Аннотация
Consumption of cassava meal affected by crude oil has significant effects on lipid and protein metabolism. The hepatoprotective action of spices is mostly attributed to the suppression of lipid oxidation and protein breakdown. This study examined the protein restoration and anti-lipidemic potential of Monodora myristica (Gaertn.) in rats fed with cassava contaminated with crude oil.The research involved 36 albino rats separated into six groups (n = 6). Group 1 (control) consumed cassava without crude oil. Group 2 received cassava with crude oil. Groups 3, 4, and 5 fed on cassava with crude oil and various extracts of M. myristica, i.e., aqueous, ethanol, and diethyl ether, respectively. Group 6 received non-ionic synthetic surfactant Tween 80. The experiment relied on standard methods.
Blood serum and liver obtained from the rats of Group 2 showed a significant (p < 0.05) increase in total cholesterol, low density lipoprotein cholesterol, triacylglycerol, and malondialdehyde, as well as a decrease in total protein, albumin, and high-density lipoprotein cholesterol. The groups that received M. myristica extracts showed a significant increase (p < 0.05) in total protein, albumin, and high-density lipoprotein cholesterol. They also had lower total cholesterol, low density lipoprotein cholesterol, triacylglycerol, and malondialdehyde as compared to Group 2, which dieted on cassava contaminated with crude oil without additives.
In this research, crude oil-contaminated cassava affected proteins and lipids in rats. Diethyl ether extract of M. myristica demonstrated the best anti-lipidemic and protein restoration.
Ключевые слова
Cassava meal, crude petroleum oil, lipids, Monodora myristica, proteinВВЕДЕНИЕ
Cassava (Manihot esculenta Crantz) is a primary food crop growing in South America. This plant is believed to have been introduced to Nigeria by Portuguese explorers and colonists at the height of the slave trade in the 16th century [1]. Due to cassava droughtresistance, its now grows in all parts of Africa. It is a perennial woody shrub that can withstand water shortages and has a starch content of up to 32% (fresh). These days, cassava is one of the most important staple food crops in sub-Saharan Africa. In some regions, its average consumption exceeds 300 kg per person annually [2].
Cassava is a remarkably adaptable crop with a wide range of applications and by-products. Its leaves can be fed to cattle as a protein feed additive. People can eat them dry or in soups. Stems are used for plant propagation and grafting. Roots are typically processed for human and industrial consumption as a good source of carbohydrates [2]. Cassava yields a variety of goods, including cassava starch, fried cassava granules, lafun (cassava flour), and garri (fried cassava).
Cassava is a source of both animal feed and biofuel. Oil spillages affect the economy by destroying vegetation, food crops, and soil fertility, which reduces food productivity [3]. However, poor crop yield and productivity decline are not the only problems related to oil spillage. Cassava grown in crude oil-impacted soils accumulates toxic hydrocarbons and heavy metals with a high consequence of transfer within the food web [4].The chemical composition of crude oil depends on the earth crust development. In fact, it is a complex of up to 6000 potentially unique hydrocarbons and metals. A rising crude oil exposure may be dangerous for people and animals [3]. Toxicants generate free radicals, and free radicals may lead to oxidative stress [5]. If followed by oxidative alteration, free radicals cause lipid peroxidation, thus harming such vital cellular components such as proteins, lipids, and DNA [6–8]. The oxidation and digestion of lipids and carbohydrates obtained with food provides most of the energy required by the human body [4].
The liver has a higher capacity for the metabolism of xenobiotics, as well as other hydrocarbons and elements found in petroleum and petrochemical goods. Akinbule et al. studied the changes in lipid profiles and health lipid quality of some Nigerian composite meals [9]. Spices such as turmeric, bay leaf, cinnamon, and cloves demonstrated a positive effect on human health. Herbs and spices are essential components of daily diet [10]. In addition, they also enhance the flavor and taste of food [11, 12]. Even in very small quantities, spices can make an average meal appealing and fragrant. For instance, the calabash nutmeg (Monodora myristica (Gaertn.)) may participate in lipid metabolism. M. myristica is a tropical tree that belongs to the Annonaceae family of flowering plants. Valuable yet neglected, M. myristica has a lot of names in African languages: ehirior ehuru (Ibo); gujiyadanmiya (Hausa), ariwo, arigbo, abolakoshe, or eyinaghose (Yoruba), and ehinawosin (Ikale). Its fruit contains seeds that are typically 1.5 cm long and covered in a white, sweet-smelling mush. Olatoye et al. reported that M. myristica seeds serve mostly as a flavoring in various foods, including soups and salads [13].
Our study is part of a research project that features the changes in lipid and protein parameters in rats fed with cassava contaminated with crude oil. However, the effect of M. myristica on the toxicity of crude oil in cassava has not been acknowledged scientifically. In future, this project may prove the efficacy of M. myristica in treating crude oil-induced lipidemia in Wistar rats. As M. myristica is a popular component of African diet, the research results may eventually find dietary application in treating people exposed to cassava foods contaminated with crude oil.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Monodora myristica (Gaertn.) and crude oil. The M. myristica and cassava were obtained from a market in Amai, Delta State, Nigeria, in October 2021. M. myristica (voucher number FHI107259) was identified at Forest Research Institute of Nigeria, Ibadan. The crude oil was obtained from a refinery in Warri that belongs to the Nigerian National Petroleum Cooperation (NNPC), Delta State, Nigeria.
Preparing cassava diet. The fresh cassava tubers that had just reached maturity were purchased in October 2021 from local farmers in Amai community, Delta State. They were washed in clean water to remove dirt and peeled. After being manually cut into smaller bits, they were rinsed in clean water, dried in an oven at 40°C, and milled. The samples included cassava meal with crude oil and cassava meal without crude oil. The rats diet consisted of dried cassava (54.64%), casein (11.26%), corn cob cellulose (5%), bone meal (2%), oyster shell (1%), Vitmin premix (1%), glucose monohydrate (5%), and salt (5%). The difference between the two groups was that the control mix without crude oil contained 10% of sucrose, which was substituted with 10% of crude oil in the experimental mix. The ingredients were mixed together manually before being fed to albino rats.
Preparing spice extracts. The dried M. myristica was crushed into fine particles in a high-speed blender. After that, we dissolved 100 g of the powder in 500 mL of the following solvents: ethanol (95% by volume), hot water (60°C), and diethyl ether (95% by volume). This stage lasted for 48 h. After being filtered through a cotton cloth, the solutions underwent a water bath at 45°C and were concentrated to dryness.
Experimental procedures. The experiment featured 36 rats that were 7–8 weeks old. The average weight of the rats were 130–140 g. The rats were allowed to acclimatize to laboratory condition for one week. After that, they were divided into six groups with six rats in each. Group 1 was normal control and included rats fed on cassava without crude oil. Group 2 was the experimental control and included rats consumed nothing but cassava contaminated with crude oil. In Group 3, the rats had a diet of cassava with crude oil plus aqueous M. myristica extract. Group 4 combined cassava with crude oil and ethanol M. myristica extract. In Group 5, the rats ate cassava with crude oil plus a diethyl ether extract of M. myristica. In Group 6, there were rats fed on cassava with crude oil plus 1 mL/kg of 5% Tween 80. The cassava meal and extracts were administered orally for 28 days using cannulas.
Ethical approval. Approval for the current study was granted by the Ethics Committee of Novena University (case No. NUO/PGD/21/890). The research followed the Ethics of Animal Research [14].
Blood collection and tissue homogenate. The rats were sacrificed on day 29 of the experiment after an overnight fast. We collected blood samples from the heart using a syringe and a needle to transfer the samples to an anticoagulant-free test tube. The clotted blood was centrifuged at 2500 rpm for 15 min to isolate serum for further examination. The serum was stored in a freezer at –4°C. One gram of liver and kidney were homogenized in 10 mL of normal saline and centrifuged at 2500 g for 15 min. The obtained supernatant was kept in a freezer at –4°C for biochemical examination.
Biochemical examination. Total cholesterol. We used the method described by Allan et al. to determine the total cholesterol (T-Chol) [15]. According to the pro-
cedure, we added 1 mL of cholesterol reagent to a labelled test tube. After that, 10 µL of each sample was added to a respective tube and mixed. The tubes with the reagent and sample mix were left to stand for 5 min. When the solution turned pinkish-red, the absorbance test took place at 530 nm after blanking.
Triacylglycerol. The triacylglycerol assay followed the method described by Young in [16]. We placed 1 mL triacylglycerol regent in labelled tubes. After that, we transferred 10 µL sample, blank (distilled water), and standard reagent into the respective tubes and mixed the contents. The tubes with reagents and samples were stored at room temperature for 5 min. The absorbance was determined at 540 nm after blanking.
High-density lipoprotein cholesterol. We applied the method specified by Badimon et al. to determine high-density lipoprotein cholesterol (HDL-C) [17]. In line with the procedure, we added precisely 500 µL diluted precipitant to 200 µL sample. The resulting mix was allowed to settle at room temperature for 10 min. After that, we centrifuged them at 4000 rpm for 10 min or at 12 000 rpm for 2 min. Subsequently, 1 mL cholesterol reagent was placed in a labelled test tube, upon which we transferred 25 µL supernatant, blank (distilled water), and standard reagent to the respective tubes. The content of each tube was thoroughly mixed for 5 min, and the values were recorded at 500 nm.
Low-density lipoprotein cholesterol. The low-density lipoprotein cholesterol (LDL-C, mg/dL) test relied on the Friedewald Equation as in [18]:
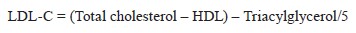
Albumin. According to Droumas et al., the albumin content was defined based on its quantitative blending to 3′,3′′,5′,5′′-tetrabromo-m-cresol-sulphoepthalein (bromocresol green) [20]. We dispensed 3 mL bromocresol green in a labelled test tube. After that, we transferred 10 μL sample into the tubes and allowed them to settle for 5 min. The absorbance for spectrophotometry was measured at 580 nm.
Total protein. We used the Tietz technique to calculate the total protein [21]. Protein forms a blue-colored complex when treated with cupric ions in an alkaline solution. The blue color is proportional to the protein concentration. According to the procedure, we transferred 1 mL reagent to blank, test, and standard tubes. Then, we added 20 µL sample to the appropriate tubes. After 30 min, we measured the absorbance at 546 nm.
Statistical analysis. The data were subjected to descriptive statistics, and the results were shown as mean ± SD and mean bars. We used the ANOVA analysis of variance and the post hoc test to identify the significant differences between the groups. The statistical analysis relied on SPSS 22.0. A statistically significant difference between the test and control groups was defined as p < 0.05.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
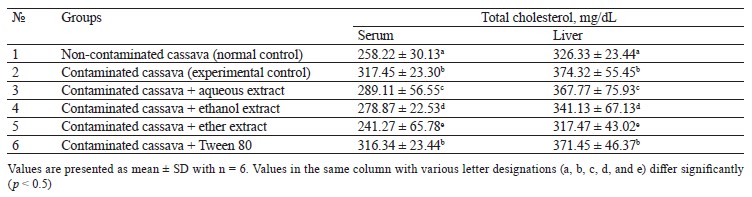
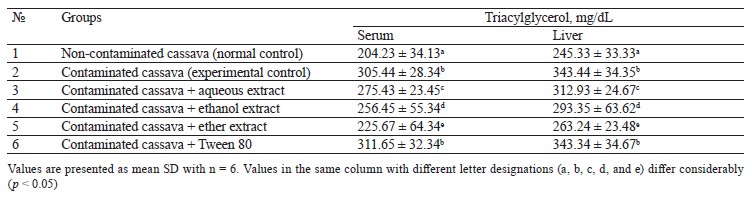
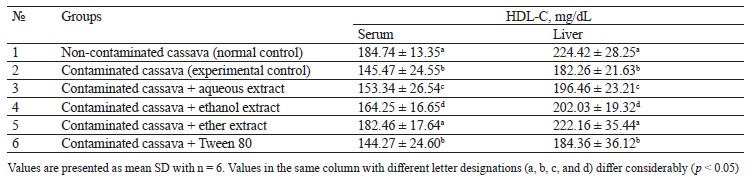
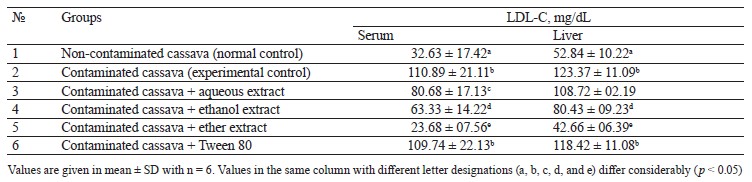
Lipid profile of rats fed with cassava containing crude oil. Tables 1–4 illustrate the changes in serum and liver total cholesterol, triacylglycerol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) in rats fed with cassava without crude oil, cassava with crude oil, and various Monodora myristica (Gaertn.) extracts. The serum and liver samples from Group 2 (cassava contaminated with crude oil) and Group 6 (cassava with crude oil plus 1 mL/kg of 5% Tween 80) were compared with Group 1. The samples demonstrated a significant (p < 0.05) increase in triacylglycerol, total cholesterol, and low-density lipoprotein cholesterol while the high-density lipoprotein cholesterol was significantly lower.
Group 3 (cassava with crude oil plus aqueous M. myristica extract), Group 4 (cassava with crude oil and ethanol M. myristica extract), and Group 5 (cassava with crude oil plus a diethyl ether M. myristica extract) were compared to Group 2. In contrast, they showed significantly lower levels of triacylglycerol, total cholesterol, and low-density lipoprotein cholesterol, whereas the level of high-density lipoprotein cholesterol increased.
Onuoha & Chukwuma reported that a higher lipid metabolism could induce generation of free radicals [3]. The cholesterol dropped due to the reduction in either the synthesis of cholesterol by hepatocytes or the fractional reabsorption in the small intestine [22].
The oil-contaminated cassava might impose a reciprocal relationship between the low and high-density cholesterols in the serum and liver of albino rats. The condition when high-density lipoprotein cholesterol is low and low-density lipoprotein cholesterol is high is the primary risk factor for coronary heart disease [22].
In this study the extracts also reduced the levels of triacylglycerol, total cholesterol, and low-density lipoprotein cholesterol in rats fed with oil-contaminated cassava.
The low total cholesterol could be explained by the substantial amount of phytochemicals in M. myristica extracts: for instance, steroids are known to inhibit absorption of dietary cholesterol. Thus, the low total cholesterol in blood serum could arise from the lipid-lowering potential of M. myristica.
The hypolipidemic mechanism may be explained by the fact that the NADPH-dependent HMG-CoA reductase activity is inhibited in cholesterol biosynthetic pathway. This result is in line with Adeyanju et al., who used Sesamum indicum to reduce hyperlipidemia in rats [23]. Likewise, Liu et al. reported that antioxidant spices enhanced the plasma lecithin cholesterol acyl transferase and hydroxyl methyl glutaryl CoA reductase, thus causing an improved degradation of cholesterol [22].
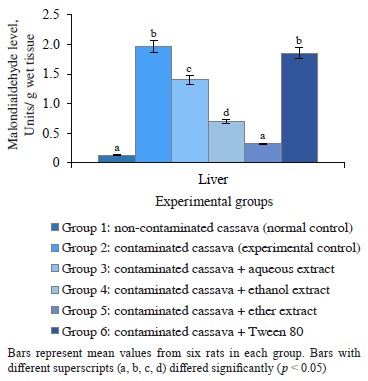
M. myristica effect on liver in cassava-fed rats. Free radicals generated by crude petroleum oil have the potential to bind with various proteins or lipids and initiate lipid peroxidation [3, 24]. In the current study, rats fed with nothing but oil-contaminated cassava had significantly (p < 0.05) high levels of malondialdehyde in their liver compared to the control. The same was true for Group 6 fed with oil-contaminated cassava and Tween 80 (Fig. 1). The peroxyl radicals generated from crude petroleum oil seemed to initiate the degradation of membrane lipids. The process triggered the generation of lipid peroxides, which, in turn, caused lipid peroxidation that resulted in a loss of cell membrane integrity and liver injury [3]. The M. myristica extracts reduced the level malondialdehyde, i.e., the lipid peroxidation end product. Therefore, the M. myristica extracts could minimize lipid peroxidation induced by cassava contaminated with crude oil.
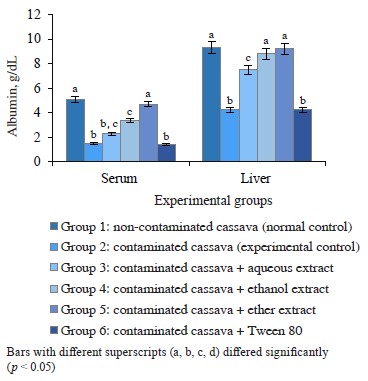
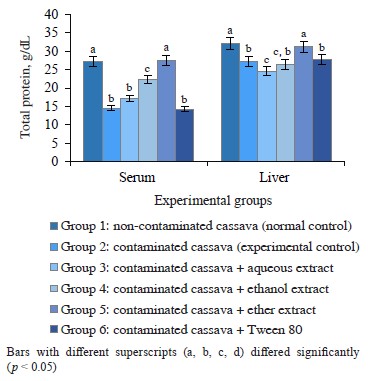
Protein in rats fed cassava with and without crude oil. Figures 2 and 3 show the albumin and total protein in the serum and liver tissue of rats that consumed
Figure 2 Albumin in rats administered cassava and without crude oil and various Monodora myristica extracts cassava with and without crude oil. The groups that received oil-contaminated cassava demonstrated a significant decrease in albumin and total protein, compared to rats fed with non-contaminated cassava. The groups that dieted on contaminated cassava with M. myristica extracts demonstrated a significant increase in albumin and total protein when compared to the group that received oil-contaminated cassava without additives. The low level of total protein and albumin in serum may be a consequence of poor diet, crude petroleum oil, liver dysfunction, or abnormality in nutrient absorption [25]. Evidently, crude oil might contain toxic compounds such as polycyclic aromatic hydrocarbons, which are important constituent of crude oil [25]. Its effect on the liver could prevent it from synthesizing enough albumins to be released into the serum. Evidently, the extracts of M. myristica were able to reverse this effect and improve albumin synthesis.
ВЫВОДЫ
A diet of cassava grown on soils contaminated with crude oil may caused the development of free radicals that coul be responsible for protein and lipid alterations in rats. The administration of various Monodora myristica (Gaertn.) extracts reversed the high levels of total cholesterol, triacylglycerol, and low-density lipoprotein cholesterol, thereby preventing chemically-induced dyslipidemia and oxidative damage to hepatocytes. Probably, the free radical scavenging properties of M. myristica could shift the demand for lipids as a substrate. Therefore, the inhibited lipid oxidation contributed to the hetoprotective effect against liver damage induced by oil-contaminated cassava. The diethyl ether extract of M. myristica revealed a better antilipidemic potential than other extracts. Farmers should be warned against planting cassava and other crops in crude oil-contaminated areas because toxic compounds could permeate into the produce. In this research, the negative effect of crude oil-contaminated cassava could be reversed by M. myristica in the diet of albino rats.
Вклад авторов
J. Okpoghono designed and wrote the draft manuscript. J.K. Ukperegbulem performed the laboratory tests and generated the data. U.B. Igue analyzed the obtained data. The manuscript was approved by all named authors.
КОНФЛИКТ ИНТЕРЕСОВ
No conflicts of interest are disclosed by the authors.
ФИНАНСИРОВАНИЕ
The study was funded by the authors, and no support was acquired from any funding agency.СПИСОК ЛИТЕРАТУРЫ
- Adeniyi VA, Akangbe JA, Kolawole AE, Ayeni MD, Olorunfemi DO. Women cassava processors’ livelihood; implications for improved processing technology usage in Nigeria. Cogent Social Sciences. 2023;9(1). https://doi.org/10.1080/23311886.2023.2191898
- Anyaegbu CN, Okpara KE, Taweepreda W, Akeju D, Techato K, Onyeneke RU, et al. Impact of climate change on cassava yield in Nigeria: An autoregressive distributed lag bound approach. Agriculture. 2023;13(1). https://doi.org/10.3390/agriculture13010080
- Onuoha SC, Chukwuma CC. Biochemical investigations of Albino rats orally exposed to Bonny light crude oil and leaf extract of Cnidoscolus aconitifolius. International Journal of Biological Studies. 2023;3(1):1–16. https://doi.org/10.47941/ijbs.1183
- Udousoro LL, Ebiekpi IE, Otong EJ. Proximate compositions anti-nutrients and heavy metals contents in edible leafy vegetables in Akwa Ibom State, Nigeria. Book of Abstracts, 2nd World Conference on Applied Science and Technology; 2018.
- Melebary SJ, Elnaggar MHR. Impact of Moringa oleifera leaf extract in reducing the effect of lead acetate toxicity in mice. Saudi Journal of Biological Sciences. 2023;30(1). https://doi.org/10.1016/j.sjbs.2022.103507
- Ji X, Guo J, Cao T, Zhang T, Liu Y, Yan Y. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Science and Human Wellness. 2023;12(6):1969–1980. https://doi.org/10.1016/j.fshw.2023.03.017
- Li J, Li X, Zhang Z, Cheng W, Liu G, Zhao G. High-fat diet aggravates the disorder of glucose metabolism caused by chlorpyrifos exposure in experimental rats. Foods. 2023;12(4). https://doi.org/10.3390/foods12040816
- Tabakaev AV, Tabakaeva OV, Piekoszewski W, Kalenik TK, Poznyakovsky VM. Antioxidant properties of edible sea weed from the Northern Coast of the Sea of Japan. Foods and Raw Materials. 2021;9(2):262–270. https://doi.org/10.21603/2308-4057-2021-2-262-270
- Akinbule OO, Onabanjo OO, Sanni SA, Adegunwa MO, Akinbule AS. Fatty acid, lipid profiles, and health lipid quality of selected Nigerian composite meals and soups. Food Chemistry. 2022;391. https://doi.org/10.1016/j.foodchem.2022.133227
- Eisenreich A, Schäfer B. Natural compounds in plant-based food. Foods. 2023;12(4). https://doi.org/10.3390/foods12040857
- Okonta CI, Okpoghono J, George BO, Joseph O, Obiejogo J. Antihyperlipidemic and antioxidant activity of Syzygium aromaticum extract in rats fed cycas diet. Tropical Journal of Natural Product Research. 2021;5(5):959–962.
- Otuaga EJ, Okpoghono J, George BO. Proximate composition, phytochemicals and antioxidant status of Banga Soup (Elaeis guineensis extract). FUPRE Journal of Scientific and Industrial Research. 2020;4(2):63–74.
- Olatoye KK, Fapojuwoa OO, Olorunsholaa JA, Ayorinde JO. Potentials of African nutmeg (Monodora myristica) as a flavourant in cookie production. International Journal of Food Studies. 2019;8(2). https://doi.org/10.7455/ijfs.v8i2.508
- Animal research ethics. A handbook of USP researchers, Volume 2. Research Office Publisher; 2009. pp 3–4.
- Allan CC, Poon IS, Chan CSG, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clinical Chemistry. 1974;20(4):470–475. https://doi.org/10.1093/clinchem/20.4.470
- Young DS, Pestaner LC, Gibberman V. Effects of drugs on clinical laboratory tests. Clinical Chemistry. 1975;21(5):1D–432D.
- Badimon JJ, Badimon L, Fuester V. Determination of HDL-cholesterol. Journal of Clinical Investigation. 1990;85:1234–1241.
- Martin SS, Blaha MJ, Elshazly MB, Brinton EA, Toth PP, McEvoy JW, et al. Friedewald-estimated versus directly measured low-density lipoprotein cholesterol and treatment implications. Journal of the American College of Cardiology. 2013;62(8):732–739. https://doi.org/10.1016/j.jacc.2013.01.079
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods in Enzymology. 1978;52:302–310. https://doi.org/10.1016/S0076-6879(78)52032-6
- Dumas BT, Waston WA, Biggs HG. Albumin standard and the measurement of serum albumin with bromocresol green. Clinica Chimica Acta. 1997;258(1):21–30. https://doi.org/10.1016/S0009-8981(96)06447-9
- Tietz NW. Clinical guide to laboratory test. Philadelphia, London: W.B. Saunders Co; 1976. 695 p.
- Liu D, Ji Y, Zhao J, Wang H, Guo Y, Wang H. Black rice (Oryza sativa L.) reduces obesity and improves lipid metabolism in C57BL/6J mice fed with high-fat diet. Journal of Functional Foods. 2020;64. https://doi.org/10.1016/j.jff.2019.103605
- Adeyanju MM, Saheed IA, Oyelekan IO, Dele-Osibanjo AT, Adelegan AA, Raimi AJ, et al. Sesamum indicum diet, prevents hyperlipidemia in experimental rats. Food Chemistry: Molecular Sciences. 2022;4. https://doi.org/10.1016/j.fochms.2022.100092
- Kamal FZ, Stanciu GD, Lefter R, Cotea VV, Niculaua M, Ababei DC, et al. Chemical composition and antioxidant activity of Ammi visnaga L. essential oil. Antioxidants. 2022;11(2). https://doi.org/10.3390/antiox11020347
- Orisakwe OE. Crude oil and public health issues in Niger Delta, Nigeria: Much ado about the inevitable. Environmental Research. 2021;194. https://doi.org/10.1016/j.envres.2021.110725