Аннотация
The wild mushroom Volvariella volvacea is widely picked and consumed in Côte d’Ivoire. However, it is highly perishable due to its high moisture content. This study aimed to determine the effects of three drying methods on the biochemical and mineral composition, as well as antioxidant properties, of V. volvacea powders.Three V. volvacea powders were obtained by sun drying, oven drying, and freeze-drying. Each powder was analyzed for its biochemical and mineral composition according to standard analytical methods. The powder methanolic extracts were analyzed for their antioxidant components by colorimetric methods or titration, while their antioxidant capacities were determined by using DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) scavenging and the ferric reducing antioxidant power.
The freeze-dried powder of V. volvacea had a lower moisture content than the oven-dried and sun-dried powders. The highest protein, ash, and fiber contents were also recorded in the freeze-dried powder. In addition, freeze-drying provided the highest contents of iron, magnesium, sodium, and potassium. Regarding the antioxidant components, the freeze-dried powder showed the highest levels of total phenolic compounds, flavonoids, and vitamin C. Similarly, freeze-drying provided the best antioxidant capacities in terms of DPPH scavenging and the ferric reducing antioxidant power.
Our study showed that freeze-drying ensured a better retention of essential nutrients and antioxidant components in the mushroom V. volvacea, while sun-drying led to greater losses of these compounds.
Ключевые слова
Mushroom, Volvariella volvacea, drying methods, mushroom powders, biochemical and nutritional properties, antioxidant capacityВВЕДЕНИЕ
In Africa, as in several developing countries around the world, wild edible mushrooms have always been an important component of foodstuffs intended for human consumption [1–4]. Several studies have reported that edible mushrooms are valuable healthy and nutritious foods, low in calories and rich in vegetable proteins, vitamins, and minerals [5–9]. In addition to their food value, they play a role in traditional medicine. Indeed, certain species of mushrooms have, among other things, antioxidant, antimicrobial, and anticancerous properties [10–13]. During rainy seasons in Côte d’Ivoire, wild edible mushrooms are collected and sold by women and even men in rural and peri-urban areas [14].
According to a recent study, the species Psathyrella tuberculata ranks first in terms of importance, followed by Volvariella volvacea and Termitomyces letestui [15]. V. volvacea has been the subject of ecological, as well as biochemical and nutritional studies in Côte d’Ivoire [4, 8, 16]. This saprophytic species, which generally grows on the trunks of dead oil palms, is rich in proteins, carbohydrates, fibers, minerals, and natural antioxidants. However, it is highly perishable in the fresh state, like other species of mushrooms, due to a high water content (over 80%) which promotes bacterial proliferation. Moreover, the oxidation of phenolic compounds under the action of enzymes, such as polyphenol oxidases, causes browning that affects the quality [17].In tropical Africa, mushrooms are traditionally sundried, which is a cheap and therefore accessible method [18]. Although this method has an undeniable advantage resulting from the conversion of ergosterol into vitamin D, its impact on the dried mushroom does not guarantee its quality in terms of health safety and nutrient retention [19]. Therefore, other methods such as oven-drying and freeze-drying seem appropriate to improve the quality of the dried mushrooms. Previous works have indicated satisfactory results for the drying of several species of mushrooms [20–22].
Thus, we aimed to analyze and compare three methods of drying the wild edible mushroom V. volvacea harvested in Côte d’Ivoire in terms of the chemical composition and antioxidant properties of the resulting products.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Volvariella volvacea collection. Fresh fruiting bodies of V. volvacea mushrooms were harvested from dead trunks of oil palms in the plantations of Dimbokro, the N’zi region (Côte d’Ivoire). Once harvested, the mushrooms were packaged in ventilated baskets and carefully transported on the same day to the laboratory for subsequent analyses.
Sampling. In the laboratory, the mushrooms were sorted and cleared of debris. Then, they were washed three times with tap water and rinsed with distilled water. Finally, the mushrooms were divided into three 200-g samples. Each of the samples was subsequently dried and ground into a powder.
Drying and obtaining V. volvacea powders. The first sample was placed on a table covered with aluminum foil and subjected to direct sunlight for 5 successive days. As soon as the sun set, the table was removed to the laboratory to avoid rehydration due to the humidity of the air. The second sample was dried in a ventilated oven (BOBASE, China) at 45°C for 48 h. The third sample was first frozen at –80°C, quickly transferred to a pre-cooled freeze-dryer (BIOBASE, China), and dried for 48 h. The freeze-dryer had a cold trap temperature of –80°C and a vacuum of 1 bar. After the application of these drying methods, the mushrooms of each sample were crushed with a flat-hammer grinding mill and sifted through a 60-mesh screen. The three powdered samples were packaged in labeled bottles, which were previously dried and hermetically sealed. These bottles were stored in a desiccator at 25°C until further use.
Biochemical and mineral composition. The moisture, ash, fat, and protein contents of the mushroom powders were determined according to the AOAC [23]. The moisture content was determined by drying a sample in an oven at 105°C to a constant weight. Fat contents were determined by continuous extraction in a Soxhlet apparatus for 4 h using hexane as a solvent. After evaporation of the solvent, the fat content was obtained by the gravimetric method. Ash was measured from the residual mass obtained after incinerating the samples at 550°C for 2 h in a muffle furnace. The protein content was calculated by nitrogen ×6.25. The contents of fibers and total carbohydrates were respectively determined gravimetrically and by difference. The energy value of each sample was estimated by multiplying protein, fat, and available carbohydrates (total carbohydrates minus fibers) by 4, 9, and 4, respectively. Total and reducing sugars were quantified using the methods of Chow & Landhäusser and Garriga et al., respectively [24, 25]. To determine the contents of different mineral elements, the ash residue of each sample (1 g) was digested with a mixture of concentrated nitric acid (14.44 mol/L), sulfuric acid (18.01 mol/L), and perchloric acid (11.80 mol/L). After cooling, the samples were filtered through Whatman filter paper No. 4. Then, each sample solution was made up to a final volume of 25 mL with distilled water. An aliquot of each solution was used to determine the contents of Zn, Cu, Fe, Ca, Mg, Mn, and Na by measuring atomic absorption [26]. P was determined colorimetrically using the method of Taussky & Shorr [27].
Antinutritional factors. Oxalate contents in the V. volvacea powder were determined according to the method described by Day & Underwood using a potassium permanganate solution (0.05 M KMnO4) [28]. Phytate contents were determined according to the method of Latta & Eskin using a Wade reagent [29].
The phenolic compounds of each V. volvacea powder were extracted with 80% (v/v) methanol. For this, 10 g of V. volvacea powder was extracted by stirring with 50 mL of 80 % (v/v) methanol at 25°C for 24 h and filtered through Whatman paper No. 4. The residue was then extracted with two additional 50 mL portions of methanol. The combined methanolic extracts were evaporated at 35°C in a Heildolph Laborota 4003 Control rotary evaporator (Schwabach, Germany) until the volume of 25 mL. The extracts obtained were used to estimate the contents of phenolic compounds.
The content of total phenolic compounds in each V. volvacea powder was determined using the Folin-Ciocalteu reagent as described by Singleton et al. [30]. The results were expressed as mg gallic acid equivalent (GAE)/100 g DW. The content of flavonoids was estimated by the method of Meda et al. using aluminum chloride [31]. The results were expressed as mg of quercetin equivalent (QE)/100 g DW. The tannin content was determined according to the method described by Bainbridge et al. using a vanillin reagent [32]. The results were expressed as mg of tannic acid equivalent (TAE)/100 g DW.
The vitamin C content in each powder was estimated by titration with 2,6-dichloroindophenol as reported by Pongracz et al. [33].
Antioxidant capacities. The capacity of the V. volvacea powders to scavenge DPPH radicals was monitored according to the method described by Hatano et al. [34]. For this, V. volvacea powder solutions (2.5 m) at various concentrations ranging from 0 to 100 mg/mL and a Trolox solution (standard reference of antioxidant) were added to 1 mL of a methanolic solution of DPPH (3 mM).

where Acontrol is the absorbance of the DPPH solution, Asample is the absorbance of the solution with the sample extract.
The concentration of each powder solution causing 50% inhibition (EC50) was estimated from the graph of the DPPH inhibition percentage against the powder solution concentration.
The ferric reducing antioxidant power of the methanolic solution of each of the V. volvacea powders or the Trolox methanolic solution was determined according to the method described by Barros et al. with slight modifications [35]. For this, 0.1 mL of the V. volvacea powder solution or the Trolox solution prepared at various concentrations (0 to 100 mg/mL) was mixed with 2.0 mL of phosphate buffer (0.2 M, pH 6.6) and then with 2 mL of 1% potassium hexacyanoferrate [K3Fe(CN)6] (w/v). The mixture was incubated at 50°C in a water bath for 20 min and then cooled. A volume of 2 mL of 10% (w/v) trichloroacetic acid was then added and the mixture was centrifuged at 3000 rpm for 10 min. Finally, 2 mL of the supernatant was mixed with 2 mL of distilled water and 0.4 mL of ferric chloride (FeCl3). A blank without a powder was prepared under the same conditions. Absorbance was measured at 700 nm against the blank. Increased absorbance indicated higher reducing power. The concentration of each powder solution causing half maximal effective concentration (EC50) was estimated from the graph of absorbance at 700 nm against the powder solution concentration.
Statistical analysis. All chemical analyses and assays were carried out in triplicate. The results were expressed as mean values ± standard deviation (SD). Analysis of variance (ANOVA) followed by Duncan’s test was performed to test for differences between the means by employing Kyplot (version 2.0 beta 15, ©1997–2001, Koichi Yoshioka) statistical software. Significance of differences was defined at the 5% level (p < 0.05).
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
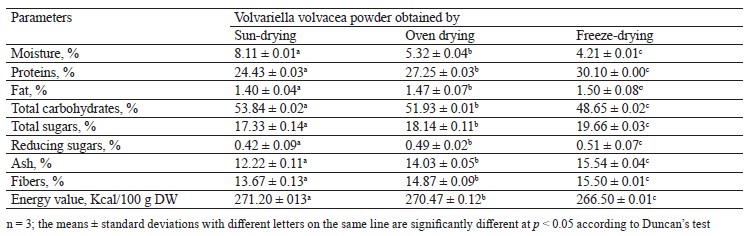
The biochemical composition and energy values of the Volvariella volvacea powders obtained by sun drying, oven drying, and freeze-drying are presented in Table 1.
In terms of moisture, freeze-drying allowed better dehydration of the mushroom V. volvacea with a powder water content of 4.21%, compared to oven drying and sun drying with the water contents of 5.32 and 8.11%, respectively. It is well-known that dehydration by conventional drying methods is an important process in the food industry because it considerably reduces the water activity which affects the microbiological stability and the physicochemical deterioration reactions [21, 36].
Various studies dedicated to the drying of edible mushrooms have indicated that freeze-drying is the best form of dehydration since it transforms water into ice and then directly into water vapor, skipping the liquid phase [21, 22, 37]. On the other hand, oven drying, which uses a uniform temperature, resulted in a higher removal of moisture compared to sun drying [22, 38]. With regard to the retention of different nutrients, freeze-drying also gave better results than oven drying. This could be due to the fluctuation of temperature for efficient moisture removal with a good retention of proteins, total and reducing sugars, ash, and fibers.
The sun-dried V. volvacea powder presented the worst results for nutrient retention. Several reports have indicated a similar trend regarding the impact of the drying method on the protein content of mushrooms [21, 22, 39, 40]. The capacity of freeze-drying to ensure a better retention of proteins could be explained by the fact that drying with hot air (sun drying and oven drying) can cause denaturation of some proteins due to relatively high temperatures, resulting in a substantial protein loss [22, 41]. In addition, the low protein content of the sun-dried and oven-dried V. volvacea powders, compared to that of the freeze-dried powder, could be partly due to the leaching of soluble proteins by the washing water and losses during browning reactions (Maillard reactions) [41, 42].
The low total and reducing sugar contents of the sundried and oven-dried powders could be attributed to the fact that some sugars are consumed by heat-induced Maillard reactions [43]. The low content of fibers in the sundried V. volvacea powder could be explained by the fact that this method required a long duration, i.e., a long exposure to the sun rays, which resulted in a rupture of the cell walls, thus causing a decrease in fibers as a betaglucan content [44, 45]. However, it is generally recognized that the method of drying does not affect the fiber content in mushrooms [20, 22].
The fat contents were quite low for the V. volvacea powders from the three drying methods, as previously reported [4, 8]. The few slight differences between the powders obtained by hot air drying and freeze-drying could be due to oxidation. The mushroom fat is mainly made up of unsaturated fatty acids which are very prone to oxidation when exposed to heat and ambient air [22, 38]. The good ash retention observed during the freeze-drying of the V. volvacea mushroom is in agreement with the results reported by Bashir et al. for the mushroom Pleurotus florida [21]. The considerable decrease in ash during the sun drying could be due to the diffusion of some minerals into the water which migrates out of the mushroom during the process [21, 45]. With respect to energy, the V. volvacea powder from sun drying recorded the highest value (271.20 ± 0.13 Kcal/100 g DW), followed by the oven-dried powder (270.47 ± 0.12 DW) and the freeze-dried powder (266.50 ± 0.01 Kcal/100 g DW). This resulted from the fact that the powder from freeze-drying has the lowest levels of available carbohydrates estimated by difference, as reported by Dunkwal et al. [46].
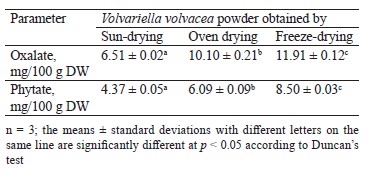
Oxalate and phytate are antinutritional factors, also called antinutrients, which interfere with the absorption of certain minerals (iron, calcium, zinc, etc.). Table 2 shows the contents of these two antinutritional factors in the V. volvacea powders.
The highest levels of oxalate and phytate were observed in the freeze-dried powder of V. volvacea with the respective values of 11.91 ± 0.12 and 8.50 ± 0.03 mg/100 g DW. The lowest contents of oxalate and phytate were recorded in the powder obtained by sun drying. It is well known that oven drying significantly reduces the antinutrient levels of vegetables, which could justify the fact that the oven-dried powder showed lower oxalate and phytate contents than the freeze-dried powder [47]. The report of Bello et al. indicated that drying oyster mushroom Pleurotus sajur-caju in the oven at 60°C considerably reduced the levels of antinutritional factors, including phytate and oxalate [48]. The low contents of these compounds in the sun-dried V. volvacea powder could be attributed to the fact that these two antinutrients are more or less soluble in water and an important amount of them could have migrated with the water that came out of the mushroom during drying. We found that the freeze-drying showed better retention of phytate and oxalate. However, the levels obtained were well below the limits reported by the WHO, which are 22.10 and 105.00 mg/100 g for phytate and oxalate, respectively [49].
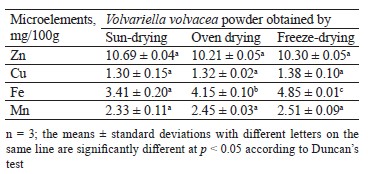
The mineral composition of each V. volvacea powder was evaluated in terms of microelements and macroelements. Table 3 presents the microelement (zinc, copper, iron, and manganese) contents of the V. volvacea powders.
Overall, only the iron content varied significantly from one drying method to another. The powder from freeze-drying had the highest iron content of 4.850 ± 0.014 mg/100 g against 4.15 ± 0.10 and 3.41 ± 0.20 mg/100 g for the oven-dried and sun-dried powders, respectively. This result was comparable to that reported by Bashir et al. for the oyster mushroom P. florida [21]. In their study, the freeze-dried powder of this mushroom showed the best iron content of 5.10 mg/100 g compared to other methods. On the other hand, Maray et al. reported that the sun drying of Pleurotus ostreatus resulted in the highest iron content of 11.6 mg/100 g [45]. The other microelements did not show any significant differences depending on the drying method. The values were around 10.30, 1.32, and 2.45 mg/100 g for zinc, copper, and manganese, respectively.
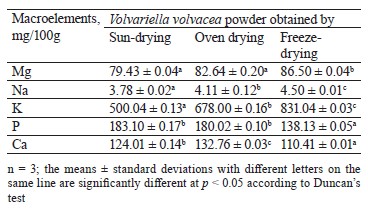
The contents of the macroelements (magnesium, sodium, potassium, phosphorus, and calcium) are presented in Table 4. These contents showed significant differences. The highest contents of most of the macroelements were obtained in the freeze-dried V. volvacea powder. Thus, freeze-drying allowed for a good retention of these minerals. This same trend was observed by Bashir et al. for the contents of magnesium, potassium, phosphorus, and calcium during the drying of the oyster mushroom P. florida [21].
In addition, potassium showed the highest content in each of the V. volvacea powders, while sodium showed the lowest content. Due to a high K/Na ratio, the V. volvacea powders may have an advantage for patients with hypertension and other cardiovascular diseases. Such a result was reported very recently for powders from the oyster mushrooms P. sajor-caju and P. djamor [50].
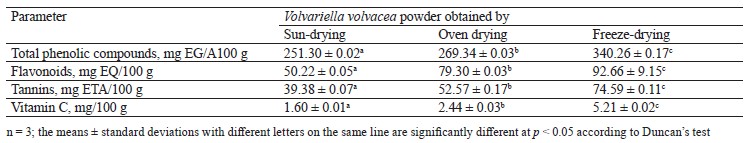
Total phenolic compounds, flavonoids, tannins and vitamin C. Table 5 shows the contents of total phenolic compounds, flavonoids, tannins, and vitamin C in the V. volvacea powders obtained by different drying methods.
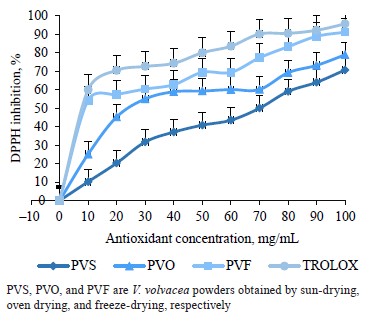
Antioxidant capacities. Two methods of in vitro assay of antioxidant activities were used to evaluate the V. volvacea powders, namely the DPPH scavenging and the ferric reducing antioxidant power. Figure 1 represents the percentage of DPPH inhibition as a function of the V. volvacea powder solution. We observed higher inhibition percentages with increased concentrations of V. volvacea powders in the following order: freezedrying > oven drying > sun-drying (Fig. 1). Trolox used as a reference antioxidant showed the highest percentage of DPPH inhibition. At 100 mg/mL, the Trolox, freeze-dried, oven-dried, and sundried powders had DPPH inhibition percentages of 95.6, 91.2, 78.9, and 70.7%, respectively.
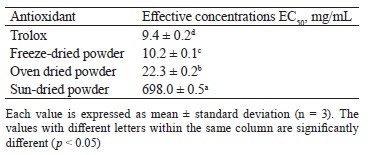
According to the results, the freeze-dried powder of V. volvacea showed the best antioxidant capacity in terms of DPPH scavenging due to its lower effective concentration EC50. The decrease in DPPH radical scavenging activity in the sun-dried and oven-dried powders could be the consequence of thermal degradation of vitamin C and some phenolic compounds, as reported by Bashir et al. [55]. Similar results were reported for the mushroom Lentinus edodes, with the lowest effective concentration obtained by its powder from freezedrying [56].
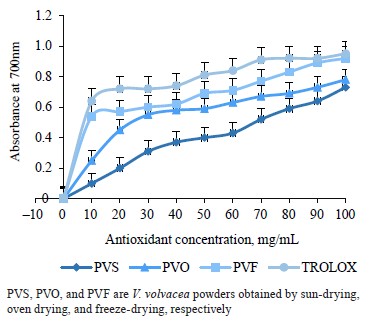
With regard to the ferric reducing antioxidant power, Fig. 2 represents the absorbance at 700 nm as a function of the concentration of the V. volvacea powder solution. We found that the reducing power expressed by increased absorbance at 700 nm was higher with increased concentrations of the V. volvacea powders. Trolox showed the highest absorbances at all concentrations, followed respectively by the powders from freezedrying, oven drying, and sun drying. At 100 mg/mL, the absorbances were 0.95, 0.92, 0.78, and 0.72 for Trolox, freeze-dried, oven-dried, and sun-dried powders, respectively.
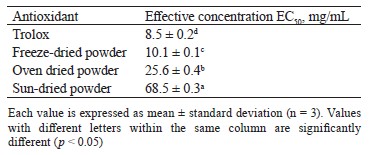
The effective concentrations (EC50) for the ferric reducing antioxidant power (Тable 7) followed the same trend as those for the DPPH trapping activities: Trolox < freeze-drying < oven drying < sun-drying. The values of effective concentrations were 10.1 ± 0.1, 25.6 ± 0.4, and 68.5 ± 0.3 mg/mL for the V. volvacea powders obtained by freeze-drying, oven drying, and sun drying, respectively.
Similar patterns were reported by some authors about the reducing power of mushroom powders from different drying methods [55]. Better antioxidant activity of the freeze-dried samples can be attributed to low temperature and vacuum used in the freeze-drying process which cause less thermal degradation and oxidation of phenolic compounds [56].
ВЫВОДЫ
Our results clearly indicate that freeze-drying is the best method of drying the mushroom Volvariella volvacea from Côte d’Ivoire because it results in a better retention of nutrients and bioactive compounds responsible for antioxidant activities. This could promote the use of the freeze-dried powder of V. volvacea to formulate functional foods and fortify certain conventional foods.
Вклад авторов
E.J.P. Kouadio designed the research concept, provided the analysis tools, wrote the manuscript, and submitted it. B.B. Koffi, O.J. Gbotognon, and S. Soro were responsible for data collection and analysis.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare no conflict of interest regarding this publication.
СПИСОК ЛИТЕРАТУРЫ
- Kotowski MA. History of mushroom consumption and its impact on traditional view on mycobiota – an example from Poland. Microbial Biosystems. 2019;4(3):1–13.
- You SW, Hoskin RT, Komarnytsky S, Moncada M. Mushrooms as functional and nutritious food ingredients for multiple applications. ACS Food Science and Technology. 2022;2(8):1184–1195. https://doi.org/10.1021/acsfoodscitech.2c00107
- El-Ramady H, Abdalla N, Badgar K, Llanaj X, Törȍs G, Hajdú P, et al. Edible mushrooms for sustainable and healthy human food: Nutritional and medicinal attributes. Sustainability. 2022;14(9). https://doi.org/10.3390/su14094941
- Anno HFA, Kouadio EJP, Konan, KH, Dué AE, Kouamé LP. Two widely consumed wild mushrooms from central Côte d’Ivoire: their proximate analysis, mineral composition and amino acids profile. Annals. Food Science and Technology. 2016;17(1):139–149.
- Rizzo G, Goggi S, Giampieri F, Baroni L. A review of mushrooms in human nutrition and health. Trends in Food Science and Technology. 2021;117:60–73. https://doi.org/10.1016/j.tifs.2020.12.025
- Zhang Y, Wang D, Chen Y, Liu T, Zhang S, Fan H, et al. Healthy function and high valued utilization of edible fungi. Food Science and Human Wellness. 2021;10(4):408–420. http://doi.org/10.1016/j.fshw.2021.04.003
- Ketnawa S, Rawdkuen S. Properties of texturized proteins from edible mushrooms by using single-screw extruder. Foods. 2023;12(6). https://doi.org/10.3390/foods12061269
- Zoho Bi FGA, Amoikon KE, Ahui-Bitty M-LB, Kouamé KG, Kati-Coulibaly S. Nutrients value of some edible mushrooms in Côte d’Ivoire. Agriculture and Biology Journal of North America. 2016;7(3):140–145.
- Rahman MdA, Roy J, Mahomud MdS. Textural and antioxidant properties of mozzarella cheese fortified with dehydrated oyster mushroom flour. Foods and Raw Materials. 2023;11(2):251–258. https://doi.org/10.21603/2308-4057-2023-2-574
- Shaffique S, Kang S-M, Kim A-Y, Imran M, Aaqil Khan M, Lee I-J. Current knowledge of medicinal mushrooms related to anti-oxidant properties. Sustainability. 2021;13(14). https://doi.org/10.3390/su13147948
- Mwangi RW, Macharia JM, Wagara IN, Bence RL. The antioxidant potential of different edible and medicinal mushroom. Biomedicine and Pharmacotherapy. 2022;147. https://doi.org/10.1016/j.biopha.2022.112621
- Bhambri A, Srivastava M, Mahale VG, Mahale S, Karn SK. Mushrooms as potential sources of active metabolites and medicines. Frontiers in Microbiology. 2022;13. https://doi.org/10.3389/fmicb.2022.837266
- Bakaytis VI, Golub OV, Miller YuYu. Fresh and processed wild Cantharellus cibarius L. growing in West Siberia: food value. Foods and Raw Materials. 2021;9(2):234–243. https://doi.org/10.21603/2308-4057-2021-2-234-243
- Koné N’GA. Yéo K, Konaté S, Linsenmair EK. Socio-economical aspects of the exploitation of Termitomyces fruit bodies in central and southern Côte d’Ivoire: Raising awareness for their sustainable use. Journal of Applied Biosciences. 2013;70:5580–5590. https://doi.org/10.4314/jab.v70i1.98759
- Soro B, Koné N’GA. Vanié-Léabo LPL, Bakayoko A, Konaté S. Koné D. Sale of wild useful mushrooms in Côte d'Ivoire: Diversity, availability and socio-economic importance. Journal of Applied Biosciences. 2022;172:17905–17918. (In French.). https://doi.org/10.35759/JABs.172.5
- Soro B. Koné N’GA. Vanié-Léabo LPL, Konaté S, Bakayoko A, Koné D. Phytogeographical and sociolinguistical patterns of the diversity, distribution, and uses of wild mushrooms in Côte d’Ivoire, West Africa. Journal of Ethnobiology and Ethnomedicine. 2019;15. https://doi.org/10.1186/s13002-019-0284-5
- Yan M, Yuan B, Xie Y, Cheng S, Huang G, Zhang W, et al. Improvement of postharvest quality, enzymes activity and polyphenoloxidase structure of postharvest Agaricus bisporus in response to high voltage electric field. Postharvest Biology and Technology. 2020;166. https://doi.org/10.1016/j.postharvbio.2020.111230
- Bastos C, Liberal A, Moldao M, Catarino L, Barros L. Ethnomycological prospect of wild edible and medicinal mushrooms from Central and Southern Africa – A review. Food Frontiers. 2023;4(2):549–575. https://doi.org/10.1002/fft2.215
- Jiang O, Zhang M, Mujumdar AS. UV induced conversion during drying of ergosterol to vitamin D in various mushrooms: Effect of different drying conditions. Trends in Food Science and Technology. 2020;105:200–210. https://doi.org/10.1016/j.tifs.2020.09.011
- Asamoa AA, Essel EA, Agbenorhevi JK, Odur IN. Effect of processing methods on the proximate composition, total phenols and antioxidant properties of two mushroom varieties. American Journal of Food and Nutrition. 2018;6(2):55–59. https://doi.org/10.12691/ajfn-6-2-4
- Bashir N, Sood M, Bandral JD. Impact of different drying methods on proximate and mineral composition of oyster mushroom (Pleurotus florida). Indian Journal of Traditional Knowledge. 2020;19(3):656–661.
- Shams R, Singh J, Dash KK, Dar AH. Comparative study of freeze drying and cabinet drying of button mushroom. Applied Food Research. 2022;2(1). https://doi.org/10.16/j.res.2022.100084
- Official Methods of Analysis, 18th edn. Washington: Association of Official Analytical Chemists; 2005.
- Chow PS, Landhäusser SM. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiology. 2004;24(10):1129–1136. https://doi.org/10.1093/treephys/24.10.1129
- Garriga M, Almaraz M, Marchiaro A. Determination of reducing sugars in extracts of Undaria pinnatifida (harvey) algae by UV-visible spectrophotometry (DNS method). Actas de Ingeniería. 2017;3:173–179.
- Milner BA, Whiteside PJ. An introduction to atomic absorption spectrophotometry. Cambridge: Pye Union Ltd; 1981. pp 43–46.
- Taussky HH, Shorr E. A microcolorimetric method for the determination of inorganic phosphorus. Journal of Biological Chemistry. 1953;202(2):675–685.
- Day RA, Underwood AL. Quantitative analysis. 5th Edition. Upper Saddle River: Prentice Hall; 1986. 701 p.
- Latta M, Eskin M. A simple and rapid method for phytate determination. Journal of Agricultural and Food Chemistry. 1980;28(6):1313–1315. https://doi.org/10.1021/jf60232a049
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology. 1999;299:152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chemistry. 2005;91(3):571–577. https://doi.org/10.1016/j.foodchem.2004.10.006
- Bainbridge Z, Tomlins K, Willings K, Westby A. Methods for assessing quality characteristics of non-grain starch staple. Part 4 advanced methods. Chatham: National Resources Institute, University of Greenwich; 1996. pp. 43–79.
- Pongracz G, Weiser H, Matzinger D. Tocopherols – antioxydant. Fat Science Technology. 1971;97:90–104.
- Hatano T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging affects. Chemical and Pharmaceutical Bulletin. 1988;36(6):2090–2097. https://doi.org/10.1248/cpb.36.2090
- Barros L, Baptista P, Ferreira ICFR. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food and Chemical Toxicology. 2007;45(9):1731–1737. https://doi.org/10.1016/j.fct.2007.03.006
- Subramaniam S, Jiao S, Zhang Z, Jing P. Impact of post-harvest processing or thermal dehydration on physiochemical, nutritional and sensory quality of shiitake mushrooms. Comprehensive Reviews in Food Science and Food Safety. 2021;20(3):2560–2595. https://doi.org/10.1111/1541-4337.12738
- Tarafdar A, Shahi CN, Singh A. Freeze-drying behaviour prediction of button mushrooms using artificial neural network and comparison with semi-empirical models. Neural Computing and Applications. 2019;31:7257–7268. https://doi.org/10.1007/s00521-018-3567-1
- Muyanja C, Kyambadde D, Namugumya B. Effect of pretreatments and drying methods on chemical composition and sensory evaluation of oyster mushroom (Pluerotus oestreatus) powder and soup. Journal of Food Processing and Preservation. 2014;38(1):457–465. https://doi.org/10.1111/j.1745-4549.2012.00794.x
- Marçal S, Sousa AS, Taofiq O, Antunes F, Morais AMMB, Freitas AC, et al. Impact of postharvest preservation methods on nutritional value and bioactive properties of mushrooms. Trends in Food Science and Technology. 2021;110:418–431. https://doi.org/10.1016/j.tifs.2021.02.007
- Rahman MA, Arif M, Md Shahjalal H, Kakon AJ, Ahmed F. Nutritional profile study of oyster mushroom (Pleurotus ostreatus) at different storage conditions. Fungal Genomics and Biology. 2022;12(5).
- Tolera KD, Abera S. Nutritional quality of Oyster Mushroom (Pleurotus ostreatus) as affected by osmotic pretreatments and drying method. Food Science and Nutrition. 2017;5(5):989–996. https://doi.org/10.1002/fsn3.484
- Kibar B. Influence of different drying methods and cold storage treatments on the postharvest quality and nutritional properties of P. ostreatus mushroom. Turkish Journal of Agriculture and Forestry. 2021;45:565–579. https://doi.org/10.3906/tar-2102-76
- Hiranpradith V, Therdthai N, Soontrunnarudrungsri A. Effect of steaming and microwave heating on taste of clear soup with split-gill mushroom powder. Foods. 2023;12(8). https://doi.org/10.3390/foods12081685
- Santhiya K, Abinaya S, Aswini R, Nitheshlee M. Assessment of button and oyster mushroom nutritional quality using various drying methods. Carpathian Journal of Food Science and Technology. 2022;14(3):171–181. https://doi.org/10.34302/crpjfst/2022.14.3.15
- Maray ARM, Mostafa MK, El-Fakhran AE-DMA. Effect of pretreatments and drying methods on physico-chemical, sensory characteristics and nutritional value of oyster mushroom. Journal of Food Processing and Preservation. 2017;42(41). https://doi.org/10.1111/jfpp.13352
- Dunkwal V, Jood S, Singh S. Physico-chemical properties and sensory evaluation of Pleurotus sajor-caju powder as influenced by pre-treatments and drying methods. British Food Journal. 2007;109(9):749–759. https://doi.org/10.1108/00070700710780715
- Khodifad C, Dhamsaniya NK. Drying of food materials by microwave energy – A review. International Journal of Current Microbiology and Applied Sciences. 2020;9(5):1950–1973. https://doi.org/10.20546/ijcmas.2020.905.223
- Bello M, Oluwamukomi MO, Enujiugha VN. Influence of drying on the antinutritional contents and antioxidant capacities of oyster mushrooms (Pleurotussajur-caju). Applied Tropical Agriculture. 2019;24(1):49–55.
- Post-harvest and pressing technology of staple food. Technical compendium of WHO Agricultural Science Bulletin. 2003;88:171–172.
- Siti-Nuramira J, Farhana R, Nabil S, Jafari SM, Raseetha S. Impact of drying methods on the quality of grey (Pleurotus sajor caju) and pink (Pleurotus djamor) oyster mushrooms. Journal of Food Measurement and Characterization. 2022;16:3331–3343. https://doi.org/10.1007/s11694-022-01435-w
- Mwangi RW, Macharia JM, Wagara IN, Bence RL. The antioxidant potential of different edible and medicinal mushrooms. Biomedicine and Pharmacotherapy. 2022;147. https://doi.org/10.1016/j.biopha.2022.112621
- Bach F, Zielinski AAF, Helm CV, Maciel GM, Pedro AC, Stafussa AP, et al. Bio compounds of edible mushrooms: in vitro antioxidant and antimicrobial activities. LWT. 2019;107:214–220. https://doi.org/10.1016/j.lwt.2019.03.017
- Tarafdar A, Shahi NC, Singh A, Sirohi R. Optimization of freeze-drying process parameters for qualitative evaluation of button mushroom (Agaricus bisporus) using response surface methodology. Journal of Food Quality. 2017;2017. https://doi.org/10.1155/2017/5043612
- Gąsecka M, Siwulski M, Magdziak Z, Budzynska S, Stuper-Szablewska K, Niedzielski P, et al. The effect of drying temperature on bioactive compounds and antioxidant activity of Leccinum scabrum (Bull.) Gray and Hericium erinaceus (Bull.) Pers. Journal of Food Science and Technology. 2020;57(2):513–525. https://doi.org/10.1007/s13197-019-04081-1
- Bashir N, Sood M, Bandral JD, Munaza B, Sharma S. Physical and antioxidant properties of oyster mushroom Pleurotus florida in response to different drying methods. Bioscience Biotechnology Research Communications. 2021;14(3):1240–1247. https://doi.org/10.21786/bbrc/14.3.49
- Zhang Z, Lv G, Pan H, Wu Y, Fan L. Effects of different drying methods and extraction condition on antioxidant properties of shiitake (Lentinus edodes). Food Science and Technology Research. 2009;15(5):547–552. https://doi.org/10.3136/fstr.15.547