Аннотация
Nanotechnology is important in food packaging because it increases shelf life, enhances food safety, and improves sensory characteristics and nutrient availability. We aimed to review scientific publications on the synthesis of nanoparticles, as well as their properties and applications in the food industry.Research and review articles published from 2020 to 2022 were obtained from the database using the keywords “nanoparticles”, “film”, and “food”. They were on the synthesis of metal and metal oxide nanoparticles and their uses in food films and coatings.
We reviewed methods for synthesizing inorganic nanoparticles from metals and their compounds (silver, zinc, iron, etc.), as well as described their antimicrobial action against foodborne pathogens. By incorporating nanoparticles into films, we can create new materials with strong antimicrobial properties in vitro. Nanoparticles can be used to develop both polymer and biopolymer films, as well as their mixtures. Composite coatings can work synergistically with metal nanoparticles to create multifunctional food packaging systems that can act as compatibilizers. Particular attention was paid to metal nanoparticles in food coatings. We found that nanoparticles reduce the rate of microbial spoilage and inhibit lipid oxidation, thereby increasing the shelf life of raw materials and ready-to-eat foods. The safety of using nanoparticles in food coatings is an important concern. Therefore, we also considered the migration of nanoparticles from the coating into the food product.
Incorporating nanoparticles into polymer and biopolymer films can create new materials with antimicrobial properties against foodborne pathogens. Such composite films can effectively extend the shelf life of food products. However, the undesirable migration of metal ions into the food product may limit the use of such films.
Ключевые слова
Nanoparticles, antibacterial effect, film, coating, packaging, food productsВВЕДЕНИЕ
The exploitation of oil resources and the production of various oil-based plastics are taking a heavy toll on the environment. Therefore, biopolymers are increasingly being used an alternative material due to their biodegradability, safety, biocompatibility, and renewability [1]. Polysaccharides, proteins, and lipids are the main components of such composites.
Films based on biodegradable materials are often supplemented with bioactive substances, such as plant extracts or particles of inorganic metals, to meet the changing needs of modern consumers [2, 3].
Biosynthesized metal-based nanomaterials are used both for medical purposes (e.g., antimicrobial coatings, drug delivery) and for catalytic water purification and environmental sensors [4–6]. Nanoparticles of inorganic metals (silver, gold, zinc oxide, titanium oxide, etc.) can act as antimicrobial agents in packaging films [7–9].
Nanoparticles of various metals, including titanium, copper, magnesium, and especially silver and gold, are known to have antimicrobial, antiviral, and antifungal properties [10, 11]. These nanoparticles are being actively studied as disinfectants, ingredients in the food industry, and as additives in clothing and medical devices [12].
He et al. described the antibacterial activity of gold nanoparticles against Escherichia coli [13]. In particular, they disrupt the membrane potential by inhibiting adenosine triphosphatase (ATPase) activity and decrease cellular ATP levels. Another effect of gold nanoparticles is the inhibited binding of transfer RNA to the ribosome subunit.
Current studies of plant tissues and extracts as reducing agents are of great interest in the field of biosynthesized antibacterial nanoparticles. Biosynthesized nanoparticles may need to be purified from pathogenic or poisonous compounds, especially when used in vivo [14]. However, toxicity problems can be eliminated by using only well-studied and qualitative plant extracts for biosynthesis [15]. For example, green tea extract from camellia leaves is a widely used reducing agent for nanoparticle biosynthesis. Vaseeharan et al. used green tea extract to obtain silver nanoparticles in the study of antibacterial activity against Vibrio harveyi [16].
Several studies have described the antifungal activity of biosynthesized nanoparticles against Aspergillus niger. Gajbhiye et al. reported the antifungal properties of biosynthesized nanomaterials against Phoma glomerata, Phoma herbarum, Fusarium semitectum, Trichoderma sp., and Candida albicans in combination with fluconazole, a triazole antifungal drug [17].
Biosynthesized nanomaterials have been applied in almost all the areas where traditional nanomaterials have been in use. One of the challenges, however, has been in separating biogenic nanoparticles from biological material, since contamination with biological cells can cause allergens and pathogens to be unintentionally introduced into the product.
There is a growing interest in the production of packaging materials with nanocomponents used as antimicrobial agents, including biocoatings for food products [18–20].
The recent growth of publications on the synthesis of nanomaterials indicates much broader possibilities of their application to be discovered. Meanwhile, researchers are fine-tuning the synthesis methods used to create new biosynthetic forms of nanomaterials. Our interest was in metal nanoparticles with antibacterial action and their application in films and coatings, including those used for food products. We aimed to review the existing literature on the synthesis of nanoparticles, as well as their properties and applications in the food industry.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
We reviewed the latest Russian and foreign publications on the synthesis of metal nanoparticles and their compounds, as well as on their uses in films and coatings for food products. The data were correlated and classified.
A film was defined as a thin material based on synthetic and/or natural raw materials in various proportions that can be used as a packaging or a separating layer.
A coating was defined as a thin material applied directly to a food product.
A packaging material was defined as a material intended for the manufacture of packaging, containers, or auxiliary means of packaging.
Publications (articles and reviews) were searched in the Scopus-indexed journals (as of April 2022) using the keywords “nanoparticles”, “film”, and “food”. The search period was limited to 2020–2022. These search criteria ensured that the publications were from credible academic sources.РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
Synthesis of nanoparticles. Nanotechnologies are important in food packaging since they increase shelf life and improve the food’s quality, safety, sensory characteristics, and nutrient availability. In the food industry, nanoparticles are used as part of active or smart packaging in the form of nanotubes, nanoemulsions, nanocomposites, nanocapsules, nanofibers, etc., which can be of both organic and inorganic origin [21]. This review covers inorganic particles obtained from transition metals (silver, iron) and metal oxides (zinc oxide, titanium dioxide). Such nanoparticles are the most promising due to their antibacterial properties and a high surface to volume ratio [22].
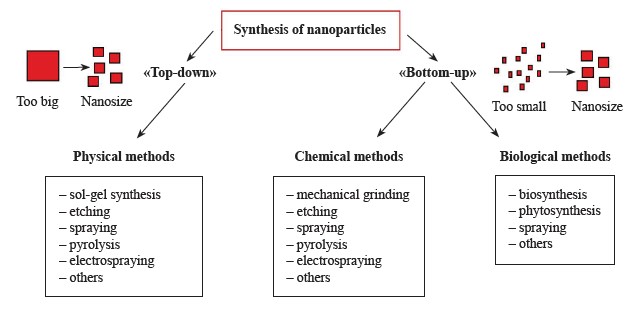
Nanoparticles can be synthesized in two ways: “bottom-up” and “top-down”. Methods for their synthesis can be divided into physical, chemical, and biological (Fig. 1).
In the bottom-up method, atoms, molecules, or small particles join together to form a nanostructured building block of nanoparticles. Its examples include physical and chemical vapor deposition, liquid state synthesis, chemical reduction, gas phase, and solvothermal methods [23]. In contrast, the top-down method involves reducing the size of the bulk material through various physical and chemical treatments. Some of the physical methods include lithography, laser ablation, sputtering, electrochemical pulse etching, and vapor deposition [24].
Zinc oxide nanoparticles obtained chemically were proposed as a superhydrophobic coating [25]. Such coatings can be used in the medical and food industries due to their high antimicrobial and hydrophobic properties, which help minimize surface contamination.
Biocompatible hybrid Ag-TiO2 nanorods were obtained by successive synthesis of TiO2 nanoparticles from titanium (IV) butoxide on a Teflon tube at 180°C for 18 h [26]. Next, they were mixed with a solution of silver nitrate at 120°C in nitrogen atmosphere and the resulting nanoparticles were stabilized in cyclohexane. Due to their ultra-small size and hybrid nature, the nanocomposites efficiently accumulated in breast cancer cells and generated large amounts of reactive oxygen species to ablate tumor cells. In addition, Ag-TiO2 nanorods showed significant antibacterial activity against Escherichia coli and Staphylococcus aureus compared to TiO2 nanoparticles, which was due to the synergistic properties of Ag and TiO2 nanoparticles.
Biological (“green”) methods for synthesizing metal nanoparticles use plant material, bacteria, fungi, algae, and even viruses [27]. These economical methods produce environmentally friendly nanoparticles from biocompatible materials, since biological molecules are used as stabilizers and protective agents.
Biosynthetic ZnO nanoparticles obtained from Aspergillus fumiga leaf extract showed high antimicrobial activity against E. coli (Gram-negative bacteria) and S. aureus (Gram-positive bacteria), as well as strong antifungal activity [28]. A. fumiga leaf extract became more active in the presence of ZnO nanoparticles. Thus, the resulting nanoparticles can act as effective antimicrobial and antioxidant agents for commercial use in biomedicine and can be used as a substrate in drug therapy.
The biosynthesis of silver nanoparticles using an extract of Ganoderma lucidum can produce crystals with an average size of 11.38 ± 5.51 nm [29]. In biological tests, colloidal nanoparticles demonstrated antimicrobial activity against S. aureus, E. coli, Pseudomonas aeruginosa, Salmonella enterica, and Candida albicans with IC50 values of 17.97, 17.06, 1.32, 54.69, and 27.78 µg/cm3, respectively. The antioxidant capacity of silver nanoparticles was evaluated using 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical reagent (IC50 = 447.120 ± 0.084 µg/cm3). In addition, colloidal silver nanoparticles had better antitumor activity against human epidermal carcinoma cells with IC50 values of 190.06 ± 3.62 μg/cm3, compared to a pure extract of G. lucidum.
Antibacterial action of metal nanoparticles. Nanoparticles are increasingly being used in the treatment of infectious diseases. In addition, they are used in the production of films and packaging that exhibit antibacterial activity against foodborne pathogens, thereby extending the product’s shelf life.
Structurally, nanoparticles are three-dimensional particles ranging from 1 to 100 nm [30]. Their physicochemical properties, which underlie their antimicrobial activity, include size, charge, surface morphology, crystal structure, and zeta potential [31]. The small size is the main advantage of nanoparticles that ensures high antimicrobial activity against intracellular bacteria since nanoparticles can easily penetrate through bacterial cell walls [32]. When nanoparticles electrostatically bind to bacterial cell walls, they damage the membrane, which ultimately leads to changes in its potential and depolarization [33]. As a result, bacterial cells lose their integrity and experience respiratory failure. Moreover, this causes imbalance within the bacteria, interrupts energy transduction, and ultimately leads to cell lysis.
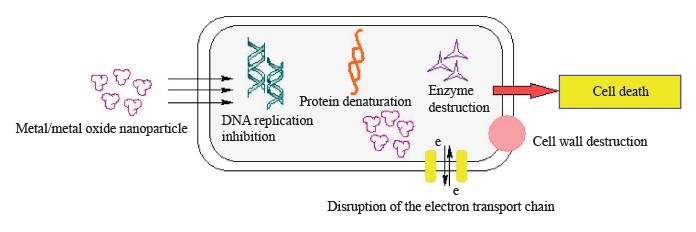
The key processes that underlie the antimicrobial action of nanoparticles include the destruction of cell walls and membranes, which leads to oxidative stress, impaired energy transduction, enzyme inhibition, photocatalysis, and interference in DNA and RNA [34] (Fig. 2).
Destruction of cell walls and membranes. Cell walls and membranes are the first line of defense for bacteria. Both act as important protective barriers, helping bacteria maintain their shape and protecting them from the external environment [35]. Nanoparticles start their antimicrobial action by causing damage to cell walls and membranes, which leads to cell lysis. The pathway of nanoparticle adsorption is determined by the components of the bacterial cell membrane [36]. Nanoparticles have a stronger antimicrobial effect against Gram-positive bacteria than against Gram-negative ones. The cell wall of Gram-negative bacteria consists of lipoproteins, lipopolysaccharides, and phospholipids which allow only macromolecules to penetrate it, forming a barrier between the cell membrane and its environment. Conversely, the cell wall of Gram-positive bacteria contains a thick layer of peptidoglycan and teichoic acid with numerous pores that allow foreign molecules to easily penetrate the membrane. This leads to membrane disruption, loss of cytoplasmic constituents, and, ultimately, to apoptosis.
The degree of destruction of cell walls and membranes depends on the size and charge of nanoparticles. In particular, smaller-sized Ag nanoparticles showed lower values of minimum inhibitory and minimum bactericidal concentrations, exhibiting great antimicrobial activity [37].
Disruption of the mitochondrial electron transport chain. Another antimicrobial mechanism of nanoparticles is that they disrupt the mitochondrial electron transport chain. Flores‐López et al. reported that the strong binding of Ag and Ag+ nanoparticles to thiol groups in cysteine residues impaired mitochondrial membrane proteins, membrane permeability, and mitochondrial functions [38]. Nanoparticles can accumulate in mitochondria, causing the mitochondrial membrane to depolarize and blocking the mitochondrial electron transport chain. This is due to the activation of an enzyme associated with nicotinamide adenine dinucleotide phosphate [39]. Blocking the mitochondrial electron transport chain further increases cellular levels of reactive oxygen species through electron transport.
Overproduction of reactive oxygen species. Mitochondria play a major role in the regulation of intracellular levels of reactive oxygen species. Therefore, the disruption of the mitochondrial electron transport chain leads to their excessive production [40]. The control over reactive oxygen species production regulates certain processes such as the initiation of defense against pathogens, programmed cell lysis, and energy generation through mitochondria.
Inhibition of proteins and enzymes. As a result of interacting with nanoparticles, vital proteins lose their important functions and enzymes become inhibited. In particular, nanoparticles cause structural changes in the proteins adsorbed on them. For example, new secondary bonds are formed and the initial bonds are destroyed during protein adsorption on Au nanoparticles, and such changes are irreversible [41]. Structural changes in proteins, which are caused by interactions between proteins and peptides binding to nanoparticles, can lead to chemical denaturation and the formation of fibrils due to thermodynamic instability. This subsequently leads to the loss of protein functions. The possibility of interaction between nanoparticles and proteins is quite high, since proteins make up to 35% of biological fluids by volume.
Oxidative damage to DNA and RNA. DNA is the fundamental molecule of a living organism. DNA strand breakage is a standard biomarker of DNA damage, natural or caused by other factors [42]. It is believed that nanoparticles can induce DNA damage by inhibiting DNA replication. Nanoparticles can change the ability of cells to repair induced DNA damage due to the overproduction of reactive oxygen species [43]. DNA is particularly susceptible to oxidative damage since the production of HO• from Fenton reactions can attack nucleic bases or sugar phosphate and lead to the fragmentation of saccharides and strand breakage. Nanoparticles can also initiate a double-strand break by modulating replication forks, which further leads to apoptosis.
Properties of nanoparticle-based films. By using nanoparticles in the production of films, scientists can create new materials with strong antimicrobial properties in vitro. Nanoparticles of metals, oxides, or their combinations have been used to develop polymer and biopolymer films, as well as their mixtures. There is a trend towards using nanomaterials based on particles of silver or zinc. The effects of metal nanoparticles on the physicochemical and antibacterial properties of films are described below.
Silver. Polyethylene nanocomposite films containing synthesized silver and titanium dioxide nanoparticles showed antibacterial efficacy against E. coli and S. aureus in the cultural research method [44]. Polyethylene films obtained by extrusion and containing Ag/TiO2/montmorillonite clay had bactericidal properties and therefore potential applications in medicine and food packaging to prevent bacterial contamination. In addition, irradiation of the composites obtained by reducing ions to nanosilver on the surface of TiO2/montmorillonite clay caused complete death to the pathogens tested. Those composites which had a smaller size due to the irradiation of surface films showed a more stable bactericidal activity.
The scientists developed a two-layer food packaging film with unique properties. Particularly, the film prevented bacterial growth, inhibited oxidative processes, increased the safety of food products, blocked UV penetration, and was oil-resistant and transparent. It had an Ag-organometallic basis with p-coumaric acid, chitosan nanoparticles, and a mixture of polyvinyl alcohol and starch. As described in [45], a two-layer film is composed of separate previously obtained components to be subsequently used as a packaging material.
An environmentally friendly method was applied for the biosynthesis of hybrid Ag/AgCl nanoparticles using Levan from Bacillus mojavensis. Such nanoparticles can be used as highly efficient antimicrobial agents against a wide range of foodborne bacteria [46]. The morphological and physicochemical characteristics of Levan Ag/AgCl nanoparticles were studied by transmission electron microscopy, X-ray diffraction, UV visual spectroscopy, dynamic light scattering, and thermogravimetric analysis. Levan-Ag/AgCl was analyzed for antibacterial activity against foodborne pathogenic bacteria (E. coli, Klebsiella pneumoniae, S. enterica, P. aeruginosa, S. aureus, Micrococcus luteus, Listeria monocytogenes, Enterococcus faecalis, Bacillus subtilis, and Bacillus thuringiensis) by diffusion on agar. The study demonstrated high antimicrobial activity of Levan-Ag/AgCl nanoparticles as a potent inhibitor against all the tested strains, with higher efficacy against Gramnegative than against Gram-positive bacteria. Due to these properties, Levan-Ag/AgCl nanoparticles can be used as a component in packaging films to increase the shelf life of beef. Their antimicrobial activity may be due to a slow release of silver ions and their interaction with negatively charged biomolecules. These biomolecules damage the cell wall, cause structural changes in the protein and its biofunction, and ultimately lead to cell death.
The size of metal and oxide nanoparticles significantly affects the transmission of light through a film [47]. A number of studies have shown that metal nanoparticles increase the hydrophobicity of film materials. For examples, silver nanoparticles caused a 77% increase in the hydrophobicity of a film based on polyvinyl alcohol and guar gum, compared to the control group.
A biocomposite consisting of sodium alginate, oxidized nanocellulose, and silver nanoparticles was introduced into a paper matrix for food packaging. It inhibited the growth of Gram-positive (S. aureus, B. subtilis) and Gram-negative (E. coli, Pseudomonas aeuroginosa) bacteria [48].
A sandwich-like chitosan nanocomposite film was produced from corn stalk as a green reducing agent, silver nanoparticles, and graphene oxide nanoparticles used for stabilization and controlled release of nanosilver in the inner layer and chitosan in the outer one [49]. The results showed that the sandwich-like film released silver nanoparticles during 14 days, with a total amount of only 1.9%. The nanocomposite film had a high antibacterial activity against E. coli and S. aureus and did not exhibit toxicity to cells.
Silver nanoparticles synthesized using Saraca asoca leaf extract were used to produce nanocomposite films based on rice starch [50]. They had a spherical shape with a diameter of 27 to 45 nm. Silver nanoparticles improved the tensile at break of rice starch films and reduced their elongation at break. In addition, the films showed lower water solubility, water-holding capacity, and vapor permeability. Silver nanoparticles in the form of colloids or discrete films exhibited high antibacterial activity against common foodborne pathogens (E. coli, S. aureus and B. subtilis).
Silver nanoparticles can be synthesized in situ in a pectin matrix using γ-irradiation at 2.5 and 5 kGy. They provide nanocomposite films with antibacterial activity against E. coli and Salmonella typhimurium, as shown by the total colony count [51].
Dash et al. studied the physicochemical, mechanical, barrier, and thermal properties of flax protein-alginate films produced with various concentrations of silver nanoparticles [52]. The study showed that all the films with silver nanoparticles had a strong antibacterial effect, compared to the control film. According to the inhibition zones, the antibacterial activity against Gramnegative bacteria (E. coli) was higher than that against Gram-positive bacteria (S. aureus).
The amount of active ions released also affects the film’s antimicrobial properties. In a study by Wang et al., silver nanoparticles in polymer films based on chitosan or polyvinyl alcohol (PVA) affected the growth of Pseudomonas fluorescens differently [53]. The study was carried out both on agar and on a model food hydrogel. In particular, the film based on PVA exhibited a stronger antimicrobial effect than the one based on chitosan. This correlated with a larger amount of silver ions released from the PVA film into hydrogel. The study showed that the strength of interaction between silver nanoparticles and the film polymer is a key factor that determines the release of antimicrobials and, therefore, the antimicrobial activity of the packaging film.
Sallak et al. created a film from corn starch containing Satureja khuzestanica essential oil and Ag-TiO2 nanocomposites (sized 30–60 nm) and assessed its antimicrobial, morphological, physical, and mechanical characteristics [54]. The film showed a reduction in S. aureus and E. coli of 3–4 and 6–7 log (CFU/mL), respectively, compared to the control.
Silver nanoparticles based on Ricinus communis leaf extract exhibited high antioxidant activity by absorbing DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals [55]. They also showed antibacterial activity against Grampositive (S. aureus) and Gram-negative (E. coli, P. aeruginosa) bacteria, as determined by agar plate diffusion.
Zinc. A nanocomposite based on a copolymer of lactic and glycolic acids and nanozinc oxide promoted the formation of long-lived reactive protein species [56]. It also caused 8-oxoguanine, a key biomarker of oxidative stress, to appear in DNA. The nanocomposite exhibited significant bacteriostatic properties, which depended on the concentration of nanoparticles. Its surface was non-toxic to eukaryotic cells and did not prevent their adhesion, growth, or division. Due to its low cytotoxicity and bacteriostatic properties, this nanocomposite can be used as a coating material in the food industry, an additive for textiles, and in biomedicine.
A nanocomposite film consisting of polylactic acid (PLA), zinc oxide (ZnO) nanoparticles, and graphene oxide (GO) is a biodegradable polymer that can be used as food packaging [57]. ZnO and GO increased the strength of the resulting packaging material and gave it antibacterial properties against E. coli and B. subtilis cells, as determined by counting viable colonies. The PLA/ZnO/GO composite was synthesized by a successive mixing of graphene oxide and zinc oxide nanoparticles in acetone under ultrasound at 40°C and a subsequent addition of a PLA solution with vigorous stirring for 3 h. The resulting composite was dried at room temperature on an acrylic plate.
A functional film based on carrageenan/agar was obtained by combining tea tree oil emulsion and zinc sulfide nanoparticles, which were uniformly dispersed in a binary polymer matrix [58]. Zinc sulfide nanoparticles improved the film’s mechanical strength, while the tea tree oil emulsion slightly reduced it. However, due to their combined use, the film retained its mechanical strength and became slightly more flexible. These components slightly increased the vapor barrier, water resistance, and thermal stability of the film. In addition, the composite membrane showed pronounced antioxidant and antibacterial activity.
Tymczewska et al. described a “green” method for obtaining active films based on gelatin/polyvinyl alcohol, black cumin cake extract, and zinc oxide nanoparticles [59]. The nanoparticles were preliminarily synthesized from zinc nitrate hexahydrate and an aqueous extract of black cumin cake in an alkaline medium at 60°C. The film had strong antibacterial properties against Gram-positive bacteria (M. luteus, L. monocytogenes, and S. aureus). Black cumin cake extract and zinc oxide nanoparticles also increased the films’ antioxidant activity, as determined by the DPPH and ABTS methods (2,2-azino-di-[3-ethylbenzthiazoline sulfonate). In addition, these components affected the physicochemical characteristics of the films. In particular, they reduced their tensile at break and vapor permeability and increased their opacity and elongation at break. These nanocomposite materials can be recommended to reduce microbial spoilage and inhibit oxidative rancidity of packaged foods.
Zinc oxide nanoparticles are also used in nanofibrous membranes for wound dressings [60]. Titanium oxide nanoparticles in silk fibers and collagen, which are synthetic membranes for skin tissue regeneration, exhibit antibacterial activity against S. aureus and E. coli [61].
In another study, a functional biodegradable food packaging film was produced from soy protein isolate, a natural antimicrobial agent (mangosteen peel extract, 10 wt.%), and functional modifiers (zinc oxide nanoparticles) at various concentrations by solution casting [62]. The triple combined composite films exhibited significantly improved mechanical properties, water vapor permeability, water solubility, UV barrier, antioxidant properties, and thermal stability. Due to the antibacterial properties of the plant extract and ZnO nanoparticles, the composite films showed excellent antibacterial action against E. coli and S. aureus.
Zinc nanoparticles were used to prepare a functional composite film based on pectin/agar [63]. Their integration significantly improved the film’s physical properties, such as mechanical and UV protective properties, without affecting its transparency too much. In addition, zinc nanoparticles had no effect on the film’s hydrophobicity, vapor barrier, and thermal properties. Finally, the composite film showed strong antibacterial activity against pathogenic foodborne bacteria E. coli and L. monocytogenes.
Lee et al. analyzed the antibacterial activity of composite films based on chicken skin gelatin/tapioca starch and zinc oxide nanoparticles (0–5%) obtained by casting [64]. They found that the inhibition zones for both Gram-positive S. aureus (16–20 mm) and Gram-negative E. coli (15–20 mm) were greater in the film with 5% zinc oxide. Overall, the films based on chicken skin gelatin and tapioca starch with 3% zinc oxide nanoparticles were found to be optimally formulated and have good physical, mechanical, and antibacterial properties.
Well-dispersed zinc oxide (ZnO) nanoparticles were placed in situ on a non-toxic natural palygorskite (PAL) nanorod to form an antibacterial composite PAL@ZnO nanorod. This nanorod was embedded in a chitosan/ gelatin-based film to obtain composite films with markedly improved mechanical properties and antibacterial activity against S. aureus and E. coli bacteria (inhibition zones of 21.82 ± 0.95 and 16.36 ± 1.64 mm, respectively) [65]. In addition, PAL@ZnO nanorods significantly improved the film’s water and heat resistance.
In another study, bionanocomposite films based on bovine gelatin supplemented with chitosan and zinc oxide nanoparticles showed better thermal stability and tensile at break, as well as lower elongation at break and solubility [66]. In addition, gelatin-based biocomposite films exhibited antibacterial properties against S. aureus and E. coli.
The physicochemical properties of nanomaterials depend on their size and shape, the key factors for biomedical applications. Hasanzadeh et al. used the hydrothermal method to obtain ZnO nanostructures of various shapes, including nanospheres, nanoplates, and nanopyramids [67]. They studied the antibacterial activity (colony count) of nanostructures against E. coli and found that ZnO nanopyramids obtained at 70°C had a stronger inhibitory effect than nanospheres and nanoplates.
Iron. Appu et al. biosynthesized chitosan-coated Fe3O4 nanocomposites by mixing solutions of iron chloride and broccoli extract while heating to be subsequently leached [68]. Next, the resulting Fe3O4 nanoparticles were mixed with a solution of chitosan in acetic acid at 60°C. The nanocomposite material was analyzed by Fourier-transform infrared spectroscopy, X-ray photoelectron spectroscopy, electron microscopy, an X-ray diffractometer, and a vibrating sample magnetometer. In addition, the nanoparticles exhibited significant antibacterial efficacy against foodborne bacterial pathogens, such as S. aureus and E. coli, with inhibition zones of 11.5 and 13.5 mm, respectively.
Yaseen et al. compared the antibacterial, antioxidant, and larvicidal activity of Alstonia scholaris and Polyalthia longifolia extracts and that of Fe2MgO4 nanoparticles based on them [69]. The study objects were active against Gram-positive (B. subtilis, S. aureus) and Gram-negative (P. aeruginosa, S. typhimurium, and E. coli) bacteria in the following order: A. scholaris-based Fe2MgO4 > P. longifolia-based Fe2MgO4 > A. scholaris extract > P. longifolia extract. A similar correlation was observed for larvicidal activity against Aedes albopictus larvae. In particular, Fe2MgO4 nanoparticles exhibited excellent larvicidal activity with an LD50 of 5–10 μg/cm3, a much lower dose than for the plant extracts. The antioxidant potential of Fe2MgO4 nanoparticles was determined by DPPH and phosphomolybdenum assays. It showed that Fe2MgO4 nanoparticles performed better than pure plant extract (controls).
Nickel. Ramji and Vishnuvarthanan created nanocomposite films based on polylactic acid with silica and nickel oxide nanoparticles in various concentrations (0.25, 0.5, 0.75, and 1 wt.%) and studied the nanoparticles’ effect on the film’s extensibility, barrier, surface color, opacity, and antibacterial properties [70]. The authors found that the films had good antibacterial activity against Gram-positive (L. monocytogenes) and Gramnegative (Salmonella) bacteria.
In another study, active nanocomposite films were obtained by incorporating nickel oxide nanoparticles (3, 6, and 9 wt.%) into chitosan-based films [71]. They were synthesized by burning the solution, and the films were obtained by casting in solvents. Fourier-transform infrared spectroscopy and X-ray diffraction analysis confirmed the formation of new interactions and an increase in crystallinity. In addition, the nanocomposite films showed good antibacterial activity against Grampositive (S. aureus) and Gram-negative (S. typhimurium) bacteria.
Titanium. According to antibacterial tests, none of the bacterial species was sensitive to TiO2 nanoparticles at any concentration under dark conditions, indicating that their antibacterial effects were due to photocatalytic reactions. Without light excitation, TiO2 nanoparticles were not toxic to bacteria, as shown by CFU values. The nanoparticles only absorbed on the bacterial cell wall. To ensure the adsorption–desorption equilibrium of TiO2 nanoparticles doped with phosphorus and fluorine (PF-TiO2), they were thoroughly mixed with bacterial suspensions under dark conditions and incubated for 15 min [72]. Light can induce charge separation in nanoparticles, and redox reactions can subsequently occur on their surface. The photocatalytic reactions confirmed the presence of hydroxyl (OH•) and superoxide (O2•-) radicals, as well as singlet oxygen.
Ezati et al. synthesized nanoparticles of titanium dioxide (TiO2) and Cu-active-TiO2 (Cu-TiO2) by the solgel method, using carboxymethylcellulose (CMC) as a nanofiller to obtain functional packaging films [73]. The nanoparticles increased the films’ mechanical and thermal stability, as well as their vapor barrier properties. Unlike the CMC/TiO2 film, the CMC/Cu-TiO2 film exhibited significant antibacterial activity against foodborne pathogenic bacteria (L. monocytogenes and E. coli) under visible light. The CMC/Cu-TiO2 film also significantly delayed the browning of bananas when stored at 25°C for 14 days under visible light. The researchers assumed that the nanoparticles exhibited photocatalytic activity in visible light. Therefore, they can be used in functional packaging films to extend the shelf life of fruits after harvest, as well as in active packaging.
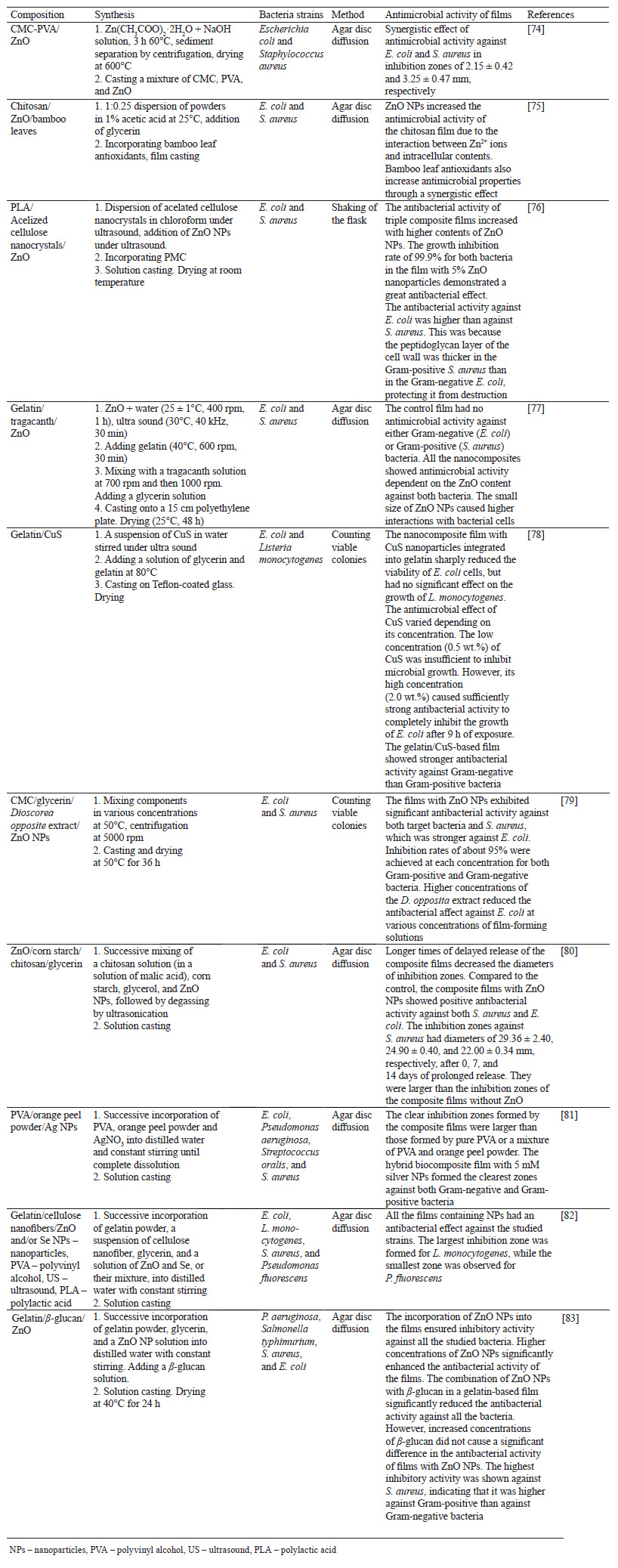
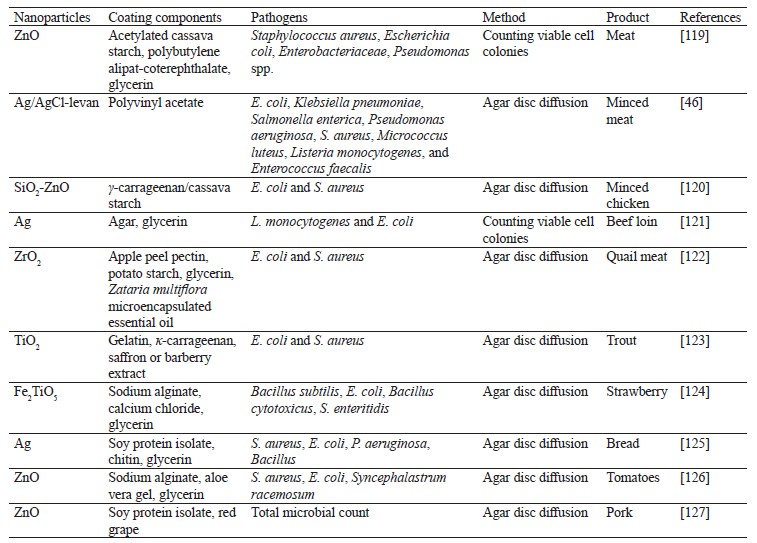
Films with antibacterial properties are mainly obtained from solutions and suspensions of film components that are molded by casting and dried under various conditions. Table 1 presents various films with metal and metal oxide nanoparticles used as antibacterial agents.
Nanoparticles incorporated in food coatings. The food industry involves a range of processes, including the preparation of raw materials, production, packaging, storage, etc. Packaging ensures food quality and safety, affecting the product’s shelf life and marketing. Traditionally, packaging performs the following functions: storage; transportation; protection from physical damage, as well as chemical or biological deterioration; and branding [84–86].
Growing consumer concern and interest in health, nutritional value, food safety, and environmental issues has driven the development of biodegradable packaging. Since synthetic packaging materials are not biodegradable, they contribute to environmental pollution, climate change, and the depletion of natural resources. Despite excellent properties, high mechanical strength, low production cost, and process optimization, these non-renewable materials have a significant environmental impact in terms of greenhouse gas emissions, as well as land and water footprints [87]. Therefore, alternative ways of obtaining biopolymers are gaining more attention, since they address environmental issues, including the end-of-life treatment of plastic film waste.
Biodegradable films are commonly made from renewable sources mainly composed of proteins, carbohydrates, and lipids. Biopolymers have been extensively studied due to their abundance, good film-forming ability, transparency, and excellent barrier properties against O2, CO2, and lipids [88].
Currently, biopolymer food packaging is produced from a variety of materials, including natural agents, plant extracts, and nanomaterials. Such technologies can work synergistically along with nanotechnologies, creating multifunctional food packaging systems of high quality that can act as compatibilizers [89].
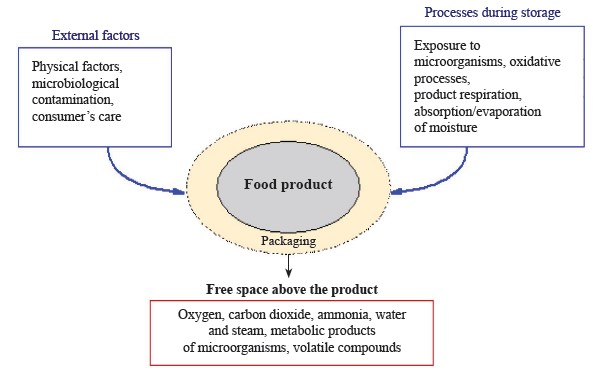
In recent years, bioactive films and smart technologies have been in wide use for packaging in the food sector [90, 91]. Plastic films have served as a barrier to protect food from heat, moisture, micro-organisms, dust, and dirt. Recent advances, such as smart packaging, active packaging, and intellectual packaging, have provided barrier films with extra functions [92–96]. These packaging systems are an effective way to extend the shelf life of foods produced through simplified processes or with a minimal use of preservatives [97, 98]. Yet, there cannot be a single package to suit all food products, since they vary greatly in chemical and microbiological components. In addition, food products are complex systems that change over time. Therefore, it is extremely important to develop optimal packaging for different types of products that meets their specific requirements. Figure 3 shows changes in the product-packaging system over time.
Cheese. Cheeses are mainly produced from pasteurized milk and usually have a low risk of microbiological contamination. However, foodborne pathogens can appear in cheese due to its improper handling during processing or due to re-opening of the packaging during storage [99]. Soft cheeses are an excellent environment for pathogenic microflora due to their high water activity, high protein content, and a moderate amount of fat [100].
Han et al. described the preparation of a nanosilver/ graphene/PLA coating and its use as a package for curd cheese [101]. Three days of storage showed no difference in sensory parameters, regardless of the type of packaging. The cheese packaged in a nanosilver/graphene/ PLA coating had minimal changes in appearance and color. It was uniform, slightly matte, white, and slightly pungent. The scientists also evaluated a possibility of reusing the active packaging.
The soft cheese samples were packaged in alginate composite coatings (ZnO nanoparticles and lemongrass essential oil) and stored at 4 ± 1°C and 75% relative humidity for 14 days [102]. The control sample packaged in a pure alginate coating became quite hard and translucent with visible signs of spoilage, while the test samples were whiter and softer, and retained the original creamy texture. The weight loss of the cheeses increased linearly with storage time due to the continuous movement of water vapor from the cheese into the environment. The pH values were monitored daily and found to be in the range of 4.45–4.57, indicating little change. However, the pH of the control sample dropped to 4.33, probably due to increased fermentation stimulated by microorganisms.
In another study, a coating with TiO2 nanoparticles or a combination of TiO2 and Ag in the PLA matrix increased the shelf life of chilled Yunnan curd from 5– 7 days to 25 days at 5 ± 1°C [103]. Yet, no changes were observed in pH, as well as physicochemical and sensory indicators. The incorporation of nanoparticles effectively inhibited the growth of microorganisms, including yeasts and molds. The authors also tested the migration of nanoparticles from the composite coating in line with the standards of the European Food Safety Authority. Although the levels of metal ion migration reached 20.04 ± 1.38 µg/kg at the end of the shelf life, those values were below the acceptable migration limit of 104 µg/kg for food contact materials.
Meat and poultry. Meat and poultry, as well as their products, are very sensitive to storage conditions. When refrigerated, they have a shelf life of only a few days due to microbial growth and lipid oxidation [104]. Large amounts of moisture and nutrients make them perishable products. Bacterial growth causes unpleasant smell and taste, as well as mucus. Moreover, lipid oxidation changes the sensory characteristics of meat and poultry, which also limits their shelf life [105].
A simple method was proposed to obtain translucent antimicrobial nanocomposite films using regenerated cellulose and zinc oxide nanoparticles. The resulting nanocomposite coatings were transparent enough for food packaging, including meat products. They showed good mechanical strength, UV barrier properties, water vapor permeability, and antimicrobial activity against six pathogenic foodborne bacteria [106]. According to the tests, adding 3 wt.% zinc oxide nanoparticles effectively inhibited the growth of pathogenic Gram-positive (Bacillus cereus, S. aureus, and L. monocytogenes) and Gram-negative (E. coli, S. typhimurium, and Vibrio parahaemolyticus) bacteria. The UV transmission and oxygen permeability of the coatings reduced by 32 and 37%, respectively.
Despite high antibacterial activity, silver nanoparticles pose potential danger to human health. Therefore, they need to be incorporated into safer compounds. Zhao et al. used iturin A to synthesize silver nanoparticles to obtain a composite that can prevent chicken from bacterial contamination [107]. The composite material showed a broader antimicrobial spectrum, a lower concentration of silver, and higher antibacterial and antifungal activity, compared to the control. When applied to paper packaging for chicken, the iturin-Ag nanoparticle complex showed no silver residue, which demonstrated its high potential for food preservation.
Minced meat was placed in composite coatings (gelatin, chickpea protein, copper sulfide nanoparticles, and microencapsulated Nigella sativa essential oil) and stored for 14 days at 4°C [108]. The microbiological tests (L. monocytogenes, S. aureus, Enterobacteriaceae, and Pseudomonas spp.) showed that the simultaneous use of microencapsulated essential oil and CuS nanoparticles had a positive synergistic effect in increasing the shelf life of minced meat, compared to other samples.
Alginate-based nanocomposite coatings containing TiO2 nanoparticles and caraway essential oil showed a high potential for extending the shelf life of beef [109]. The researchers studied their effect on chemical processes (changes in pH, total volatile basic nitrogen, peroxide value, and thiobarbituric acid-reactive substances) and microbial spoilage (changes in viable enterobacteria, lactic acid bacteria, L. monocytogenes, and Pseudomonas spp.) during 24 days of storage (4°C). The active coatings significantly reduced lipid oxidation, microbial growth, and total volatile basic nitrogen. They also improved the beef’s sensory properties, retained its color for longer, and increased its shelf life from 4 to 16 days.
Hybrid chitosan-ZnO nanoparticles in combination with clove essential oil were found to have a good potential in active packaging materials [110]. The scanning electron microscope images showed a uniform distribution of the components with their minimal aggregation in the nanocomposite coatings. These components improved the film’s tensile at break (~ 39.82%), film hydrophobicity (~ 35.36%), ability to block ultraviolet light, water vapor barrier (~ 84.64%), and oxygen barrier (~ 57.66%), compared to the control film. In addition, they increased antioxidant activity and antibacterial activity against P. aeruginosa, S. aureus, and E. coli. The nanocomposite coatings extended the shelf life of chicken meat up to 5 days when stored at 8 ± 2°C. The researchers also determined the total migration limit in distilled water, 3% acetic acid, and 50% ethanol. According to the results, the coatings had a total migration rate below the allowable limit of 1000 µg/dL for model systems. The overall migration limit increased in the following order depending on the solvent: distilled water < 50% ethanol < 3% acetic acid.
Fish and seafood. Like meat and its products, fish and seafood have a short shelf life that is limited to a greater extent by microbiological spoilage.
Paidari and Ahari studied the effect of nanosilver and nanoclay incorporated into packaging materials on the growth of pathogens (V. parahaemolyticus, S. aureus, and E. coli) in shrimp stored for 6 days at 4°C [111]. They found that nanopackaging reduced the number of colonies by more than one logarithmic cycle, which is a significant reduction (p < 0.05). A synergistic effect was also observed for packaging with both nanosilver and nanoclay particles. Plasma-mass spectroscopy showed no migration of coating components (Ag, Al, and Si) in the shrimp samples on day 6 of the experiments.
Incorporating anthocyanins into packaging materials helps control the process of food spoilage. For example, pH-sensitive and antibacterial coatings based on chitosan/polyvinyl alcohol/ZnO nanoparticles and containing extracts of purple potato or rosella helped monitor the degree of freshness in shrimp by changing the color from purple to light green [112]. The coatings absorbed total volatile nitrogen released from the spoiled shrimp, which caused hydroxide ions to form and induce discoloration of the anthocyanins in the coating matrix. Therefore, such coatings have promising potential as active and intelligent packaging materials for food.
Efatian et al. studied a possibility of using a composite coating for packaging Nile tilapia fish stored at 4 and –20°C for 5 and 10 days [113]. The coating consisted of low-density polyethylene, silver nanoparticles, or a mixture of silver, copper, and titanium oxide nanoparticles. Antibacterial tests showed that the PNP/Ag + Cu/ TiO2 nanocomposite coating exhibited strong antibacterial activity against E. coli and L. monocytogenes. The researchers also evaluated the chemical characteristics (pH, protein and fat concentrations, free fatty acid profile) of the coated fish samples, as well as the migration of nanoparticles. According to the results, the samples packaged in Ag + Cu coatings had the least changes in pH and a free fatty acid profile, compared to fresh samples (p < 0.05). The migration of Ag and Cu nanoparticles from the coating into the tilapia samples was negligible, with Ag and Cu releases of < 2.0 and < 10 μg/kg, respectively.
Fresh fruits. Fresh fruits contain minerals, vitamins, phenolic compounds, and other nutrients. However, if not properly preserved, they quickly deteriorate and have a shelf life of only 5–7 days after harvest [114]. Currently, various types of processing (drying, jamming, etc.) are used to maintain post-harvest quality, but such processing leads to a loss of nutrients. However, coatings incorporated with nanoparticles can improve the preservation of these perishable products and extend their shelf life.
Strawberry fruits were packaged in multifunctional thin films (polylactic acid, antibacterial agents, and silver nanoparticles obtained from mango peel extract) and stored for 7 days at room temperature [115]. The films showed strong antimicrobial properties against E. coli and S. aureus. As we know, consumers are concerned about the migration of nanomaterials from food containers into food products posing potential risk to health. In this study, however, the total amount of silver migration was within the standard range throughout the storage period. The membrane also exhibited lower cytotoxicity, and the cells exposed to the composite film had an 80% survival.
The blotting paper layer of the packaging material incorporated with a mixture of plant extracts and silver nanoparticles as an antibacterial, anti-mold, anti-yeast, and anti-viral functional additive increased the shelf life of perishable products [116]. The coated paper showed good antibacterial activity against E. coli and Motlagh S. aureus. Freshly harvested tomatoes and coriander leaves packaged in the coated paper retained their quality characteristics for 15 and 30 days. However, when stored in plastic bags or outdoors, they showed partial spoilage and wilting.
Silver nanoparticles were obtained from an acidic polysaccharide isolated from the fruits of Rosa roxburghii [117]. This polysaccharide promoted the reduction of AgNO3 into composites of Ag nanoparticles by a one-stage, environmentally friendly process. The resulting composites exhibited excellent antimicrobial activity against S. aureus and E. coli. The coating based on silver nanoparticles with chitosan showed a preservative effect on cherry tomatoes.
Paper packaging incorporated with silver nanoparticles bound with iturin A was used to prevent bacterial spoilage of chicken and protect oranges from fungal contamination [107].
Ready-to-eat products. Ready-to-eat foods, including ready-made salads, are the most vulnerable to microbiological spoilage. Their shelf life under standard conditions of 4 ± 2°C is no more than 24 h.
Motlagh et al. studied the effect of polyethylene packages containing silver nanoparticles in various concentrations (0.02 and 0.04%) on the shelf life, appearance, as well as microbiological and sensory properties of Olivier salad on days 2, 12, and 22 of storage [118]. According to the tests, the total count of microorganisms and molds increased with longer storage time and decreased with higher concentrations of nanoparticles.
In addition, E. coli decreased over time in all three packages, while the concentration of nanoparticles had no effect on the coliforms. Sensory characteristics were better in the samples stored in the packages with silver nanoparticles. The most optimal shelf life was 14 days in a package with 404.93 ppm of silver nanoparticles. The migration of silver ions from the package into the salad was measured by atomic absorption and was found to be within acceptable limits.
Table 2 summarizes the key characteristics of coatings based on nanoparticles that have not been covered above.
Although increasing the shelf life of food products, metals and their oxides can affect human health due to toxic effects [128, 129]. Therefore, their migration into products is undesirable and must be carefully controlled. As noted above, most studies have been devoted to antibacterial nanoparticles of silver and zinc in films and coatings. Therefore, the migration of coating components into food systems has also been described using these nanoparticles.
After ingestion, food products can release nanoparticles into the gastrointestinal environment [130]. According to in vitro studies, silver nanoparticles can disrupt the function of intestinal apical epithelial cells [131]. Moreover, they can induce inflammatory changes and oxidative stress in intestinal cells [132].
Other studies determined the toxicity of nanoparticles of metals and their oxides depending on the dose of exposure. They found that low concentrations of nanoparticles had no detrimental effect in vitro or in vivo. Particularly, the values of the starting point of transcriptomics for silver nanoparticles were significantly lower than the concentrations associated with changes in the calcium channel, oxidative stress, and membrane potential [128]. According to [133], ZnO nanoparticles are able to pass through the intestinal barrier and move from the intestinal lumen to hemolymph. However, no general toxicity was observed, and no oxidative stress was found in hemolymph cells (hemocytes).
Thus, there is still a lack of studies on the effect of films with nanoparticles of metals and their compounds on ready-to-eat products, so this area is worth pursuing in further research. The existing data are contradictory and therefore they cannot clearly indicate whether films and coatings with metal nanoparticles are safe or not, including those that are in contact with food.
ВЫВОДЫ
Nanotechnology is a rapidly developing field of science. Researchers have been searching for ways to create nanoparticles with specified parameters using environmentally friendly methods, including biosynthesis based on plant extracts, plant parts, bacteria, and viruses. Such nanoparticles are widely used in films for various purposes. Nanoparticles of metals and metal oxides can act as antibacterial agents that are active even at low concentrations.Nanoparticles have unique properties associated with their small size. They can attach and electrostatically bind to bacterial cell walls, causing membrane damage and, eventually, cell lysis. This explains their superior antimicrobial activity.
We studied the potential of incorporating nanoparticles into polymer and biopolymer films to obtain new materials with strong antimicrobial properties in vitro. We saw a trend towards a widespread use of silver or zinc nanoparticles, compared to other types of metal nanoparticles. The films under study exhibited antibacterial properties against major food pathogens, such as Escherichia coli, Staphylococcus aureus, Listeria monocytogenes, Pseudomonas aeruginosa, Streptococcus oralis, Salmonella typhimurium, etc. Many of them also showed improved physicochemical properties, such as water vapor permeability, water solubility, UV barrier, antioxidant properties, and others.
Bioactive coatings with nanoparticles of metals or their compounds are increasingly being studied as potential packaging for food raw materials and ready-to-eat products. Such composite films can extend the product’s shelf life by reducing the rate of microbial spoilage and lipid oxidation. In addition, they have no, or only a slight, effect on the sensory characteristics of food products.
However, the migration of metal ions into a food product is highly undesirable, which may prevent composite films with metal nanoparticles from wide use. Further research into the migration of nanoparticles from the film into the food matrix might show if such films can be safely used for products with different chemical compositions.
КОНФЛИКТ ИНТЕРЕСОВ
The author declare no conflict of interest.
ФИНАНСИРОВАНИЕ
This study was financially supported by the Russian Science Foundation (RSF) (project No. 23-26-00056).СПИСОК ЛИТЕРАТУРЫ
- Chesnokova NYu, Prikhodko YuV, Kuznetsova AA, Kushnarenko LV, Gerasimova VA. Anthocyanin films in freshness assessment of minced fish. Food Processing: Techniques and Technology. 2021;51(2):349–362. (In Russ.). https://doi.org/10.21603/2074-9414-2021-2-349-362
- Bhargava N, Sharanagat VS, Mor RS, Kumar K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends in Food Science and Technology. 2020;105:385–401. https://doi.org/10.1016/j.tifs.2020.09.015
- Koosha M, Hamedi S. Intelligent Chitosan/PVA nanocomposite films containing black carrot anthocyanin and bentonite nanoclays with improved mechanical, thermal and antibacterial properties. Progress in Organic Coatings. 2019;127:338–347. https://doi.org/10.1016/j.porgcoat.2018.11.028
- Gubitosa J, Rizzi V, Lopedota A, Fini P, Laurenzana A, Fibbi G, et al. One pot environmental friendly synthesis of gold nanoparticles using Punica Granatum Juice: A novel antioxidant agent for future dermatological and cosmetic applications. Journal of Colloid and Interface Science. 2018;521:50–61. https://doi.org/10.1016/j.jcis.2018.02.069
- Rolima WR, Pelegrinoa MT, de Araújo Lima B, Ferraza LS, Costa FN, Bernardesd JS, et al. Green tea extract mediated biogenic synthesis of silver nanoparticles: Characterization, cytotoxicity evaluation and antibacterial activity. Applied Surface Science. 2019;463:66–74. https://doi.org/10.1016/j.apsusc.2018.08.203
- Piñón-Segundo E, Mendoza-Muñoz N, Quintanar-Guerrero D. Nanoparticles as dental drug-delivery systems. In: Subramani K, Ahmed W, editors. Nanobiomaterials in clinical dentistry. A volume in micro and nano technologies. Elsevier; 2019. pp. 567–593. https://doi.org/10.1016/B978-0-12-815886-9.00023-1
- Qin Y, Liu Y, Yuan L, Yong H, Liu J. Preparation and characterization of antioxidant, antimicrobial and pH-sensitive films based on chitosan, silver nanoparticles and purple corn extract. Food Hydrocolloids. 2019;96:102–111. https://doi.org/10.1016/j.foodhyd.2019.05.017
- Sun J, Jiangm H, Wu H, Tong C, Pang J, Wu C. Multifunctional bionanocomposite films based on konjac glucomannan/chitosan with nano-ZnO and mulberry anthocyanin extract for active food packaging. Food Hydrocolloids. 2020;107. https://doi.org/10.1016/j.foodhyd.2020.105942
- Gmoshinski IV, Ananyan MA, Shipelin VA, Riger NA, Trushina EN, Mustafina OK, et al. Effect of dihydroquercetin on the toxic properties of nickel nanoparticles. Foods and Raw Materials. 2023;11(2):232–242. https://doi.org/10.21603/2308-4057-2023-2-572
- Khan SA, Noreen F, Kanwal S, Iqbal A, Hussain G. Green synthesis of ZnO and Cu-doped ZnO nanoparticles from leaf extracts of Abutilon indicum, Clerodendrum infortunatum, Clerodendrum inerme and investigation of their biological and photocatalytic activities. Materials Science and Engineering: C. 2018;82:46–59. https://doi.org/10.1016/j.msec.2017.08.071
- Rai M, Yadav A, Gad A. Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances. 2009;27(1):76–83. https://doi.org/10.1016/j.biotechadv.2008.09.002
- Marambio-Jones C, Hoek EMV. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. Journal of Nanoparticle Research. 2010;12:1531–1551. https://doi.org/10.1007/s11051-010-9900-y
- Cui Y, Zhao Y, Tian Y, Zhang W, Lü X, Jiang X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials. 2012;33(7):2327–2333. https://doi.org/10.1016/j.biomaterials.2011.11.057
- Dhayalan M, Denison MIJ, Ayyar M, Gandhi NN, Krishnan K, Abdulhadi B. Biogenic synthesis, characterization of gold and silver nanoparticles from Coleus forskohlii and their clinical importance. Journal of Photochemistry and Photobiology B: Biology. 2018;183:251–257. https://doi.org/10.1016/j.jphotobiol.2018.04.042
- Kumar KP, Paul W, Sharma CP. Green synthesis of gold nanoparticles with Zingiber officinale extract: Characterization and blood compatibility. Process Biochemistry. 2011;46(10):2007–2013. https://doi.org/10.1016/j.procbio.2011.07.011
- Vaseeharan B, Ramasamy P, Chen JC. Antibacterial activity of silver nanoparticles (AgNps) synthesized by tea leaf extracts against pathogenic Vibrio harveyi and its protective efficacy on juvenile Feneropenaeus indicus. Letters in Applied Microbiology. 2010;50(4):352–356. https://doi.org/10.1111/j.1472-765X.2010.02799.x
- Gajbhiye M, Kesharwani J, Ingle A, Gade A, Rai M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine: Nanotechnology, Biology, and Medicine. 2009;5(4):382–386. https://doi.org/10.1016/j.nano.2009.06.005
- Tankhiwale R, Bajpai SK. Preparation, characterization and antibacterial applications of ZnO-nanoparticles coated polyethylene films for food packaging. Colloids and Surfaces B: Biointerfaces. 2012;90:16–20. https://doi.org/10.1016/j.colsurfb.2011.09.031
- Bang Y-J, Shankar S, Rhim J-W. In situ synthesis of multi-functional gelatin/resorcinol/silver nanoparticles composite films. Food Packaging and Shelf Life. 2019;22. https://doi.org/10.1016/j.fpsl.2019.100399
- Liu J, Ma Z, Liu Y, Zheng X, Pei Y, Tang K. Soluble soybean polysaccharide films containing in-situ generated silver nanoparticles for antibacterial food packaging applications. Food Packaging and Shelf Life. 2022;31. https://doi.org/10.1016/j.fpsl.2021.100800
- Perera KY, Jaiswal S, Jaiswal AK. A review on nanomaterials and nanohybrids based bio-nanocomposites for food packaging. Food Chemistry. 2022;376. https://doi.org/10.1016/j.foodchem.2021.131912
- Khodashenas B, Ghorbani HR. Synthesis of silver nanoparticles with different shapes. Arabian Journal of Chemistry. 2019;12(8):1823–1838. https://doi.org/10.1016/j.arabjc.2014.12.014
- Dzulkharnien NSF, Rohani R. A review on current designation of metallic nanocomposite hydrogel in biomedical applications. Nanomaterials. 2022;12(10). https://doi.org/10.3390/nano12101629
- Rosli NA, Teow YH, Mahmoudi E. Current approaches for the exploration of antimicrobial activities of nanoparticles. Science and Technology of Advanced Materials. 2021;22(1):885–907. https://doi.org/10.1080/14686996.2021.1978801
- Shaikh S, Nazam N, Rizvi SMD, Ahmad K, Baig MH, Lee EJ, et al. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. International Journal of Molecular Sciences. 2019;20(10). https://doi.org/10.3390/ijms20102468
- Beyth N, Houri-Haddad Y, Domb A, Khan W, Hazan R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evidence-Based Complementary and Alternative Medicine. 2015;2015. https://doi.org/10.1155/2015/246012
- Jamkhande PG, Ghule NW, Bamer AH, Kalaskar MG. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. Journal of Drug Delivery Science and Technology. 2019;53. https://doi.org/10.1016/j.jddst.2019.101174
- Balasooriya ER, Jayasinghe CD, Jayawardena UA, Ruwanthika RWD, de Silva RM, Udagama PV. Honey mediated green synthesis of nanoparticles: New era of safe nanotechnology. Journal of Nanomaterials. 2017;2017. https://doi.org/10.1155/2017/5919836
- Lyu J, Xing S, Meng Y, Wu N, Yin C. Flexible superhydrophobic ZnO coating harvesting antibacterial and washable properties. Materials Letters. 2022;314. https://doi.org/10.1016/j.matlet.2022.131730
- Hou Y, Mushtaq A, Tang Z, Dempsey E, Wu Y, Lu Y, et al. ROS-responsive Ag-TiO2 hybrid nanorods for enhanced photodynamic therapy of breast cancer and antimicrobial applications. Journal of Science: Advanced Materials and Devices. 2022;7(2). https://doi.org/10.1016/j.jsamd.2022.100417
- Maťátková O, Michailidu Ja, Miškovská A, Kolouchová I, Masák J, Čejková A. Antimicrobial properties and applications of metal nanoparticles biosynthesized by green methods. Biotechnology Advances. 2022;58. https://doi.org/10.1016/j.biotechadv.2022.107905
- Chinnapaiyan M, Selvam Y, Bassyouni F, Ramu M, Sakkaraiveeranan C, Samickannian A, et al. Nanotechnology, green synthesis and biological activity application of zinc oxide nanoparticles incorporated argemone mxicana leaf extract. Molecules. 2022;27(5). https://doi.org/10.3390/molecules27051545
- Dat TD, Viet ND, Dat NM, My PLT, Thinh DB, Thy LTM, et al. Characterization and bioactivities of silver nanoparticles green synthesized from Vietnamese Ganoderma lucidum. Surfaces and Interfaces. 2021;27. https://doi.org/10.1016/j.surfin.2021.101453
- Singh P, Garg A, Pandit S, Mokkapati VRSS, Mijakovic I. Antimicrobial effects of biogenic nanoparticles. Nanomaterials. 2018;8(12). https://doi.org/10.3390/nano8121009
- Liu Z, Persson S, Sánchez-Rodríguez C. At the border: the plasma membrane–cell wall continuum. Journal of Experimental Botany. 2015;66(6):1553–1563. https://doi.org/10.1093/jxb/erv019
- Swaminathan M, Sharma NK. Antimicrobial activity of the engineered nanoparticles used as coating agents. In: Martínez LMT, Kharissova OV, Kharisov BI, editors. Handbook of ecomaterials. Cham: Springer; 2017. pp. 1–15. https://doi.org/10.1007/978-3-319-48281-1_1-1
- Dong Y, Zhu H, Shen Y, Zhang W, Zhang L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio natriegens. PLoS ONE. 2019;14(9). https://doi.org/10.1371/journal.pone.0222322
- Flores-López LZ, Espinoza-Gómez H, Somanathan R. Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. Journal of Applied Toxicology. 2018;39(1):16–26. https://doi.org/10.1002/jat.3654
- Dai Y, Wang Z, Zhao J, Xu L, Xu L, Yu X, et al. Interaction of CuO nanoparticles with plant cells: internalization, oxidative stress, electron transport chain disruption, and toxicogenomic responses. Environmental Science: Nano. 2018;5(10):2269–2281. https://doi.org/10.1039/C8EN00222C
- Muntean DM, Sturza A, Dănilă MD, Borza C, Duicu OM, Mornoș C. The role of mitochondrial reactive oxygen species in cardiovascular injury and protective strategies. Oxidative Medicine and Cellular Longevity. 2016;2016. http://doi.org/10.1155/2016/8254942
- Liu J, Peng Q. Protein-gold nanoparticle interactions and their possible impact on biomedical applications. Acta Biomaterialia. 2017;55:13–27. https://doi.org/10.1016/j.actbio.2017.03.055
- Wang LL, Hu C, Shao LQ. The antimicrobial activity of nanoparticles: present situation and prospects for the future. International Journal of Nanomedicine. 2017;12:1227–1249. https://doi.org/10.2147/IJN.S121956
- Kaur P, Nene AG, Sharma D, Somani PR, Tuli HS. Synergistic effect of copper nanoparticles and antibiotics to enhance antibacterial potential. Bio-Materials and Technology. 2019;1(1):33–47.
- Oliani WL, Pusceddu FH, Parra DF. Silver-titanium polymeric nanocomposite non ecotoxic with bactericide activity. Polymer Bulletin. 2022;79:10949–10968. https://doi.org/10.1007/s00289-021-04036-7
- Zhang M, Zheng Y, Jin Y, Wang D, Wang G, Zhang X, et al. Ag@MOF-loaded p-coumaric acid modified chitosan/chitosan nanoparticle and polyvinyl alcohol/starch bilayer films for food packing applications. International Journal of Biological Macromolecules. 2022;202:80–90. https://doi.org/10.1016/j.ijbiomac.2022.01.074
- Haddar A, Ayed EB, Sila A, Putaux J-L, Bougatef A, Boufic S. Hybrid levan–Ag/AgCl nanoparticles produced by UV-irradiation: Properties, antibacterial efficiency and application in bioactive poly(vinyl alcohol) films. RSC Advance. 2021;11:38990–39003. https://doi.org/10.1039/D1RA07852F
- Gasti T, Hiremani VD, Kesti ShS, Vanjeri VN, Goudar N, Masti SP, et al. Physicochemical and antibacterial evaluation of poly (vinyl alcohol)/guar gum/silver nanocomposite films for food packaging applications. Journal of Polymers and the Environment. 2021;29:3347–3363. https://doi.org/10.1007/s10924-021-02123-4
- Adel AM, Al-Shemy MT, Diab MA, El-Sakhawy M, Toro RG, Montanari R, et al. Fabrication of packaging paper sheets decorated with alginate/oxidized nanocellulose-silver nanoparticles bio-nanocomposite. International Journal of Biological Macromolecules. 2021;181:612–620. https://doi.org/10.1016/j.ijbiomac.2021.03.182
- Gu B, Jiang Q, Luo B, Liu C, Ren J, Wang X, et al. A sandwich-like chitosan-based antibacterial nanocomposite film with reduced graphene oxide immobilized silver nanoparticles. Carbohydrate Polymers. 2021;260. https://doi.org/10.1016/j.carbpol.2021.117835
- Khurshid S, Arif S, Ali TM, Iqbal HM, Shaikh M, Khurshid H, et al. Effect of silver nanoparticles prepared from Saraca asoca leaf extract on morphological, functional, mechanical, and antibacterial properties of rice starch films. Starch – Stärke. 2022;74(5–6). https://doi.org/10.1002/star.202100228
- Ardjoum N, Shankar S, Chibani N, Salmieri S, Lacroix M. In situ synthesis of silver nanoparticles in pectin matrix using gamma irradiation for the preparation of antibacterial pectin/silver nanoparticles composite films. Food Hydrocolloids. 2021;121. https://doi.org/10.1016/j.foodhyd.2021.107000
- Dash KK, Kumar A, Kumari S, Malik MA. Silver nanoparticle incorporated flaxseed protein-alginate composite films: Effect on physicochemical, mechanical, and thermal properties. Journal of Polymers and the Environment. 2021;29:3649–3659. https://doi.org/10.1007/s10924-021-02137-y
- Wang L, Periyasami G, Aldalbahi A, Fogliano V. The antimicrobial activity of silver nanoparticles biocomposite films depends on the silver ions release behaviour. Food Chemistry. 2021;359. https://doi.org/10.1016/j.foodchem.2021.129859
- Sallak N, Moghanjoughi AM, Ataee M, Anvar A, Golestan L. Antimicrobial biodegradable film based on corn starch/Satureja khuzestanica essential oil/Ag–TiO2 nanocomposites. Nanotechnology. 2021;32(40). https://doi.org/10.1088/1361-6528/ac0a15
- Mintiwab A, Jeyaramraja PR. Evaluation of phytochemical components, antioxidant and antibacterial activities of silver nanoparticles synthesized using Ricinus communis leaf extracts. Vegetos. 2021;34:606–618. https://doi.org/10.1007/s42535-021-00244-8
- Burmistrov DE, Simakin AV, Smirnova VV, Uvarov OV, Ivashkin PI, Kucherov RN, et al. Bacteriostatic and cytotoxic properties of composite material based on ZnO nanoparticles in PLGA obtained by low temperature method. Polymers. 2022;14(1). https://doi.org/10.3390/polym14010049
- Ching LW, Keesan FWM, Muhamad II. Optimization of ZnO/GO nanocomposite-loaded polylactic acid active films using response surface methodology. Journal of King Saud University – Science. 2022;34(3). https://doi.org/10.1016/j.jksus.2022.101835
- Saedi S, Shokri M, Priyadarshi R, Rhim J-Wh. Carrageenan-based antimicrobial films integrated with sulfur-coated iron oxide nanoparticles (Fe3O4@SNP). ACS Applied Polymer Materials. 2021;3(10):4913–4923. https://doi.org/10.1021/acsapm.1c00690
- Tymczewska A, Furtado BU, Nowaczyk J, Hrynkiewicz K, Szydłowska-Czerniak A. Functional properties of gelatin/polyvinyl alcohol films containing black cumin cake extract and zinc oxide nanoparticles produced via casting technique. International Journal of Molecular Sciences. 2022;23(5). https://doi.org/10.3390/ijms23052734
- Abolhassani S, Alipour H, Alizadeh A, Nemati MM, Najafi H, Alavi O. Antibacterial effect of electrospun polyurethane-gelatin loaded with honey and ZnO nanoparticles as potential wound dressing. Journal of Industrial Textiles. 2022;5(1S):954S–968S. https://doi.org/10.1177/15280837211069871
- Khalid H, Iqbal H, Zeeshan R, Nasir M, Sharif F, Akram M, et al. Silk fibroin/collagen 3D scaffolds loaded with TiO2 nanoparticles for skin tissue regeneration. Polymer Bulletin. 2021;78:7199–7218. https://doi.org/10.1007/s00289-020-03475-y
- Huang X, Zhou X, Dai Q, Qin Z. Antibacterial, antioxidation, uv-blocking, and biodegradable soy protein isolate food packaging film with mangosteen peel extract and ZnO nanoparticles. Nanomaterials. 2021;11(12). https://doi.org/10.3390/nano11123337
- Roy S, Rhim J-W. Preparation of pectin/agar-based functional films integrated with zinc sulfide nano petals for active packaging applications. Colloids and Surfaces B: Biointerfaces. 2021;207. https://doi.org/10.1016/j.colsurfb.2021.111999
- Lee SW, Said NS, Sarbon NM. The effects of zinc oxide nanoparticles on the physical, mechanical and antimicrobial properties of chicken skin gelatin/tapioca starch composite films in food packaging. Journal of Food Science and Technology. 2021;58(11):4294–4302. https://doi.org/10.1007/s13197-020-04904-6
- Ding J, Hui A, Wang W, Yang F, Kang Y, Wang A. Multifunctional palygorskite@ZnO nanorods enhance simultaneously mechanical strength and antibacterial properties of chitosan-based film. International Journal of Biological Macromolecules. 2021;189:668–677. https://doi.org/10.1016/j.ijbiomac.2021.08.107
- Ahmad AA, Sarbon NM. A comparative study: Physical, mechanical and antibacterial properties of bio-composite gelatin films as influenced by chitosan and zinc oxide nanoparticles incorporation. Food Bioscience. 2021;43. https://doi.org/10.1016/j.fbio.2021.101250
- Hasanzadeh Y, Hamidinezhad H, Ashkarran AA. Shape dependent antibacterial activity of various forms of ZnO nanostructures. BioNanoScience. 2021;11:893–900. https://doi.org/10.1007/s12668-021-00870-1
- Appu M, Lian Z, Zhao D, Huang J. Biosynthesis of chitosan-coated iron oxide (Fe3O4) hybrid nanocomposites from leaf extracts of Brassica oleracea L. and study on their antibacterial potentials. 3 Biotech. 2021;11. https://doi.org/10.1007/s13205-021-02820-w
- Yaseen MW, Sufyan M, Nazir R, Naseem A, Shah R, Sheikh AA, et al. Simple and cost-effective approach to synthesis of iron magnesium oxide nanoparticles using Alstonia scholaris and Polyalthia longifolia leaves extracts and their antimicrobial, antioxidant and larvicidal activities. Applied Nanoscience. 2021;11:2479–2488. https://doi.org/10.1007/s13204-021-02051-8
- Ramji V, Vishnuvarthanan M. Influence of NiO supported silica nanoparticles on mechanical, barrier, optical and antibacterial properties of polylactic acid (PLA) bio nanocomposite films for food packaging applications. Silicon. 2022;14:531–538. https://doi.org/10.1007/s12633-020-00839-x
- Marand SA, Almasi H, Marand NA. Chitosan-based nanocomposite films incorporated with NiO nanoparticles: Physicochemical, photocatalytic and antimicrobial properties. International Journal of Biological Macromolecules. 2021;190:667–678. https://doi.org/10.1016/j.ijbiomac.2021.09.024
- Al-Tayyar NA, Youssef AM, Al-hindi R. Antimicrobial food packaging based on sustainable Bio-based materials for reducing foodborne Pathogens: A review. Food Chemistry. 2020;310. https://doi.org/10.1016/j.foodchem.2019.125915
- Ezati P, Riahi Z, Rhim J-W. CMC-based functional film incorporated with copper-doped TiO2 to prevent banana browning. Food Hydrocolloids. 2022;122. https://doi.org/10.1016/j.foodhyd.2021.107104
- Helmiyati H, Hidayat ZSZ, Sitanggang IFR, Liftyawati D. Antimicrobial packaging of ZnO–Nps infused into CMC–PVA nanocomposite films effectively enhances the physicochemical properties. Polymer Testing. 2021;104. https://doi.org/10.1016/j.polymertesting.2021.107412
- Liu J, Huang J, Hu Z, Li G, Hu L, Chen X, et al. Chitosan-based films with antioxidant of bamboo leaves and ZnO nanoparticles for application in active food packaging. International Journal of Biological Macromolecules. 2021;189:363–369. https://doi.org/10.1016/j.ijbiomac.2021.08.136
- Yu F, Fei X, He Y, Li H. Poly(lactic acid)-based composite film reinforced with acetylated cellulose nanocrystals and ZnO nanoparticles for active food packaging. International Journal of Biological Macromolecules. 2021;186:770–779. https://doi.org/10.1016/j.ijbiomac.2021.07.097
- Shahvalizadeh R, Ahmadi R, Davandeh I, Pezeshki A, Seyed Moslemi SA, Karimi S, et al. Antimicrobial bio-nanocomposite films based on gelatin, tragacanth, and zinc oxide nanoparticles – Microstructural, mechanical, thermo-physical, and barrier properties. Food Chemistry. 2021;354. https://doi.org/10.1016/j.foodchem.2021.129492
- Roy S, Rhim J-W. Gelatin-based film integrated with copper sulfide nanoparticles for active packaging applications. Applied Science. 2021;11(14). https://doi.org/10.3390/app11146307
- Li X, Ren Z, Wang R, Liu L, Zhang J, Ma F, et al. Characterization and antibacterial activity of edible films based on carboxymethyl cellulose, Dioscorea opposita mucilage, glycerol and ZnO nanoparticles. Food Chemistry. 2021;349. https://doi.org/10.1016/j.foodchem.2021.129208
- Lian R, Cao J, Jiang X, Rogachev AV. Physicochemical, antibacterial properties and cytocompatibility of starch/chitosan films incorporated with zinc oxide nanoparticles. Materials Today Communications. 2021;27. https://doi.org/10.1016/j.mtcomm.2021.102265
- Rathinavel S, Saravanakumar SS. Development and analysis of silver nano particle influenced PVA/natural particulate hybrid composites with thermo-mechanical properties. Journal of Polymers and the Environment. 2021;29:1894–1907. https://doi.org/10.1007/s10924-020-01999-y
- Tymczewska A, Furtado BU, Nowaczyk J, Hrynkiewicz K, Szydłowska-Czerniak A. Functional properties of gelatin/polyvinyl alcohol films containing black cumin cake extract and zinc oxide nanoparticles produced via casting technique. International Journal of Molecular Sciences. 2022;23(5). https://doi.org/10.3390/ijms23052734
- Azari SSh, Alizadeh A, Roufegarinejad L, Asefi N, Hamishehkar H. Preparation and characterization of gelatin/β-glucan nanocomposite film incorporated with ZnO nanoparticles as an active food packaging system. Journal of Polymers and the Environment. 2021;29:1143–1152. https://doi.org/10.1007/s10924-020-01950-1
- Drago E, Campardelli R, Pettinato M, Perego P. Innovations in smart packaging concepts for food: An extensive review. Foods. 2020;9(11). https://doi.org/10.3390/foods9111628
- Risch SJ. Food packaging history and innovations. Journal of Agricultural Food Chemistry. 2009;57(18):8089–8092. https://doi.org/10.1021/jf900040r
- Shaikh S, Yaqoob M, Aggarwal P. An overview of biodegradable packaging in food industry. Current Research in Food Science. 2021;4:503–520. https://doi.org/10.1016/j.crfs.2021.07.005
- Brizga J, Hubacek K, Feng K. The unintended side effects of bioplastics: Carbon, land, and water footprints. One Earth. 2020;3(4):515–516. https://doi.org/10.1016/j.oneear.2020.09.004
- Etxabide A, Uranga J, Guerrero P, de la Caba K. Development of active gelatin films by means of valorisation of food processing waste: A review. Food Hydrocolloids. 2017;68:192–198. https://doi.org/10.1016/j.foodhyd.2016.08.021
- Said NS, Howell NK, Sarbon NM. A review on potential use of gelatin-based film as active and smart biodegradable films for food packaging application. Food Reviews International. 2021;39(2):1063–1085. https://doi.org/10.1080/87559129.2021.1929298
- Sohail M, Sun D-W, Zhu Z. Recent developments in intelligent packaging for enhancing food quality and safety. Critical Reviews in Food Science and Nutrition. 2018;58(15):2650–2662. https://doi.org/10.1080/10408398.2018.1449731
- Nogueira GF, Soares CT, Cavasini R, Fakhouri FM, de Oliveira RA. Bioactive films of arrowroot starch and blackberry pulp: Physical, mechanical and barrier properties and stability to pH and sterilization. Food Chemistry. 2019;275:417–425. https://doi.org/10.1016/j.foodchem.2018.09.054
- Vanderroost M, Ragaert P, Devlieghere F, de Meulenaer B. Intelligent food packaging: The next generation. Trends in Food Science and Technology. 2014;39(1):47–62. https://doi.org/10.1016/j.tifs.2014.06.009
- Kerry JP, O’Grady MN, Hogan SA. Past, current and potential utilisation of active and intelligent packaging systems for meat and muscle-based products: A review. Meat Science. 2006;74(1):113–130. https://doi.org/10.1016/j.meatsci.2006.04.024
- Han J-W, Ruiz-Garcia L, Qian J-P, Yang X-T. Food packaging: A comprehensive review and future trends. Comprehensive Reviews in Food Science and Food Safety. 2018;17(4):860–877. https://doi.org/10.1111/1541-4337.12343
- Schaefera D, Cheung WM. Smart packaging: Opportunities and challenges. Procedia CIRP. 2018;72:1022–1027. https://doi.org/10.1016/j.procir.2018.03.240
- Müller P, Schmid M. Intelligent packaging in the food sector: A brief overview. Foods. 2019;8(1). https://doi.org/10.3390/foods8010016
- Janjarasskul T, Suppakul P. Active and intelligent packaging: The indication of quality and safety. Critical Reviews in Food Science and Nutrition. 2018;58(5):808–831. https://doi.org/10.1080/10408398.2016.1225278
- Wicochea-Rodríguez JD, Chalier P, Ruiz T, Gastaldi E. Active food packaging based on biopolymers and aroma compounds: How to design and control the release. Frontiers in Chemistry. 2019;7. https://doi.org/10.3389/fchem.2019.00398
- Youssef AM, EL-Sayed SM, EL-Sayed HS, Salama HH, Dufresne A. Enhancement of Egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film. Carbohydrate Polymers. 2016;151:9–19. https://doi.org/10.1016/j.carbpol.2016.05.023
- Al-Nabulsi A, Osaili T, Sawalha A, Olaimat AN, Albiss BA, Mehyar G, et al. Antimicrobial activity of chitosan coating containing ZnO nanoparticles against E. coli O157:H7 on the surface of white brined cheese. International Journal of Food Microbiology. 2020;334. https://doi.org/10.1016/j.ijfoodmicro.2020.108838
- Peter A, Cozmuta LM, Nicula C, Cozmuta AM, Talasman CM, Drazic G, et al. Chemical and organoleptic changes of curd cheese stored in new and reused active packaging systems made of Ag-graphene-TiO2-PLA. Food Chemistry. 2021;363. https://doi.org/10.1016/j.foodchem.2021.130341
- Motelica L, Ficai D, Oprea O, Ficai A, Trusca R-D, Andronescu E, et al. Biodegradable alginate films with zno nanoparticles and citronella essential oil – A novel antimicrobial structure. Pharmaceutics. 2021;13(7). https://doi.org/10.3390/pharmaceutics13071020
- Li W, Li L, Zhang H, Yuan M, Qin Y. Evaluation of PLA nanocomposite films on physicochemical and microbiological properties of refrigerated cottage cheese. Journal of Food Processing and Preservation. 2018;42(1). https://doi.org/10.1111/jfpp.13362
- Hosseinzadeh S, Partovi R, Talebi F, Babaei A. Chitosan/TiO2 nanoparticle/Cymbopogon citratus essential oil film as food packaging material: Physico-mechanical properties and its effects on microbial, chemical, and organoleptic quality of minced meat during refrigeration. Journal of Food Processing and Preservation. 2020;44(7). https://doi.org/10.1111/jfpp.14536
- Marcous A, Rasouli S, Ardestani F. Low-density polyethylene films loaded by titanium dioxide and zinc oxide nanoparticles as a new active packaging system against Escherichia coli O157:H7 in fresh calf minced meat. Packaging Technology and Science. 2017;30(11):693–701. https://doi.org/10.1002/pts.2312
- Saedi S, Shokri M, Kim JT, Shin GH. Semi-transparent regenerated cellulose/ZnONP nanocomposite film as a potential antimicrobial food packaging material. Journal of Food Engineering. 2021;307. https://doi.org/10.1016/j.jfoodeng.2021.11066
- Zhao X, Wang K, Ai C, Yan L, Jiang C, Shi J. Improvement of antifungal and antibacterial activities of food packages using silver nanoparticles synthesized by iturin A. Food Packaging and Shelf Life. 2021;28. https://doi.org/10.1016/j.fpsl.2021.100669
- Rasul NH, Asdagh A, Pirsa S, Ghazanfarirad N, Sani IK. Development of antimicrobial/antioxidant nanocomposite film based on fish skin gelatin and chickpea protein isolated containing Microencapsulated Nigella sativa essential oil and copper sulfide nanoparticles for extending minced meat shelf life. Materials Research Express. 2022;9. https://doi.org/10.1088/2053-1591/ac50d6
- Sayadi M, Langroodi AM, Amiri S, Radi M. Effect of nanocomposite alginate-based film incorporated with cumin essential oil and TiO2 nanoparticles on chemical, microbial, and sensory properties of fresh meat/beef. Food Science and Nutrition. 2022;10(5):1401–1413. https://doi.org/10.1002/fsn3.2724
- Gasti T, Dixit S, Hiremani VD, Chougale RB, Masti SP, Vootla SK, et al. Chitosan/pullulan based films incorporated with clove essential oil loaded chitosan-ZnO hybrid nanoparticles for active food packaging. Carbohydrate Polymers. 2022;277. https://doi.org/10.1016/j.carbpol.2021.118866
- Paidari S, Ahari H. The effects of nanosilver and nanoclay nanocomposites on shrimp (Penaeus semisulcatus) samples inoculated to food pathogens. Journal of Food Measurement and Characterization volume. 2021;15:3195–3206. https://doi.org/10.1007/s11694-021-00905-x
- Liu J, Huang J, Ying Y, Hu L, Hu Y. pH-sensitive and antibacterial films developed by incorporating anthocyanins extracted from purple potato or roselle into chitosan/polyvinyl alcohol/nano-ZnO matrix: Comparative study. International Journal of Biological Macromolecules. 2021;178:104–112. https://doi.org/10.1016/j.ijbiomac.2021.02.115
- Efatian H, Ahari H, Shahbazzadeh D, Nowruzi B, Yousefi S. Fabrication and characterization of LDPE/silver-copper/titanium dioxide nanocomposite films for application in Nile Tilapia (Oreochromis niloticus) packaging. Journal of Food Measurement and Characterization. 2021;15:2430–2439. https://doi.org/10.1007/s11694-021-00836-7
- Yinzhe R, Shaoying Z. Effect of carboxymethyl cellulose and alginate coating combined with brewer yeast on postharvest grape preservation. International Scholarly Research Notices. 2013;2013. https://doi.org/10.1155/2013/871396
- Cheng J, Lin X, Wu X, Liu Q, Wan S, Zhang Y. Preparation of a multifunctional silver nanoparticles polylactic acid food packaging film using mango peel extract. International Journal of Biological Macromolecules. 2021;188:678–688. https://doi.org/10.1016/j.ijbiomac.2021.07.161
- Chaitanya Kumari S, Naga Padma P, Anuradha K. Green silver nanoparticles embedded in cellulosic network for fresh food packaging. Journal of Pure and Applied Microbiology. 2021;15(3):1236–1244. https://doi.org/10.22207/JPAM.15.3.13
- Wang L, Tian Y, Zhang P, Li C, Chen J. Polysaccharide isolated from Rosa roxburghii Tratt fruit as a stabilizing and reducing agent for the synthesis of silver nanoparticles: antibacterial and preservative properties. Journal of Food Measurement and Characterization. 2022;16:1241–1251. https://doi.org/10.1007/s11694-021-01248-3
- Motlagh NV, Aghazamani J, Gholami R. Investigating the effect of nano-silver contained packaging on the olivier salad shelf-life. BioNanoScience. 2021;11:838–847. https://doi.org/10.1007/s12668-021-00876-9
- Phothisarattana D, Wongphan P, Promhuad K, Promsorn J, Harnkarnsujarit N. Blown film extrusion of PBAT/TPS/ZnO nanocomposites for shelf-life extension of meat packaging. Colloids and Surfaces B: Biointerfaces. 2022;214. https://doi.org/10.1016/j.colsurfb.2022.112472
- Praseptiangga D, Widyaastuti D, Panatarani C, Joni IM. Development and characterization of semi-refined iota carrageenan/ SiO2-ZnO bionanocomposite film with the addition of cassava starch for application on minced chicken meat packaging. Foods. 2021;10(11). https://doi.org/10.3390/foods10112776
- Hong S-I, Cho Y, Rhim J-W. Effect of Agar/AgNP composite film packaging on refrigerated beef loin quality. Membranes. 2021;11(10). https://doi.org/10.3390/membranes11100750
- Sani IK, Geshlaghi SP, Pirsa S, Asdagh A. Composite film based on potato starch/apple peel pectin/ZrO2 nanoparticles/ microencapsulated Zataria multiflora essential oil; investigation of physicochemical properties and use in quail meat packaging. Food Hydrocolloids. 2021;117. https://doi.org/10.1016/j.foodhyd.2021.106719
- Sani MA, Tavassoli M, Salim SA, Azizi-lalabadi M, McClements DJ. Development of green halochromic smart and active packaging materials: TiO2 nanoparticle- and anthocyanin-loaded gelatin/κ-carrageenan films. Food Hydrocolloids. 2022;124. https://doi.org/10.1016/j.foodhyd.2021.107324
- Rizzotto F, Vasiljevic ZZ, Stanojevic G, Dojcinovic MP, Jankovic-Castvan I, Vujancevic JD, et al. Antioxidant and cell-friendly Fe2TiO5 nanoparticles for food packaging application. Food Chemistry. 2022;390. https://doi.org/10.1016/j.foodchem.2022.133198
- Koshy RR, Reghunadhan A, Mary SK, Sadanandan S, Jose S, Thomas S, et al. AgNP anchored carbon dots and chitin nanowhisker embedded soy protein isolate films with freshness preservation for active packaging. Food Packaging and Shelf Life. 2022;33. https://doi.org/10.1016/j.fpsl.2022.100876
- Abdel Aziz MS, Salama HE. Development of alginate-based edible coatings of optimized UV-barrier properties by response surface methodology for food packaging applications. International Journal of Biological Macromolecules. 2022;212:294–302. https://doi.org/10.1016/j.ijbiomac.2022.05.107
- Ran R, Chen S, Su Y, Wang L, He S, He B, et al. Preparation of pH-colorimetric films based on soy protein isolate/ZnO nanoparticles and grape-skin red for monitoring pork freshness. Food Control. 2022;137. https://doi.org/10.1016/j.foodcont.2022.108958
- Xu K, Mittal K, Ewald J, Rulli S, Jakubowski JL, George S, et al. Transcriptomic points of departure calculated from human intestinal cells exposed to dietary nanoparticles. Food and Chemical Toxicology. 2022;170. https://doi.org/10.1016/j.fct.2022.113501
- Wang S, Kang X, Alenius H, Wong SH, Karisola P, El-Nezami H. Oral exposure to Ag or TiO2 nanoparticles perturbed gut transcriptome and microbiota in a mouse model of ulcerative colitis. Food and Chemical Toxicology. 2022;169. https://doi.org/10.1016/j.fct.2022.113368
- Liu Y, Huang Y, Mou Z, Li R, Hossen A, Dai J, et al. Characterization and preliminary safety evaluation of nano-SiO2 isolated from instant coffee. Ecotoxicology and Environmental Safety. 2021;224. https://doi.org/10.1016/j.ecoenv.2021.112694
- Cornu R, Chrétien C, Pellequer Y, Martin H, Béduneau A. Small silica nanoparticles transiently modulate the intestinal permeability by actin cytoskeleton disruption in both Caco-2 and Caco-2/HT29-MTX models. Archives of Toxicology. 2020;94:1191–1202. https://doi.org/10.1007/s00204-020-02694-6
- Xu K, Basu N, George S. Dietary nanoparticles compromise epithelial integrity and enhance translocation and antigenicity of milk proteins: An in vitro investigation. NanoImpact. 2021;24. https://doi.org/10.1016/j.impact.2021.100369
- Alaraby M, Annangi B, Hernández A, Creus A, Marcos R. A comprehensive study of the harmful effects of ZnO nanoparticles using Drosophila melanogaster as an in vivo model. Journal of Hazardous Materials. 2015;296:166–174. https://doi.org/10.1016/j.jhazmat.2015.04.053