Аннотация
Comparative studies that feature the physiology of wild and domestic animals replenish the fundamental knowledge in the field of biology and adaptive potential, thus increasing the efficiency of domestication. Semi-free conditions and artificial environment create prerequisites for epidemics and stress. However, early detection can prevent critical situations. This research provides new data on moose biology and physiology by establishing age and sex hematological parameters.The study featured moose blood samples (n = 55) obtained in the Kirov Region in the northeast of European Russia. Hematological tests relied on a veterinary version of a MicroCC-20 Plus automatic analyzer (High Technology).
This research was the first of its kind to introduce a comparative hematological analysis of local European moose according to age and sex. Adults and calves demonstrated significant differences (p < 0.05) in red blood cells, hemoglobin, hematocrit, mean corpuscular volume, mean concentration hemoglobin, mean corpuscular hemoglobin concentration, platelet distribution width, red blood cell distribution width, platelet crit, platelets, leukocytes, and eosinophils. Females and males also had significant differences (p < 0.05) in red blood cells, hemoglobin, mean corpuscular volume, red blood cell distribution width, platelet distribution width, platelets, and eosinophil content. The single- and multivariate analysis made it possible to establish the effect of physiological factors on the blood parameters in moose.
The hematological values were in line with the most indicators reported in other publications on wild artiodactyls. The existing differences in blood parameters depended on the species, habitat, food supply, age, and sex.
Ключевые слова
Alces alces, moose females, moose males, adult animals, calves, hematology, erythrocytes, leukocytes, plateletsВВЕДЕНИЕ
The European moose (Alces alces, Linnaeus, 1758) is one of the largest representatives of the taiga fauna. The species has a significant ecological variability and acclimatizes quite easily to new habitats. The moose is a traditional game species in many countries [1–3]. Nowadays, moose are bred in semi-free conditions and artificial habitats. Their complete commercial development is possible only on farms. Moose are a source of meat and milk, which has a chemical composition of a high-quality food product. Moose skin can be processed into top-grade suede. In addition, moose can be used for transport purposes.
Moose possess numerous valuable qualities, including rapid growth and maturation. Under normal conditions, the fecundity approximates 1.5 calves per mature female. This indicator depends on the habitat, climate, and individual characteristics. Both females and males become breedable at 18 months [4].
An important advantage is that moose experience no forage competition from farm animals. In their yearround free-ranging, they rely on trees and shrubs of a certain composition and age. Moose diet varies from habitat to habitat and from season to season [4].
The bottle-neck of moose breeding is the veterinary and sanitary measures against non-communicable and contagious diseases. Thus, moose farming requires not only hunting and biotechnical procedures, but also a number of veterinary measures.
Peripheral blood is highly sensitive to environmental conditions. As a result, it can be used to assess the health, adaptive capabilities, and stability of moose organism. Hematology currently occupies a leading place in veterinary science as it links physiology with clinical evidence. By establishing the effect of age, sex, and season on blood parameters, veterinarians can detect the slightest changes in animal physiology [5]. In addition, hematological studies are important because blood performs numerous and complex functions. Diagnostic hematology relies on the morphology, quantity, and ratio of blood cells. These studies include the diagnostics of hematopoietic and other organs and tissues because their malfunction affects blood morphology and biochemistry [5]. Hematology allows veterinarians to detect deviations from the norm. These methods can also be used to assess the state of wild animal populations [6].
Therefore, hematological methods are important tools in studying the health of individual animals and whole populations. They provide such valuable input data as challenging breeding conditions, semi-free or artificial habitat, acclimatization, population decline, epizootics, etc.
The first moose blood studies belonged to Ponder, who reported them as mammals with the largest red blood cells. Knorre & Knorre studied the concentration of red blood cells and hemoglobin in moose blood at the Pechoro-Ilychsky Reserve in the Komi Republic, Russia [7, 8]. Indeed, all publications report the large size of moose red blood cells, as well as their low concentration, which indicates a relatively low oxygen transportation [9].
Very few modern studies in moose hematology are now available. As a rule, they rely on insufficient sampling, provide no sex or age differences, or involve only animals kept in captivity [6, 10–11]. However unrepresentative, these studies are of considerable scientific value.
The purpose of this research was to obtain new data in the field of biology and physiology of moose and to establish hematological parameters for various age and sex groups.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Moose blood samples (n = 55) were collected from 13 young females, 11 adult females, 10 young males, and 21 adult males.
The animals were caught in 2011–2020 during hunting seasons between October and December. All the animals were clinically healthy and demonstrated no signs of disease at the time of sampling.
The body weight varied as follows: 127.0–185.0 kg in young females, 363.0–432.0 kg in adult females, 178.0– 201.5 in young males, and 280.0–420.5 in adult males.
The sampling covered the experimental game farm at the Professor Zhitkov Russian Research Institute of Game Management and Fur Farming. It is located in the southern taiga of the north-east of European Russia, namely, in Slobodsky, Zuevsky, and Belokholunsky districts of the Kirov Region with the geographical center in the village of Rogovoe (58°33’04”N, 50°43’42”E). The total area of the experimental farm is 66 250 ha. The climate is continental, with moderately cold winters and warm summers. All the animals were wild and roamed freely within the farm, feeding on local vegetation.
Blood samples were obtained by cutting the jugular vein (Venae jugularis L.) immediately after shooting: 4 cm3 of blood was collected into UNIVAC vacuum tubes with dipotassium ethylenediaminetetraacetic acid (K2EDTA) anticoagulant. The tubes were kept refrigerated until sent to the laboratory.
The hematological studies took place on days 1–3 using a veterinary modification of a MicroCC-20 Plus automatic analyzer (High Technology, USA). The leukocyte formula was calculated by the microscopy of blood smears stained with dye-fixative eosin methylene blue according to May-Grunwald and azure-eosin dye according to Romanovsky (MiniMed-M-G, Russia).
Each blood sample was studied for red blood cells, hemoglobin, hematocrit, mean corpuscular volume, mean concentration hemoglobin, mean corpuscular hemoglobin concentration, red blood cell distribution width, platelet distribution width, platelet crit, platelet count, white blood cells, and eosinophils.
The statistical analysis involved MS Excel (Office 2019), Statgraphics (19-X64) software, and standard methods [12]. The mean (M), standard deviation (SD), median (Me), and the 25th and 75th percentiles served to describe the samples. The Mann-Whitney U test and the Kruskal-Wallis H test were applied to compare the groups. Single- and multivariate analysis of variance made it possible to assess the effect of age, sex, and body weight on the hematological parameters. The effect was considered significant at p < 0.05.РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
Blood analysis assesses the state of health or the course of a pathological process in the animal body. In the recent past, veterinary practice did not rely on hematology because blood testing methods were underdeveloped, the equipment was lacking, and no data on blood composition in wild species were available. Moreover, veterinary science possessed very poor evidence about the relationship between the qualitative composition of blood and the course of pathological processes. Blood testing opened up good prospects for understanding various pathologies and their control. Blood changes can facilitate diagnosis and prognosis.
Thus, the baseline values for various hemogram parameters can indicate the state of specimen and populations of game animals that are exposed to the environment conditions, pathogenic microorganisms, parasites, toxins, etc.
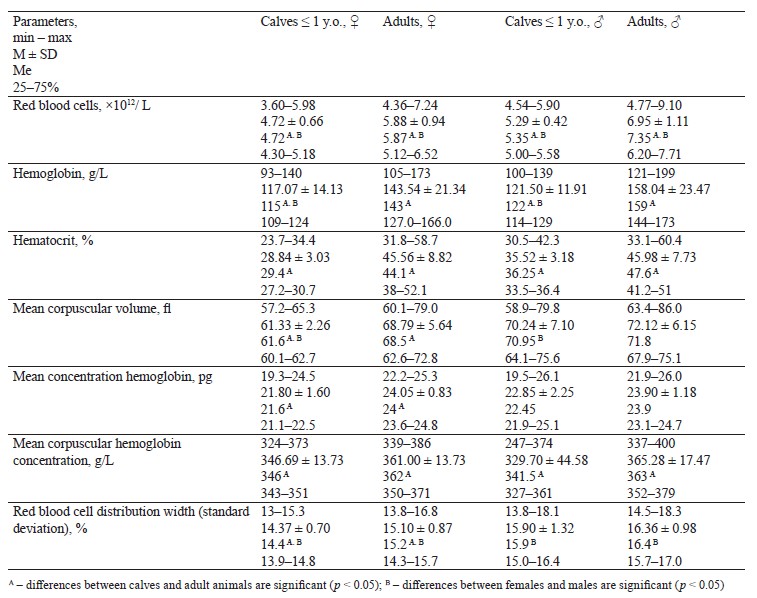
The research delivered reliable data on red blood cells, while blood cells, and platelets of wild moos depending on sex and age. Table 1 structures the red blood cell count for adult wild moose and calves (n = 55).
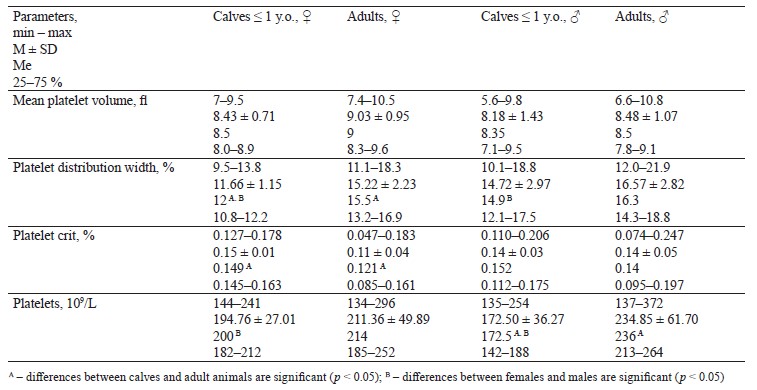
Table 2 features platelet parameters in adult wild moose and calves (n = 55).
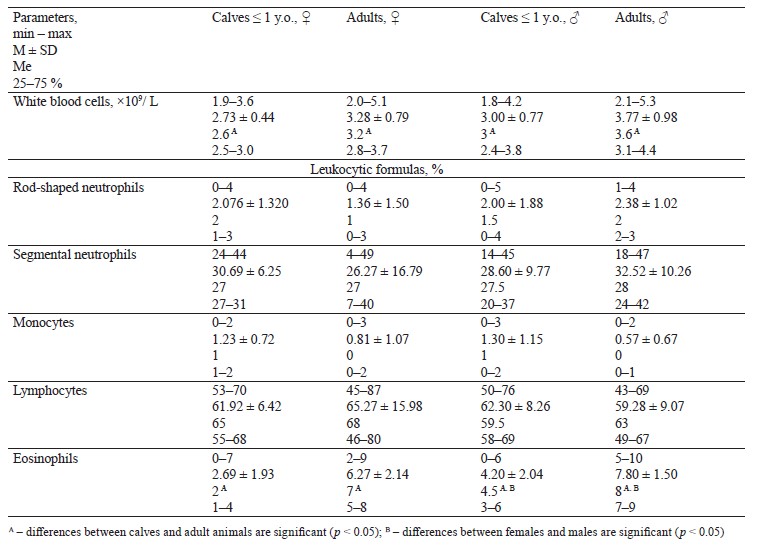
Table 3 describes while blood cells and leukocytic formula for adult wild moose and calves.
Our single- and multivariate analysis revealed the effect of age, sex, and weight on blood parameters. Sex and age proved to have a significant effect on red blood cells (p = 0.00, p = 0, respectively), mean corpuscular volume (p = 0.00, p = 0.00), red blood cell distribution width (p = 0.00, p = 0.03), platelet distribution width (p = 0.00, p = 0.00), and eosinophils (p = 0.00, p = 0). Age had a significant effect on hemoglobin (p = 0.00), hematocrits (p = 0.00), mean concentration hemoglobin (p = 0.00), mean corpuscular hemoglobin concentration (p = 0.00), platelets (p = 0.00), white blood cells (p = 0.00), and monocytes (p = 0.02).
The moose of different sex and age differed significantly in body weight, which affected the following hematological parameters: red blood cells (p = 0.00), hemoglobin (p = 0.00), hematocrits (p = 0.00), mean corpuscular volume (p = 0.00), mean concentration hemoglobin (p = 0.00), red blood cell distribution width (p = 0.00), platelet distribution width (p = 0.00), platelets (p = 0.00), white blood cells (p = 0.00), and eosinophils (p = 0.00).
Respiratory phylo- and ontogenesis of blood in vertebrates has one common trend: erythrocytes lose their nucleus, lifespan increases, and size and shape change [13]. In precocial species, blood becomes denuclearized as early as in embryogenesis. Non-nucleated erythrocytes break down glucose to lactic acid, and this metabolic process is responsible for the gas transport function in mammals [14].
The respiratory function of blood also depends on the development of the skeleton and the musculoskeletal system [15]. In newborn mammals, the entire red bone marrow is engaged in hematopoiesis. In mature animals, a certain part of red bone marrow is replaced by yellow adipose marrow [16]. In newborn reindeer, the weight of the bone marrow is 13% body weight and 58.3% skeleton weight; 60% of bone marrow is in the peripheral skeleton [14].
Between birth and puberty, blood volume, red blood cells, and hemoglobin increase together with live weight gain. In wild animals, the mean blood volume per unit of body weight hardly changes during postnatal ontogenesis. In domestic ungulates, body weight in ontogenesis increases faster than the mean blood volume and hemoglobin.
A large volume of circulating blood and its high hemoglobin content allow wild ungulates to endure the intense muscle load and the high energy exchange associated with physical activity [14]. Korzhuev formulated this phenomenon as a greater “provision” of wild animals with blood [15].
The blood of wild ungulates undergoes significant changes in the first six months of life because calves need to prepare for winter [16]. Like other wild ungulates, moose grow very fast after birth: in fact, their body weight increases by ≥ 10 times over the summer [9]. Newborn calves have a significant blood content, which may reach 10.6–16.2% body weight [9]. During the first post-natal week, the concentration of red blood cells and hemoglobin decreases because the plasma volume increases in a matter of days, while the count of red blood cells remains the same or decreases slightly. The blood becomes less saturated with cells, while its total volume goes up [9]. Moose calves are reported to eat clay and drink rusty marsh water to consume ferrous iron. This need to replenish the iron supply during intensive hematopoiesis is an important ecological feature [9]. Newborn moose, like reindeer, demonstrated a high blood percentage but no anemia [9, 17].
After this temporary inhibition, the hematopoietic activity increases by the time calves are 30 days old. The count of red blood cells increases by an average of 48.9%, and erythrocytes and hemoglobin soon regain their initial count. Since the weight of moose calves rapidly increases during this period, the mean volume of blood and hemoglobin drops by 15 and 25%, respectively [9]. The rapid growth was reported by Knorre & Knorre and Petrov [8, 18]. In addition, the fast growth rate increases together with the activity of hematopoietic organs: one-year-old calves and adult moose have the same blood-to-body weight ratio.
In wild moose, blood volume keeps pace with body weight much better than in domestic animals. According to Petrov, it takes the same time for calf’s skeleton to increase by 10 times as it takes its heart to grow by 30 times [19]. A larger heart means a larger blood volume. Thus, adult moose have much larger mean blood content than cows, sheep, and horses: it is 9–12% in moose and only 6–9% in farm animals [20]. In this respect, the moose is similar to the reindeer and the argali. However, these wild ungulates have much higher hemoglobin (1 g per 1 kg body weight). Moose make poor long-distance runners, and their low hemoglobin can be explained by their lower mobility.
To sum it up, all processes inside animal organisms affect the morphological, biochemical, and immunological parameters of blood. External factors also have a significant effect. However, the changes that take place in blood composition as a result of external and internal factors cannot be assessed without a reliable reference range. Still, this range should be considered as a rough guide, not an iconic reference because free-ranging wild animals seldom make a large sample.
Veterinary laboratories of the early XXI century saw a rapid automatization. Unlike traditional methods, automatic hematology analyzers are highly accurate and efficient. They illustrate cell distribution as histograms and require very little biological material. Modern analyzers cover 20 parameters, some of which cannot be established by microscopy, e.g., red blood cell distribution width, platelet distribution width, mean corpuscular hemoglobin concentration, mean platelet volume, etc. These parameters have significant clinical and diagnostic value [21]. In addition, automatic hematology analyzers are more objective compared to the manual method. Thus, automated blood testing offers new diagnostic possibilities for wildlife studies.
Erythrocyte indicators. Red blood cells are one of the most important erythrocyte indicators. Automated blood tests provide coefficient of variation for this parameter below 1% [21]. Polycythemia, or erythrocytosis, is a high red blood cell count caused by various processes. The list of physiological causes usually includes prolonged physical stress and high altitudes while pathological causes involve chronic lung diseases, kidney diseases associated with erythropoietin, chronic heart failure, tumors, etc. Relative polycythemia develops if the volume of circulating plasma increases, e.g., as a result of long diarrhea and vomiting. It can also be caused by redistribution and deposition of blood or its superfluous release from the blood pool, as well as by acute hypoxia and stress, accompanied by an increase in blood norepinephrine, adrenaline, and glucocorticoids.
Erythropenia is a low red blood count caused by various anemias, kidney diseases associated with low erythropoietin, infectious and autoimmune diseases, hyper- and hypofunction of the thyroid gland, hemoglobinuria, hemoglobinopathies, radiation sickness, etc.
Hematocrit represents the volume percentage of red blood cells. Hematocrit levels that are too high can indicate symptomatic erythrocytosis or erythremia, trauma, shock, or dehydration caused by severe diarrhea, vomiting, or burn disease. Low hematocrit levels indicate severe blood loss, various anemias, or an increase in circulating blood volume.
Hemoglobin reflects the concentration of hemoglobin in the blood. Automatic hematological analyzers provide the variation coefficient below 2% [21]. Hemoglobin levels that are too high may reflect physiological changes and diseases associated with high red blood cell count. Low hemoglobin is typical of anemia and may be caused by blood loss, impaired hematopoiesis, or hemolysis. Though anemia can be an independent disease, it is usually a symptom of another chronic disease.
Mean cell volume, or mean corpuscular volume, is calculated by dividing the total red blood cells by their number. In an automated blood test, it indicates the volume of the entire cell population. The mean cell volume can have a normal value even in case of a pronounced macro- and microcytosis. Then, the real sizes of erythrocytes can be determined by analyzing histograms of cell distribution in the population. Microcytic, normocytic, and macrocytic anemias depend on the mean cell volume [21].
Mean concentration of hemoglobin, or mean cell hemoglobin, in an erythrocyte is calculated by dividing the hemoglobin concentration by the number of red blood cells per unit volume. The clinical significance of this parameter is similar to the blood color index. Anemias are divided into normochromic, hypochromic, and hyperchromic ones, depending on mean cell hemoglobin.
Mean cell hemoglobin concentration, or mean corpuscular hemoglobin concentration, reflects the degree of saturation of erythrocytes with hemoglobin. It is the ratio of hemoglobin values to hematocrit, multiplied by 100. The mean corpuscular hemoglobin concentration is a concentration index that does not depend on the cell volume, which makes it a sensitive indicator of a hemoglobin formation problem.
An increase in the mean corpuscular hemoglobin concentration may be associated with a microspherocytic hemolytic anemia unless it is a technical error. Low mean corpuscular hemoglobin concentrations accompany impaired hemoglobin synthesis in cases such as hemoglobinopathies and iron deficiency anemia.
Red blood cell distribution width is an indicator of erythrocyte volume heterogeneity and anisocytosis. It determines the degree of anisocytosis, i.e., fluctuations in the size of red blood cells [21].
In laboratory practice, the abovementioned red blood cell parameter usually serves to diagnose anemia of various etiologies. However, red blood cell distribution width appeared to be a reliable laboratory indicator that can be used to diagnose diseases that are not associated with anemia. Thus, automatic hematological analyzers provide erythrocyte indices and histograms that can serve as a useful diagnostic tool for a wide range of pathologies.
The hematological parameters obtained in this research somewhat differed from the results reported by foreign and domestic scientists, but the trend between adult animals and calves was similar in most indicators.
We established significant differences (p < 0.05) for all red blood cell parameters between calves and mature animals. Females and males in both age groups also demonstrated significant differences.
The Pechoro-Ilychsky Reserve (Komi Republic) conducted a comprehensive study of hematological parameters in moose. According to Kochanov, moose hardly differed in red blood cells: calves – 5.52×106 per 1 mm3, one-year-olds – 5.63×106 per 1 mm3, adult females – 5.41×106 per 1 mm3 [22]. The hemoglobin percentage slightly increased with age and reached 8.99, 9.25, and 9.7 g%, respectively. Knorre & Knorre reported data similar to our findings on red blood cells in one-year-olds and adult moose [8]. Adult moose had a gradual decrease in hemoglobin, which dropped from 11.5 to 8.16 g%, unlike three-month-old calves. Irzhak reported bigger numbers for one-month-olds in spring: their red blood cell count was by 2 million/mm3 higher than in adults (6.2 and 4.2 million, respectively), and the hemoglobin was by 0.7 g% higher (8 and 7.3 g%, respectively) [23].
Norwegian moose had higher indicators than those obtained in this research. Calves had by 25% more red blood cells and by 20.47% more hemoglobin while adult moose had hemoglobin by 6.22% more than in our work [6]. Adult female American moose (Alces americanus shirasi, Nelson 1914) in northwestern Wyoming, USA, exceeded our results in the red blood cell count by 26.72% and hemoglobin by 13.96% [10]. Adult female California wapiti (Cervus elaphus nannodes, Merriam 1905) that live in California, USA, had more hemoglobin by 17.35% and more hematocrit by 13.7% [24]. The difference could probably be explained by their habitat. Most of the territory of Norway is more than 490 m above sea level; the highest point of Wyoming is 4207 m while the state of California is 4421 m above sea level. In the Kirov Region, the difference in absolute heights ranges from 56 to 337 m. In all the abovementioned studies, the mean corpuscular volume, mean concentration hemoglobin, and mean corpuscular hemoglobin concentration were almost identical with our results.
The black-and-white cattle (Bos taurus, Linnaeus, 1758) from Tajikistan mountains also showed a higher red blood cell count in adult males: it was by 7.55% higher than our data for the moose from the Kirov Region [25]. Yaks (Bos mutus, Przewalski, 1883) also had a higher total red blood cell count [25]. For young yak females and males, the figures were higher by 28.6 and 18.2%, respectively, while the hemoglobin count was higher by 16.67% for young females and by 7.58% for young males. The hypsometric levels of Tajikistan mountains range from 300 to 7495 m above sea level.
The West Caucasian tur (Capra caucasica severtzovi, Menzbier 1887) from the Karachay-Cherkessia had the total red blood cell count 50% as high as that of the moose from the Kirov Region [26]. Their mean corpuscular hemoglobin concentration also was by 13.5% higher. The pastures of the Karachay-Cherkessia Republic are 1650 m above sea level.
Domesticated Nenets reindeer (Rangifer tarandus, Linnaeus 1758) in the Yamalo-Nenets Autonomous Region had the total red blood cell count in the peripheral blood by 18.2% higher than in this research [27]. The Transuralian area is 200–500 m above sea level.
Adult free-range wild reindeer in southwestern Norway showed higher red blood cell counts by 38.23% and hemoglobin by 12.72% [28]. However, they had lower figures for the mean erythrocyte volume, mean concentration hemoglobin, and red blood cell distribution width by 37.28, 32.09, and 8.875, respectively. The hematocrit and mean corpuscular hemoglobin concentration coincided with our results.
The moose from the Pechoro-Ilychsky Reserve of the Komi Republic had very similar blood parameters to those reported in this study [11]. The Reserve is 150– 175 m above sea level.
Animals use different anatomical, physiological and biochemical means to adapt to different heights, i.e., to the amount of oxygen available [29]. High altitudes trigger certain transformations in the oxygen transport system. The partial pressure of oxygen is not enough to saturate the blood, so the total oxygen-transportation capacity increases. The low capacity of hemoglobin to carry oxygen requires more circulating respiratory pigment. The regulatory effect of 2,3-diphosphoglycerate increases to facilitate the release of oxygen by hemoglobin in the tissues. Behavioral and physiological adaptations include low muscle activity and low oxygen demand, as well as increased heart rate and breathing, which increases the amount of oxygen delivered to tissues. However, adaptive shifts that occur at the molecular level eliminate this phenomenon. First, a greater red blood cell count increases the oxygen-carrying capacity. Second, hemoglobin gives more oxygen to tissues, because the concentration of 2,3-DPG in erythrocytes increases [29].
Climate is as important as altitude. The review above included animals that inhabited different climatic zones, from the Mediterranean, California, and subtropical Tajikistan to the Yamalo-Nenets area in the Far North with its sharply continental climate.
Most animals are excellent survivors under harsh winter conditions. The reindeer would simply not be able to live in a warm habitat because it lacks sweat glands. However, these animals maintain a high level of hemoglobin during the winter period: it reaches 1.5 per 100 kg of live weight, which is 0.6–1.2 kg in other animals, and its blood volume reaches 11% live weight [30, 31].
Moose demonstrate significant age-related differences in blood counts. Some studies [11, 32] report physiological anemia as normal for young animals that are low in red blood cells, hemoglobin, hematocrit, and leukocytes. Males have a higher red blood cell count than females because red blood cells increase testosterone under the action of erythropoietin. As a result, male moose increase their blood volume before the rut season, which presupposes fights and wounds. By the beginning of the rut season, they develop mature antlers [33, 34]. The percentage of red blood cell weight and hemoglobin increases. Fluctuations in hematocrit can also be caused by different water intake, intestinal disorders, etc. [34].
Our sampling was performed by hunting. The chaserelated stress and hyperventilation can cause spleen contraction and an increase in hematocrit by 10% or more, as well as in red blood cells and hemoglobin [35].
Platelet indicators. Automated hematological blood tests also provide valuable information on platelets, their mean volume, distribution width, and crit.
Total platelet count, or platelets, is an important indicator that can detect thrombocytosis, an increase in platelets typical of chronic myeloproliferative diseases, acute and chronic inflammatory processes, amyloidosis, blood loss, malignant neoplasms, and hemolytic anemia. Thrombocytopenia is a decrease in platelets caused by their destruction and inhibited formation [5].
Mean platelet volume describes the size of platelets based on their volumetric distribution. Mean platelet volume is inversely proportional to platelet count, which maintains hemostasis and constant mass. Consequently, the more numerous they are, the smaller they get. A pathology might violate this proportion. Intensive thrombocytopoiesis, increased aging, and various activators can change the proportions between the mean volume and count.
Platelet crit represents the ratio of mean platelet volume and total blood volume. It depends on the number of platelets and their size. In clinical practice, this indicator assesses the risk of bleeding and thrombosis. Platelet distribution width reflects the size heterogeneity in terms of size, i.e., the anisocytosis degree. Platelet aggregates, microerythrocytes, and platelet fragments may increase this indicator. Myeloproliferative diseases also affect platelet distribution width [21].
This research established significant (p < 0.05) age differences in terms of mean platelet distribution width and platelet crit between young and adult females. Another correlation included platelet count in young and adult males. We also revealed significant sex differences in mean platelet distribution width between young females and males, as well as in platelet count in adults.
The moose from the Kirov Region differed in platelet parameters from Norwegian moose in that adult Russian moose had a larger platelet count by 27.28% [6]. The platelets in young Norwegian moose exceeded our figures by 19.31%. The same dynamics was typical of the mean platelet volume: our data were higher by 22.34% in calves and 32.31% in adults. Norwegian adult female moose also had fewer platelets by 26.75% [10].
Leukocyte indicators. Leukocyte count and composition are the most important leukocyte indicators. Leukogramic changes often precede the clinical signs of certain diseases and indicate pathological processes. Leukocytosis is an increase in the number of leukocytes. It may be the result of leukemia, acute inflammatory and infectious processes, myocardial infarction, malignant tumors, burns, thrombosis of peripheral arteries accompanied by gangrene, uremia, significant blood loss, toxic poisoning, and ionizing radiation. Leukopenia is a decrease in the leukocyte count. It accompanies viral diseases, aplastic anemia, agranulocytosis, some acute leukemias, radiation sickness, systemic diseases, endocrine pathology, chronic gastritis, colitis, and cholecystitis [5, 21]. All leukocytes perform the protective function, but each type does this in a special way.
We established significant (p < 0.05) age differences in leukocytes and eosinophils in females and males, as well as a certain gender-related difference in eosinophils.
We also studied the lymphocytic profile of moose blood. In moose, lymphocytes are mostly medium in size, with fewer small and large varieties. Some young females had 90% of lymphocytes. Segmented neutrophils had polysegmented nuclei and poorly visible granularity. Kizhina et al. obtained similar data on lymphocytes and cell morphology for moose from Karelia [36].
They reported that moose had a lymphocytic blood profile with 85% lymphocytes, which averaged 54.50 ± 17.03%.
We detected significant differences in the leukoformula content of eosinophils, which had a large number of small granules in their composition, between young and adult females, as well as between young and adult males. Adult moose had a much greater eosinophil count than domestic or young animals. Apparently, these cells were parasitic infection frontliners. Some moose had as many as 23–27% of eosinophils, but we did not exclude them from the statistics.
According to Kochanova, moose experience a rather sharp increase in leukocytes when they approach their first birthday, but this indicator stabilizes as they grow older: it is 7460 per 1 mm3 in young animals, 10 600 per 1 mm3 in one-year-olds, and 10 840 in adult female moose [22].
Knorre & Knorre detected a sharp decrease in leukocytes in adult moose, which dropped from 8625 to 4000, compared with three-month-old calves [8].
However, Rostal et al. reported no differences in leukocytes between adult and young moose below one year old (3.2×109/L) [6]. In their research, adult moose had approximately the same ratio of lymphocytes and neutrophils: calves moved from a predominantly lymphocytic (51%) and lower neutrophilic (33%) leukocyte profile to a more even distribution of 45% neutrophils and 42% lymphocytes in adult moose. These age-related changes in the ratio of neutrophils and lymphocytes were recorded in other ruminant species and, probably, reflected the maturation of the immune system [32, 37–38].
According to Shideler, Californian wapiti had 60% (44–74%) of lymphocytes in adult females and 29% (5– 45%) in adult males [24].
Two-year-old black-and-white male yaks from Tajikistan highlands had 7.32 ± 0.49×109/L of leukocytes [25]. Eight-month-old female yaks had 6.11 ± 0.72×109/L of leukocytes, while for males it was 6.24 ± 0.33×109/L. Two-year-old female yaks had 6.84 ± 0.44×109/L while for males it was 6.51 ± 0.311×109/L.
Bagirov et al. reported 13.3×109/L leukocytes for the West Caucasian tur and 12.4×109/L for domestic goats of the Karachay breed [26]. Novak et al. reported 6.09 ± 0.21×109/L leukocytes for adult domesticated reindeer [27]. Domesticated reindeer that inhabited the Yakutian taiga had 4.20 ± 0.95×109/L leukocytes in winter and 45.2 ± 1.8% lymphocytes [39].
The differences between the abovementioned data and those obtained in this research probably depend on the species, individual characteristics, and environmental conditions. Protein digestion depends on the proteolytic enzymes of neutrophils, while the lipolytic enzymes of lymphocytes affect fat digestion. Myogenic leukocytosis is more typical of animals that live in the Caucasus or the highlands of Tajikistan because intensive muscular activity requires redistribution of blood cells caused by the changes in vasomotors and metabolism.
Reshetnikov linked the drop in lymphocytes in autumn with the poor winter diet [40]. The sharp changes under the weather conditions triggered a stress response to the challenges of winter foraging, which released lymphocytes into the peripheral blood.
No information is currently available on the effect of different methods of moose blood sampling on the hematological parameters. However, a series of studies of white-tailed deer (Odocoileus virginianus, Zimmermann 1780) showed that if blood was sampled immediately after immobilization, the hemogram variations did not depend on the trapping method [41–44]. Therefore, we made no difference for blood sampling methods.
Our baseline hematology data for this moose population had some limitations that should be considered in comparative studies. They included the differences in hematological analyzers, laboratory diagnostics, and animal habitats [45]. In addition, a delay of ≥ 72 h between the blood sampling and laboratory analysis could affect the blood composition [46]. Probably, this time gap caused the decrease in platelets, leukocytes, lymphocytes, monocytes, and basophils, as well as the associated increase in hematocrit. Thus, our results should be interpreted with these factors in mind.
The moose blood profile had seasonal differences. Thus, the red blood cell parameters were higher in autumn than in spring because animals prepare for winter forage [34].
Thus, the hematological values we obtained in this study follow the patterns reported by other studies of wild artiodactyls. The differences depended on the species, sex, age, climat, environment, and food supply.
ВЫВОДЫ
1. The research revealed the hematological parameters for the European moose (Alces alces, Linnaeus 1758) of different sex and age from the Kirov Region, Russia. Adults and calves demonstrated significant differences (p < 0.05) in red blood cells, hemoglobin, hematocrit, mean corpuscular volume, mean concentration hemoglobin, mean corpuscular hemoglobin concentration, platelet distribution width, red blood cell distribution width, platelet crit, platelets, leukocytes, and eosiphils. Females and males had significant differences (p < 0.05) in red blood cells, hemoglobin, mean corpuscular volume, platelet distribution width, red blood cell distribution width, platelets, and eosinophils.
2. The single- and multivariate analysis established the effect of such physiological factors as age, sex, and weight on blood parameters. Sex and age had a significant effect on red blood cells, mean corpuscular volume, platelet distribution width, red blood cell distribution width, and eosinophils. Age had a significant effect on hemoglobin, hematocrit, mean concentration hemoglobin, mean corpuscular hemoglobin concentration, platelets, leukocytes, and monocytes. Weight affected erythrocytes, hemoglobin, hematocrit, mean corpuscular volume, mean concentration hemoglobin, platelet distribution width, red blood cell distribution width, platelets, leukocytes, and eosinophils.
3. By comparing the physiology of wild and domestic animals, scientists replenished the fundamental and practical knowledge in the field of biology and the adaptive potential of species, thus increasing the efficiency of their domestication. Animals kept in semi-free conditions and artificial habitats may fall victim to epidemics and stress. As a result, any disease must be detected at the earliest stage possible. Modern automated devices make it possible to monitor the physiological state of animals and register the very first signs of various health issues.
Вклад авторов
All the authors contributed equally to the study and bear equal responsibility for information published in this article.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare that there is no conflict of interests regarding the publication of this article.
БЛАГОДАРНОСТИ
We are grateful to hunters of Russian Research Institute of Game Management and Fur Farming for their help in collecting biological material. We also thank Dr. Boris Zarubin for his help and assistance with data collection.ФИНАНСИРОВАНИЕ
This research was partially supported by the Russian Academy of Sciences (RAS) FSZZ-2019-0001 (АААА-А19-119020190132-5). It was performed on the premises of the Professor Zhitkov Russian Research Institute of Game Management and Fur Farming (VNIIOZ profl Zhitkov).СПИСОК ЛИТЕРАТУРЫ
- Litvinov VF, Podoshvelev DA, Kovalev NA. Breeding and resettlement of game animals in Belarus. Relevant Issues of Nature Management, Hunting, and Fur Farming: Proceedings of the International scientific and practical conference dedicated to the 95th anniversary of B.M. Zhitkov All-Russian Research Institute of Hunting and Fur Breeding; 2017; Kirov. Kirov: B.M. Zhitkov All-Russian Research Institute of Hunting and Fur Breeding; 2017. p. 387–390. (In Russ.).
- Likhatskiy Yu, Likhatskiy E, Atamanov P. Semi-free breeding of ungulates in the Deer nature reserve. Relevant Issues of Nature Management, Hunting, and Fur Farming: Proceedings of the International scientific and practical conference dedicated to the 95th anniversary of B.M. Zhitkov All-Russian Research Institute of Hunting and Fur Breeding; 2017; Kirov. Kirov: B.M. Zhitkov All-Russian Research Institute of Hunting and Fur Breeding; 2017. p. 391–394. (In Russ.).
- Urosevic IM, Deutz A, Petrovic J, Ristic AZ, Mirceta J. Deer farming in European Union and Serbia: Veterinary legislation perspective. International Symposium on Animal Science 2016; 2016; Belgrade. Belgrade – Zemun: University of Belgrade; 2016. p. 391–398.
- Veber AEh, Simakov AF, Chuvʹyurova NI, Chalyshev AV, Badlo LP, Kochan TI, et al. Physiology of nutrition and metabolism in moose. Syktyvkar: Komi Scientific Center of the Ural Branch of the Russian Academy of Sciences; 1992. 126 p. (In Russ.).
- Azaubaeva GS. Blood pattern in animals and birds. Kurgan: Zauralʹe; 2004. 167 p. (In Russ.).
- Rostal MK, Evans AL, Solberg EJ, Arnemo JM. Hematology and serum chemistry reference ranges of free-ranging moose (Alces alces) in Norway. Journal of Wildlife Diseases. 2012;48(3):548–559. https://doi.org/10.7589/0090-3558-48.3.548
- Ponder Е. The mammalian red cell and the properties of haemolytic systems. Protoplasma. 1935;22:492–494.
- Knorre EP, Knorre EK. Physiological characteristics of the moose. Proceedings of the Pechoro-Ilych State Reserve. 1959;7:133–167. (In Russ.).
- Irzhak LI. Moose blood physiology. Proceedings of the Pechoro-Ilych State Reserve. 1964;11:61–66. (In Russ.).
- Becker SA, Kauffman MJ, Anderson SH. Nutritonal condition of adult female Shiras moose in northwest Wyoming. Alces. 2010;46:151–166.
- Moyseenko NA. Com ponents of red blood in young moose. Alces. 2002;2:93–97.
- Ivanter EhV, Korosov AV. Elementary biometrics. Petrozavodsk: PetrGU; 2005. 104 p. (In Russ.).
- Mogalev NP. Nuclear-cytoplasmic ratio and shape of erythroid cells in reindeer embryos. Archives of Anatomy, Histology, and Embryology. 1987;92(5):45–48. (In Russ.).
- Chermnykh NA. Ecological physiology of the reindeer. Ekaterinburg: UrO RAN; 2008. 196 p. (In Russ.).
- Korzhuev PA. Hemoglobin. Comparative Physiology and Biochemistry. Moscow: Nauka; 1964. 287 p. (In Russ.).
- Korzhuev PA. The respiratory function of blood and the vertebrate skeleton. Uspekhi Sovremennoi Biologii. 1955;39(2):163–195. (In Russ.).
- Gorodetsky VK. Ecological and physiological parameters of reindeer blood. Abstract cand. bio. sci. diss. Moscow: Moscow Order of Lenin and the Order of the Red Banner of Labor State University. M.V. Lomonosov; 1962. 18 p. (In Russ.).
- Petrov AK. Moose vs. cattle: growth and development. Abstract dr. bio. sci. diss. Moscow: Moscow Veterinary Academy; 1958. 28 p. (In Russ.).
- Petrov A. K. Patterns of heart development in moose. Zoologicheskiy Zhurnal. 1961;40(3):447–453. (In Russ.).
- Irzhak LI. Morphology of mammalian erythrocytes and the age-related changes in red blood cells. Biophysics, Biochemistry, and Pathology of Erythrocytes: Conference proceedings. Krasnoyarsk; 1960. p. 64–68. (In Russ.).
- Egorova EN, Pustovalova RA, Gorshkova MA. Clinical and diagnostic significance of red blood cell indices, defined automatic hematological analyzers. Volga Medical Journal. 2014;12(3):34–41. (In Russ.).
- Kochanova NE. Moose metabolism in summer. Proceedings of the Pechoro-Ilych State Reserve. 1964;11:31–54. (In Russ.).
- Irzhak LI. Physiology of the moose in the Pechoro-Ilychsky Reserve. News of the Komi Branch of the All-Union Geographical Society. 1963;(8):88–89. (In Russ.).
- Shideler SE, Stoops MA, Gee NA, Tell LA. Hematologic values for Tule elk (Cervus elaphus nannodes). Journal of Wildlife Diseases. 2002;38(3):589–597. https://doi.org/10.7589/0090-3558-38.3.589
- Kosilov VI, Irgashev TA, Shabunova BK, Akhmedov D. Clinical and hematological parameters of black-spotted cattle of different genotypes and yaks under the mountainous conditions of Tadzhikistan. Izvestia Orenburg State Agrarian University. 2015;51(1):112–115. (In Russ.).
- Bagirov VA, Aybazov MM, Mamontova TV. Morphometric and biological indicators of the west C. Caucasica. Proceedings of the Stavropol Research Institute of Animal Husbandry and Forage Production. 2014;3(7):33–40. (In Russ.).
- Novak G, Bodrova L. Hematologic parameters of blood in reindeers with different types of feeding. Bulletin of the BSSA named after V.R. Filippov. 2014;36(3):22–27. (In Russ.).
- Miller AL, Evans AL, Os Ø, Arnemo JM. Biochemical and hematologic reference values for free-ranging, chemically immobilized wild Norwegian Reindeer (Rangifer tarandus tarandus) during early winter. Journal of Wildlife Diseases. 2013;49(2):221–228. https://doi.org/10.7589/2012-04-115
- Hochachka P, Somero J. Biochemical adaptation strategy. Moscow: Mir; 1977. 398 p. (In Russ.).
- Lyakh SP. Adaptation of microorganisms to low temperatures. Moscow: Nauka; 1976. 160 p. (In Russ.).
- Sroslova GA, Postnova MV, Zimina YuA. Features of adaptation of living organisms. Science of VolSU. Natural Sciences. 2017;7(4):32–38. (In Russ.). https://doi.org/10.15688/jvolsu11.2017.4.5
- Taylor JA, Feldman BF, Zinki JG, Jain NC. Leukocyte responses in ruminants. In: Feldman BF, Zinkl JG, Jain NC, editors. Schalm’s veterinary hematology. Wiley; 2000. pp. 391–393.
- Irzhak LI. Respiratory function of blood in mammals. Moscow; Leningrad: Nauka; 1964. 183 p. (In Russ.).
- Marma BB. Veterinary and physiological observations of moose in a zoo. Proceedings of the Pechoro-Ilych State Reserve. 1967;12:74–86. (In Russ.).
- Brenner KV, Gurtler H. Further investigations on metabolic and hematological reactions of pigs restraint by means of a rope round the upper jaw. Journal of Experimental Veterinary Medicine. 1981;35:401–407.
- Kizhina AG, Uzenbaeva LB, Panchenko DV, Ilyukha VA. Morphofunctional organization of blood cells in Nordic Cervidae. Dynamics of the game animals populations in Northern Europe: Book of abstracts. The 7th International symposium; 2018; Petrozavodsk. Petrozavodsk: KRC RAS; 2018. p. 52–54. (In Russ.).
- Thorn NE, Feldman BF, Zinki JG, Jain NC. Hematology of the deer. In: Feldman BF, Zinkl JG, Jain NC, editors. Schalm’s veterinary hematology. Wiley; 2000. pp. 1179–1183.
- Vegad JL, Feldman BF, Zinki JG, Jain NC. Normal blood values of the water buffalo (Bubalus bubalis). In: Feldman BF, Zinkl JG, Jain NC, editors. Schalm’s veterinary hematology. Wiley; 2000. pp. 1085–1088.
- Koryakina LP, Maksimov VI, Machakhtyrov GN. Morphophysiological parameters and enzyme blood profile in domestic reindeer by seasons in the taiga and mountain taiga zones of Yakutia. Agrarian Bulletin of the Urals. 2008;43(1):48–50. (In Russ.).
- Reshetnikov IS. Effect of natural conditions and seasons on the reindeer thymus lymphocytes. Adaptations to Natural Conditions: Proceedings of the VI All-Union Conference on Ecological Physiology; 1982; Syktyvkar. Syktyvkar: Komi filiala AN SSSR; 1982. p. 46. (In Russ.).
- Kocan AA, Glenn BL, Thedford TR, Doyle R, Waldrup K, Kubat G, et al. Effects of chemical immobilization on hematologic and serum chemical values in captive white-tailed deer. Journal of the American Veterinary Medical Association. 1981;179(11):1153–1156.
- Wolfe G, Kocan AA, Thedford TR, Barron SJ. Hematologic and serum chemical values of adult female Rocky Mountain elk from New Mexico and Oklahoma. Journal of Wildlife Diseases. 1982;18(2):223–227. https://doi.org/10.7589/0090-3558-18.2.223
- Mautz WW, Seal US, Boardman CB. Blood serum analysis of chemically restrained white-tailed deer. Journal of Wildlife Management. 1980;44:343–351.
- Wesson JA, Sanlon PF, Kirkpatrick RL, Mosby HS. Influence of chemical immobilization and physical restraint on packed cell volume, total protein, glucose, and blood urea nitrogen in blood in white-tailed deer. Canadian Journal of Zoology. 1979;57(4):756–767. https://doi.org/10.1139/z79-093
- Kazakova MS, Lugovskaya SA, Dolgov VV. The reference values of indicators of total blood analysis of adult working population. Clinical Laboratory Diagnostics. 2012;(6):43–49. (In Russ.).
- Bleul U, Sorbiraj A, Bostedt H. Effects of duration and storage temperature on cell counts of bovine blood samples as determined by an automated haematology analyser. Comparative Clinical Pathology. 2002;11:211–216. https://doi.org/10.1007/s005800200021