitamins and minerals in the development of functional and preventive food products. They are also used as ingredients in meat, dairy, bakery and flour products, as well as in food additives and beverages, to provide potential health benefits and essential nutrients.
Studies have proven the functional effectiveness of food products containing brown seaweed and its derivatives. The incorporation of seaweed components into functional foods could contribute to global food security. More research is needed to develop new competitive products based on seaweed and to investigate them for the presence of substances hazardous to humans and the environment.
Ключевые слова
Brown seaweed, bioactive substances, bioactivity, functional foods, functional ingredients, essential nutrientsВВЕДЕНИЕ
Nowadays, there is an increasing interest in the production of healthy food products, with special attention paid to balanced therapeutic and functional products. This is associated with their positive impact on human health and preventative action against various diseases. Food formulators focus on the use of novel and renewable sources of commercial food raw materials that are rich in bioactive substances with therapeutic potential. Brown seaweed is a promising source of bioactive components since it has a high growth rate, a large increase in biomass, and an abundance of fermentable carbohydrates. In addition, seaweed does not need fresh water for cultivation.
Based on pigmentation, seaweeds belong to three different groups: Phaeophyta (brown algae), Chlorophyta (green algae), and Rhodophyta (red algae) [1–3]. Brown seaweeds, which number over 2000 species, have predominated in global seaweed production (some of the most common of them are shown in Fig. 1) [4]. From 1950 to 2019, seaweed production annually increased by 11% to reach about 35 million tons [5]. In 2019, two main brown seaweeds (Laminaria saccharina and Undaria pinnatifida) accounted for about 47% of global seaweed production [6].
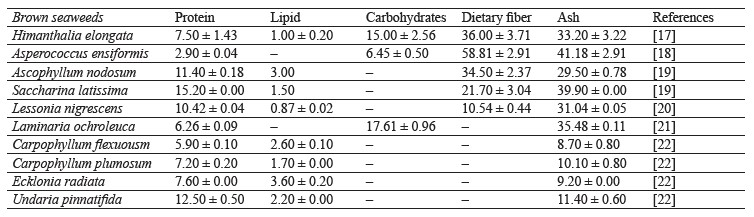
Brown seaweeds are rich in carbohydrates, proteins, polyunsaturated fatty acids, and dietary fibers (Table 1), while being very low in lipids [10]. They are a plant source of vitamins, minerals (micro- and macronutrients), bioactive molecules and enzymes, as well as iodine [11]. These components may vary in content depending on the conditions of seaweed growth: temperature, water salinity, light exposure, degree of surfacing, depth of growth, type of substrate, and other factors [12, 13]. These changes are associated with the influence of external factors on the processes of photosynthesis, respiration, and permeability of seaweed shells [14–16].
Seaweeds are consumed in small quantities and therefore cannot be considered a major source of energy. However, brown seaweeds are a source of extracts rich in nutrients and bioactive substances. They have high potential for use as food additives and/or ingredients to enhance the nutritional and biological value of food products.
In this review, we analyzed and systematized data on the therapeutic and nutritional potential of the functional components of brown seaweeds. In the first part, we described their bioactive components, including polysaccharides, dietary fibers, phenolic compounds, pigments, lipids (fatty acids), proteins, peptides, amino acids, vitamins, and minerals. Based on current scientific publications, we evaluated possible uses of these compounds in the treatment and prevention of various diseases. The second part of the review is devoted to the use of bioactive substances extracted from brown seaweeds in food technologies for the production of foods with functional and preventative action.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
This study was carried out in the Chemistry and Technology of Marine Bioresources Laboratory at Murmansk State Technical University. We systematized data from original research articles and reviews on seaweeds, their bioactive components, as well as their nutritional and therapeutic potential and applications. For this, we employed a number of methods. First, bibliometric analysis was performed to determine the relevance of literature sources and clean the data of irrelevant or repeated sources. Then, the key scientific publications selected were exposed to in-depth analysis and systematization. A logical search strategy was employed for exploratory analysis to select publications covering the entire range of research problems, from the description of properties of brown seaweeds to their consumption [23]. The search terms (keywords) were broad to provide maximum coverage and included various combinations with the term “brown seaweed”. The search was limited to publications from 2010 to 2023 on three databases: Web of Science, Scopus, and eIBRARY.RU. The publication citation ranking was used to select publications from the generated list for in-depth analysis.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
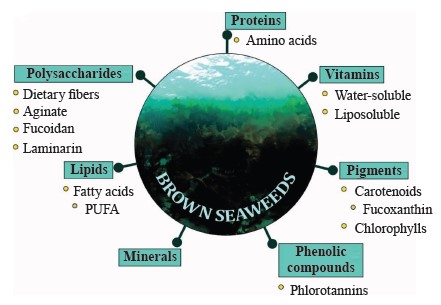
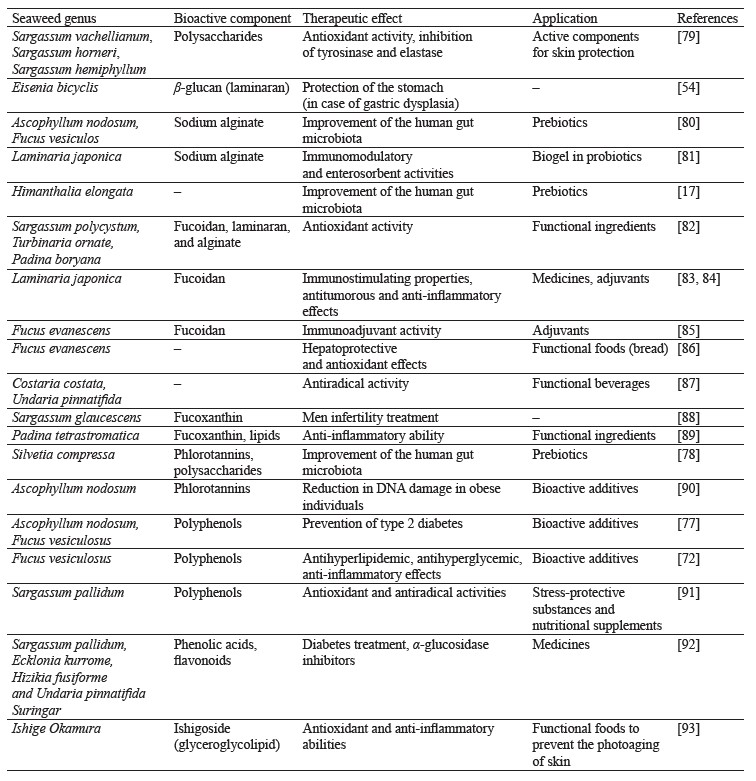
Bioactive components of brown seaweeds and their therapeutic potential. Brown seaweeds have a unique chemical composition and are rich in various bioactive substances (
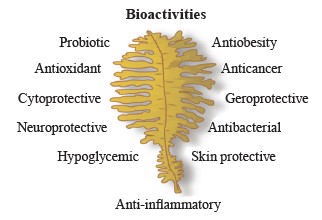
Functional components extracted from brown seaweed have high potential in treating a number of chronic diseases due to their antioxidant, anticoagulant, antiglycemic, antitumorous, and neuroprotectant activities [26–32] (Fig. 3). These properties are key to potential nutraceutical and therapeutic applications of brown seaweeds [33, 34].
Figure 3 shows the main components of brown seaweeds that have biological activity and are widely used in various technologies.
Polysaccharides. Seaweed is an important source of polysaccharides, which are more diverse than those in land plants [35, 36]. Most carbohydrates in seaweed are sulfated and unsulfated polysaccharides. Brown seaweed is rich in polysaccharides such as laminarin, alginate, and fucoidan, which consist of monosaccharides such as glucose, rhamnose, galactose, fucose, xylose, mannose, as well as glucuronic and mannuronic acids [24, 37].
According to clinical studies, seaweed-derived bioactive components are effective in the prevention and treatment of COVID-19. Sulfated polysaccharides and polyunsaturated fatty acids obtained from seaweed also exhibit immunostimulating and antitumorous effects [33, 37, 38].
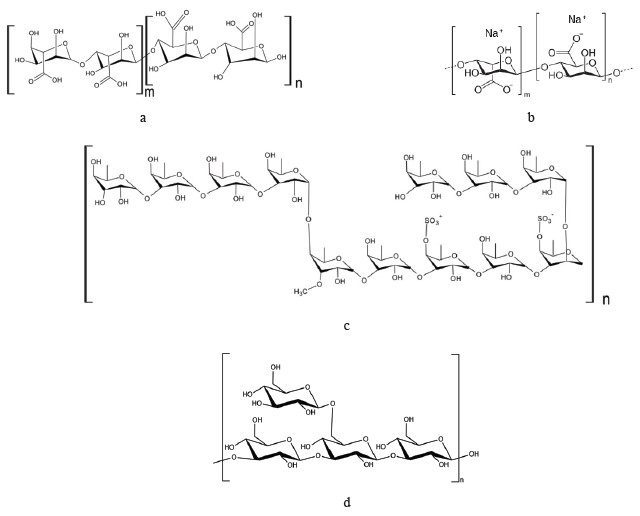
Alginates (or alginic acid) (Figs. 4a and b), which are part of cell membranes, are the most common polysaccharide in brown seaweeds. Some of them contain up to 70% of alginic acid on dry basis [39]. Alginates can be obtained by chemical extraction, by microwave radiation, ultrasonic extraction, or a combination of enzymatic and traditional chemical extraction [40]. Alginates are extracted from such genera as Laminaria, Ecklonia, Ascophyllum, Durvillaea, Lessonia, Macrocystis, Sargassum, and Turbinaria [41]. Laminaria and Macrocystis are currently the main sources of alginates [42]. Alginates are widely used in the food and pharmaceutical industries to prevent bowel diseases and regulate blood sugar levels. Placebo-controlled studies in humans have shown that alginates have a positive effect on the appetite by increasing a feeling of fullness and reducing energy consumption by the body while maintaining its functionality [43–46].
Alginates are also able to form hydrogels in the presence of metal cations. They are widely used in the food and pharmaceutical industries due to their high waterholding capacity and good adsorption properties [32].
Fucoidan (Fig. 4c) is a fucose-containing sulfated cell wall polysaccharide that protects brown seaweed against environmental impacts. Its content and composition vary depending on the species, as well as the season and stage of seaweed growth [47, 48]. Fucoidan can be extracted by conventional chemical, microwave, ultrasonic, or enzyme extraction [49]. Saccharina latissima and Fucus evanescens are the most suitable sources of fucoidans [50].
Brown seaweed-derived fucoidan is the most promising anticancer agent due to its powerful antitumor activity against various types of cancer [51]. Also, fucoidan has a hypoglycemic effect and is therefore used to treat diabetes mellitus and prevent its complications [46, 52].
Fucoidan extracted from brown seaweed can also be used in aquaculture as a functional bioactive component in the diets of both fish and shellfish [53].
Laminarin. Beta-glucan (β-glucan), which is a polymer of glucose, is contained in the cell walls of plants, cereals, fungi, seaweed, and some species of bacteria [54]. Brown seaweed is the main source of laminarin (or laminaran), a type of β-(1→3)-glucan containing β-(1→6)-related branches (Fig. 4d) [55]. Laminarin was first discovered in kelp species [56]. It belongs to dietary fibers and is not digested in the upper gastrointestinal tract. Laminarin helps reduce the risk of colon cancer, obesity, and diabetes [47]. Oxidation and reduction processes enhance the antitumorous, antioxidant, and anti-inflammatory properties of laminarin [32]. In 2020, the beta-glucan market amounted to $403.8 million, and it is estimated to grow annually by 7.6% to reach $628.3 million by 2026 [57].
Dietary fibers. The human gut microbiota plays an enormous role in general health and disease prevention. Numerous current studies are aiming to strengthen the immune system with the help of the gut microbiota and to treat a number of diseases, such as diabetes, cancer, and obesity [58]. Polysaccharides obtained from brown seaweed have been found effective in stimulating the gut microorganisms. Seaweeds contain 25–70% of total dietary fibers, of which 50–80% are soluble fibers [59]. Laminarin and fucoidan are typical soluble dietary fibers in brown seaweeds, while cellulose belongs to insoluble dietary fibers [60]. Sulfated polysaccharides, which are dietary fibers in seaweeds, are rarely found in terrestrial plants [61].
Dietary fibers have a prebiotic effect on human health. Brown seaweeds of the genera Ecklonia, Sargassum, Laminaria, Ascophyllum, Fucus, Undaria, Saccorhiza, and Porphyra have this effect due to the presence of polysaccharides, including dietary fibers (carbohydrates) which are not digested by human digestive enzymes. On the other hand, they are a nutrient substrate that stimulates the growth of beneficial microbiota (e.g., Lactobacillus, Bifidobacterium, and Faecalibacterium) [62, 63]. The prebiotic potential of seaweed has been confirmed by studies using a human colon model in vitro [17, 64]. As prebiotic compounds, dietary fibers induce an immune response by increasing the microbial activity of the gastrointestinal tract. This leads to fermentation and production of short-chain fatty acids, which, in turn, has several positive physiological effects. Seaweed’s dietary fibers have antioxidant, anti-inflammatory, anticoagulant, and antiviral activities [65–68]. Ajanth Praveen et al. reviewed the structure of various seaweed polysaccharides, new methods their extraction and purification, as well as their immunomodulatory effects on the gut microbiota [69].
Dietary fibers of brown seaweed are widely used in food technology, mainly as thickeners, emulsifiers, gelling agents, and prebiotics [70]. Sulfated polysaccharides (dietary fiber) have been shown to play an important role in enhancing seaweed’s antioxidant, immunomodulatory, anticarcinogenic, antiviral, and antimicrobial activities [41]. Dietary fibers extracted from brown seaweed show an excellent ability to swell and retain water due to the hydrophilic characteristics of sulfated polysaccharides [71]. This property is used in the production of meat products.
Phenolic compounds are among the most important bioactive components of seaweed. They include phenolic acids, tannins, flavonoids, catechins, and phlorotannins. Their composition varies depending on the type of seaweed. Brown seaweed contains mainly phlorotannins, which are complex polymers made up of phloroglucin links (1,3,5-trihydroxybenzene). Polyphenols account for 2 to 30% of seaweed’s dry weight. Phenolic compounds in brown seaweed have been shown to have antihyperlipidemic and antihyperglycemic effects [72, 73]. Phlorotannins exhibit antioxidant, anti-inflammatory, antimicrobial, cytotoxic, and antitumorous activities [74, 75]. They can also be used as anti-aging agents [76]. Phlorotannins play a major role in cell wall creation and perform protective functions [56].
People with prediabetes, overweight, and obesity are recommended to consume extracts of brown seaweeds (Ascophyllum nodosum and Fucus vesiculosus), which contribute to a significant reduction in body weight, waist circumference, and overall fat mass, as well as have beneficial effects on insulin secretion [77]. Numerous studies have shown a positive effect of phlorotannins and polysaccharides from Silvetia compressa on the growth of probiotic bacteria and therefore the human microbiota [78]. The therapeutic effects and possible applications of polysaccharides and phenolic compounds from brown seaweeds are shown in Table 2.
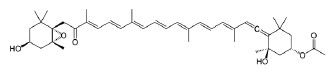
Pigments. There are three groups of pigments in seaweed: chlorophylls, carotenoids, and phycobiliprote- ins [94, 95]. Brown seaweed contains chlorophylls “a”, “c1 and c2”, and fucoxanthin (Fig. 5), which give the cells the brown color, as well as β-carotene, neofucoxanthin, and other carotenoids [96]. Chlorophyll pigments have positive effects on human health. In particular, they contribute to chelation with some chemical carcinogens and mutagens, lower the risk of cancer, and exhibit high antioxidant activity [95]. Pigments can be extracted from seaweed by solvent extraction, liquid extraction under pressure, or microwave extraction [97].
Fucoxanthin is among the most common carotenoids in brown seaweed. It is absent in terrestrial plants [26]. Fucoxanthin exhibits strong antioxidant action against oxidative stress [45]. It is safe to use and has no side effects. Fucoxanthin protects the cardiovascular system and has anti-inflammatory, anti-cancerous, and neuroprotective effects. Furthermore, it is an effective chelator of toxic and heavy metals [98]. Fucoxanthin’s antioxidant activity is associated with its neuroprotective, photoprotective, and hepatoprotective effects [26].
Lipids, fatty acids. Fatty acids with two or more double bonds are necessary for normal cell function, and they play a key role in cellular and tissue metabolism, regulating membrane fluidity, electron and oxygen transport, as well as temperature adaptation [99].
Lipids of seaweed mainly contain fatty acids with a long carbohydrate chain. Some brown seaweeds have a high content of total lipids in the range of 10–20 wt%. In particular, lipids account for 11.91 ± 2.00 wt% in Dictyota bartayresii, 10.80 ± 0.99 wt% in Dictyota dichotoma, 11.73 ± 0.49 wt% in Spatoglossum macrodontum, 12.8, 13.4, and 10.9 wt% (in April, May, and July, respectively) in Costaria costata, and 15.59 wt% (January) in Cystoseira hakodatensis [100]. Essential fatty acids and polyunsaturated fatty acids are found in large quantities in brown seaweed [101]. The main omega-3 polyunsaturated fatty acids are eicosapentaenoic acid (20:5 n-3), stearidonic acid (18:4 n-3), and α-linolenic acid (18:3 n-3), while the main omega-6 polyunsaturated fatty acid is arachidonic acid (20:4 n-6). Polyunsaturated fatty acids account for 51.28% of all fatty acids in Saccharina japonica. Arachidonic acid (C20:4 n-6) varies from 10.55% (Undaria pinnatifida) to 14.87% (Sargassum horneri), and eicosapentaenoic acid ranges from 8.36% (C. costata) to 13.04% (Saccharina japonica) [102].The content of eicosapentaenoic acid is also high in A. nodosum (6.85%) and S. latissima (4.67%) [19]. Sargassum pallidum extract contains n-6 polyunsaturated fatty acids (41.3%) with C18 and C20 carbon atoms and such n-3 polyunsaturated fatty acids as α-linolenic (18:2; 7.8%), stearidonic (18:4; 7.3%), and eicosapentaenoic (20:5; 3.5%) acids Since the human body has a low ability to synthesize docosahexaenoic acid from linoleic acid, high intake of long-chain fatty acids and more unsaturated forms of linoleic acid (eicosapentaenoic and docosahexaenoic acids) is recommended to prevent cardiovascular disease [100].
Lipids containing n-6 and n-3 long-chain fatty acids are known to prevent atherosclerosis [103]. The lipid complex of S. pallidum introduced into the diet may have a hypolipidemic effect, restoring the liver’s lipid metabolism and esterifying function, as well as regulating the lipoprotein content in plasma. The combined action of n-3 and n-6 polyunsaturated fatty acids in S. pallidum’s lipid complex has an antioxidant effect on
the organism [104]. S. japonica’s lipid complex reduces dyslipidemia and hypercholesterolemia, as well as normalizes the ratio of fatty acids in total lipids of blood plasma and erythrocyte membranes due to the presence of various polyunsaturated fatty acids [105].
Proteins, peptides, and amino acids. The protein content in brown seaweed ranges from 5.00 to 19.66%. For example, Chnoospora minima, Dictyota menstrualis, Padina gymnospora, and Sargassum vulgare contain from 10 to 15% of protein dry weight. Brown seaweed contains all essential amino acids in the quantities recommended by the Food and Agriculture Organization of the United Nations (FAO). Its levels of aspartic and glutamic amino acids, which give seaweed a unique taste and aroma, are higher than those in red or green seaweed [4, 47, 101, 106]. Peptides derived from seaweed are known to exhibit antioxidant properties. They are also effective in treating cardiovascular diseases and diseases associated with metabolic syndrome [47].
Glycoproteins obtained from brown seaweed have unique biological properties. For example, glycoprotein isolated from U. pinnatifida has an antioxidant effect and is active against inflammatory diseases and Alzheimer’s disease due to cholinesterase inhibition [106, 107]. This glycoprotein inhibits the formation of toxic β-amyloid peptides by inhibiting β-secretase. In addition, it does not exhibit cytotoxicity in primary hippocampal cells and protects the cells from natural death. This glycoprotein was also shown to inhibit inflammatory mediators and nitric oxide, so it can be used as a dietary supplement to prevent inflammatory pathologies. Glycoprotein isolated from the brown seaweed Laminaria japonica has an antiproliferative effect on HT-29 colon cancer cells [108]. Glycoprotein was also shown to stimulate gastrointestinal cell growth in mice [109].
Brown seaweeds contain lectins, glycoproteins capable of reversibly and specifically binding to sugar residues [110]. Lectins are capable of specific recognition and can bind sugars (lactose, mannose, galactose, N-acetylgalactosamine, and N-acetylglucosamine) by non-covalent interactions. Lectin-carbohydrate interactions play an important role in such biological processes as cell adhesion, agglutination, opsonization, complement activation, and phagocytosis. Due to the specificity of mannose binding, lectins from brown seaweed are used to decipher and characterize complex mannose-containing glycans from the glycocalyx covering both normal and transformed cells. Lectins are also widely used as effective agents against the human immunodeficiency virus [111].
Seaweed lectins, often called phycolectins, are similar to plant lectins, but they also differ in some physical and chemical properties and have a unique carbohydrate specificity [112]. Phycolectins are monomeric proteins with a low molecular weight and an isoelectric point (pI) in the range of 4 to 6 [113]. Over 800 lectins from seaweeds have now been identified, of which 61% are from red seaweed, 22% from green seaweed, and 17% from brown seaweed. However, only about 40 lectins have been identified, purified, and sequenced [114].
Seaweed lectins are attracting attention because of their antiviral activity. Lectins can prevent the virus’s invasion into host cells and spreading there. This is where they differ from most traditional antiviral agents which block the life cycle of a virus once it has entered the cell. In addition, lectins act as surface markers for tumor cell recognition, transmembrane signal transduction, cell adhesion, mitotic apoptosis, and cytotoxicity. Therefore, lectins can be used in cancer diagnosis and therapy [110].
Noteworthily, the consumption of brown seaweed that has not undergone deep processing can reduce the availability and digestibility of protein due to high contents of soluble fibers and polyphenols. For use in food, the protein of brown seaweed should be separated from non-protein components [115, 116]. Proteins can be extracted from seaweed by using enzymes, microwaves, ultrasound, pulsed electric fields, or supercritical fluids [4]. Enzymes that decompose polysaccharides are used to release flavor components that impart umami flavor, such as peptides and amino acids [117].
Vitamins. Seaweed contains both water- and fatsoluble vitamins. Brown seaweed is an excellent source of vitamins A, B1, B2, B3, B12, C, D, E, as well as pantothenic and folic acids.
Brown seaweed is rich in vitamins B1 and B2. In particular, U. pinnatifida and S. japonica contain 0.3 and 0.24 mg B1/100 g dry weight, respectively, as well as 1.35 and 0.85 mg of B2/100 g dry weight, respectively [118, 119]. According to [120], U. pinnatifida has even higher contents of vitamins B1 and B2, namely 5 mg of B1/100 g dry weight and 11.7 mg of B2/100 g dry weight. Vitamins B1 and B2 are contained in smaller amounts in Eisenia arborea, namely 0.06–0.12 and 0.65– 0.92 mg/100 g dry weight, respectively [121]. Brown seaweed is also rich in vitamin C. Its contents in U. pinnatifida, S. latissima, and F. vesiculosus are 14.58, 61, and 40.9–51.7 mg/100 g dry weight, respectively [122–124].
Brown seaweed has a higher content of α-tocopherol, as well as β- and γ-tocopherols, compared to red and green seaweeds containing only α-tocopherol. The largest amount of vitamin E was found in Macrocystis pyrifera (132.77 mg/100 g) [119]. In Durvillaea antarctica and U. pinnatifida, the content of vitamin E amounted to 84.0 ± 0.5 mg/kg dry weight and 0.63 mg/100 g dry weight, respectively [125, 126].
Seaweed contains only provitamins of vitamin A. The brown seaweed S. japonica has a high content of β-carotene with vitamin A activity (2.99 mg/100 g dry weight calculated as 481 IU/100 g dry weight) [119]. According to [126], the content of vitamin A in U. pinnatifida is 4.73 IU/kg dry weight.
The bioavailability of vitamins is primarily related to their solubility, which ensures their absorption in the intestine. The bioavailability and absorption of some seaweed fat-soluble vitamins depends on whether they are taken with fat-containing foods or not. Fat-soluble vitamins are absorbed in the same way as dietary lipids [119]. Also, vitamins that are bound to fiber or some other carbohydrates in foods are less available than those taken in pure form.
Seaweeds are an important source of antioxidants since they can generate necessary compounds for protection against oxidation [127, 128]. Antioxidant activity is determined by several factors, such as the antioxidant’s internal chemical activity against radicals, the location and reactivity of radicals, the antioxidant’s concentration and interaction with other antioxidants, etc. Antioxidant compounds in seaweed include vitamin E (α-tocopherol), vitamin C (ascorbic acid), vitamin B1, and nicotinic acid [119, 129]. Vitamins with strong antioxidant capacity can act as therapeutic agents to protect against cancer [51, 130].
Seaweed is the only non-animal source of vitamin B12, which is important for vegetarians. Cobalamin is not synthesized in higher plants and is not required for their metabolism, so vegetables and fruits are low in this vitamin. Vitamin B12 deficiency is a common consequence of vegetarian and vegan diets that leads to pernicious anemia, a disease characterized by impaired hematopoiesis. Vitamin B12 is present in brown seaweeds of the genera Ascophyllum and Laminaria. Vitamin B12 also slows down the aging process [131, 132].
Vitamin E found in seaweed is a strong antioxidant that prevents the formation of free radicals. As reported in [133], vitamin E improves the condition of blood vessels and reduces their damage. Vitamin E has also been shown to lower the risk of lung and cervix uteri cancer by interacting with genotoxic radicals, reducing mutagenic activity, inhibiting the formation of carcinogenic nitrosamines, and protecting cell membranes from peroxidation [134, 135]. α-tocopherol is able to bind free radicals through the phenol group and plays an important role in the oxidation of biological membranes, lipoproteins, and fatty deposits, controlling or reducing lipid levels [136].
Ascorbic acid contained in seaweed is another effective antioxidant [119]. Due to its ability to neutralize free radicals, it is believed to play an important role in preventing cancer. In addition, ascorbic acid has prooxidant properties [137]. Several studies have established a correlation between vitamin C intake and lower incidence of stomach cancer [134, 138, 139]. They have also found a possible association with a decreased risk of developing cancer of the oral cavity, pharynx, lungs, and gallbladder in men. Vitamin C intake helps lower blood pressure in patients with hypertension, hyperlipidemia, and diabetes. A combination of vitamin C with other antioxidants (vitamin E, β-carotene) can provide a synergistic antihypertensive effect [140].
Minerals. Seaweed can accumulate micro- and macronutrients contained in seawater, which gave rise to the term “marine organic drugs”. Brown seaweed absorbs minerals better than green or red seaweed due to its high content of alginic acid and its salts [141]. In brown seaweed, minerals and micronutrients account for 14– 45% dry weight, depending on seasonal and climatic variations [142]. The mineral content in seaweed is up to 36% dry weight, which is 10–100 times higher than that of fruits and vegetables. Thus, seaweed can make an important contribution to the daily mineral intake [143].
Brown seaweeds Laminaria digitata, U. pinnatifida, and F. vesiculosus are rich in minerals that are thought to improve glycemic control. These minerals include potassium (K; 2–15% dry weight), calcium (Ca; 0.1– 3.0% dry weight), and magnesium (Mg; 0.1–1.5% dry weight) [31, 122, 144, 145]. These seaweeds also contain zinc (Zn) and chromium (Cr) in the amounts of 0.004– 0.020 and 0.02–0.05%, respectively, due to improved circulating glucose levels [31, 122, 145–147].
According to scientific estimates, about one-third of the world’s population is at risk of zinc deficiency, especially children under the age of five who need zinc to support their growth [148]. Zinc exhibits therapeutic effects in several chronic diseases, such as atherosclerosis, some cancers, autoimmune diseases, Alzheimer’s and other neurodegenerative disorders, diabetes, depression, Wilson’s disease, as well as aging [149]. Chromium is required for energy production from blood sugar, as well as for insulin function and lipid metabolism [150]. A daily intake of one gram of U. pinnatifida can meet the recommended physiological requirement for chromium [143].
Seaweed is one of the most important sources of calcium and phosphorus. It contains large amounts of Ca, Mg, Na, P, Zn, and I. Compared to other mineral-rich foods, seaweed contains more Ca, Cr, I, Fe, Mg, P, Se, Zn, K, and Na. However, it has a lower copper content compared to other foods such as raw meat or mushrooms [151]. The content of calcium in the brown seaweeds A. nodosum (575.0 mg/100 g raw weight) and L. digitata (364.7 mg/100 g raw weight) exceeds that of whole milk (115.0 mg/100 g raw weight). Thus, these seaweeds can be used as a source of calcium to prevent or treat osteoporosis in growing children, as well as in pre- and postmenopausal women. In addition, the non-digestible prebiotic carbohydrates in seaweed can increase calcium absorption and bioavailability [152–154]. F. vesiculosus also contains Ca and Mg in much higher concentrations than many other products. Particularly, its Ca values (2.175 mg per 100 g dry weight) are almost 20 times as high as in whole milk, while its Mg concentration (994 mg per 100 g) is about 5 times as high as in peanuts. The iron (Fe) content in F. vesiculosus can reach 49– 52 mg per 100 g dry weight, so this seaweed may be useful in providing daily iron intake and preventing iron deficiency anemia [141, 154].
It has also been shown that one gram of seaweed can cover up to 57.6 % of the recommended daily intake of selenium, with the brown seaweeds M. pyrifera and S. japonica having the highest content [143].
Brown seaweeds are able to accumulate higher concentrations of sodium (Na) ranging from 0.4 to 9% dry weight and potassium (K) ranging from 2 to 15% dry weight than green seaweeds. The Na/K ratio in brown seaweeds is quite low (0.3–1.5), so they can support human cardiovascular health by reducing blood pressure [155].
Mg and Ca play a key role in the health of bones and teeth. In addition, Mg is also involved in cellular metabolism and enzyme systems, while Ca is involved in the regulation of heartbeat, nerve impulse transmission, muscle contraction, blood clotting, and activation of insulin and thyroid hormone calcitonin [156]. Brown seaweeds have been shown to contain higher amounts of calcium than tofu or cabbage [143].
Brown seaweeds are a source of iodine, especially in the genus Laminaria, which can accumulate iodine in amounts exceeding its content in seawater more than 30 000 times [157]. Iodine content in different seaweeds consumed as food can vary significantly (from 0.1 to 30 mg/g dry weight). Iodine is an important trace element necessary for the production of thyroid hormones thyroxine and triiodothyronine, which stimulate both metabolic regulation and normal development of the body. The bioavailability of iodine from brown seaweed is quite high, ranging from 31 to 90%, as shown by in vivo studies. However, excessive iodine intake can cause thyroid disorders and lead to both hyperthyroidism and hypothyroidism [158].
Uses of brown seaweeds in food technology. Among brown seaweeds, F. evanescens, S. japonica, S. latissima, L. digitata, and U. pinnatifida have the largest application in the food industry [159].
Seaweed is obtained in their natural habitat or grown on special farms. The world production of brown seaweed increased from 13 000 tons in 1950 to 16.4 million tons in 2019. Its average annual growth in 1950–2019 was higher than the growth of global aquaculture of all species. In 2019, the brown seaweeds Laminaria saccharina and U. pinnatifida accounted for 47.3% of the world’s seaweed production [5]. The world market for seaweed products amounted to $4.7 billion in 2021 and is expected to reach $6.4 billion by 2026, with an average annual growth of 6.3% [160].
Seaweed is mainly cultivated and processed in East and Southeast Asia, where it is commonly used as a food product. Although it is a traditional product in the coastal communities, many countries consider seaweed a niche or a novel product and therefore consume it in small quantities. Functionally, seaweed is consumed:
– as part of vegetarian diet, as well as in therapeutic or preventative nutrition;
– as a seaweed-based food additive;
– for gastronomic purposes in exotic dishes of oriental cuisine;
– as organic, ecological bio-products, whose production is environmentally-friendly and reduces emissions of greenhouse gases; and
– in social nutrition as an affordable balanced product for the growing population, etc.
In addition to nutritional purposes, seaweed is also used to produce feed for farm animals (including aquaculture), pharmaceuticals and nutraceuticals, cosmetics, textiles, biofertilizers/biostimulants, bio-packing, biofuels, etc. [159, 161–163].
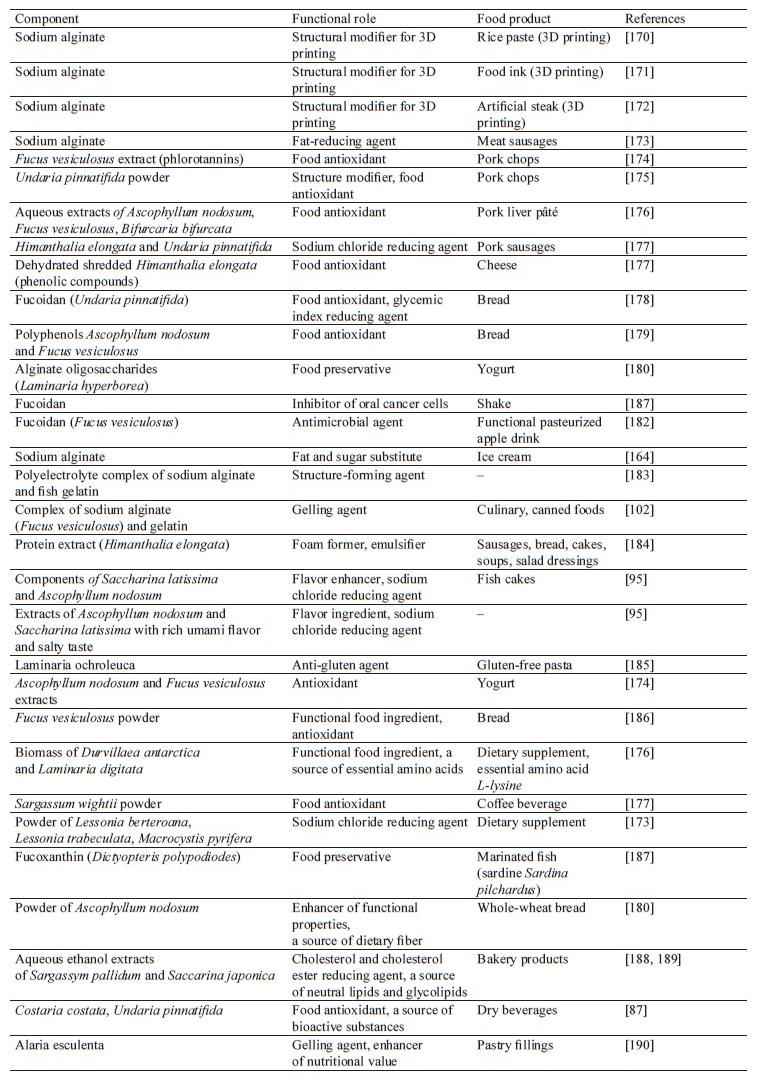
Brown seaweeds are widely used as a functional ingredient in food production to improve health and reduce the risk of developing diet-related diseases [24]. Due to their physical and chemical properties, as well as biological activity, brown seaweeds can be used for nutraceutical purposes [152]. Brown seaweeds and their components (polysaccharides, protein extracts, etc.) are increasingly used in food technologies [164–167]. Many regions where the use of seaweed is limited for various reasons are showing interest in functional foods, including seaweed products [168, 169]. The functional roles and uses of bioactive components of brown seaweeds in food products are shown in Table 3.
In 2021, the global market of functional foods reached more than $180 billion, and it is expected to grow by almost 3% per year in 2022–2027 [179].
Noteworthily, consumers are conditioned to use traditional, familiar products. Therefore, people in those countries where seaweed is not commonly used as a food and is not part of traditional cuisine are neophobic towards this product [176, 184]. However, seaweed is often used in sophisticated, trendy cuisine due to its unique sensory properties. Its chemical composition and functional effects also contribute to its growing popularity [191].
The main types of products where seaweed or seaweed-derived bioactive substances are used as functional ingredients are reviewed below.
Meat products. Modern meat products containing bioactive ingredients have a balance of nutritional and functional properties [35, 192].
F. vesiculosus is rich in phlorotannins, polyphenolic compounds with high antioxidant activity. F. vesiculosus extracts are used as natural preservatives in pork chops to protect their lipids and proteins from oxidation during storage [193].
Extracts of the brown seaweeds A. nodosum, F. vesiculosus, and Bifurcaria bifurcata, which are high in natural antioxidants, provide chilled pâté of lean pork liver with oxidative stability similarly to the synthetic antioxidant tert-butyl-4-hydroxytoluene [194].
Replacing sodium chloride with edible seaweed (Himanthalia elongata and U. pinnatifida) in meat sausages can lower the risk of chronic diseases by reducing the salt content [195].
Alginates are widely used as thickening and stabilizing agents to reduce the fat content in various restructured meat products. Their stabilizing properties are due to their ability to form complexes with proteins [42]. Replacing pork speck with sodium alginate in meat sausages decreases the fat content and the energy value of the final product [182].
The addition of dietary fiber derived from seaweed can clearly improve the quality, nutritional properties, and taste characteristics of processed meat products [4]. Seaweeds can also enhance the ability of sausages to bind water/lipids and contribute to a thinner and denser gel-like matrix of meat protein [196]. The addition of laminarin and fucoidan significantly delays lipid oxidation in pork chops during storage [197]. Further, dietary fiber derived from brown seaweed can be a real alternative to phosphates in the production of meat sausages [198]. Phosphate-free sausages treated with dietary fiber have an improved quality profile, which meets the demand for healthier meat products. Dietary fiber improves the texture of phosphate-free sausages and effectively slows down lipid oxidation during storage.
Dairy products. Bioactive components of brown seaweeds (H. elongata, Laminaria ochroleuca, U. pinnatifida) are used in the production of various dairy products to improve their quality indicators. In particular, phenolic compounds exhibit antioxidant properties and can therefore increase the antioxidant activity of cheese when added to the milk clot. Introducing dehydrated seaweed can also enhance the retention of whey in the milk clot and has a positive effect on the color and texture of cheese [199].
Alginate oligosaccharides have a potential antifungal effect against certain yeasts that cause milk spoilage. In yogurt starter culture, alginate oligosaccharides extracted from Laminaria hyperborea decreased the growth of microorganisms Candida parapsilosis, Debaryomyces hansenii, and Meyerozyma guilliermodii. Thus, these oligomers can ensure safe storage of dairy and milk-containing products whose shelf life is reduced by yeast [200].
The use of bioactive food foam containing sodium alginate can replace fat and sugar in ice cream. Such functional products can benefit consumers who are overweight, obese, or have other weight-related complications [201].
Bakery and flour products. In bakery and flour products (bread, noodles, cakes, cookies, etc.), seaweed is usually used in the form of fine powder [202–204]. Seaweed forms stable mixtures and emulsions with dough, improving the functional properties of the end products. Added to bakery and flour products, seaweed decreased the color values of lightness, redness, and yellowness [205].
When added to bread, polyphenol-rich brown seaweeds (A. nodosum and F. vesiculosus) significantly reduce carbohydrate digestion compared with the control bread. However, the heat treatment of seaweed in bread during baking lowers its polyphenol content, which may reduce the seaweed’s ability to inhibit carbohydrates digestion in vitro [206].
Bread made from flour enriched with fucoidan extracted from the brown seaweed U. pinnatifida has a significantly high specific volume and softer crumb. Its improved quality is associated with a high production of CO2 during proofing. Fucoidan’s antitumorous activity in vitro is preserved even after baking [207]. The brown seaweed F. vesiculosus can be added to bread as a natural antioxidant, as well as to increase its nutritional value. The addition of F. vesiculosus powder increased the longitudinal viscosity of the dough, which decreased its sponginess at the end of proofing, compared to a typical wheat bread formulation [208].
Food additives are used to achieve a certain technological or sensory effect [209]. The growing consumer interest in natural products has led to an increased demand for natural food additives among food and beverage manufacturers. These additives are believed to have health benefits and are used as functional ingredients and natural sources of soluble dietary fiber.
Seaweed has a naturally salty taste due to its high content of minerals such as potassium, which can be used as a healthy substitute for sodium to reduce the risk of cardiovascular disease [95].
Food hydrocolloids produced from brown seaweed are biopolymers that are widely used as thickeners (in soups, gravies, salad dressings, sauces, and fillings), moisture-holding agents, stabilizers, emulsifiers, and gelling agents (in jam, jelly, marmalade, restructured foods, and low-fat products) [42, 166]. The global hydrocolloid market amounted to $9.7 billion in 2020 and is estimated to reach $13.36 billion by 2026 [210].
3D food printing is a new technology that can produce any food product a consumer might desire. For example, it can be used to develop a product with the exact nutritional value, the most beneficial nutrients, and without the ingredients a consumer is allergic to. It can even predict or personalize the taste, color, shape, and size of the food product [211]. Hydrocolloids are added to facilitate extrusion during 3D printing. Sodium alginate determines rheological properties and therefore is widely used in 3D food printing [212].
Drinks. Brown seaweed components are added to provide drinks with functional properties. For example, fucoidan, a water-soluble polysaccharide, is added to beverages such as tea, coffee, fruit drinks, etc. Fucoidanrich seaweed is non-toxic and has antioxidant activity. Functional tea was developed from the brown seaweed Sargassum binderi. It was supplemented with lemon essence to mask the seaweed’s unpleasant taste and smell and thus improve its consumer acceptance [213]. Another example is a functional pasteurized apple drink, in which fucoidan obtained from the brown seaweed F. vesiculosus exhibited biological activity as an antimicrobial agent against Listeria monocytogenes and Salmonella enterica serovar Typhimurium [214].
ВЫВОДЫ
The production of functional food products based on brown seaweeds has enormous potential due to their unique biochemical composition. Brown seaweeds are rich in polysaccharides, dietary fiber, proteins, vitamins, minerals, and other nutrients, which contributes to high consumer interest. A number of authoritative in vitro studies have proven the effectiveness of food products based on brown seaweeds. Developing new competitive products is an important step in promoting seaweeds further and making them commercially viable. Also, more studies are needed to determine the safety of brown seaweeds during their harvesting, cultivation, and processing, including the environmental impact. In particular, new functional foods based on brown seaweeds should be thoroughly examined for the presence of pollutants, allergens, heavy metals or other substances that may pose a risk to both humans and the environment.
The incorporation of seaweeds into functional foods and a daily diet could potentially contribute to global food security in the future.
Вклад авторов
All the authors contributed equally to the study and bear equal responsibility for the information published in this article.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare no conflict of interest.
БЛАГОДАРНОСТИ
The study was performed at the Laboratory of Chemistry and Technology of Marine Bioresources established with the financial support of the Russian Ministry of Science and Higher Education (Agreement No. 075-032021-088/4). S.R. Derkach thanks the Russian Science Foundation (Project No.22-16-20046) and the Ministry of Education and Science of the Murmansk Region (Agreement No. 103) for their support.
ФИНАНСИРОВАНИЕ
This research was funded by the Russian Science Foundation (RSF) (Project No. 21-73-00191 “Obtaining polyelectrolyte complexes based on underutilized biopolymers of marine origin for biomedical purposes”).СПИСОК ЛИТЕРАТУРЫ
- Levine I. Algae: A way of life and health. In: Fleurence J, Levine I, editors. Seaweed in health and disease prevention. Academic Press; 2016. pp. 1–5. https://doi.org/10.1016/B978-0-12-802772-1.00001-4
- Peng Y, Hu J, Yang B, Lin X-P, Zhou X-F, Yang X-W, et al. Chemical composition of seaweeds. In: Tiwari BK, Troy DJ, editors. Seaweed sustainability. Food and non-food applications. Academic Press; 2015. pp. 79–124. https://doi.org/10.1016/B978-0-12-418697-2.00005-2
- Paymulina AV, Potoroko IYu, Naumenko NV, Motovilov OK. Sonochemical microstructuring of sodium alginate to increase its effectiveness in bakery. Food Processing: Techniques and Technology. 2023;53(1):13–24. (In Russ.). https://doi.org/10.21603/2074-9414-2023-1-2411
- Raja K, Kadirvel V, Subramaniyan T. Seaweeds, an aquatic plant-based protein for sustainable nutrition – A review. Future Foods. 2022;5 https://doi.org/10.1016/j.fufo.2022.100142
- Cai J, Lovatelli A, Aguilar-Manjarrez J, Cornish L, Dabbadie L, Desrochers A, et al. Seaweeds and microalgae: An overview for unlocking their potential in global aquaculture development. Rome: FAO; 2021. 48 p. https://doi.org/10.4060/cb5670en
- Webb P, Somers NK, Thilsted SH. Seaweed's contribution to food security in low- and middle-income countries: Benefits from production, processing and trade. Global Food Security. 2023;37. https://doi.org/10.1016/j.gfs.2023.100686
- Aminina MN. Biochemical bases of rational use of brown algae. Actual problems of biodiversity and nature management: Materials of the second all-Russian scientific-practical conference; 2019; Kerch. Simferopol: PP “Arial” LLC; 2019. p. 262–266. (In Russ.). https://www.elibrary.ru/DSYNXO
- Khotimchenko YuS, Silachev DN, Katanaev VL. Marine natural products from the Russian pacific as sources of drugs for neurodegenerative diseases. Marine Drugs; 2022;20(11). https://doi.org/10.3390/md20110708
- Obluchinskaya ED. Phytochemicals and technological study of the Barents Sea algae. Proceedings of the Kola Science Center of the Russian Academy of Sciences. 2020;11(4–7):178–198. (In Russ.). https://doi.org/10.37614/2307-5252.2020.11.4.008
- Ferdouse F, Holdt SL, Smith R, Murúa P, Yang Z. The global status of seaweed production, trade and utilization. Rome: FAO; 2018. 124 p.
- Imchen T. Nutritional value of seaweeds and their potential to serve as nutraceutical supplements. Phycologia. 2021;60(6):534–546. https://doi.org/10.1080/00318884.2021.1973753
- Obluchinskaya ED. The influence of environmental factors on the content of polysaccharides in Fucus vesiculosus L. Chemistry of Plant Raw Material. 2011;(3):47–51. (In Russ.). https://www.elibrary.ru/OHSUJV
- Bogolitsyn K, Parshina A, Ivanchenko N, Polomarchuk D. Seasonal variations in the chemical composition of Arctic brown macroalgae. Algal Research. 2023;72. https://doi.org/10.1016/j.algal.2023.103112
- Galysheva YuA, Khristoforova NK. Environments and macrobenthos in the Vostok Bay (Japan Sea) in conditions of anthropogenic impact. Izvestiya TINRO. 2007;149:270–309. (In Russ.). https://www.elibrary.ru/JJYWVT
- Kravchenko AO, Byankina Barabanova AO, Glazunov VP, Yakovleva IM, Yermak IM. Seasonal variations in a polysaccharide composition of Far Eastern red seaweed Ahnfeltiopsis flabelliformis (Phyllophoraceae). Journal of Applied Phycology. 2018;30:535–545. https://doi.org/10.1007/s10811-017-1262-8
- Sharma S, Neves L, Funderud J, Mydland LT, Øverland M, Horn SJ. Seasonal and depth variations in the chemical composition of cultivated Saccharina latissimi. Algal Research. 2018;32:107–112. https://doi.org/10.1016/j.algal.2018.03.012
- Lopez-Santamarina A, Cardelle-Cobas A, Mondragon AC, Sinisterra-Loaiza L, Miranda JM, Cepeda A. Evaluation of the potential prebiotic effect of Himanthalia elongata, an Atlantic brown seaweed, in an in vitro model of the human distal colon. Food Research International. 2022;156. https://doi.org/10.1016/j.foodres.2022.111156
- Poza AM, Fernández C, Latour EA, Raffo MP, Dellatorre FG, Parodi ER, et al. Optimization of the rope seeding method and biochemical characterization of the brown seaweed Asperococcus ensiformis. Algal Research. 2022;64. https://doi.org/10.1016/j.algal.2022.102668
- Samarasinghe MB, van der Heide ME, Weisbjerg MR, Sehested J, Sloth JJ, Bruhn A, et al. A descriptive chemical analysis of seaweeds, Ulva sp., Saccharina latissima and Ascophyllum nodosum harvested from Danish and Icelandic waters. Animal Feed Science and Technology. 2021;278. https://doi.org/10.1016/j.anifeedsci.2021.115005
- Meng W, Mu T, Sun H, Garcia-Vaquero M. Evaluation of the chemical composition and nutritional potential of brown macroalgae commercialised in China. Algal Research. 2022;64. https://doi.org/10.1016/j.algal.2022.102683
- Flórez-Fernández N, Torres MD, González-Muñoz MJ, Domínguez H. Recovery of bioactive and gelling extracts from edible brown seaweed Laminaria ochroleuca by non-isothermal autohydrolysis. Food Chemistry. 2019;277:353–361. https://doi.org/10.1016/j.foodchem.2018.10.096
- Zhang R, Yuen AKL, de Nys R, Masters AF, Maschmeyer T. Step by step extraction of bio-actives from the brown seaweeds, Carpophyllum flexuosum, Carpophyllum plumosum, Ecklonia radiata and Undaria pinnatifida. Algal Research. 2020;52. https://doi.org/10.1016/j.algal.2020.102092
- Roe D, Booker F, Day M, Zhou W, Allebone-Webb S, Hill NAO, et al. Are alternative livelihood projects effective at reducing local threats to specified elements of biodiversity and/or improving or maintaining the conservation status of those elements? Environmental Evidence. 2015;4. https://doi.org/10.1186/s13750-015-0048-1
- Ghosh S, Sarkar T, Pati S, Kari ZA, Edinur HA, Chakraborty R. Novel bioactive compounds from marine sources as a tool for functional food development. Frontiers in Marine Science. 2022;9. https://doi.org/10.3389/fmars.2022.832957
- Jagtap AS, Manohar CS, Ayyapankutty AMT, Meena SN. Antioxidant and antiglycemic properties of macroalgae, an underutilized blue economy bioresource in India. Russian Journal of Marine Biology. 2021;47(6):489–497. https://doi.org/10.1134/S1063074021060067
- Miyashita K, Beppu F, Hosokawa M, Liu X, Wang S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Archives of Biochemistry and Biophysics. 2020;686. https://doi.org/10.1016/j.abb.2020.108364
- Digala P, Saravanan M, Dhanraj M, Pamarthi J, Muralidharan S, Narikimelli A, et al. Optimized extraction of sulfated polysaccharide from brown seaweed Sargassum polycystum and their evaluation of anti-cancer and wound healing potential. South African Journal of Botany. 2022;151(B):345–359. https://doi.org/10.1016/j.sajb.2022.03.015
- Urrea-Victoria V, Furlan CM, dos Santos DYAC, Chow F. Antioxidant potential of two Brazilian seaweeds in response to temperature: Pyropia spiralis (red alga) and Sargassum stenophyllum (brown alga). Journal of Experimental Marine Biology and Ecology. 2022;549. https://doi.org/10.1016/j.jembe.2022.151706
- Pereira L, Valado A. The seaweed diet in prevention and treatment of the neurodegenerative diseases. Marine Drugs. 2021;19(3). https://doi.org/10.3390/md19030128
- Silva J, Alves C, Freitas R, Martins A, Pinteus S, Ribeiro J, et al. Antioxidant and neuroprotective potential of the brown seaweed Bifurcaria bifurcata in an in vitro Parkinson’s disease model. Marine Drugs. 2019;17(2). https://doi.org/10.3390/md17020085
- Obluchinskaya ED, Pozharitskaya ON, Zakharov DV, Flisyuk EV, Terninko II, Generalova YuE, et al. The biochemical composition and antioxidant properties of Fucus vesiculosus from the Arctic region. Marine Drugs. 2022;20(3). https://doi.org/10.3390/md20030193
- Kartik A, Akhil D, Lakshmi D, Gopinath KP, Arun J, Sivaramakrishnan R, et al. A critical review on production of biopolymers from algae biomass and their applications. Bioresource Technology. 2021;329. https://doi.org/10.1016/j.biortech.2021.124868
- Ziyaei K, Ataie Z, Mokhtari M, Adrah K, Daneshmehr MA. An insight to the therapeutic potential of algae-derived sulfated polysaccharides and polyunsaturated fatty acids: Focusing on the COVID-19. International Journal of Biological Macromolecules. 2022;209(A):244–257. https://doi.org/10.1016/j.ijbiomac.2022.03.063
- You L, Gong Y, Li L, Hu X, Brennan C, Kulikouskaya V. Beneficial effects of three brown seaweed polysaccharides on gut microbiota and their structural characteristics: An overview. International Journal of Food Science and Technology. 2020;55(3):1199–1206. https://doi.org/10.1111/ijfs.14408
- Gullón B, Gagaoua M, Barba FJ, Gullón P, Zhang W, Lorenzo JM. Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends in Food Science and Technology. 2020;100:1–18. https://doi.org/10.1016/j.tifs.2020.03.039
- Bayu A, Warsito MF, Putra MY, Karnjanakom S, Guan G. Macroalgae-derived rare sugars: Applications and catalytic synthesis. Carbon Resources Conversion. 2021;4:150–163. https://doi.org/10.1016/j.crcon.2021.04.002
- Reynolds D, Huesemann M, Edmundson S, Sims A, Hurst B, Cady S, et al. Viral inhibitors derived from macroalgae, microalgae, and cyanobacteria: A review of antiviral potential throughout pathogenesis. Algal Research. 2021;57. https://doi.org/10.1016/j.algal.2021.102331
- Assef ANB, da Costa BB, Moreira TA, do Carmo LD, de Souza TFG, Alencar NMN, et al. Antitumor and immunostimulating sulfated polysaccharides from brown algae Dictyota caribaea. Carbohydrate Polymer Technologies and Applications. 2021;2. https://doi.org/10.1016/j.carpta.2021.100142
- Leandro A, Pacheco D, Cotas J, Marques JC, Pereira L, Gonçalves AMM. Seaweed’s bioactive candidate compounds to food industry and global food security. Life. 2020;10(8). https://doi.org/10.3390/life10080140
- Okolie CL, Mason B, Mohan A, Pitts N, Udenigwe CC. Extraction technology impacts on the structure-function relationship between sodium alginate extracts and their in vitro prebiotic activity. Food Bioscience. 2020;37. https://doi.org/10.1016/j.fbio.2020.100672
- Tanna B, Mishra A. Nutraceutical potential of seaweed polysaccharides: structure, bioactivity, safety, and toxicity. Comprehensive Reviews in Food Science and Food Safety. 2019;18(3):817–831. https://doi.org/10.1111/1541-4337.12441
- Rhein-Knudsen N, Meyer AS. Chemistry, gelation, and enzymatic modification of seaweed food hydrocolloids. Trends in Food Science and Technology. 2021;109:608–621. https://doi.org/10.1016/j.tifs.2021.01.052
- Wang M, Zhou J, Selma-Royo M, Simal-Gandara J, Collado MC, Barba FJ. Potential benefits of high-added-value compounds from aquaculture and fish side streams on human gut microbiota. Trends in Food Science and Technology. 2021;112:484–494. https://doi.org/10.1016/j.tifs.2021.04.017
- Wang X, Wang X, Jiang H, Cai C, Li G, Hao J, et al. Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: An overview. Carbohydrate Polymers. 2018;195:601–612. https://doi.org/10.1016/j.carbpol.2018.05.003
- Cherry P, O’Hara C, Magee PJ, McSorley EM, Allsopp PJ. Risks and benefits of consuming edible seaweeds. Nutrition Reviews. 2019;77(5):307–329. https://doi.org/10.1093/nutrit/nuy066
- Bermano G, Stoyanova T, Hennequart F, Wainwright CL. Seaweed-derived bioactives as potential energy regulators in obesity and type 2 diabetes. Advances in Pharmacology. 2020;87:205–256. https://doi.org/10.1016/bs.apha.2019.10.002
- Lafarga T, Acién-Fernández FG, Garcia-Vaquero M. Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal Research. 2020;48. https://doi.org/10.1016/j.algal.2020.101909
- Li N, Wang C, Georgiev MI, Bajpai VK, Tundis R, Simal-Gandara J, et al. Advances in dietary polysaccharides as anticancer agents: Structure-activity relationship. Trends in Food Science and Technology. 2021;111:360–377. https://doi.org/10.1016/j.tifs.2021.03.008
- Okolie CL, Mason B, Mohan A, Pitts N, Udenigwe CC. The comparative influence of novel extraction technologies on in vitro prebiotic-inducing chemical properties of fucoidan extracts from Ascophyllum nodosum. Food Hydrocolloids. 2019;90:462–471. https://doi.org/10.1016/j.foodhyd.2018.12.053
- Bittkau KS, Neupane S, Alban S. Initial evaluation of six different brown algae species as source for crude bioactive fucoidans. Algal Research. 2020;45. https://doi.org/10.1016/j.algal.2019.101759
- Sakthivel R, Devi KP. Antioxidant, anti-inflammatory and anticancer potential of natural bioactive compounds from seaweeds. Studies in Natural Products Chemistry. 2019;63:113–160. https://doi.org/10.1016/B978-0-12-817901-7.00005-8
- Wen Y, Gao L, Zhou H, Ai C, Huang X, Wang M, et al. Opportunities and challenges of algal fucoidan for diabetes management. Trends in Food Science and Technology. 2021;111:628–641. https://doi.org/10.1016/j.tifs.2021.03.028
- Abdel-Latif HMR, Dawood MAO, Alagawany M, Faggio C, Nowosad J, Kucharczyk D. Health benefits and potential applications of fucoidan (FCD) extracted from brown seaweeds in aquaculture: An updated review. Fish and Shellfish Immunology. 2022;122:115–130. https://doi.org/10.1016/j.fsi.2022.01.039
- Desamero MJ, Kakuta S, Chambers JK, Uchida K, Hachimura S, Takamoto M, et al. Orally administered brown seaweed-derived β-glucan effectively restrained development of gastric dysplasia in A4gnt KO mice that spontaneously develop gastric adenocarcinoma. International Immunopharmacology. 2018;60:211–220. https://doi.org/10.1016/j.intimp.2018.05.002
- Cui Y, Zhu L, Li Y, Jiang S, Sun Q, Xie E, et al. Structure of a laminarin-type β-(1→3)-glucan from brown algae Sargassum henslowianum and its potential on regulating gut microbiota. Carbohydrate Polymers. 2021;255. https://doi.org/10.1016/j.carbpol.2020.117389
- Gupta S, Abu-Ghannam N. Bioactive potential and possible health effects of edible brown seaweeds. Trends in Food Science and Technology. 2011;22(6):315–326. https://doi.org/10.1016/j.tifs.2011.03.011
- Beta-glucan market by source (cereal, mushroom, yeast and seaweed), application (food & beverages, personal care, pharmaceuticals, dietary supplements, and animal feed), category (soluble and insoluble), and region – Global forecast to 2026 [Internet]. [cited 2023 Feb 07]. Available from: https://www.marketresearch.com/MarketsandMarkets-v3719/Beta-Glucan-Source-Cereal-Mushroom-14370237
- Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: An integrative view. Cell. 2012;148(6):1258–1270. https://doi.org/10.1016/j.cell.2012.01.035
- de Jesus Raposo MD, de Morais AMMB, de Morais RMSC. Emergent sources of prebiotics: Seaweeds and microalgae. Marine Drugs. 2016;14(2). https://doi.org/10.3390/md14020027
- Jiménez-Escrig A, Sánchez-Muniz FJ. Dietary fibre from edible seaweeds: Chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutrition Research. 2000;20(4):585–598. https://doi.org/10.1016/S0271-5317(00)00149-4
- Huang W, Tan H, Nie S. Beneficial effects of seaweed-derived dietary fiber: Highlights of the sulfated polysaccharides. Food Chemistry. 2022;373. https://doi.org/10.1016/j.foodchem.2021.131608
- Lopez-Santamarina A, Miranda JM, Mondragon AC, Lamas A, Cardelle-Cobas A, Franco CM, et al. Potential use of marine seaweeds as prebiotics: A review. Molecules. 2020;25(4). https://doi.org/10.3390/molecules25041004
- Deng Z, Wu N, Wang J, Zhang Q. Dietary fibers extracted from Saccharina japonica can improve metabolic syndrome and ameliorate gut microbiota dysbiosis induced by high fat diet. Journal of Functional Foods. 2021;85. https://doi.org/10.1016/j.jff.2021.104642
- Cronin P, Joyce SA, O’Toole PW, O’Connor EM. Dietary fibre modulates the gut microbiota. Nutrients. 2021;13(5). https://doi.org/10.3390/nu13051655
- Charoensiddhi S, Franco C, Su P, Zhang W. Improved antioxidant activities of brown seaweed Ecklonia radiata extracts prepared by microwave-assisted enzymatic extraction. Journal of Applied Phycology. 2015;27:2049–2058. https://doi.org/10.1007/s10811-014-0476-2
- Suleria HAR, Gobe G, Masci P, Osborne SA. Marine bioactive compounds and health promoting perspectives; innovation pathways for drug discovery. Trends in Food Science and Technology. 2016;50:44–55. https://doi.org/10.1016/j.tifs.2016.01.019
- Jin W, Zhang Q, Wang J, Zhang W. A comparative study of the anticoagulant activities of eleven fucoidans. Carbohydrate Polymers. 2013;91(1):1–6. https://doi.org/10.1016/j.carbpol.2012.07.067
- Lozano I, Wacyk JM, Carrasco J, Cortez-San Martín MA. Red macroalgae Pyropia columbina and Gracilaria chilensis: Sustainable feed additive in the Salmo salar diet and the evaluation of potential antiviral activity against infectious salmon anemia virus. Journal of Applied Phycology. 2016;28:1343–1351. https://doi.org/10.1007/s10811-015-0648-8
- Ajanth Praveen M, Karthika Parvathy KR, Balasubramanian P, Jayabalan R. An overview of extraction and purification techniques of seaweed dietary fibers for immunomodulation on gut microbiota. Trends in Food Science and Technology. 2019;92:46–64. https://doi.org/10.1016/j.tifs.2019.08.011
- Pradhan B, Bhuyan PP, Patra S, Nayak R, Behera PK, Behera C, et al. Beneficial effects of seaweeds and seaweed-derived bioactive compounds: Current evidence and future prospective. Biocatalysis and Agricultural Biotechnology. 2022;39. https://doi.org/10.1016/j.bcab.2021.102242
- Gómez-Ordóñez E, Jiménez-Escrig A, Rupérez P. Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Research International. 2010;43(9):2289–2294. https://doi.org/10.1016/j.foodres.2010.08.005
- Murray M, Dordevic AL, Cox KHM, Scholey A, Ryan L, Bonham MP. Study protocol for a double-blind randomised controlled trial investigating the impact of 12 weeks supplementation with a Fucus vesiculosus extract on cholesterol levels in adults with elevated fasting LDL cholesterol who are overweight or have obesity. BMJ Open. 2018;8. https://doi.org/10.1136/bmjopen-2018-022195
- Mildenberger J, Stangeland JK, Rebours C. Antioxidative activities, phenolic compounds and marine food allergens in the macroalgae Saccharina latissima produced in integrated multi-trophic aquaculture systems. Aquaculture. 2022;546. https://doi.org/10.1016/j.aquaculture.2021.737386
- Kaushalya KGD, Gunathilake KDPP. Encapsulation of phlorotannins from edible brown seaweed in chitosan: Effect of fortification on bioactivity and stability in functional foods. Food Chemistry. 2022;377. https://doi.org/10.1016/j.foodchem.2021.132012
- Murray M, Dordevic AL, Ryan L, Bonham MP. Phlorotannins and macroalgal polyphenols: Potential as functional food ingredients and role in health promotion. In: Rani V, Yadav UCS, editors. Functional food and human health. Singapore: Springer; 2018. pp. 27–58. https://doi.org/10.1007/978-981-13-1123-9_3
- Obluchinskaya ED, Daurtseva AV, Pozharitskaya ON, Flisyuk EV, Shikov AN. Natural deep eutectic solvents as alternatives for extracting phlorotannins from brown algae. Pharmaceutical Chemistry Journal. 2019;53(3):243–247. https://doi.org/10.1007/s11094-019-01987-0
- Vodouhè M, Marois J, Guay V, Leblanc N, Weisnagel SJ, Bilodeau J-F, et al. Marginal impact of brown seaweed Ascophyllum nodosum and Fucus vesiculosus extract on metabolic and inflammatory response in overweight and obese prediabetic subjects. Marine Drugs. 2022;20(3). https://doi.org/10.3390/md20030174
- Vázquez-Rodríguez B, Santos-Zea L, Heredia-Olea E, Acevedo-Pacheco L, Santacruz A, Gutiérrez-Uribe JA, et al. Effects of phlorotannin and polysaccharide fractions of brown seaweed Silvetia compressa on human gut microbiota composition using an in vitro colonic model. Journal of Functional Foods. 2021;84. https://doi.org/10.1016/j.jff.2021.104596
- Jesumani V, Du H, Pei P, Zheng C, Cheong K-L, Huang N. Unravelling property of polysaccharides from Sargassum sp. as an anti-wrinkle and skin whitening property. International Journal of Biological Macromolecules. 2019;140:216–224. https://doi.org/10.1016/j.ijbiomac.2019.08.027
- Sokolan NI, Kuranova LK. Obtaining carbohydrate biopolymers (prebiotics) from brown algae. IOP Conference Series: Earth and Environmental Science. 2021;625. https://doi.org/10.1088/1755-1315/625/1/012019
- Kuznetsova TA, Makarenkova ID, Koneva EL, Aminina NM, Yakush EV. Effect of probiotic product containing bifidobacteria and biogel from brown algae on the intestinal microflora and parameters of innate immunity in mice with experimental drug dysbacteriosis. Problems of Nutrition. 2015;84(1):73–79. (In Russ.). https://www.elibrary.ru/TMLCUF
- Mohd Fauziee NA, Chang LS, Wan Mustapha WA, Nor AR, Lim SJ. Functional polysaccharides of fucoidan, laminaran and alginate from Malaysian brown seaweeds (Sargassum polycystum, Turbinaria ornata and Padina boryana). International Journal of Biological Macromolecules. 2021;167:1135–1145. https://doi.org/10.1016/j.ijbiomac.2020.11.067
- An E-K, Hwang J, Kim S-J, Park H-B, Zhang W, Ryu J-H, et al. Comparison of the immune activation capacities of fucoidan and laminarin extracted from Laminaria japonica. International Journal of Biological Macromolecules. 2022;208:230–242. https://doi.org/10.1016/j.ijbiomac.2022.03.122
- Ni L, Wang L, Fu X, Duan D, Jeon Y-J, Xu J, et al. In vitro and in vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica. International Journal of Biological Macromolecules. 2020;156:717–729. https://doi.org/10.1016/j.ijbiomac.2020.04.012
- Kuznetsova TA, Smolina TP, Makarenkova ID, Ivanushko LA, Persiyanova EV, Ermakova SP, et al. Immunoadjuvant activity of fucoidans from the brown alga Fucus evanescens. Marine Drugs. 2020;18(3). https://doi.org/10.3390/md18030155
- Fedyanina LN, Lyakh VA, Smertina ES. Evaluation of the effectiveness of preventive effect of bread with extract of brown seaweed. Bulletin of KSAU. 2018;140(5):275–280. (In Russ.). https://www.elibrary.ru/MIGXNR
- Tabakaev AV, Tabakaeva OV. Instant drinks based on extracts of Japan Sea brown algae and fruit and berry juices as functional products. Problems of Nutrition. 2022;91(4):107–114. (In Russ.). https://doi.org/10.33029/0042-8833-2022-91-4-107-114
- Wang P-T, Sudirman S, Hsieh M-C, Hu J-Y, Kong Z-L. Oral supplementation of fucoxanthin-rich brown algae extract ameliorates cisplatin-induced testicular damage in hamsters. Biomedicine and Pharmacotherapy. 2022;125. https://doi.org/10.1016/j.biopha.2020.109992
- Sharma PP, Chonche MJ, Mudhol S, Muthukumar SP, Baskaran V. Anti-inflammatory efficacy of brown seaweed (Padina tetrastromatica) in 3T3-L1 adipocytes and low-dose LPS induced inflammation in C57BL6 mice. Algal Research. 2023;71. https://doi.org/10.1016/j.algal.2023.103027
- Baldrick FR, McFadden K, Ibars M, Sung C, Moffatt T, Megarry K, et al. Impact of a (poly)phenol-rich extract from the brown algae Ascophyllum nodosum on DNA damage and antioxidant activity in an overweight or obese population: A randomized controlled trial. The American Journal of Clinical Nutrition. 2018;108(4):688–700. https://doi.org/10.1093/ajcn/nqy147
- Fomenko SE. Use of extract from the brown seaweed of Sargassum pallidum for restoration of parameters of antioxidant system on model the stress – influences. Health. Medical Ecology. Science. 2018;75(3):59–65. (In Russ.). https://doi.org/10.5281/zenodo.1488038
- Xie X, Chen C, Fu X. Screening α-glucosidase inhibitors from four edible brown seaweed extracts by ultra-filtration and molecular docking. LWT. 2021;138. https://doi.org/10.1016/j.lwt.2020.110654
- Xiao Z, Yang S, Liu Y, Zhou C, Hong P, Sun S, et al. A novel glyceroglycolipid from brown algae Ishige okamurae improve photoaging and counteract inflammation in UVB-induced HaCaT cells. Chemico-Biological Interactions. 2022;351. https://doi.org/10.1016/j.cbi.2021.109737
- Manivasagan P, Bharathiraja S, Moorthy MS, Mondal S, Seo H, Lee KD, et al. Marine natural pigments as potential sources for therapeutic applications. Critical Reviews in Biotechnology. 2018;38(5):745–761. https://doi.org/10.1080/07388551.2017.1398713
- Aryee ANA, Agyei D, Akanbi TO. Recovery and utilization of seaweed pigments in food processing. Current Opinion in Food Science. 2018;19:113–119. https://doi.org/10.1016/j.cofs.2018.03.013
- Dawes C. Macroalgae systematics. In: Fleurence J, Levine I, editors. Seaweed in health and disease prevention. Academic Press; 2016. pp. 107–148. https://doi.org/10.1016/B978-0-12-802772-1.00004-X
- Chen K, Roca M. In vitro bioavailability of chlorophyll pigments from edible seaweeds. Journal of Functional Foods. 2018;41:25–33. https://doi.org/10.1016/j.jff.2017.12.029
- Lourenço-Lopes C, Fraga-Corral M, Jimenez-Lopez C, Carpena M, Pereira AG, Garcia-Oliveira P, et al. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends in Food Science and Technology. 2021;117:163–181. https://doi.org/10.1016/j.tifs.2021.03.012
- Cardozo KHM, Guaratini T, Barros MP, Falcão VR, Tonon AP, Lopes NP, et al. Metabolites from algae with economical impact. Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology. 2007;146(1–2):60–78. https://doi.org/10.1016/j.cbpc.2006.05.007
- Miyashita K, Mikami N, Hosokawa M. Chemical and nutritional characteristics of brown seaweed lipids: A review. Journal of Functional Foods. 2013;5(4):1507–1517. https://doi.org/10.1016/j.jff.2013.09.019
- Ganesan AR, Tiwari U, Rajauria G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Science and Human Wellness. 2019;8(3):252–263. https://doi.org/10.1016/j.fshw.2019.08.001
- Susanto E, Fahmi AS, Hosokawa M, Miyashita K. Variation in lipid components from 15 species of tropical and temperate seaweeds. Marine Drugs. 2019;17(11). https://doi.org/10.3390/md17110630
- Fomenko SE, Kushnerova NF, Sprygin VG, Drugova ES, Lesnikova LN, Merzlyakov VYu, et al. Lipid composition, content of polyphenols, and antiradical activity in some representatives of marine algae. Russian Journal of Plant Physiology. 2019;66(6):452–460. (In Russ.). https://doi.org/10.1134/S0015330319050051
- Fomenko SE, Kushnerova NF, Sprygin VG, Drugova ES, Merzluakov VYu, Lesnikova LN. Lipid complex from the brown seaweed Sargassum pallidum (turner) C. Agardh as a hypolipidemic and antioxidant agent for a high fat diet in experiment. Chemistry of Plant Raw Materials. 2021;(4):381–392. (In Russ.). https://doi.org/10.14258/jcprm.2021049411
- Kushnerova NF. Correction of the lipid composition of blood plasma and erythrocyte’s membranes by lipid complex from extract of brown algae Saccharina japonica at experimental dyslipidemia. Health. Medical Ecology. Science. 2018;75(3):65–73. (In Russ.). https://doi.org/10.5281/zenodo.1488050
- Wang L, Park Y-J, Jeon Y-J, Ryu BM. Bioactivities of the edible brown seaweed, Undaria pinnatifida: A review. Aquaculture. 2018;495:873–880. https://doi.org/10.1016/j.aquaculture.2018.06.079
- Rafiquzzaman SM, Kim EY, Lee JM, Mohibbullah M, Alam B, Moon IS, et al. Anti-Alzheimers and anti-inflammatory activities of a glycoprotein purified from the edible brown alga Undaria pinnatifida. Food Research International. 2015;77:118–124. https://doi.org/10.1016/j.foodres.2015.08.021
- Go H, Hwang H-J, Nam T-J. A glycoprotein from Laminaria japonica induces apoptosis in HT-29 colon cancer cells. Toxicology in Vitro. 2010;24(6):1546–1553. https://doi.org/10.1016/j.tiv.2010.06.018
- Go H, Hwang H-J, Nam T-J. Glycoprotein extraction from Laminaria japonica promotes IEC-6 cell proliferation. International Journal of Molecular Medicine. 2009;24(6):819–824. https://doi.org/10.3892/ijmm_00000298
- Abraham A, Rafeeq CM, Karim R, Rubeena AS. Aquatic lectins: An overview (a paradigm). In: Elumalai P, Vaseeharan B, Lakshmi S, editors. Aquatic lectins: Immune defense, biological recognition and molecular advancements. Singapore: Springer; 2022. pp. 3–21. https://doi.org/10.1007/978-981-19-0432-5_1
- Barre A, Simplicien M, Benoist H, van Damme EJM, Rougé P. Mannose-specific lectins from marine algae: Diverse structural scaffolds associated to common virucidal and anti-cancer properties. Marine Drugs. 2019;17(8). https://doi.org/10.3390/md17080440
- Singh RS, Thakur SR, Bansal P. Algal lectins as promising biomolecules for biomedical research. Critical Reviews in Microbiology. 2015;41(1):77–88. https://doi.org/10.3109/1040841X.2013.798780
- Teixeira EH, Arruda FVS, do Nascimento KS, Carneiro VA, Nagano CS, da Silva BR, et al. Biological applications of plants and algae lectins: An overview. In: Chang C-F, editor. Carbohydrates. Comprehensive studies on glycobiology and glycotechnology. IntechOpen; 2012. https://doi.org/10.5772/50632
- Hwang H-J, Han J-W, Jeon H, Han JW. Induction of recombinant lectin expression by an artificially constructed tandem repeat structure: A case study using Bryopsis plumosa mannose-binding lectin. Biomolecules. 2018;8(4). https://doi.org/10.3390/biom8040146
- Abdollahi M, Axelsson J, Carlsson N-G, Nylund GM, Albers E, Undeland I. Effect of stabilization method and freeze/thaw-aided precipitation on structural and functional properties of proteins recovered from brown seaweed (Saccharina latissima). Food Hydrocolloids. 2019;96:140–150. https://doi.org/10.1016/j.foodhyd.2019.05.007
- Geada P, Moreira C, Silva M, Nunes R, Madureira L, Rocha CMR, et al. Algal proteins: Production strategies and nutritional and functional properties. Bioresource Technology. 2021;332. https://doi.org/10.1016/j.biortech.2021.125125
- Jensen S, Ólafsdóttir A, Einarsdóttir B, Hreggviðsson GÓ, Guðmundsson H, Jónsdóttir LB, et al. New wave of flavours – On new ways of developing and processing seaweed flavours. International Journal of Gastronomy and Food Science. 2022;29. https://doi.org/10.1016/j.ijgfs.2022.100566
- Gil MN, Torres AI, Commendatore MG, Marinho C, Arias A, Giarratano E, et al. Nutritive and xenobiotic compounds in the alien algae Undaria pinnatifida from Argentine Patagonia. Archives of Environmental Contamination and Toxicology. 2015;68:553–565. https://doi.org/10.1007/s00244-014-0090-y
- Škrovánková S. Seaweed vitamins as nutraceuticals. Advances in Food and Nutrition Research. 2011;64:357–369. https://doi.org/10.1016/B978-0-12-387669-0.00028-4
- MacArtain P, Gill CIR, Brooks M, Campbell R, Rowland IR. Nutritional value of edible seaweeds. Nutrition Reviews. 2007;65(12):535–543. https://doi.org/10.1301/nr.2007.dec.535-543
- Hernandez-Carmona G, Carrillo-Domínguez S, Arvizu-Higuera DL, Rodríguez-Montesinos YE, Murillo-Álvarez JI, Muñoz-Ochoa M, et al. Monthly variation in the chemical composition of Eisenia arborea J.E. Areschoug. Journal of Applied Phycology. 2009;21:607–616. https://doi.org/10.1007/s10811-009-9454-5
- Tabakaeva OV, Tabakaev AV. Biologically active agents of potential trade brown seaweed of the Far East Region. Problems of Nutrition. 2016;85(3):126–132. (In Russ.). https://www.elibrary.ru/WFGBEB
- Sappati PK, Nayak B, VanWalsum GP, Mulrey OT. Combined effects of seasonal variation and drying methods on the physicochemical properties and antioxidant activity of sugar kelp (Saccharina latissima). Journal of Applied Phycology. 2019;31:1311–1332. https://doi.org/10.1007/s10811-018-1596-x
- Tkachenko A, Mitra M, Schwarz J, Waguespack Y, Brooks C. The content of carotenes, ascorbic acid and tocopherols in selected seaweeds. International Journal Algae. 2011;13(1):63–73. https://doi.org/10.1615/InterJAlgae.v13.i1.50
- Uribe E, Vega-Gálvez A, Vargas N, Pasten A, Rodríguez K, Ah-Hen KS. Phytochemical components and amino acid profile of brown seaweed Durvillaea antarctica as affected by air drying temperature. Journal of Food Science and Technology. 2018;55:4792–4801. https://doi.org/10.1007/s13197-018-3412-7
- Taboada MC, Millán R, Miguez MI. Nutritional value of the marine algae wakame (Undaria pinnatifida) and nori (Porphyra purpurea) as food supplements. Journal of Applied Phycology. 2013;25:1271–1276. https://doi.org/10.1007/s10811-012-9951-9
- Chakraborty K, Anusree M, Makkar F. Antioxidant activity of brown seaweeds. Journal of Aquatic Food Product Technology. 2017;26(4):406–419.
- Rodríguez-Bernaldo de Quirós A, Frecha-Ferreiro S, Vidal-Pérez AM, López-Hernández J. Antioxidant compounds in edible brown seaweeds. European Food Research and Technology. 2010;231:495–498. https://doi.org/10.1007/s00217-010-1295-6
- O’Sullivan AM, O’Callaghan YC, O’Grady MN, Queguineur B, Hanniffy D, Troy DJ, et al. In vitro and cellular antioxidant activities of seaweed extracts prepared from five brown seaweeds harvested in spring from the west coast of Ireland. Food Chemistry. 2011;126(3):1064–1070. https://doi.org/10.1016/j.foodchem.2010.11.127
- Hussain E, Wang L-J, Jiang B, Riaz S, Butt GY, Shi D-Y. A review of the components of brown seaweeds as potential candidates in cancer therapy. RSC advances. 2016;6:12592–12610. https://doi.org/10.1039/C5RA23995H
- Baweja P, Kumar S, Sahoo D, Levine I. Biology of seaweeds. In: Fleurence J, Levine I, editors. Seaweed in health and disease prevention. Academic Press; 2016. pp. 41–106. https://doi.org/10.1016/B978-0-12-802772-1.00003-8
- Watanabe F, Yabuta Y, Bito T, Teng F. Vitamin B12-containing plant food sources for vegetarians. Nutrients. 2014;6(5):1861–1873. https://doi.org/10.3390/nu6051861
- Matanjun P, Mohamed S, Muhammad K, Mustapha NM. Comparison of cardiovascular protective effects of tropical seaweeds, Kappaphycus alvarezii, Caulerpa lentillifera, and Sargassum polycystum, on high-cholesterol/high-fat diet in rats. Journal of Medicinal Food. 2010;13(4):792–800. https://doi.org/10.1089/jmf.2008.1212
- El-Shaibany A, Al-Habori M, Al-Maqtari T, Al-Mahbashi H. The Yemeni brown algae Dictyota dichotoma exhibit high in vitro anticancer activity independent of its antioxidant capability. BioMed Research International. 2020;2020. https://doi.org/10.1155/2020/242569
- Aklakur M. Natural antioxidants from sea: A potential industrial perspective in aquafeed formulation. Reviews in Aquaculture. 2018;10(2):385–399. https://doi.org/10.1111/raq.12167
- Karthikeyan R, Somasundaram ST, Manivasagam T, Balasubramanian T, Anantharaman P. Hepatoprotective activity of brown alga Padina boergesenii against CCl4 induced oxidative damage in Wistar rats. Asian Pacific Journal of Tropical Medicine. 2010;3(9):696–701. https://doi.org/10.1016/S1995-7645(10)60168-X
- Wang T, Li Z, Yuan F, Lin H, Pavase TR. Effects of brown seaweed polyphenols, α‐tocopherol, and ascorbic acid on protein oxidation and textural properties of fish mince (Pagrosomus major) during frozen storage. Journal of the Science of Food and Agriculture. 2017;97(4):1102–1107. https://doi.org/10.1002/jsfa.7835
- Kraan S. Pigments and minor compounds in algae. In: Dominquez H, editor. Functional ingredients from algae for foods and nutraceuticals. Woodhead Publishing; 2013. pp. 205–251. https://doi.org/10.1533/9780857098689.1.205
- Wang J, Fan Y, Qian J, Wang S, Li Y, Xu M, et al. Relationship between dietary fiber and vitamin c intake and oral cancer. Frontiers in Public Health. 2022;10. https://doi.org/10.3389/fpubh.2022.880506
- Miazek K, Beton K, Śliwińska A, Brożek-Płuska B. The effect of β-carotene, tocopherols and ascorbic acid as anti-oxidant molecules on human and animal in vitro/in vivo studies: A Review of research design and analytical techniques used. Biomolecules. 2022;12(8). https://doi.org/10.3390/biom12081087
- Kalinchenko SYu, Smykalova AS, Vorslov LO. Brown seaweed preparations: Biological properties, potential for using in medicine and dietetics. Nutrition. 2019;9(1):25–32. (In Russ.). https://doi.org/10.20953/2224-5448-2019-1-25-32
- Holdt SL, Kraan S. Bioactive compounds in seaweed: Functional food applications and legislation. Journal of Applied Phycology. 2011;23:543–597. https://doi.org/10.1007/s10811-010-9632-5
- Muñoz LI, Díaz NF. Minerals in edible seaweed: Health benefits and food safety issues. Critical Reviews in Food Science and Nutrition. 2020;62(6):1592–1607. https://doi.org/10.1080/10408398.2020.1844637
- Bogolitsyn K, Kaplitcin P, Kashina E, Ivanchenko N, Kokryatskaya N, Ovchinnikov D. Peculiarities of the mineral composition of the brown algae in the white and Barents Seas. Chemistry of Plant Raw Materials. 2014;(1):243–250. (In Russ.). https://www.elibrary.ru/SNNCYJ
- Aminina NM. Comparative description of brown algae from the coastal zone of Far East. Izvestiya TINRO. 2015;182:258–268. (In Russ.). https://www.elibrary.ru/UYADST
- Zaharudin N, Tullin M, Pekmez CT, Sloth JJ, Rasmussen RR, Dragsted LO. Effects of brown seaweeds on postprandial glucose, insulin and appetite in humans – A randomized, 3-way, blinded, cross-over meal study. Clinical Nutrition. 2021;40(3):830–838. https://doi.org/10.1016/j.clnu.2020.08.027
- Voron'ko NG, Derkach SR, Kuchina YuA, Sokolan NI, Kuranova LK, Obluchinskaya ED. Influence of added gelatin on the rheological properties of a Fucus vesiculosus extract. Food Bioscience. 2019;29:1–8. https://doi.org/10.1016/j.fbio.2019.03.002
- Cakmak I, Kutman UB. Agronomic biofortification of cereals with zinc: A review. European Journal of Soil Science. 2018;69(1):172–180. https://doi.org/10.1111/ejss.12437
- Chasapis CT, Ntoupa P-SA, Spiliopoulou CA, Stefanidou ME. Recent aspects of the effects of zinc on human health. Archives of Toxicology. 2020;94:1443–1460. https://doi.org/10.1007/s00204-020-02702-9
- Saha N, Mollah MZI, Alam MF, Rahman MS. Seasonal investigation of heavy metals in marine fishes captured from the Bay of Bengal and the implications for human health risk assessment. Food Control. 2016;70:110–118. https://doi.org/10.1016/j.foodcont.2016.05.040
- Mohamed S, Hashim SN, Rahman HA. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends in Food Science and Technology. 2012;23:(2):83–96. https://doi.org/10.1016/j.tifs.2011.09.001
- Brown EM, Allsopp PJ, Magee PJ, Gill CIR, Nitecki S, Strain CR, et al. Seaweed and human health. Nutrition Reviews. 2014;72(3):205–216. https://doi.org/10.1111/nure.12091
- Peñalver R, Lorenzo JM, Ros G, Amarowicz R, Pateiro M, Nieto G. Seaweeds as a functional ingredient for a healthy diet. Marine Drugs. 2020;18(6). https://doi.org/10.3390/md18060301
- Lorenzo JM, Agregán R, Munekata PES, Franco D, Carballo J, Şahin S, et al. Proximate composition and nutritional value of three macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Marine Drugs. 2017;15(11). https://doi.org/10.3390/md15110360
- Circuncisão AR, Catarino MD, Cardoso SM, Silva AMS. Minerals from macroalgae origin: Health benefits and risks for consumers. Marine Drugs. 2018;16(11). https://doi.org/10.3390/md16110400
- Mann J, Truswell S. Essentials of human nutrition. 5th ed. Oxford: Oxford University Press; 2017. 720 p.
- Siahaan EA, Pangestuti R, Kim S-K. Seaweeds: Valuable ingredients for the pharmaceutical industries. In: Rampelotto PH, Trincone A, editors. Grand challenges in marine biotechnology. Cham: Springer; 2018. pp. 49–95. https://doi.org/10.1007/978-3-319-69075-9_2
- Blikra MJ, Henjum S, Aakre I. Iodine from brown algae in human nutrition, with an emphasis on bioaccessibility, bioavailability, chemistry, and effects of processing: A systematic review. Comprehensive Reviews in Food Science and Food Safety. 2022;21(2):1517–1536. https://doi.org/10.1111/1541-4337.12918
- Milinovic J, Mata P, Diniz M, Noronha JP. Umami taste in edible seaweeds: The current comprehension and perception. International Journal of Gastronomy and Food Science. 2021;23. https://doi.org/10.1016/j.ijgfs.2020.100301
- Algae products market by type (lipids, carotenoids, carrageenan, alginate, algal protein), facility type, form (liquid, solid), source (brown algae, green algae, red algae, blue-green algae), and region – Global forecast to 2026 [Internet]. [cited 2023 Feb 07]. Available from: https://www.marketresearch.com/MarketsandMarkets-v3719/Algae-Products-Type-Lipids-Carotenoids-30142703
- Morais T, Inácio A, Coutinho C, Ministro M, Cotas J, Pereira L, et al. Seaweed potential in the animal feed: A review. Journal of Marine Science and Engineering. 2020;8(8). https://doi.org/10.3390/jmse8080559
- Buschmann AH, Camus C, Infante J, Neori A, Israel Á, Hernández-González MC, et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. European Journal of Phycology. 2017;52(4):391–406. https://doi.org/10.1080/09670262.2017.1365175
- Hurtado AQ. Genetic resources for farmed seaweeds. Rome: FAO; 2022. 88 p.
- Mouritsen OG, Rhatigan P, Pérez-Lloréns JL. The rise of seaweed gastronomy: Phycogastronomy. Botanica Marina. 2019;62(3):195–209. https://doi.org/10.1515/bot-2018-0041
- Koyande AK, Chew KW, Manickam S, Chang J-S, Show P-L. Emerging algal nanotechnology for high-value compounds: A direction to future food production. Trends in Food Science and Technology. 2021;116:290–302. https://doi.org/10.1016/j.tifs.2021.07.026
- The state of world fisheries and aquaculture 2022. Towards blue transformation. Rome: FAO; 2022. 266 p.
- Nova P, Martins AP, Teixeira C, Abreu H, Silva JG, Silva AM, et al. Foods with microalgae and seaweeds fostering consumers health: A review on scientific and market innovations. Journal of Applied Phycology. 2020;32:1789–1802. https://doi.org/10.1007/s10811-020-02129-w
- Blikra MJ, Altintzoglou T, Løvdal T, Rognså G, Skipnes D, Skåra T, et al. Seaweed products for the future: Using current tools to develop a sustainable food industry. Trends in Food Science and Technology. 2021;118:765–776. https://doi.org/10.1016/j.tifs.2021.11.002
- Bizzaro G, Vatland AK, Pampanin DM. The One-Health approach in seaweed food production. Environment International. 2022;158. https://doi.org/10.1016/j.envint.2021.106948
- Liu Y, Tang T, Duan S, Qin Z, Li C, Zhang Z, et al. Effects of sodium alginate and rice variety on the physicochemical characteristics and 3D printing feasibility of rice paste. LWT. 2020;127. https://doi.org/10.1016/j.lwt.2020.109360
- Park SM, Kim HW, Park HJ. Callus-based 3D printing for food exemplified with carrot tissues and its potential for innovative food production. Journal of Food Engineering. 2020;271. https://doi.org/10.1016/j.jfoodeng.2019.109781
- Tsai C-R, Lin Y-K. Artificial steak: A 3D printable hydrogel composed of egg albumen, pea protein, gellan gum, sodium alginate and rice mill by-products. Future Foods. 2022;5. https://doi.org/10.1016/j.fufo.2022.100121
- Véliz K, Toledo P, Araya M, Gómez MF, Villalobos V, Tala F. Chemical composition and heavy metal content of Chilean seaweeds: Potential applications of seaweed meal as food and feed ingredients. Food Chemistry. 2023;398. https://doi.org/10.1016/j.foodchem.2022.133866
- O'Sullivan AM, O'Grady MN, O'Callaghan YC, Smyth TJ, O'Brien NM, Kerry JP. Seaweed extracts as potential functional ingredients in yogurt. Innovative Food Science and Emerging Technologies. 2016;37:293–299. https://doi.org/10.1016/j.ifset.2016.07.031
- Nagai NF, Lorenzo JM, Ranalli N, Pérez-Álvarez JÁ, Sepulveda N, Domínguez R, et al. Use of seaweed powder (Undaria sp.) as a functional ingredient in low-fat pork burgers. Algal Research. 2022;67. https://doi.org/10.1016/j.algal.2022.102862
- Hoffmann SL, Kohlstedt M, Jungmann L, Hutter M, Poblete-Castro I, Becker J, et al. Cascaded valorization of brown seaweed to produce L-lysine and value-added products using Corynebacterium glutamicum streamlined by systems metabolic engineering. Metabolic Engineering. 2021;67:293–307. https://doi.org/10.1016/j.ymben.2021.07.010
- Boutheina B, Leila K, Besbes N, Messina CM, Santulli A, Saloua S. Evaluation of the qualitative properties and consumer perception of marinated sardine Sardina pilchardus: The effect of fucoxanthin addition. International Journal of Gastronomy and Food Science. 2023;31. https://doi.org/10.1016/j.ijgfs.2022.100611
- Tuorila H, Hartmann C. Consumer responses to novel and unfamiliar foods. Current Opinion in Food Science. 2020;33:1–8. https://doi.org/10.1016/j.cofs.2019.09.004
- Functional food market – growth, trends, covid-19 impact, and forecasts (2022–2027) [Internet]. [cited 2023 Feb 07]. Available from: https://www.mordorintelligence.com/industry-reports/global-functional-food-market#:~:text=The%20Global%20Functional%20Food%20Market,the%20forecast%20period%202021%2D2026
- Lamont T, McSweeney M. Consumer acceptability and chemical composition of whole-wheat breads incorporated with brown seaweed (Ascophyllum nodosum) or red seaweed (Chondrus crispus). Journal of the Science of Food and Agriculture. 2021;101(4):1507–1514. https://doi.org/10.1002/jsfa.10765
- Chen P-H, Chiang P-C, Lo W-C, Su C-W, Wu C-Y, Chan C-H, et al. A novel fucoidan complex-based functional beverage attenuates oral cancer through inducing apoptosis, G2/M cell cycle arrest and retarding cell migration/invasion. Journal of Functional Foods. 2021;85. https://doi.org/10.1016/j.jff.2021.104665
- Kang Z-L, Wang T, Li Y, Li K, Ma H. Effect of sodium alginate on physical-chemical, protein conformation and sensory of low-fat frankfurters. Meat Science. 2020;162. https://doi.org/10.1016/j.meatsci.2019.108043
- Derkach SR, Kolotova DS, Voron’ko NG, Obluchinskaya ED, Malkin AYa. Rheological properties of fish gelatin modified with sodium alginate. Polymers. 2021;13(5). https://doi.org/10.3390/polym13050743
- Garcia-Vaquero M, Lopez-Alonso M, Hayes M. Assessment of the functional properties of protein extracted from the brown seaweed Himanthalia elongata (Linnaeus) S. F. Gray. Food Research International. 2017;99(3):971–978. https://doi.org/10.1016/j.foodres.2016.06.023
- Fradinho P, Raymundo A, Sousa I, Domínguez H, Torres MD. Edible Brown seaweed in gluten-free pasta: Technological and nutritional evaluation. Foods. 2019;8(12). https://doi.org/10.3390/foods8120622
- Losada-Lopez C, Dopico DC, Faína-Medín JA. Neophobia and seaweed consumption: Effects on consumer attitude and willingness to consume seaweed. International Journal of Gastronomy and Food Science. 2021;24. https://doi.org/10.1016/j.ijgfs.2021.100338
- Kumar Y, Tarafdar A, Kumar D, Badgujar PC. Effect of Indian brown seaweed Sargassum wightii as a functional ingredient on the phytochemical content and antioxidant activity of coffee beverage. Journal of Food Science and Technology. 2019;56(10):4516-4525. https://doi.org/10.1007/s13197-019-03943-y
- Smertina ES, Fedyanina LN, Lyakh VA, Kurapova KF. Prospects for the development of bakery products enriched with seaweed lipid fractions. Bread Products. 2022;(9):52–56. (In Russ.). https://doi.org/10.32462/0235-2508-2022-31-9-52-56
- Fedyanina LN, Smertina ES, Lyakh VA, Koptienko EO. Use of seaweed extract as an innovative ingredient in the bakery recipe. Bread Products. 2019;(5):54–57. (In Russ.). https://doi.org/10.32462/0235-2508-2019-28-5-54-57
- Krekhnova AP, Efimova MV, Efimov AA. Development of technology of confectionery fillings with brown and red algae used as polyfunctional additives. Bulletin of Kamchatka State Technical University. 2019;(47):24–32. (In Russ.). https://doi.org/10.17217/2079-0333-2019-47-24-32
- Reboleira J, Freitas R, Pinteus S, Silva J, Alves C, Pedrosa R, et al. Brown seaweeds. In: Nabavi SM, Silva AS, editors. Nonvitamin and nonmineral nutritional supplements. Academic Press; 2019. pp. 171–176. https://doi.org/10.1016/B978-0-12-812491-8.00024-2
- Manzoor S, Masoodi FA, Rashid R, Naqash F, Ahmad M. Oleogels for the development of healthy meat products: A review. Applied Food Research. 2022;2(2). https://doi.org/10.1016/j.afres.2022.100212
- Agregán R, Barba FJ, Gavahian M, Franco D, Khaneghah AM, Carballo J, et al. Fucus vesiculosus extracts as natural antioxidants for improvement of physicochemical properties and shelf life of pork patties formulated with oleogels. Journal of the Science of Food and Agriculture. 2019;99(10):4561–4570. https://doi.org/10.1002/jsfa.9694
- Agregán R, Franco D, Carballo J, Tomasevic I, Barba FJ, Gómez B, et al. Shelf life study of healthy pork liver pâté with added seaweed extracts from Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcate. Food Research International. 2018;112:400–411. https://doi.org/10.1016/j.foodres.2018.06.063
- Vilar EG, Ouyang H, O'Sullivan MG, Kerry JP, Hamill RM, O'Grady MN, et al. Effect of salt reduction and inclusion of 1% edible seaweeds on the chemical, sensory and volatile component profile of reformulated frankfurters. Meat Science. 2020;161. https://doi.org/10.1016/j.meatsci.2019.108001
- Cofrades S, Benedí J, Garcimartin A, Sánchez-Muniz FJ, Jimenez-Colmenero F. A comprehensive approach to formulation of seaweed-enriched meat products: From technological development to assessment of healthy properties. Food Research International. 2017;99:1084–1094. https://doi.org/10.1016/j.foodres.2016.06.029
- Moroney NC, O’Grady MN, O’Doherty JV, Kerry JP. Effect of a brown seaweed (Laminaria digitata) extract containing laminarin and fucoidan on the quality and shelf-life of fresh and cooked minced pork patties. Meat Science. 2013;94(3):304–311. https://doi.org/10.1016/j.meatsci.2013.02.010
- Yuan D, Xu Y, Kong B, Cao C, Zhang F, Xia X, et al. Application of seaweed dietary fiber as a potential alternative to phosphates in frankfurters with healthier profiles. Meat Science. 2023;196. https://doi.org/10.1016/j.meatsci.2022.109044
- del Olmo A, Picon A, Nuñez M. Cheese supplementation with five species of edible seaweeds: Effect on microbiota, antioxidant activity, colour, texture and sensory characteristics. International Dairy Journal. 2018;84:36–45. https://doi.org/10.1016/j.idairyj.2018.04.004
- Bouillon GA, Gåserød O, Rattray FP. Evaluation of the inhibitory effect of alginate oligosaccharide on yeast and mould in yoghurt. International Dairy Journal. 2019;99. https://doi.org/10.1016/j.idairyj.2019.104554
- Nooshkam M, Varidi M, Alkobeisi F. Licorice extract/whey protein isolate/sodium alginate ternary complex-based bioactive food foams as a novel strategy to substitute fat and sugar in ice cream. Food Hydrocolloids. 2023;135. https://doi.org/10.1016/j.foodhyd.2022.108206
- Arufe S, Sineiro J, Moreira R. Determination of thermal transitions of gluten-free chestnut flour doughs enriched with brown seaweed powders and antioxidant properties of baked cookies. Heliyon. 2019;5(6). https://doi.org/10.1016/j.heliyon.2019.e01805
- Oh H, Lee P, Kim SY, Kim Y-S. Preparation of cookies with various native seaweeds found on the Korean coast. Journal of Aquatic Food Product Technology. 2020;29(2):167–174. https://doi.org/10.1080/10498850.2019.1707925
- Palmieri N, Forleo MB. An explorative study of key factors driving Italian consumers’ willingness to eat edible seaweed. Journal of International Food and Agribusiness Marketing. 2022;34(4):433–455. https://doi.org/10.1080/08974438.2021.1904082
- Quitral V, Sepúlveda M, Gamero-Vega G, Jiménez P. Seaweeds in bakery and farinaceous foods: A mini-review. International Journal of Gastronomy and Food Science. 2022;28. https://doi.org/10.1016/j.ijgfs.2021.100403
- Wilcox MD, Cherry P, Chater PI, Yang X, Zulali M, Okello EJ, et al. The effect of seaweed enriched bread on carbohydrate digestion and the release of glucose from food. Journal of Functional Foods. 2021;87. https://doi.org/10.1016/j.jff.2021.104747
- Koh HSA, Lim SEV, Lu J, Zhou W. Bioactivity enhancement of fucoidan through complexing with bread matrix and baking. LWT. 2020;130. https://doi.org/10.1016/j.lwt.2020.109646
- Arufe S, Della Valle G, Chiron H, Chenlo F, Sineiro J, Moreira R. Effect of brown seaweed powder on physical and textural properties of wheat bread. European Food Research and Technology. 2018;244:1–10. https://doi.org/10.1007/s00217-017-2929-8
- General standard for food additives. Codex stan 192-1995. FAO, WHO; 2021. 502 p.
- Hydrocolloids market by type (gelatin, xanthan gum, carrageenan, LBG, Alginate, agar, pectin, guar gum, gum arabic, MCC, and CMC), Source (botanical, microbial, animal, seaweed, and synthetic), function, application, and region – Global forecast to 2026 [Internet]. [cited 2023 Feb 13]. Available from: https://www.marketsandmarkets.com/Market-Reports/hydrocolloid-market-1231.html
- Donn P, Prieto MA, Mejuto JC, Cao H, Simal-Gandara J. Functional foods based on the recovery of bioactive ingredients from food and algae by-products by emerging extraction technologies and 3D printing. Food Bioscience. 2022;49. https://doi.org/10.1016/j.fbio.2022.101853
- Dankar I, Haddarah A, Omar FEL, Sepulcre F, Pujolà M. 3D printing technology: The new era for food customization and elaboration. Trends in Food Science and Technology. 2018;75:231–242. https://doi.org/10.1016/j.tifs.2018.03.018
- Lim SJ, Mustapha WAW, Maskat MY. Seaweed tea: Fucoidan-rich functional food product development from Malaysian brown seaweed, Sargassum binderi. Sains Malaysiana. 2017;46(9):1573–1579. https://doi.org/10.17576/jsm-2017-4609-28
- Poveda-Castillo GC, Rodrigo D, Martínez A, Pina-Pérez MC. Bioactivity of fucoidan as an antimicrobial agent in a new functional beverage. Beverages. 2018;4(3). https://doi.org/10.3390/beverages4030064