Аннотация
Allium sativum L. protease still remains largely understudied although new varieties of garlic appear quite often, e.g., lanang garlic. This study tested the antibacterial effect of garlic and the effectiveness of various A. sativum proteases as meat tenderizers.The research involved powder extracts of four varieties of A. sativum: kating, lanang, black garlic, and sin-chung. The degradation kinetics was defined based on the Lineweaver-Burk equation. The degradation zones were measured using sodium dodecyl sulphate poly acrylamide gel electrophoresis (SDS-PAGE). Scan electron microscopy served to test the changes in meat connective tissue.
Lanang demonstrated the largest inhibition zones against Escherichia coli (9.75 ± 0.15 mm) and Staphylococcus aureus (1.04 mm). Sin-chung protease degraded beef protein with the highest Vmax of 0.1818 μg/μL/min at 10–22 KDa (small peptide, troponin C, and troponin I), 25–40 KDa (myosin light chain, troponin T, α- and β-tropomyosin, actin), and 100–140 KDa (protein C). The same garlic variety degraded mutton meat protein at 10–17 KDa (small peptide) and 25–40 KDa (myosin light chain, troponin T, α- and β-tropomyosin, actin) with Vmax of 0.1135 μg/μL/min.
All four A. sativum proteases proved to be quite effective meat tenderizers.
Ключевые слова
Allium sativum protease, lanang garlic, kating garlic, black garlic, sin-chung garlicВВЕДЕНИЕ
The benefits of garlic (Allium sativum L.) remain a relevant scientific issue, even though some of them have become a long-established concept. Almost all researchers agree on the role of A. sativum as a source of prose inhibitors and antibiotics [1–11]. Garlic is known for its antifungal, antibacterial, hypolipidemic, anti-atherosclerosis, and anticarcinogenic properties, not to mention that garlic is a world-famous culinary spice [8, 12–19].
This research aimed at identifying the connection between the A. sativum protease inhibitor and various scientifically-established concepts regarding the role of protease in A. sativum plants. Proteases are important for the metabolic processes of plant cell growth [20–22]. Researchers have reached no consensus about the relationship between A. sativum protease and its inhibitors. The role of A. sativum protease remains quite vague because its effectiveness is often disguised by the functions of other spices [23, 24]. For instance, the effect of A. sativum protease as a meat tenderizer cannot be separated from other ingredients in the marinating process [23, 25]. Figure 1 proves that very few studies report the role of A. sativum protease as a meat tenderizer. We collected around 750 papers published in 2010–2023 that featured A. sativum and searched for A. sativum protease only to find some 82 results (0.1%). Thus, the poor scientific coverage of A. sativum protease and its properties became the background for this research.
In Indonesia, A. sativum is represented by such varieties as black garlic, kating, and sin-chung. Lanang garlic, or single garlic, is an accidental new variety that appeared as a result of unsuitable planting environment in the Sarangan area, Magetan, East Java. Lanang is more of a medicinal plant than a culinary spice. Poor shoot growth in the canopy inhibits the clove budding, which results in a single big garlic clove. Such growth is suspected to be due to the effect of plant protease on the formation of plant cells [22]. In this regard, the new variety represents a prospective material for protease studies. We compared lanang garlic and its properties with other three varieties of A. sativum by testing their effect on the inhibition of Gram-negative (Escherichia coli) and Gram-positive (Staphylococcus aureus) bacteria.
The research objectives included the following tasks:
– to test the inhibitory power of Gram-positive and Gramnegative bacteria of the four varieties of A. sativum; and
– to compare the effectiveness of A. sativum protease in four garlic varieties.
We measured the effectiveness based on the following parameters:
1. Degradation kinetics. This parameter included such measurements as the maximal speed (Vmax) of protein degradation in beef and mutton substrates and the Michaelis Menten constant (KM);
2. Meat protein degradation zone. It involved sodium dodecyl sulphate poly acrylamide gel electrophoresis (SDS-PAGE); and
3. Effect of A. sativum
protease on muscle connective tissue, perimysium, endomysium, and collagen in mutton and beef using scan electron microscopy.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
We purchased lanang, kating, black garlic, sin-chung, beef, and mutton at a local supermarket. The pure bacterial cultures of Eschericia coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923) were obtained from Merck KGaA, Darmstadt, Germany. The control samples were beef and mutton without Allium sativum L., while experimental samples included beef and mutton meat with different varieties of A. sativum, namely lanang, kating, black garlic, sin-chung.
Sample treatment. To powder the garlic samples, we peeled the garlic skin and cut each clove into three pieces. After that, we baked it in an oven at 70°C for 72 h. After cooling it at room temperature (25°C) for 15 min, we pulverized the mass with a blender and sieved it. Finally, we re-ovened the powder at 70°C for 24 h.
We extracted 100 g of dried garlic powder using water (100 mL) for 72 h, then filtered and evaporated it in an evaporator for 1 h to obtain a thick extract. The final extract concentration used for analysis was 20% (20 mg/mL).
The beef and mutton were sliced into thin slices (4 cm×4 cm×2 mm). The meat was smeared with 20% garlic extract as the weight of the garlic extract to the weight of the meat. After letting it rest for 30 min, we stored the meat for 60 min at 30–35°C to prevent thermal changes. Untreated meat served as control. The protein degradation kinetics analysis (SDS PAGE) took place at 30 and 60 min, but the scan electron microscopy test was performed at 60 min.
Bacterial inhibition test. After extracting 100 g of the dry garlic powder for 72 h, we filtered and evaporated it in an evaporator for 1 h. The procedure was followed by the water bath. The resulting thick extract had a concentration of 20% (20 mg/mL) each.
The strains of E. coli (ATCC 25922) and S. aureus (ATCC 25923) were taken at 200 µL and spread out with a sterile spreader glass in a petri dish containing Muller Hinton Agar and Mannitol Salt Agar. Disk blanks were impregnated with 15 µL of stock extract. One disk served as a negative control whereas the other disc was filled with sample extracts. After incubating them at 37°C for 24 h, we observed the diameter of the inhibition zone in line with the procedure we described in [26].
Protein content. We used the biuret method with a UV-Vis spectrophotometer at λ = 595 nm to determine the protein content in the meat samples [27].
Sodium dodecyl sulphate poly acrylamide gel electrophoresis (SDS-PAGE). We performed the analysis of protein degradation using SDS-PAGE in line with the
procedure specified by the Association of Official Analytical Chemists [28]. The analysis involved acrylamide gel electrophoresis: top (5% stacking gel) and bottom (12% separating gel).
Scanning electron microscopy. This procedure relied on Běhalová et al. [29]. The meat structure was analyzed using a scanning electron microscope (ZEISS, type EVOMA 10). The image was displayed using a secondary electron (SE) detector.
Maximal speed (Vmax) and Michaelis Menten constant (KM). The kinetics of protein degradation involved the Lineweaver-Burk equation, which is the inverse of the Michaelis Menten equation [30]. The relationship between reaction rate V and substrate concentration S changed to 1/V and 1/S:
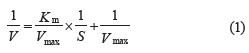
where 1/V is the y-axis and 1/S is the x-axis; y = bx + a; Vmax = 1/a; Km = Vmax × b.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
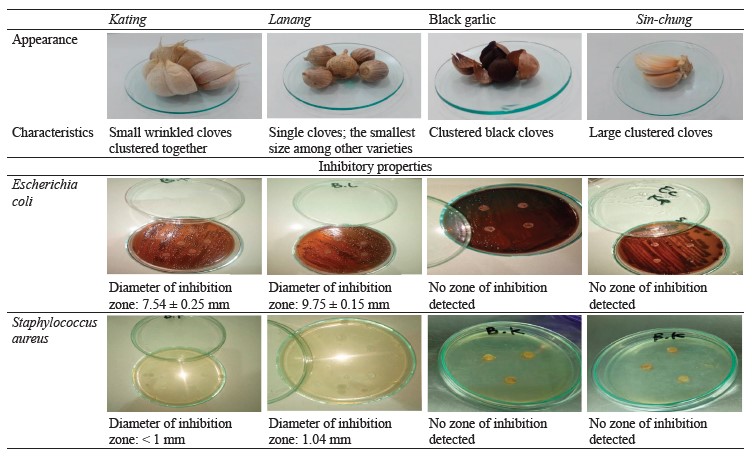
Bacterial inhibition. Lanang garlic had the greatest bacterial inhibition among all Allium sativum L. varieties, both in relation to Gram-negative and Gram-positive bacteria. The inhibition power for Gram-negative Eschericia coli was 9.75 ± 0.15 mm. For Gram-positive Staphylococcus aureus, the inhibition zone was 1.04 mm.
Kating garlic was able to inhibit E. coli with an inhibition zone of 7.54 ± 0.25 mm. For S. aureus, it did not exceed 1 mm. Black garlic and sin-chung garlic extracts yielded no inhibition results.
Table 1 sums up the results for all the A. sativum varieties in this research.
Lanang demonstrated the best inhibition results for Gram-negative and Gram-positive bacteria. It might owe its effectiveness to homogenate allicin (S-(2-propenyl)2-propene-1-sulfinothionate), which is known for its antibacterial activity [31]. Kating also inhibited E. coli and S. aureus, but to a much lesser extent. Black garlic and sin-chung produced no inhibition effect at a concentration of 20 mg/mL. Probably, the process of forming active bacterial substances in the cell enlargement process and the growth area were far from optimal [32].
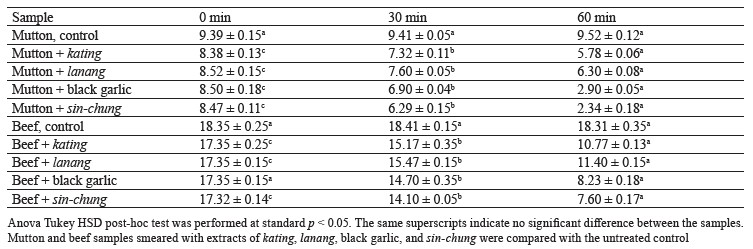
Kinetics of meat protein degradation. The control samples of beef and mutton, which were untreated with garlic extracts, showed no significant difference (p > 0.05). The mutton samples treated with extracts of kating, lanang, black garlic, and sin-chung, on the contrary, demonstrated significant differences (p < 0.05) after 30 and 60 min of processing. The same was true for the experimental beef (p < 0.05).
The sin-chung garlic extract was able to reduce the protein content, followed by black garlic and kating. Lanang, however, showed little effect on the degradation of meat protein. Table 2 illustrates the changes in protein levels for the mutton and beef samples.
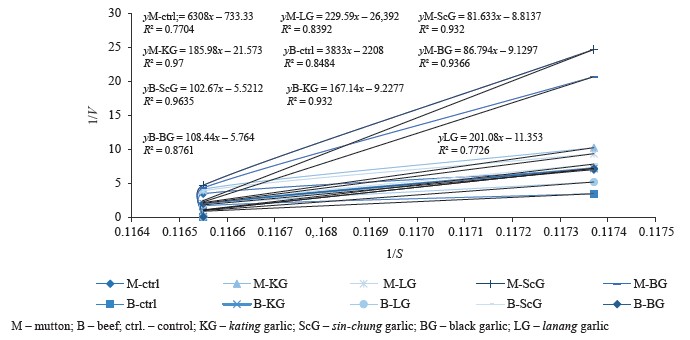
Protein degradation kinetics was analyzed using the Lineweaver-Burk equation. Figure 2 shows the relationship of velocity (1/V) to substrate degradation (1/S). Figure 3 illustrates the changes in the concentration of protein substrate (S).
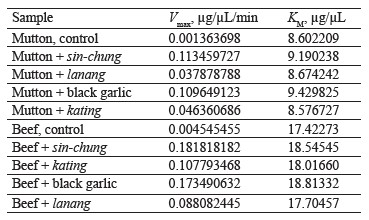
In the mutton samples, sinchung protease had the highest Vmax in the protein degradation process of 0.1135 µg/µL/min using a substrate of 9.19 µg/µL. The lanang protease showed the lowest Vmax, namely 0.0378 µg/µL/min. The results for black garlic and kating were 0.1096 and 0.0464 µg/µL/min, respectively. The sin-chung protease had the best degrading results for beef protein with Vmax of 0.1818 µg/µL/min and substrates ranging from 18.54 µg/µL. The black garlic protease produced Vmax of 0.1735 µg/µL/min while kating protease was 0.1078 µg/µL/min. The lanang protease had the lowest effect: 0.088 µg/µL/min (Table 3).
The process of forming antibacterial active substances does not always coincide with the formation of plant cells during cell formation/growth process, which involves plant proteases. This research succeeded in proving that black garlic and sin-chung were in that phase. In both cases, protease tenderized the meat. The sin-chung variety had the highest protein degradation Vmax in mutton (0.1135 µg/µL/min) and beef (0.1818 µg/µL/min). Black garlic protease had the second-best result, followed by kating and lanang. The obtained results confirm those reported by Bar et al. regarding the formation of anti-bacterial substances and Sharma & Gayen regarding the growth process of A. sativum [22, 32].
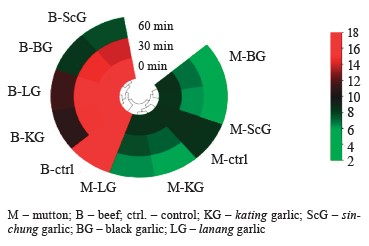
Meat protein degradation zone. To measure the protein degradation zone, we smeared the mutton and beef samples with extracts of kating, lanang, black garlic, and sin-chung at 30 and 60 min and compared the obtained results with those for the untreated control samples.
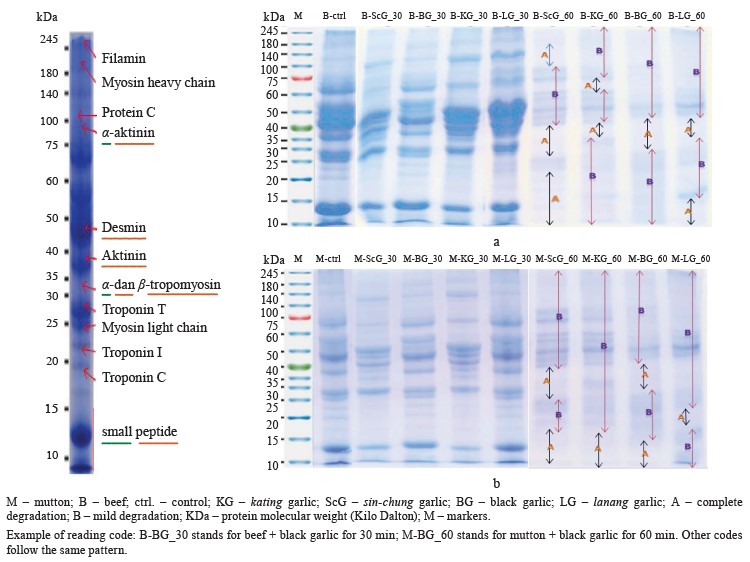
In beef (Fig. 4a), the garlic extracts produced no mild degradation within the first 30 min. Mild degradation is marked by a change in color from blue to purple while complete degradation means a loss of color. Both were clearly visible after 60 min. The sin-chung protease demonstrated complete degradation in the area of 10–22 KDa (small peptide, troponin C, and troponin I), 25–40 KDa (myosin light chain, troponin T, α- and β-tropomyosin, actin), and 100–140 KDa (Protein C). Other areas showed mild degradation only.
The kating protease was able to degrade proteins in the 35–40 KDa (actin) and 60–62 KDa zones. Other areas were only slightly degraded. The black garlic protease produced a degradation effect in the 30–42 KDa zone (α- and β-tropomyosin, actin). Meanwhile, lanang had the smallest degradation area of 10–17 KDa (small peptide) and 40–50 KDa (desmin).
In mutton (Fig. 4b), the proteases produced no degradation effect within the first 30 min. For all garlic varieties, degradation started at 60 min. Lanang was able to degrade only 20–23 KDa (troponin I). Sin-chung had a degradation zone at 10–17 KDa (small peptide) and 25– 40 KDa (myosin light chain, troponin T, α- and β-tropomyosin, actin). The back garlic protease was in the 10–15 KDa and 35–40 KDa ranges. Kating was effective in the 10–17 KDa range.
Therefore, all four A. sativum proteases proved to be effective meat tenderizers. For beef, the sin-chung extract succeeded in degrading proteins in a fairly wide area of 10–22 KDa (small peptide, troponin C, troponin I), 25–40 KDa (myosin light chain, troponin T, α- and β-tropomyosin, actin), and 100–140 KDa (protein C). As for the mutton samples, the sin-chung extract managed to degrade only 10–17 KDa (small peptide) and 25–40 KDa (myosin light chain, troponin T, α- and β-tropomyosin, actin). Meanwhile, lanang had the smallest degradation zone: 10–17 KDa (small peptide) and 40–50 KDa (desmin) for beef and 20–23 KDa (troponin I) for mutton.
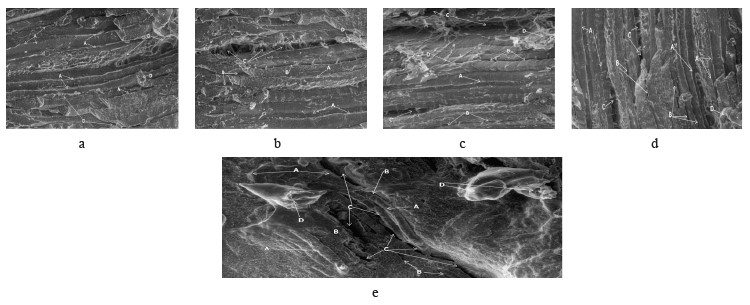
Effect of A. sativum protease on meat connective tissue. Collagen is the most abundant component in muscle tissue, where it forms perimysium and endomysium. Perimysium separates muscle fibers while endomysium coats them. Perimysium and endomysium release muscle tissue in the form of tears or cracks.
Figure 5 illustrates the effect of A. sativum protease on beef connective tissue. In the control meat (Fig. 5a), muscle tissue remained tight, and collagen dominated. In the sample treated with black garlic (Fig. 5b), endomysium connective tissue looked elongated and wide, with perimysium predominating. Some strong muscle tissue remained, and collagen was no longer predominant. The lanang protease (Fig. 5c) affected endomysium and perimysium at several points. Strong-bound muscle tissue predominated, but collagen was reduced. Kating (Fig. 5d) resulted in dominant perimysium. Small endomysium tissue was visible in some areas, and strong muscle tissue was detected in all directions. Sin-chung (Fig. 5e) was able to change the dominance of collagen and muscle tissue, and they became less dominant. Large endomysium predominated at several points, although perimysium was visible only on one side.
All four A. sativum proteases were able to separate myofibers from the perimysium, which is the most vulnerable tissue. This experiment was able to catalyze the effect of perimysium as it separated muscle fibers in muscle connective tissue. Perimysium is a fascicle that can be classified into primary, secondary, and tertiary fascicles, based on the diameter [33].
Sin-chung and black garlic had a prominent effect on the formation of dominant endomysium, which is the first step in meat tenderizing. Probably, when endomysium detached from sarcomere, it surrounded the muscle fibers of basal lamina, proteoglycans, collagen, and lamina. As a result, the endomysium formation left tears or cracks on the meat surface. The obtained results were consistent with those reported by Swasdison & Mayne regarding endomysium formation [34]. The control samples (Figs. 5a and 6a) revealed no endomysium and perimysium tissue because the cross-linked tissue was still strong in muscle tissue and collagen.
ВЫВОДЫ
In this research, the lanang garlic variety demonstrated the greatest antibacterial properties: its inhibition zone was 9.75 ± 0.15 mm against Escherichia coli and 1.04 mm against Staphylococcus aureus. Black garlic and sin-chung demonstrated no inhibitory power, probably, because the process of forming anti-bacterial substances does not always coincide with the process of plant growth, which involves plant proteases.
All four Allium sativum L. proteases proved to be effective meat tenderizers. The sin-chung extract possessed the most effective plant protease in this process. Its protease was able to degrade beef protein with the highest Vmax of 0.1818 µg/µL/min in the 10–22 KDa range (small peptide, troponin C, and troponin I), 25–40 KDa (myosin light chain, troponin T, α- and β-tropomyosin, actin), and 100–140 KDa (protein C). In mutton, it was effective only in the 10–17 KDa (small peptide) and 25– 40 KDa (myosin light chain, troponin T, α- and β-tro- pomyosin, actin) ranges with Vmax of 0.1135 µg/µL/min. The lanang protease showed the weakest protease enzyme activity: a small degradation zone in the area of 10–17 KDa (small peptide) and 40–50 KDa (desmin) with Vmax of 0.0881 µg/µL/min for beef. For the mutton samples, its result was 20–23 KDa.
Вклад авторов
All the authors were equally involved in the research analysis and manuscript writing.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare no conflict of interests regarding the publication of this article.
СПИСОК ЛИТЕРАТУРЫ
- Batiha GE-S, Beshbishy AM, Wasef LG, Elewa YHA, Al-Sagan AA, Abd El-Hack ME, et al. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients. 2020;12(3). https://doi.org/10.3390/nu12030872
- Chadha J, Harjai K, Chhibber S. Repurposing phytochemicals as anti‐virulent agents to attenuate quorum sensing‐regulated virulence factors and biofilm formation in Pseudomonas aeruginosa. Microbial Biotechnology. 2022;15(6):1695–1718. https://doi.org/10.1111/1751-7915.13981
- Hong TT, Dat TTH, Hoa NP, Dung TTK, Huyen VTT, Bui LM, et al. Expression and characterization of a new serine protease inhibitory protein in Escherichia coli. Biomedical Research and Therapy. 2020;7(2):3633–3644. https://doi.org/10.15419/bmrat.v7i2.590
- Rodrigues C, Percival SS. Immunomodulatory effects of glutathione, garlic derivatives, and hydrogen sulfide. Nutrients. 2019;11(2). https://doi.org/10.3390/nu11020295
- Winiarczyk K, Gębura J. Activity of selected hydrolytic enzymes in Allium sativum L. anthers. Plant Physiology and Biochemistry. 2016;102:37–42. https://doi.org/10.1016/j.plaphy.2016.02.018
- Ain NU, Riaz S, Abrar S, Ahmad M, Khan Z, Hafiz S, et al. Horizontal gene transfer and antibacterial effect of Allium sativum (garlic) on methicillin-resistant Staphylococcus aureus. Annals of Pakistan Institute of Medical Sciences. 2017;13(2):186–190.
- Chtita S, Belhassan A, Bakhouch M, Taourati AI, Aouidate A, Belaidi S, et al. QSAR study of unsymmetrical aromatic disulfides as potent avian SARS-CoV main protease inhibitors using quantum chemical descriptors and statistical methods. Chemometrics and Intelligent Laboratory Systems. 2021;210. https://doi.org/10.1016/j.chemolab.2021.104266
- Ismail RM, Saleh AHA, Ali KS. GC-MS analysis and antibacterial activity of garlic extract with antibiotic. Journal of Medicinal Plants Studies. 2020;8(1):26–30.
- Kshirsagar MM, Dodamani AS, Karibasappa GN, Vishwakarma PK, Vathar JB, Sonawane KR, et al. Antibacterial activity of garlic extract on cariogenic bacteria: An in vitro study. AYU. 2018;39(3):165–168.
- Parthipan P, Elumalai P, Narenkumar J, Machuca LL, Murugan K, Karthikeyan OP, et al. Allium sativum (garlic extract) as a green corrosion inhibitor with biocidal properties for the control of MIC in carbon steel and stainless steel in oilfield environments. International Biodeterioration and Biodegradation. 2018;132:66–73. https://doi.org/10.1016/j.ibiod.2018.05.005
- Piletti R, Zanetti M, Jung G, de Mello JMM, Dalcanton F, Soares C, et al. Microencapsulation of garlic oil by β-cyclodextrin as a thermal protection method for antibacterial action. Materials Science and Engineering: C. 2019;94:139–149. https://doi.org/10.1016/j.msec.2018.09.037
- Boutasknit A, Ait-Rahou Y, Anli M, Ait-El-Mokhtar M, Ben-Laouane R, Meddich A. Improvement of garlic growth, physiology, biochemical traits, and soil fertility by Rhizophagus irregularis and compost. Gesunde Pflanzen. 2021;73:149–160. https://doi.org/10.1007/s10343-020-00533-3
- Mengesha W, Tesfaye A, Djene M. Evaluation of fungicides on the control of garlic rust (Pucinnia allii) in Eastern Ethiopia. International Journal of Emerging Technology and Advanced Engineering. 2016;6(1):27–33.
- Khubber S, Hashemifesharaki R, Mohammadi M, Gharibzahedi SMT. Garlic (Allium sativum L.): A potential unique therapeutic food rich in organosulfur and flavonoid compounds to fight with COVID-19. Nutrition Journal. 2020;19. https://doi.org/10.1186/s12937-020-00643-8
- Gorinstein S, Jastrzebski Z, Namiesnik J, Leontowicz H, Leontowicz M, Trakhtenberg S. The atherosclerotic heart disease and protecting properties of garlic: contemporary data. Molecular Nutrition and Food Research. 2007;51(11):1365–1381. https://doi.org/https://doi.org/10.1002/mnfr.200700064
- Kritchevsky D. The effect of dietary garlic on the development of cardiovascular disease. Trends in Food Science and Technology. 1991;2:141–144. https://doi.org/10.1016/0924-2244(91)90658-6
- Devrim E, Durak I. Is garlic a promising food for benign prostatic hyperplasia and prostate cancer? Molecular Nutrition and Food Research. 2007;51(11):1319–1323. https://doi.org/10.1002/mnfr.200600302
- Sumiyoshi H, Wargovich MJ. Chemoprevention of 1, 2-dimethylhydrazine-induced colon cancer in mice by naturally occurring organosulfur compounds. Cancer Research. 1990;50(16):5084–5087.
- Cao Y, Gu W, Zhang J, Chu Y, Ye X, Hu Y, et al. Effects of chitosan, aqueous extract of ginger, onion and garlic on quality and shelf life of stewed-pork during refrigerated storage. Food Chemistry. 2013;141(3):1655–1660. https://doi.org/10.1016/j.foodchem.2013.04.084
- Aoki-Shioi N, Nagai Y, Deshimaru M, Terada S. Precursor genes of Bowman-Birk-type serine proteinase inhibitors comprise multiple inhibitory domains to promote diversity. Biochimica et Biophysica Acta (BBA) – General Subjects. 2023;1867(1). https://doi.org/10.1016/j.bbagen.2022.130248
- Guo R, Zhao J, Wang X, Guo C, Li Z, Wang Y, et al. Constitutive expression of a grape aspartic protease gene in transgenic Arabidopsis confers osmotic stress tolerance. Plant Cell, Tissue and Organ Culture. 2015;121:275–287. https://doi.org/10.1007/s11240-014-0699-6
- Sharma P, Gayen D. Plant protease as regulator and signaling molecule for enhancing environmental stress-tolerance. Plant Cell Reports. 2021;40:2081–2095. https://doi.org/10.1007/s00299-021-02739-9
- Al-Hadidy YI, Oleiwi SD, Khalaf AA, Saleh HM. The effectiveness of adding apple cider vinegar and garlic to chicken meat kebabs as an antimicrobial and its role in improving its sensory and physiochemical properties. Kirkuk University Journal for Agricultural Sciences. 2023;14(1):117–130. https://doi.org/10.58928/ku23.14110
- Igwegbe AO, Kassum AL, Maina FJ, Bristone C, Abubakar F, Imam HO, et al. Effects of sodium citrate and garlic on pH and microbial stability of smoked-dried meat stored at ambient temperatures. International Journal of Environmental Health Research. 2020;79.
- Tkacz K, Modzelewska-Kapituła M, Petracci M, Zduńczyk W. Improving the quality of sous-vide beef from Holstein-Friesian bulls by different marinades. Meat Science. 2021;182. https://doi.org/10.1016/j.meatsci.2021.108639
- Budianto B, Arumsari AG, Inayah N, Fatmaningrum, Suparmi A. Comparison of the effectiveness of leaf extracts of Anredera cordifolia, Psidium guajava and Pogostemon cablin on inhibitory power over Escherichia coli bacteria. Vitae. 2021;28(3). https://doi.org/10.17533/udea.vitae.v28n3a345386
- Manzoor M, Singh J, Ray A, Gani A. Recent advances in analysis of food proteins. In: Gani A, Ashwar BA, editors. Food biopolymers: Structural, functional and nutraceutical properties. Cham: Springer; 2021. pp. 269–298. https://doi.org/10.1007/978-3-030-27061-2_12
- Official Methods of Analysis of the Association of Official Analytical Chemists. 18th ed. Washington: Association of Official Analytical Chemists Washington; 2010.
- Běhalová H, Tremlová B, Kalčáková L, Pospiech M, Dordevic D. Assessment of the effect of secondary fixation on the structure of meat products prepared for scanning electron microscopy. Foods. 2020;9(4. https://doi.org/10.3390/foods9040487
- Vizcaíno AJ, Galafat A, Sáez MI, Martínez TF, Alarcón FJ. Partial characterization of protease inhibitors of Ulva ohnoi and their effect on digestive proteases of marine fish. Marine Drugs. 2020;18(6). https://doi.org/10.3390/md18060319
- Ankri S, Miron T, Rabinkov A, Wilchek M, Mirelman D. Allicin from garlic strongly inhibits cysteine proteinases and cytopathic effects of Entamoeba histolytica. Antimicrobial Agents and Chemotherapy. 1997;41(10):2286–2288. https://doi.org/10.1128/AAC.41.10.2286
- Bar M, Binduga UE, Szychowski KA. Methods of isolation of active substances from garlic (Allium sativum L.) and its impact on the composition and biological properties of garlic extracts. Antioxidants. 2022;11(7). https://doi.org/10.3390/antiox11071345
- Astruc T. Connective tissue: Structure, function, and influence on meat quality. In: Dikeman M, Devine C, editors. Encyclopedia of Meat Sciences. Academic Press; 2014. pp. 321–328. https://doi.org/10.1016/B978-0-12-384731-7.00186-0
- Swasdison S, Mayne R. Formation of highly organized skeletal muscle fibers in vitro. Comparison with muscle development in vivo. Journal of Cell Science. 1992;102(3):643–652. https://doi.org/10.1242/jcs.102.3.643
- Batiha GE-S, Beshbishy AM, Wasef LG, Elewa YHA, Al-Sagan AA, Abd El-Hack ME, et al. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients. 2020;12(3). https://doi.org/10.3390/nu12030872
- Chadha J, Harjai K, Chhibber S. Repurposing phytochemicals as anti‐virulent agents to attenuate quorum sensing‐regulated virulence factors and biofilm formation in Pseudomonas aeruginosa. Microbial Biotechnology. 2022;15(6):1695–1718. https://doi.org/10.1111/1751-7915.13981
- Hong TT, Dat TTH, Hoa NP, Dung TTK, Huyen VTT, Bui LM, et al. Expression and characterization of a new serine protease inhibitory protein in Escherichia coli. Biomedical Research and Therapy. 2020;7(2):3633–3644. https://doi.org/10.15419/bmrat.v7i2.590
- Rodrigues C, Percival SS. Immunomodulatory effects of glutathione, garlic derivatives, and hydrogen sulfide. Nutrients. 2019;11(2). https://doi.org/10.3390/nu11020295
- Winiarczyk K, Gębura J. Activity of selected hydrolytic enzymes in Allium sativum L. anthers. Plant Physiology and Biochemistry. 2016;102:37–42. https://doi.org/10.1016/j.plaphy.2016.02.018
- Ain NU, Riaz S, Abrar S, Ahmad M, Khan Z, Hafiz S, et al. Horizontal gene transfer and antibacterial effect of Allium sativum (garlic) on methicillin-resistant Staphylococcus aureus. Annals of Pakistan Institute of Medical Sciences. 2017;13(2):186–190.
- Chtita S, Belhassan A, Bakhouch M, Taourati AI, Aouidate A, Belaidi S, et al. QSAR study of unsymmetrical aromatic disulfides as potent avian SARS-CoV main protease inhibitors using quantum chemical descriptors and statistical methods. Chemometrics and Intelligent Laboratory Systems. 2021;210. https://doi.org/10.1016/j.chemolab.2021.104266
- Ismail RM, Saleh AHA, Ali KS. GC-MS analysis and antibacterial activity of garlic extract with antibiotic. Journal of Medicinal Plants Studies. 2020;8(1):26–30.
- Kshirsagar MM, Dodamani AS, Karibasappa GN, Vishwakarma PK, Vathar JB, Sonawane KR, et al. Antibacterial activity of garlic extract on cariogenic bacteria: An in vitro study. AYU. 2018;39(3):165–168.
- Parthipan P, Elumalai P, Narenkumar J, Machuca LL, Murugan K, Karthikeyan OP, et al. Allium sativum (garlic extract) as a green corrosion inhibitor with biocidal properties for the control of MIC in carbon steel and stainless steel in oilfield environments. International Biodeterioration and Biodegradation. 2018;132:66–73. https://doi.org/10.1016/j.ibiod.2018.05.005
- Piletti R, Zanetti M, Jung G, de Mello JMM, Dalcanton F, Soares C, et al. Microencapsulation of garlic oil by β-cyclodextrin as a thermal protection method for antibacterial action. Materials Science and Engineering: C. 2019;94:139–149. https://doi.org/10.1016/j.msec.2018.09.037
- Boutasknit A, Ait-Rahou Y, Anli M, Ait-El-Mokhtar M, Ben-Laouane R, Meddich A. Improvement of garlic growth, physiology, biochemical traits, and soil fertility by Rhizophagus irregularis and compost. Gesunde Pflanzen. 2021;73:149–160. https://doi.org/10.1007/s10343-020-00533-3
- Mengesha W, Tesfaye A, Djene M. Evaluation of fungicides on the control of garlic rust (Pucinnia allii) in Eastern Ethiopia. International Journal of Emerging Technology and Advanced Engineering. 2016;6(1):27–33.
- Khubber S, Hashemifesharaki R, Mohammadi M, Gharibzahedi SMT. Garlic (Allium sativum L.): A potential unique therapeutic food rich in organosulfur and flavonoid compounds to fight with COVID-19. Nutrition Journal. 2020;19. https://doi.org/10.1186/s12937-020-00643-8
- Gorinstein S, Jastrzebski Z, Namiesnik J, Leontowicz H, Leontowicz M, Trakhtenberg S. The atherosclerotic heart disease and protecting properties of garlic: contemporary data. Molecular Nutrition and Food Research. 2007;51(11):1365–1381. https://doi.org/https://doi.org/10.1002/mnfr.200700064
- Kritchevsky D. The effect of dietary garlic on the development of cardiovascular disease. Trends in Food Science and Technology. 1991;2:141–144. https://doi.org/10.1016/0924-2244(91)90658-6
- Devrim E, Durak I. Is garlic a promising food for benign prostatic hyperplasia and prostate cancer? Molecular Nutrition and Food Research. 2007;51(11):1319–1323. https://doi.org/10.1002/mnfr.200600302
- Sumiyoshi H, Wargovich MJ. Chemoprevention of 1, 2-dimethylhydrazine-induced colon cancer in mice by naturally occurring organosulfur compounds. Cancer Research. 1990;50(16):5084–5087.
- Cao Y, Gu W, Zhang J, Chu Y, Ye X, Hu Y, et al. Effects of chitosan, aqueous extract of ginger, onion and garlic on quality and shelf life of stewed-pork during refrigerated storage. Food Chemistry. 2013;141(3):1655–1660. https://doi.org/10.1016/j.foodchem.2013.04.084
- Aoki-Shioi N, Nagai Y, Deshimaru M, Terada S. Precursor genes of Bowman-Birk-type serine proteinase inhibitors comprise multiple inhibitory domains to promote diversity. Biochimica et Biophysica Acta (BBA) – General Subjects. 2023;1867(1). https://doi.org/10.1016/j.bbagen.2022.130248
- Guo R, Zhao J, Wang X, Guo C, Li Z, Wang Y, et al. Constitutive expression of a grape aspartic protease gene in transgenic Arabidopsis confers osmotic stress tolerance. Plant Cell, Tissue and Organ Culture. 2015;121:275–287. https://doi.org/10.1007/s11240-014-0699-6
- Sharma P, Gayen D. Plant protease as regulator and signaling molecule for enhancing environmental stress-tolerance. Plant Cell Reports. 2021;40:2081–2095. https://doi.org/10.1007/s00299-021-02739-9
- Al-Hadidy YI, Oleiwi SD, Khalaf AA, Saleh HM. The effectiveness of adding apple cider vinegar and garlic to chicken meat kebabs as an antimicrobial and its role in improving its sensory and physiochemical properties. Kirkuk University Journal for Agricultural Sciences. 2023;14(1):117–130. https://doi.org/10.58928/ku23.14110
- Igwegbe AO, Kassum AL, Maina FJ, Bristone C, Abubakar F, Imam HO, et al. Effects of sodium citrate and garlic on pH and microbial stability of smoked-dried meat stored at ambient temperatures. International Journal of Environmental Health Research. 2020;79.
- Tkacz K, Modzelewska-Kapituła M, Petracci M, Zduńczyk W. Improving the quality of sous-vide beef from Holstein-Friesian bulls by different marinades. Meat Science. 2021;182. https://doi.org/10.1016/j.meatsci.2021.108639
- Budianto B, Arumsari AG, Inayah N, Fatmaningrum, Suparmi A. Comparison of the effectiveness of leaf extracts of Anredera cordifolia, Psidium guajava and Pogostemon cablin on inhibitory power over Escherichia coli bacteria. Vitae. 2021;28(3). https://doi.org/10.17533/udea.vitae.v28n3a345386
- Manzoor M, Singh J, Ray A, Gani A. Recent advances in analysis of food proteins. In: Gani A, Ashwar BA, editors. Food biopolymers: Structural, functional and nutraceutical properties. Cham: Springer; 2021. pp. 269–298. https://doi.org/10.1007/978-3-030-27061-2_12
- Official Methods of Analysis of the Association of Official Analytical Chemists. 18th ed. Washington: Association of Official Analytical Chemists Washington; 2010.
- Běhalová H, Tremlová B, Kalčáková L, Pospiech M, Dordevic D. Assessment of the effect of secondary fixation on the structure of meat products prepared for scanning electron microscopy. Foods. 2020;9(4. https://doi.org/10.3390/foods9040487
- Vizcaíno AJ, Galafat A, Sáez MI, Martínez TF, Alarcón FJ. Partial characterization of protease inhibitors of Ulva ohnoi and their effect on digestive proteases of marine fish. Marine Drugs. 2020;18(6). https://doi.org/10.3390/md18060319
- Ankri S, Miron T, Rabinkov A, Wilchek M, Mirelman D. Allicin from garlic strongly inhibits cysteine proteinases and cytopathic effects of Entamoeba histolytica. Antimicrobial Agents and Chemotherapy. 1997;41(10):2286–2288. https://doi.org/10.1128/AAC.41.10.2286
- Bar M, Binduga UE, Szychowski KA. Methods of isolation of active substances from garlic (Allium sativum L.) and its impact on the composition and biological properties of garlic extracts. Antioxidants. 2022;11(7). https://doi.org/10.3390/antiox11071345
- Astruc T. Connective tissue: Structure, function, and influence on meat quality. In: Dikeman M, Devine C, editors. Encyclopedia of Meat Sciences. Academic Press; 2014. pp. 321–328. https://doi.org/10.1016/B978-0-12-384731-7.00186-0
- Swasdison S, Mayne R. Formation of highly organized skeletal muscle fibers in vitro. Comparison with muscle development in vivo. Journal of Cell Science. 1992;102(3):643–652. https://doi.org/10.1242/jcs.102.3.643