Аннотация
In this study, we aimed to investigate the impact of three different post-packaging pasteurization temperatures (55, 65, and 75°C) on the physicochemical (pH, drip loss, texture profile, and color), microbial (lactic acid bacteria, mesophilic and psychrotrophic bacteria, as well as mold and yeast), and sensory (odor, taste, texture, color, slime, exudates, swelling, and overall acceptability) characteristics of vacuum-packed beef ham during 30 days of storage at two different temperatures (5 and 12°C).Lactic acid bacteria and total mesophilic and psychrotrophic counts were reduced to zero by post-packaging pasteurization at 65 and 75°C. Higher post-packaging pasteurization temperatures resulted in a significant increase in drip loss in the treated samples at 65 and 75°C, as well as a small rise in pH in all the samples. Furthermore, higher post-packaging pasteurization temperatures decreased lightness, yellowness, and h° values while increasing redness and ΔE. During post-packaging pasteurization, Chroma remained constant. The textural profile analysis revealed that post-packaging pasteurization and storage had a significant impact on the texture of beef ham. The sensory analysis showed no changes after post-packaging pasteurization in the samples, and the sensory parameters remained stable during their storage at 65 and 75°C.
Finally, our investigation showed that 65°C is an optimal post-packaging pasteurization temperature for increasing the shelf-life of beef ham under refrigeration.
Ключевые слова
Ready-to-eat foods, meat products, thermal pasteurization, post-packaging pasteurization, shelf-life, lactic acid bacteriaВВЕДЕНИЕ
Ready-to-eat products are really popular in modern lifestyles because they are easy to prepare. Meat products like ham are preferred among ready-to-eat foods due to their excellent nutritional, protein, vitamin, and mineral contents. Processed meats are highly perishable and have a short shelf-life despite the preservation technologies employed in these products. Because of their low salt content of 2%, pH of 6, and water activity of 0.9, as well as high protein, water, and nutrient contents, these products are highly susceptible to contamination by spoilage microorganisms, particularly lactic acid bacteria species [1–3]. This can happen during manipulation processes before the final packaging, such as slicing [4].
Symptoms of lactic acid bacteria spoilage include in-package souring, swelling, slime, milky exudates, off-flavors, off-odors, and discoloration [3]. The food industry is constantly concerned about lactic acid bacteria spoilage because it causes economic problems for producers who have to recall their products and may also harm their reputation [2]. On the one hand, meat companies attempt to create a high-quality product with financial advantages. On the other hand, people are becoming more conscious of the hazards of artificial additives used in ready-to-eat products [5]. Therefore, post-package pasteurization may be used to improve microbial safety and extend the shelf-life of vacuumpacked meat products. This method is easier to use and it is less expensive than other techniques used by meat companies. Post-package pasteurization is a thermal process known as a low-temperature decontamination method that can increase the shelf-life of ready-to-eat meat products [4, 6, 7].
Most previous studies used post-package pasteurization to control Listeria monocytogenes, Escherichia coli, Salmonella spp., and coliforms on a variety of products [6, 8–11]. To the best of our knowledge, there is a lack of research on the impact of different temperatures of post-package pasteurization on controlling spoilage bacteria, primarily lactic acid bacteria, in beef ham. Moreover, there are few studies on the quality changes during the post-package pasteurization process, primarily sensory attributes, which is limited to Vahabi Anaraki et al., who applied post-package pasteurization to turkey breast at 80°C for 5.5 min [4]. The heating process can cause changes in the quality of meat, such as denaturation, fiber shrinkage, and collagen solubilization [12]. Therefore, choosing an appropriate heating temperature is a critical step in producing a high-quality product.
According to previous studies [8, 13–16], the temperatures selected for post-package pasteurization ranged between 50 and 95°C. Since higher thermal treatments cause more quality changes, we attempted to find an optimum temperature in lower thermal ranges that could efficiently eliminate bacteria while still ensuring product quality. For this, we examined the effect of post-package pasteurization at different temperatures on the microbial, physical, chemical, and sensory quality of fully cooked, vacuum-packed 90% beef ham. In addition, we evaluated post-package pasteurization’s effect on the shelf-life of beef ham during 30 days of storage at temperatures of 5 and 12°C, which are the average house and market temperatures.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
In this study, we evaluated the impact of postpackage pasteurization on the microbial activity (lactic acid bacteria, aerobic mesophilic and psychrotrophic bacteria, mold, and yeast) and on the physicochemical changes (pH, drip loss, color coordinates, texture profile) and sensory analysis in vacuum-packed 90% beef ham at three post-package pasteurization temperatures (55, 65, and 75°C) and during 30 days of storage at two storage temperatures (5 and 12°C).
Cooked ham model. Sliced cooked hams were prepared using traditional techniques in an industrial meat factory in Amol, Iran (Kalleh Company). According to the manufacturer’s instructions, the hams were produced from boneless beef cuts (90%), salt (1.5%), sodium polyphosphate (0.3%), ascorbic acid (0.05%), sodium nitrite (0.01%), seasonings (1.14%), water (2%), and wheat starch (5%). After mixing the ingredients, the mixture was tumbled (2–4°C) and packed into an artificial casing and cooked until the core temperature reached 72°C, which was measured by inserting a thermometer (Model Testo 103, Germany) into the center of a sample pack. After cooking, the product was immediately cooled in a refrigerator (4°C) for about 2 h until the core temperature reached 5°C. The hams were then sliced (~ 0.2×11×14 cm) by using a slicing machine and vacuum-packed in polyethylene-polyamide-polyethylene (100 μ diameter) bags weighing around 300 g (~ 300 g sliced ham per package).
Heat treatment. The vacuum-packed hams were divided into four groups: control (non-pasteurized samples) and experimental pasteurized samples that were transported to saturated steam chambers at three different temperatures of 60, 70, and 80°C until the core temperature reached 55, 65, and 75°C, respectively. The temperature was measured by inserting a thermometer (Model Testo 103, Germany) into the center of a sample pack. Each treatment group required 60 min to reach the core temperature. The packs were then cooled in a refrigerator (4°C) for about 2 h until the core temperature reached 5°C. Following that, both pasteurized and non-pasteurized samples were transferred to the laboratory and refrigerated for 30 days at two temperatures of 5 and 12°C. For each category (pasteurized and non-pasteurized) of treatments, 30 out of a total of 120 packed hams were used. All analyses were carried out at least three times on days 0, 15, and 30 at temperatures of 5 and 12°C.
pH measurement. The pH of the product was calculated according to the procedure described by Khorsandi [17]. After mixing 10 g of a chopped sample with 90 mL of distilled water of room temperature, we measured pH with a digital pH meter (Model C860, Consort, Belgium). On each measurement day, the pH meter was calibrated at room temperature using buffers with pH of 4.01 and 6.97. All pH measurements were replicated three times.
Drip loss measurement. Drip loss was recorded on each slice of the sample immediately after packaging or processing at both storage temperatures by removing the cover of the hams, drying the exudates with a paper towel, and reweighing the hams with the wrapping. The drip loss was calculated as follows [18]:
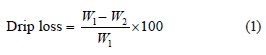
where W1 is the meat weight at packaging, g; W2 is the meat weight at sampling day, g.
The measurements were replicated at least three times.
Microbiological analysis. All microbiological analyses were performed according to Menéndez et al. [19]. Before each microbial analysis, sample packages were sterilized with 70% ethanol and opened aseptically using a sterile scalpel. Ham samples (25 g) were transferred aseptically to 0.1% sterile peptone water (225 mL) and homogenized in a stomacher (Lab Blender, Seward, London, UK) for 2 min. Further decimal dilutions were made using the same diluent, and they were then examined for aerobic mesophilic and psychrotrophic microorganisms, lactic acid bacteria, molds, and yeasts under the following conditions: lactic acid bacteria on Man Rogosa Sharpe agar at 30°C for 3 days; mesophilic and psychrotrophic bacteria on plate count agar at 30°C for 48 h and 10°C for 7 days, respectively; yeasts and molds on Rose Bengal with chloramphenicol agar at 25°C for 5 days. The tests were run in duplicate on agar plates using three samples for each treatment, and the results were represented as logarithms of the number of colony-forming units (log CFU/g).
Color analysis. The color coordinates (CIELAB) were calculated using a Minolta CR-400 colorimeter (Minolta Camera Co., Osaka, Japan; an aperture of 8 mm), illuminant D65, and a 2° observation angle, as described in [20]. L* denotes lightness and ranges from black (0) to white (100); a* denotes redness and ranges from (+) red to (–) green; and b* denotes yellowness and ranges from (+) yellow to (–) blue. A standard Minolta reflector plate was used to calibrate the colorimeter before each color measurement. Total color difference (ΔE), saturation index or Chroma (C*), and hue angle (h°), which indicate the divergence from the color space’s true red axis, were determined as follows [21]:
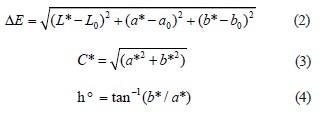
Where L*, a*,aand( b* /are) the measured values of treated and control hams on different days, and L0, a0, and b0 are the standard color parameters. In L*, a*, and b* color spaces, ∆E is the difference between two colors [22]. The Chroma or saturation scale runs from 0 for fully unsaturated (neutral gray, black, or white) to 100 for extremely high Chroma or “color purity”. The hue angle (h°) of the saturated color in the color space ranges from 0° (red), 90° (yellow), 180° (green), 270° (blue), and 360° (red) [21].
Texture profile analysis. The textural properties of hams were evaluated using a texture analyzer (M350-10CT, Testometric, UK) controlled by the comprehensive WinTest Analysis software. Samples were fixed on the central part of the texture profile analysis plate at room temperature and compressed twice to 50% of their original height through a 2-bite mechanism with a P/4 cylindrical probe (4.0 mm in diameter), a load cell of 50 kg, and a crosshead speed of 60 mm/min. The rest time between the two compressions was 10 s. The following parameters were determined: hardness, springiness, adhesiveness, cohesiveness, gumminess, and chewiness. The measurements were repeated at least three times at different points for each sample.
Sensory analysis. The sensory analysis was performed as described in [23], with eight trained panelists. The following sensory attributes were evaluated: odor, taste, texture, slime formation, pack swelling, color, exudates, and overall acceptability. The panelists rated the sensory attributes on a five-point hedonic scale: 1 for “extremely bad”, 2 for “bad”, 3 for “moderate”, 4 for “good”, and 5 for “extremely good”. The evaluations of every characteristic were repeated three times. The sensory evaluations were performed on days 0, 15, and 30 at both storage temperatures (5 and 12°C).
Statistical analysis. To evaluate the differences (p < 0.05), all the data were analyzed using one-way ANOVA. Duncan’s multiple range tests (p < 0.05) were performed to evaluate whether there were changes in the means of the treatments. The SPSS software was used for the analysis (SPSS 23.0 for Windows, SPSS Inc., Chicago, IL, USA).
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
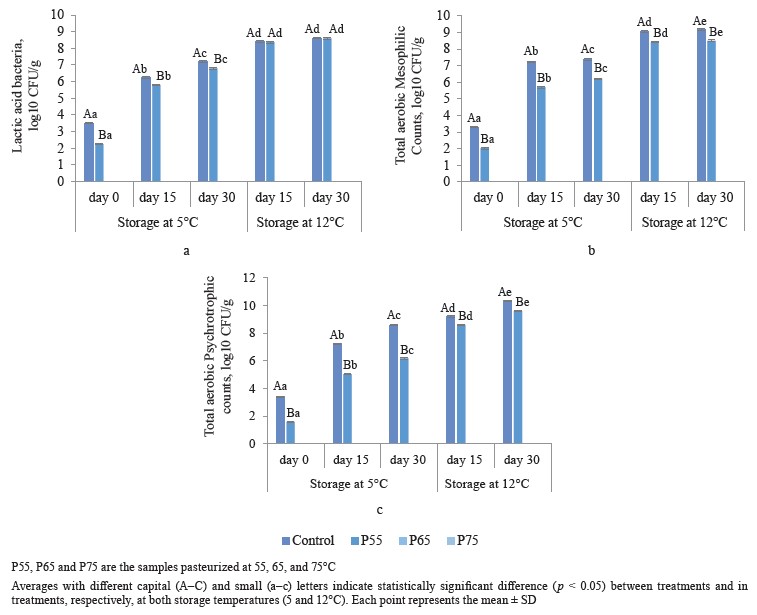
Microbiological analysis. Figure 1 depicts total aerobic mesophilic counts, total psychrotrophic counts, and lactic acid bacteria counts after post-package pasteurization and under different storage conditions. As can be seen in Fig. 1a, post-package pasteurization considerably reduced the lactic acid bacteria counts of the treated samples. No colony of lactic acid bacteria was counted during 30 days of storage in the samples pasteurized at 65 (P65) and 75°C (P75). The initial count of lactic acid bacteria was 3.5 log10 CFU/g in the control samples. Compared to the control group, this amount significantly reduced to 2.25 log10 CFU/g in the P55 samples (p < 0.05) (Fig. 1a). These results of lactic acid bacteria are similar to those reported by [3] and [24], who obtained a one-log reduction in the value of Lactobacillus sakei, Lactobacillus plantarum, Leu- conostoc mesentroiedes, and Leuconostoc curvatus in vacuum-packed sausages in less than 60 s at 55 and 57°C.
We found that on the first day of storage, the value of log10 CFU/g of mesophilic bacteria in non-pasteuri- zed samples was 3.3 log10 CFU/g. Compared to the nonpasteurized group, this amount significantly reduced (p < 0.05) to 2.04 log10 CFU/g in the P55 ham and reached zero in the P65 and P75 samples (Fig. 1b). Similarly, Pingen et al. observed 1.75 log10 CFU/g reductions in total aerobic mesophilic counts at 53°C in pork loins, and Jeong et al. observed a 3.67 log10 CFU/g reduction in total aerobic mesophilic counts after 45 min of post-package pasteurization treatment at 61°C in pork ham [9, 25].
In our study, the initial count of psychrotrophic bacteria in the control group was 3.4 log10 CFU/g on the first day of storage. Compared to the control group, postpackage pasteurization significantly reduced total psy- chrotrophic counts to 1.6 log10 CFU/g in the P55 ham and zero in the P65 and P75 samples (p < 0.05) (Fig. 1c). Our total psychrotrophic counts results were consistent with those of [10], who reported a 4 log10 CFU/g reduction in psychrotrophic counts in lamb loins after the post-package pasteurization process at 60°C for 6 h.
There were no mold or yeast counts after postpackage pasteurization or during 30 days of storage at 5 or 12°C in either the non-pasteurized or pasteurized samples. The number of bacteria in our study increased significantly during storage in both control and the P55 samples (p < 0.05). The control samples had a significantly higher lactic acid bacteria count during 30 days of storage at 5°C, but at 12°C, this amount increased to 8.6 log10 CFU/g at the end of storage for both the control and the P55 ham (p < 0.05) (Fig. 1a).At both storage temperatures (5 and 12°C), the amount of increase in total aerobic mesophilic counts and total psychrotrophic counts was constantly higher in the control group than in the P55 ham (Fig. 1b and c). Post-package pasteurization at 65 and 75°C effectively eliminated the number of lactic acid bacteria, total aerobic mesophilic counts, and total psychrotrophic counts by 3.5, 3.3, and 3.4 log10 CFU/g, respectively, whereas postpackage pasteurization at 55°C significantly eliminated these bacteria by 1.25, 1.26, and 1.8 log10 CFU/g, respectively. Furthermore, post-package pasteurization inhibited the growth of these microorganisms in both the P65 and P75 samples during cold storage (5 and 12°C), further increasing the product’s safety.
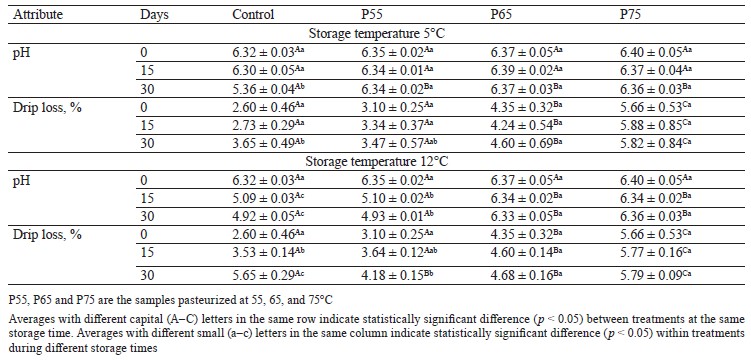
pH values. Table 1 shows variations in pH values of the treatments as a result of post-package pasteurization and cold storage. As can be seen, the pH of pasteurized hams increased with a higher post-package pasteurization temperature, but this trend was not statistically significant (p > 0.05). This could be related to a decrease in muscle acidic groups and the formation of free hydrogen sulfide as thermal temperatures rise [26]. This finding was consistent with the findings of [27] and [28], who discovered a slight increase in the pH of chicken and pork samples after heat treatment.
Drip loss analysis. Table 1 shows changes in drip loss in the samples caused by post-package pasteurization and during cold storage. Higher temperatures of post-package pasteurization increased the drip loss rate of the P65 and P75 samples, which was also significant between the experimental groups (p < 0.05). This increase in drip loss could be due to thermal treatment, which causes myofibrillar protein structures in muscle to become more rigid in the longitudinal axis due to protein denaturation. As a result, more muscle sarcoplasmic fluid is released due to this procedure [23]. Our findings agree with those of [8] in pork loin. Drip loss increased considerably in the control and P55 treatments during cold storage at both storage temperatures (p < 0.05) (Table 1). Because the waterholding capacity of muscle proteins is highly pH-dependent, this can be explained by a decrease in pH in these samples around the isoelectric point of proteins (particularly myosin with PI = 5.4) [4, 29].
Wang et al. and Ozaki et al. reported a rising trend in drip loss in their samples during storage, which is consistent with our findings [30, 31]. According to our data, post-package pasteurization significantly increased drip loss in the P65 and P75 samples, which is a downside to selecting high temperatures. This parameter rose during cold storage in both the control and the P55 ham sample, which was connected to lactic acid bacteria growth and a pH drop.
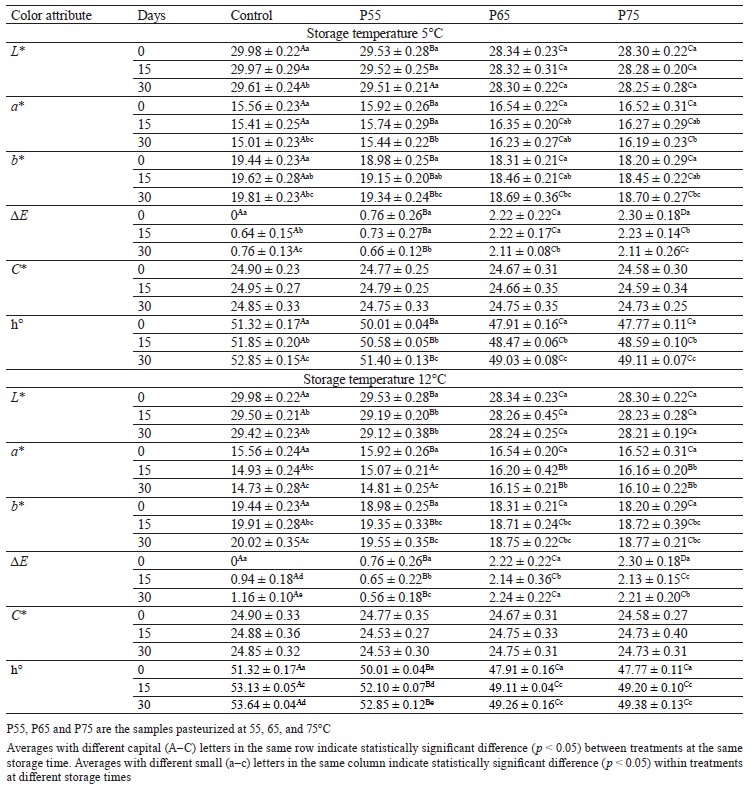
Color analysis. The L* and b* values. Table 2 shows changes in the color coordinates (CIELAB) (L*, a*, and b*) of all the samples. The L* and b* values of the pasteurized samples followed the same trend, decreasing considerably as the temperature increased up to 65°C and then leveling out at 75°C (p < 0.05). One possible explanation for the L* value’s decrease is higher drip loss in these samples, which causes moisture loss and may result in a decrease in lightness [32]. Reduced b* values may be attributed to metmyoglobin denaturation, which finally results in brown meat coloring because of ferrihemochrome production [33]. Several investigations have shown comparable findings for the L* value and b* value during post-package pasteurization [9, 16, 34]. In our study, only in the control samples (at both storage temperatures) and P55 samples (only at 12°C) did the L* value decrease significantly during cold storage (p < 0.05) (Table 2). This phenomenon could be explained by a loss of the waterholding capacity in the meat emulsion or a decrease in the pH of the samples [35, 36].
The b* value, on the other hand, increased significantly during cold storage in all the samples (p < 0.05) (Table 2). The increasing b* values observed in the control and P55 samples can be explained by pigment and lipid oxidation as a result of pH reduction [18, 37]. However, in the P65 and P75 samples, this increase could be attributed to lipid oxidation caused by released non-heme iron groups as thermal temperatures rise [38]. The b* values during the storage period in our study agreed with those obtained in [37] and [18]. Post-package pasteurization significantly increased the L* and b* values of the pasteurized samples, as well as induced darkness and blueness, according to our findings.
The a* value. Unlike the L* and b* values, the a* values of the pasteurized samples increased significantly as the post-package pasteurization temperature rose up to 65°C before leveling off at 75°C (p < 0.05). The cured red color formation in meats (Fe2+-nitrosylhemochrom pigment) as a result of the thermal process is related to the increasing redness during post-package pasteurization [25]. Our findings were consistent with those in [11] for fillet fish heated from 50 to 70°C. Conversely, during both storage temperatures, a significant reduction (p < 0.05) was observed for a* values in all the samples (Table 2), which could be attributed to the nitrosylmyoglobin pigment oxidation and metmyoglobin formation [39, 40]. Furthermore, nonenzymatic browning as a result of oxidative reactions upon heating can be responsible for a* value changes in the P65 and P75 samples, which is consistent with previous studies in sausage and pork ham [35, 41, 42].
Higher post-package pasteurization temperatures (primarily up to 65°C) were found to induce the cure meat color in beef ham, which is a desirable quality for consumer preference. This indicates that 65°C is a suitable temperature for post-package pasteurization.
Total color difference (∆E), Chroma (C*), and hue angle (h°). As can be seen in Table 2, post-package pasteurization significantly increased (p < 0.05) the total color differences of the pasteurized samples, ranging from 0.76 to 2.3, when compared to the nonpasteurized (control) samples. According to Fernández-López et al., the actual visual difference of the product occurs when the total color difference of samples is greater than 3 [22]. Increasing the post-package pasteurization temperatures reduced the h° value of all the pasteurized samples from 50.01 to 47.77 (p < 0.05) (Table 2). There were no significant differences in Chroma between the pasteurized treatments during post-package pasteurization (p > 0.05) (Table 2). This might be due to insufficient thermal temperatures, which could not affect the Chroma value.
The total color differences of the non-pasteurized samples increased significantly at both cold storage temperatures, whereas this parameter decreased significantly in all the pasteurized samples (except P65 at 12°C) (p < 0.05) (Table 2). However, according to Fernández-López et al., this reduction was not detectable by the untrained eye [22].
The hue angle results for all the pasteurized and non-pasteurized samples showed a significant increase (p < 0.05) at the two cold storage temperatures (Table 2), with values ranging from 47.77 to 53.64, indicating an increase in yellowness during storage. However, we observed no change in the Chroma values in any of the treatments during storage at both temperatures (p > 0.05) (Table 2).
According to the hue angle data, higher thermal temperatures caused more redness in the samples. Furthermore, it can be assumed that temperatures in our study were not high enough to have a significant impact on the Chroma or ∆E that could be detected.
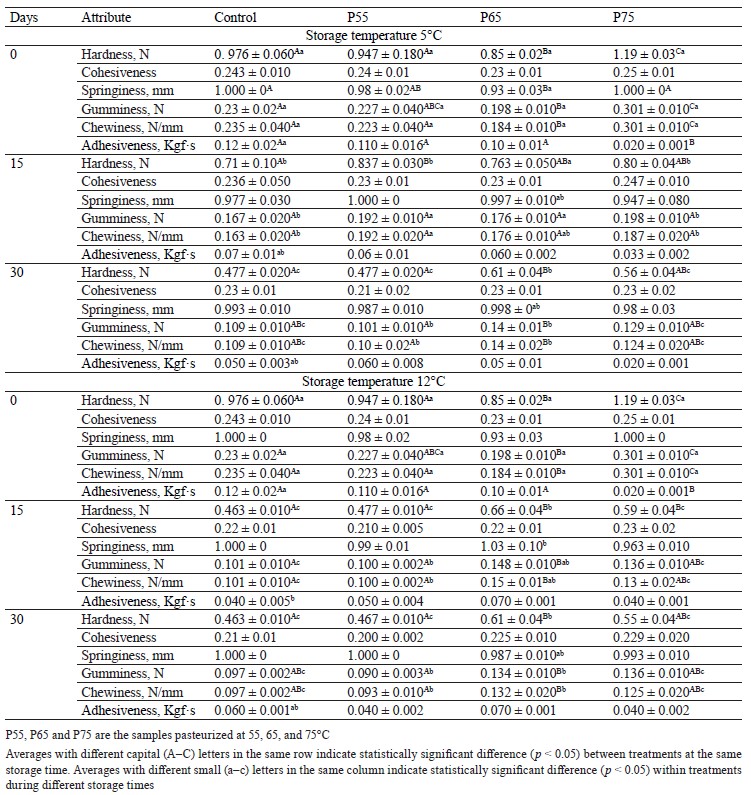
Textural profile analysis. Hardness, gumminess, and chewiness. Table 3 shows the effects of various heating and storage temperatures on the texture parameters of beef ham. Post-package pasteurization had a significant effect on the hardness, gumminess, and chewiness of the samples. Compared to the control groups, there was a significant decrease in hardness, gumminess, and chewiness in the pasteurized samples up to 65°C, followed by a significant increase in these parameters at 75°C (p < 0.05). Bertola et al. discovered the lowest hardness values at 60 and 64°C and relatively higher hardness values between 81 and 90°C [13]. In addition, Tornberg reported an increase in meat toughness during heating between 65 and 80°C caused by connective tissue shrinkage. The increase in meat tenderness during heating up to 65°C is caused by sarcoplasmic protein aggregation, gelation of fibers and fiber bundles, and decreased fracture stress [43].
Furthermore, this author connected a reduction in shear force to a weakening of collagenous connective tissue, and Shen et al. suggested that changes in the myofibrillar protein structure during heating are another factor that leads to meat hardness at temperatures above 70°C [11, 43].
The decrease in gumminess during post-package pasteurization is also caused by the heat denaturation of myosin, whereas the increase in gumminess can be related to the unfolding of actin and sarcoplasmic proteins [44]. Rabeler and Feyissa and Rigdon et al. observed a similar trend for gumminess when heating between 50 and 95°C [16, 45].
The decreased chewiness during post-package pasteurization could be attributed to the loss of the myosin structure, whereas the increased chewiness can be related to gel formation due to the denaturation of connective tissue and collagen that begins at temperatures above 70°C [14, 46, 47]. Roldán et al. found a similar trend for chewiness during post-package pasteurization in lamb loins [10].
Furthermore, these texture parameters declined during the two cold storage periods in all the samples. In general, the degradation of proteins and lipids by microorganisms or enzymatic activity, as well as the loosening of myofibrils, the degeneration of connective tissue, and the reduction of actin-myosin junctions, are associated with decreased hardness and increased softness of meat during storage [36, 48, 49]. In our study, this decreased trend was coincidental with the increase in the number of lactic acid bacteria, total aerobic mesophilic counts, and total psychrotrophic counts in the control and P55 samples (Fig. 1a, b, and c). Thus, decreased gumminess during storage could be due to ionic reaction loss [42]. Additionally, decreased gumminess and chewiness in the control and P55 samples might be due to the degradation of meat protein due to pH reduction, while decreased gumminess and chewiness in both P65 and P75 samples could be due to proteolysis caused by chemical and enzymatic activity and lipid oxidation during the thermal treatment [11, 50, 51].
Our results for hardness, gumminess, and chewiness during storage were consistent with those of [28, 36], [42, 48], and [28, 48], respectively. We found that the firmness of the treated samples decreased as a result of post-package pasteurization up to 65°C. However, the P65 samples had the highest firmness at the end of day 30 of refrigerated storage at both 5 and 12°C. According to these findings, 65°C could be considered the optimum post-package pasteurization temperature for maintaining the firmness quality of beef ham during cold storage.
Adhesiveness and cohesiveness. As shown in Table 3, none of the post-package pasteurization temperatures affected the adhesiveness and cohesiveness of the pasteurized samples, with the exception of the P55 ham sample, which had the lowest value when compared to the other treatments (p < 0.05). These results were consistent with those of D’sa et al. for adhesiveness and Rabeler and Feyissa for cohesiveness [15, 16].
The adhesiveness and cohesiveness of all the samples remained unchanged during cold storage (p
˃ 0.05). Thus, meat products such as ham cannot have high adhesiveness because they must have a firm texture and not stick when touched [52]. Rahman et al. relates ionic interaction loss to the cohesiveness of the samples during storage [49]. Similar findings have been observed for adhesiveness in fish and sausage and for cohesiveness during cold storage in sausage [48, 52, 53].
It has been reported that adhesiveness and cohesiveness can play an important role in product slicing and that increasing adhesiveness and cohesiveness results in increased product stickiness, which is an undesirable attribute for consumer preference [54]. In our study, post-package pasteurization had no effect on the product’s adhesiveness or cohesiveness, and these parameters remained unchanged during cold storage.
Springiness. Post-package pasteurization had no effect on the springiness of the samples (p ˃ 0.05) (Table 3). However, when compared to the control and P55 samples, the P65 samples had the lowest springiness (p < 0.05), which can be attributed to collagen and sarcoplasmic transitions that occurred between 65 and 67°C [43]. A similar result was observed for springiness during the heating of chicken breast meat at 50–90°C [16]. Similarly, another study discovered that the springiness of the samples treated at 65°C was lower than that of the samples treated at 72°C [27].
The springiness of the samples did not change significantly at any of the storage temperatures (p ˃ 0.05). Since an increase in springiness has been linked to a decrease in pH, while a decrease in springiness has been linked to an increase in drip loss, our results for springiness during storage may be related to the interaction of these factors [48, 55]. Feng et al. found no significant changes in the springiness of sausages after 58 days of refrigerated storage [48]. Our findings revealed that post-package pasteurization had no effect on the springiness of the samples. Additionally, this parameter remained constant across all storage temperatures (5 and 12°C), suggesting that postpackage pasteurization can preserve the texture quality of beef ham.
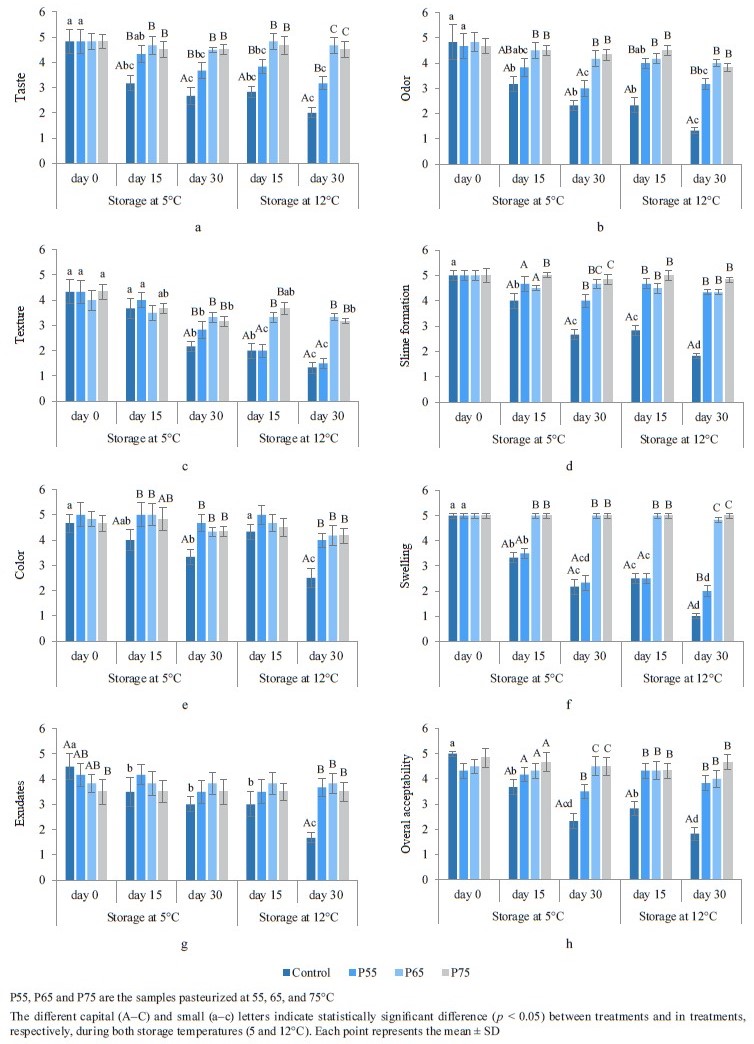
Sensory analysis. Taste, odor, and texture. Figure 2 depicts the sensory analysis of the samples after postpackage pasteurization and during cold storage. Postpackage pasteurization had no effect on the taste, odor, or texture of the samples, according to the panelists (p ˃ 0.05) (Figs. 2a–c), which was consistent with previous post-package pasteurization studies [27, 56] on sausage and pork. The sensory scores of taste and odor in the control and the P55 samples decreased significantly (p < 0.05) during cold storage at both 5 and 12°C, reaching 1 at the end of storage (Figs. 2a and b). The decreased taste and odor scores during storage could be attributed to an increase in microbial growth, free fatty acid formation, and oxidative rancidity [52]. Odors and off-tastes become noticeable when bacterial counts, particularly lactic acid bacteria, reach 7 logs, according to our findings and other studies [17, 52].
The sensory scores of texture decreased significantly at both storage temperatures in the control, P55, and P75 samples, according to the panelists (p < 0.05). However, these scores did not fall below 3 (Fig. 2c). Lower texture scores in the control and P55 samples can be related to a drop in pH, which changes muscle protein interaction and, as a result, changes textural structures [57]. The decrease in product firmness during storage has been attributed to enzymatic and microbiological activity [49]. It could also be due to the residual proteolysis activity of the protease after thermal heating during cold storage in the P75 hams [51]. Our findings were compatible with those reported by Feki et al. for sausage and Sani et al. for lamb meat [52, 58]. Our study revealed that postpackage pasteurization had no effect on the taste, odor, or texture of the product. These findings also demonstrated that taste and odor in the P65 and P75 samples, as well as texture in P65, could be preserved during both cold storage periods.
Slime formation, color, and swelling. As depicted in Figs. 2d–f, post-package pasteurization had no noticeable impact on slime formation, color, or swelling in the samples (p ˃ 0.05). These results were consistent with those of Vahabi Anaraki et al. [4].
Slime formation, color, and swelling scores in the control samples, as well as swelling scores in the P55 samples, decreased significantly (p < 0.05) at both storage temperatures, and those scores fell below 3. Slime formation and pack swelling during storage are linked to the growth of lactic acid bacteria, primarily Leuconostoc mesenteroides [59]. The decreased color score during storage can be related to pigment and lipid oxidation, which causes non-enzymatic browning of lipids and amino acids [52]. The results of color analysis in our study agree with those of Feki et al. and Sani et al. in beef sausage and lamb meat, respectively [52, 58]. Our study showed no statistically significant differences in these sensory qualities in the pasteurized samples. Furthermore, only the non-pasteurized samples had lower scores for slime formation and color after 30 days of cold storage (5 and 12°C), and pack swelling scores only decreased in the control and P55 samples.
Exudates. As displayed in Fig. 2g, the exudate scores for the pasteurized samples showed a pattern of decreasing acceptability as the post-package pasteurization temperatures rose. Heat treatment-induced protein denaturation is related to an increase in exudates [60].
The exudates scores decreased significantly during cold storage in the control and P55 ham samples (p < 0.05). Although the decrease in P55 was lower than in the control group and remained above 3, while the scores in the control group reached 4 at 5°C and fell below 2 at 12°C, this decline could be attributed to a decrease in pH, which caused protein denaturation and aggregation disarray, resulting in moisture loss [50]. Our results for exudates agree with those reported by Vahabi Anaraki et al. in turkey breast [4]. According to our results, post-package pasteurization had a negative effect on the exudates score of the pasteurized samples. After 30 days of cold storage at both 5 and 12°C, the score for this parameter did not decrease in either the P65 or P75 samples, reflecting the absence of microbial growth in these samples.
Overall acceptability. As illustrated in Fig. 2h, the overall acceptability of the P55, P65, and P75 ham samples remained from “moderate” to “very good” and never fell below 3. In the control samples, however, the scores dropped significantly to “bad” and “very bad”, and on the 30th day of cold storage, the overall acceptability fell below 3 at 5°C and below 2 at 12°C (p < 0.05). The higher sensory quality of the P65 and P75 samples was due to the lack of spoilage microorganisms in these samples, and the higher overall acceptability scores for the P55 ham, compared to the control samples, might be due to the inhibitory effect of post-package pasteurization on microbial growth in these samples. According to our findings, we can conclude that postpackage pasteurization can maintain the sensory characteristics of beef ham in pasteurized samples stored at 5 and 12°C.
ВЫВОДЫ
Our study revealed that post-package pasteurization could significantly reduce number of lactic acid bacteria, total aerobic mesophilic counts, and total psychrotrophic counts. Furthermore, we found that postpackage pasteurization significantly increased drip loss and exudates in the treated samples. Fortunately, postpackage pasteurization had no negative effect on the texture, color analysis, or sensory properties of beef ham, such as taste, odor, slime, swelling, color, texture, or overall acceptability. Also, it could significantly maintain these qualities in the P65 and P75 samples until the end of day 30 during cold storage at both 5 and 12°C. According to our findings, 65°C was the optimum temperature for post-package pasteurization application in 90% beef ham.Our study indicated that post-package pasteurization can be easily applied on an industrial scale, and the only downside to it is increased drip loss during the heating of the product. This issue could be resolved by using a different starch or phosphate in the formulation of this product to increase the water-holding capacity during post-package pasteurization.
Вклад авторов
The authors were equally involved in writing the manuscript and are equally responsible for any potential plagiarism.КОНФЛИКТ ИНТЕРЕСОВ
The authors declare no potential conflict of interest.БЛАГОДАРНОСТИ
The authors would like to express their thanks to the Kalleh Meat Products Company in Iran for providing financial support for this study.СПИСОК ЛИТЕРАТУРЫ
- Corrêa JAF, dos Santos JVG, Evangelista AG, Pinto ACSM, de Macedo REF, Luciano FB. Combined application of phenolic acids and essential oil components against Salmonella Enteritidis and Listeria monocytogenes in vitro and in ready-to-eat cooked ham. LWT. 2021;149. https://doi.org/10.1016/j.lwt.2021.111881
- Vinnikova L, Synytsia O, Shlapak H, Azarova N, Glushkov O. Decrease of repeated contamination of packed delicious meat products. EUREKA: Life Sciences. 2019;(5):58–63. https://doi.org/10.21303/2504-5695.2019.00996
- Abhari K, Jafarpour D, Shekarforoush SS. Effects of in-package pasteurization on preventing spoilage in emulsion vacuum packaged sausages during refrigerated storage. Foods and Raw Materials. 2018;6(1):40–46. https://doi.org/10.21603/2308-4057-2018-1-40-46
- Vahabi Anaraki N, Abbasvali M, Bonyadian M. Effects of post‐packaging pasteurization process on microbial, chemical, and sensory qualities of ready‐to‐eat cured vacuum‐packed Turkey breast. Journal of Food Safety. 2020;40. https://doi.org/10.1111/jfs.12776
- Horita CN, Baptista RC, Caturla MYR, Lorenzo JM, Barba FJ, Sant’Ana AS. Combining reformulation, active packaging and non-thermal post-packaging decontamination technologies to increase the microbiological quality and safety of cooked ready-to-eat meat products. Trends in Food Science and Technology. 2018;72:45–61. https://doi.org/10.1016/j.tifs.2017.12.003
- Wang Q, Zhang M, Adhikari B, Cao P, Yang C-H. Effects of various thermal processing methods on the shelf-life and product quality of vacuum-packaged braised beef. Journal of Food Process Engineering. 2019;42(4). https://doi.org/10.1111/jfpe.13035
- Huang L, Hwang C-A, Fang T. Improved estimation of thermal resistance of Escherichia coli O157:H7, Salmonella spp., and Listeria monocytogenes in meat and poultry – The effect of temperature and fat and A global analysis. Food Control. 2019;96:29–38. https://doi.org/10.1016/j.foodcont.2018.08.026
- Hwang S-I, Lee E-J, Hong G-P. Effects of temperature and time on the cookery properties of sous-vide processed pork loin. Food Science of Animal Resources. 2019;39(1):65–72. https://doi.org/10.5851/kosfa.2019.e4
- Jeong K, Hyeonbin O, Shin SY, Kim Y-S. Effects of sous-vide method at different temperatures, times and vacuum degrees on the quality, structural, and microbiological properties of pork ham. Meat Science. 2018;143:1–7. https://doi.org/10.1016/j.meatsci.2018.04.010
- Roldán M, Antequera T, Martín A, Mayoral AI, Ruiz J. Effect of different temperature–time combinations on physicochemical, microbiological, textural and structural features of sous-vide cooked lamb loins. Meat Science. 2013;93(3):572–578. https://doi.org/10.1016/j.meatsci.2012.11.014
- Shen S, Chen Y, Dong X, Liu F, Cai W, Wei J, et al. Changes in food quality and microbial composition of Russian sturgeon (Acipenser gueldenstaedti) fillets treated with low temperature vacuum heating method during storage at 4°C. Food Research International. 2020;138. https://doi.org/10.1016/j.foodres.2020.109665
- Haghighi H, Belmonte AM, Masino F, Minelli G, Lo Fiego DP, Pulvirenti A. Effect of time and temperature on physicochemical and microbiological properties of sous vide chicken breast fillets. Applied Sciences. 2021;11(7). https://doi.org/10.3390/app11073189
- Bertola NC, Bevilacqua AE, Zaritzky NE. Heat treatment effect on texture changes and thermal denaturation of proteins in beef muscle. Journal of Food Processing and Preservation. 1994;18(1):31–46. https://doi.org/10.1111/j.1745-4549.1994.tb00240.x
- Cai W, Wei J, Chen Y, Dong X, Zhang J, Bai F, et al. Effect of low‐temperature vacuum heating on physicochemical properties of sturgeon (Acipenser gueldenstaedti) fillets. Journal of the Science of Food and Agriculture. 2020;100(12):4583–4591. https://doi.org/10.1002/jsfa.10517
- D'sa EM, Harrison MA, Williams SE, Broccoli MH. Effectiveness of two cooking systems in destroying Escherichia coli O157:H7 and Listeria monocytogenes in ground beef patties. Journal of Food Protection. 2000;63(7):894–899. https://doi.org/10.4315/0362-028X-63.7.894
- Rabeler F, Feyissa AH. Kinetic modeling of texture and color changes during thermal treatment of chicken breast meat. Food and Bioprocess Technology. 2018;11:1495–1504. https://doi.org/10.1007/s11947-018-2123-4
- Khorsandi A, Eskandari MH, Aminlari M, Shekarforoush SS, Golmakani MT. Shelf-life extension of vacuum packed emulsion-type sausage using combination of natural antimicrobials. Food Control. 2019;104:139–146. https://doi.org/10.1016/j.foodcont.2019.04.040
- Dang TT, Rode TM, Skipnes D. Independent and combined effects of high pressure, microwave, soluble gas stabilization, modified atmosphere and vacuum packaging on microbiological and physicochemical shelf life of precooked chicken breast slices. Journal of Food Engineering. 2021;292. https://doi.org/10.1016/j.jfoodeng.2020.110352
- Menéndez RA, Rendueles E, Sanz JJ, Santos JA, García-Fernández MC. Physicochemical and microbiological characteristics of diverse Spanish cured meat products. CyTA – Journal of Food. 2018;16(1):199–204. https://doi.org/10.1080/19476337.2017.1379560
- Contador R, Ortiz A, del Rosario Ramírez M, García-Torres S, López-Parra MM, Tejerina D. Physico-chemical and sensory qualities of Iberian sliced dry-cured loins from various commercial categories and the effects of the type of packaging and refrigeration time. LWT. 2021;141. https://doi.org/10.1016/j.lwt.2021.110876
- Hamdi M, Nasri R, Dridi N, Moussa H, Ashour L, Nasri M. Improvement of the quality and the shelf life of reduced-nitrites turkey meat sausages incorporated with carotenoproteins from blue crabs shells. Food Control. 2018;91:148–159. https://doi.org/10.1016/j.foodcont.2018.03.048
- Fernández-López J, Lucas-González R, Viuda-Martos M, Sayas-Barberá E, Navarro C, Haros CM, et al. Chia (Salvia hispanica L.) products as ingredients for reformulating frankfurters: Effects on quality properties and shelf-life. Meat Science. 2019;156:139–145. https://doi.org/10.1016/j.meatsci.2019.05.028
- Herman RA, Adzitey F, Teye GA. The shelf life of coarse beef sausage using dawadawa (Parkia biglobosa) pulp powder as an extender. Journal of Postharvest Technology. 2019;7(2):62–68.
- Franz CMAP, von Holy A. Thermotolerance of meat spoilage lactic acid bacteria and their inactivation in vacuum-packaged Vienna sausages. International Journal of Food Microbiology. 1996;29(1):59–73. https://doi.org/10.1016/0168-1605(95)00022-4
- Pingen S, Sudhaus N, Becker A, Krischek C, Klein G. High pressure as an alternative processing step for ham production. Meat Science. 2016;118:22–27. https://doi.org/10.1016/j.meatsci.2016.03.014
- Ku S-K, Kim J, Kim S-M, Yong HI, Kim B-K, Choi Y-S. Combined effects of pressure cooking and enzyme treatment to enhance the digestibility and physicochemical properties of spreadable liver sausage. Food Science of Animal Resources. 2022;42(3):441–454. https://doi.org/10.5851/kosfa.2022.e14
- Ji D-S, Kim J-H, Yoon D-K, Kim J-H, Lee H-j, Cho W-Y, et al. Effect of different storage-temperature combinations on Longissimus dorsi quality upon sous-vide processing of frozen/thawed pork. Food Science of Animal Resources. 2019;39(2):240–254. https://doi.org/10.5851/kosfa.2019.e19
- Wang Z, Shi Y, Zhou K, Zhou H, Li X, Li C, et al. Effects of different thermal temperatures on the shelf life and microbial diversity of Dezhou-braised chicken. Food Research International. 2020;136. https://doi.org/10.1016/j.foodres.2020.109471
- Sorapukdee S, Jansa S, Tangwatcharin P. Partial replacement of pork backfat with konjac gel in Northeastern Thai fermented sausage (Sai Krok E-san) to produce the healthier product. Asian-Australasian Journal of Animal Sciences. 2019;32(11):1763–1775. https://doi.org/10.5713/ajas.18.0811
- Wang Z-C, Yan Y, Fang Z, Nisar T, Sun L, Guo Y, et al. Application of nitric oxide in modified atmosphere packaging of tilapia (Oreschromis niloticus) fillets. Food Control. 2019;98:209–215. https://doi.org/10.1016/j.foodcont.2018.11.043
- Ozaki MM, Munekata PES, Jacinto-Valderrama RA, Efraim P, Pateiro M, Lorenzo JM, et al. Beetroot and radish powders as natural nitrite source for fermented dry sausages. Meat Science. 2021;171. https://doi.org/10.1016/j.meatsci.2020.108275
- Zhang L, Du H, Zhang P, Kong B, Liu Q. Heterocyclic aromatic amine concentrations and quality characteristics of traditional smoked and roasted poultry products on the northern Chinese market. Food and Chemical Toxicology. 2020;135. https://doi.org/10.1016/j.fct.2019.110931
- Lee S, Choi Y-S, Jo K, Jeong HG, Yong HI, Kim T-K, et al. Processing characteristics of freeze-dried pork powder for meat emulsion gel. Food Science of Animal Resources. 2021;41(6):997–1011. https://doi.org/10.5851/kosfa.2021.e51
- Aleson-Carbonell L, Fernández-López J, Pérez-Alvarez JA, Kuri V. Functional and sensory effects of fibre-rich ingredients on breakfast fresh sausages manufacture. Food Science and Technology International. 2005;11(2):89–97. https://doi.org/10.1177/1082013205052003
- Alirezalu K, Hesari J, Nemati Z, Munekata PES, Barba FJ, Lorenzo JM. Combined effect of natural antioxidants and antimicrobial compounds during refrigerated storage of nitrite-free frankfurter-type sausage. Food Research International. 2019;120:839–850. https://doi.org/10.1016/j.foodres.2018.11.048
- Cao J, Wang Q, Ma T, Bao K, Yu X, Duan Z, et al. Effect of EGCG-gelatin biofilm on the quality and microbial composition of tilapia fillets during chilled storage. Food Chemistry. 2020;305. https://doi.org/10.1016/j.foodchem.2019.125454
- Santovito E, Elisseeva S, Cruz-Romero MC, Duffy G, Kerry JP, Papkovsky DB. A Simple sensor system for onsite monitoring of O2 in vacuum-packed meats during the shelf life. Sensors. 2021;21(13). https://doi.org/10.3390/s21134256
- Zhang Y, Tian X, Jiao Y, Wang Y, Dong J, Yang N, et al. Free iron rather than heme iron mainly induces oxidation of lipids and proteins in meat cooking. Food Chemistry. 2022;382. https://doi.org/10.1016/j.foodchem.2022.132345
- Cava R, García-Parra J, Ladero L. Effect of high hydrostatic pressure processing and storage temperature on food safety, microbial counts, colour and oxidative changes of a traditional dry-cured sausage. LWT. 2020;128. https://doi.org/10.1016/j.lwt.2020.109462
- Yu HH, Kim YJ, Park YJ, Shin D-M, Choi Y-S, Lee N-K, et al. Application of mixed natural preservatives to improve the quality of vacuum skin packaged beef during refrigerated storage. Meat Science. 2020;169. https://doi.org/10.1016/j.meatsci.2020.108219
- Alirezalu K, Hesari J, Yaghoubi M, Khaneghah AM, Alirezalu A, Pateiro M, et al. Combined effects of ε-polylysine and ε-polylysine nanoparticles with plant extracts on the shelf life and quality characteristics of nitrite-free frankfurter-type sausages. Meat Science. 2021;172. https://doi.org/10.1016/j.meatsci.2020.108318
- Ran M, He L, Li C, Zhu Q, Zeng X. Quality changes and shelf-life prediction of cooked cured ham stored at different temperatures. Journal of Food Protection. 2021;84(7):1252–1264. https://doi.org/10.4315/JFP-20-374
- Tornberg E. Effects of heat on meat proteins – Implications on structure and quality of meat products. Meat Science. 2005;70(3):493–508. https://doi.org/10.1016/j.meatsci.2004.11.021
- Truong BQ, Buckow R, Nguyen MH, Stathopoulos CE. High pressure processing of barramundi (Lates calcarifer) muscle before freezing: The effects on selected physicochemical properties during frozen storage. Journal of Food Engineering. 2016;169:72–78. https://doi.org/10.1016/j.jfoodeng.2015.08.020
- Rigdon M, Thippareddi H, McKee RW, Thomas CL, Stelzleni AM. Texture of fermented summer sausage with differing pH, endpoint temperature, and high pressure processing times. Meat and Muscle Biology. 2020;4(1). https://doi.org/10.22175/mmb.9476
- Villacís M, Rastogi NK, Balasubramaniam VM. Effect of high pressure on moisture and NaCl diffusion into turkey breast. LWT – Food Science and Technology. 2008;41(5):836–844. https://doi.org/10.1016/j.lwt.2007.05.018
- Kumar M, Sharma BD. The storage stability and textural, physico-chemical and sensory quality of low-fat ground pork patties with Carrageenan as fat replacer. International Journal of Food Science and Technology. 2003;39(1):31–42. https://doi.org/10.1111/j.1365-2621.2004.00743.x
- Feng C-H, Wang W, Makino Y, García-Martín JF, Alvarez-Mateos P, Song X-Y. Evaluation of storage time and temperature on physicochemical properties of immersion vacuum cooled sausages stuffed in the innovative casings modified by surfactants and lactic acid. Journal of Food Engineering. 2019;257:34–43. https://doi.org/10.1016/j.jfoodeng.2019.03.023
- Rahman MS, Seo J-K, Zahid MdA, Park J-Y, Choi S-G, Yang H-S. Physicochemical properties, sensory traits and storage stability of reduced-fat frankfurters formulated by replacing beef tallow with defatted bovine heart. Meat Science. 2019;151:89–97. https://doi.org/10.1016/j.meatsci.2019.01.011
- Lau ATY, Arvaj L, Strange P, Goodwin M, Barbut S, Balamurugan S. Effect of cranberry pomace on the physicochemical properties and inactivation of Salmonella during the manufacture of dry fermented sausages. Current Research in Food Science. 2021;4:636–645. https://doi.org/10.1016/j.crfs.2021.09.001
- Akoğlu I, Bıyıklı M, Akoğlu A, Kurhan Ş. Determination of the quality and shelf life of sous vide cooked turkey cutlet stored at 4 and 12°C. Brazilian Journal of Poultry Science. 2018;20(1):001–008. https://doi.org/10.1590/1806-9061-2017-0571
- Feki A, Sellem I, Hamzaoui A, Amar WB, Mellouli L, Zariat A, et al. Effect of the incorporation of polysaccharide from Falkenbergia rufolanosa on beef sausages for quality and shelf life improvement. LWT. 2021;143. https://doi.org/10.1016/j.lwt.2021.111139
- Sutikno LA, Bashir KMI, Kim H, Park Y, Won NE, An JH, et al. Improvement in physicochemical, microbial, and sensory properties of common squid (Todarodes pacificus Steenstrup) by superheated steam roasting in combination with smoking treatment. Journal of Food Quality. 2019;2019. https://doi.org/10.1155/2019/8721725
- Safaei F, Abhari K, Khosroshahi NK, Hosseini H, Jafari M. Optimisation of functional sausage formulation with konjac and inulin: using D-Optimal mixture design. Foods and Raw Materials. 2019;7(1):177–184. https://doi.org/10.21603/2308-4057-2019-1-177-184.
- Jung E-Y, Yun I-R, Go G, Kim G-D, Seo H-W, Joo S-T, et al. Effects of radix puerariae extracts on physicochemical and sensory quality of precooked pork sausage during cold storage. LWT – Food Science and Technology. 2012;46(2):556–562. https://doi.org/10.1016/j.lwt.2011.11.007
- Inmanee P, Kamonpatana P, Pirak T. Ohmic heating effects on Listeria monocytogenes inactivation, and chemical, physical, and sensory characteristic alterations for vacuum packaged sausage during post pasteurization. LWT. 2019;108:183–189. https://doi.org/10.1016/j.lwt.2019.03.027
- Shin D-J, Lee HJ, Lee D, Jo C, Choe J. Fat replacement in chicken sausages manufactured with broiler and old laying hens by different vegetable oils. Poultry Science. 2020;99(5):2811–2818. https://doi.org/10.1016/j.psj.2020.01.008
- Sani MA, Ehsani A, Hashemi M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. International Journal of Food Microbiology. 2017;251:8–14. https://doi.org/10.1016/j.ijfoodmicro.2017.03.018
- Samelis J, Kakouri A. Growth inhibitory and selective pressure effects of sodium diacetate on the spoilage microbiota of frankfurters stored at 4°C and 12°C in Vacuum. Foods. 2021;10(1). https://doi.org/10.3390/foods10010074
- Hassoun A, Aït-Kaddour A, Sahar A, Cozzolino D. Monitoring thermal treatments applied to meat using traditional methods and spectroscopic techniques: A review of advances over the last decade. Food and Bioprocess Technology. 2021;14:195–208. https://doi.org/10.1007/s11947-020-02510-0