Аннотация
Hyptis suaveolens L. is a medical and food plant that is commonly used to treat various microbial infections in humans in many countries of the world. We aimed to study the aqueous and ethanol extracts of H. suaveolens leaves to determine their antibacterial, in-vitro antioxidant, and phytochemical potentials for traditional medicine by using chemical analysis.The aqueous and ethanol extracts inhibited the tested bacteria species with zones of 0–15 and 10–29 mm, respectively. On the typed culture isolates, the inhibition zones were 8–25 and 16–32 mm for the aqueous and ethanol extracts, respectively.
The minimum inhibitory concentrations of the aqueous and ethanol extracts were not different, while the minimum bactericidal concentrations for the aqueous extract was higher than that for the ethanol extract. The screened phytochemicals were qualitatively and quantitatively present in both extracts, except for saponins which were absent in the aqueous extract. The free radical scavenging activity in the aqueous and ethanol extracts was 1.44 ± 0.50 and 1.57 ± 1.40 mg of ascorbic acid/1 g dry leaves, respectively. The ferric reduction was 1.19 ± 0.40 and 1.69 ± 0.18 mg of ascorbic acid/1 g dry leaves in the aqueous and ethanol extracts, respectively. Hydroxyl scavenging was 65.0 ± 0.9 and 0.43 ± 0.50 mg of ascorbic acid/1 g dry leaves for the aqueous and ethanol extracts, respectively.
The present research suggests that the extract of H. suaveleons can be applied as a controlling antibacterial growth agent against Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumoniae, Escherichia coli, Streptococcus pneumoniae and other bacterial pathogens. It is noteworthy that the ethanol extract was more effective than the aqueous one in terms of the antibacterial, phytochemical and antioxidant activities.
Ключевые слова
Health care, inhibition, plant extract, antioxidant properties, Hyptis suaveolens L., phytochemicals, pathogenic bacteriaВВЕДЕНИЕ
Medicinal plant-based remedies are used to prevent or treat disease and to maintain health. Some are also utilized as foods by many ethnic tribes of the world. Antibacterial agents derived from plants have been found effective against the growth and reproduction of bacteria. The growing resistance of bacteria to existing antibiotics has made it necessary to search for a new way of destroying or reducing them [1]. Due to this resistance, antibiotics become ineffective in treating disease. Therefore, microbiologists and other related natural and chemical scientists, such as biochemists, botanists, and biotechnologists, are deploying their knowledge and energy to discover ways to curb this trend [2].
Different tribes in the developing countries, such as Nigeria, Ghana, Kenya, Togo, and Niger, depend predominantly on herbal remedies to sustain most of the health challenges they encounter. Anand and Gokulakrishnan found that traditional healers in many regions of the world, including China and India, have used plants with medicinal properties to treat diseases, heal wounds, and prevent infections [3]. Gema Nieto noted that many of these plants are also consumed as food [4]. The effectiveness of herbal medicine in treating microbial infections is down to the bioactive compounds, such as flavonoids, phenols, phlobatannins, tannins, saponins, steroids, and glycosides, which have diverse physiological and pharmacological responses in the body [5].
Phytochemicals, which are compounds found in plants, have been shown to have a protective effect against chronic diseases and to be effective in treating dangerous body conditions and neurodegenerative diseases. These bio-active compounds can be found by extracting molecules from plants and studying their medicinal properties [6]. These are non-nutritive chemicals that protect human beings from various diseases.
According to literature, extracts from the leaves of Hyptis suaveolens L. can heal disorders such as hemorrhoids, abscesses, and swellings. Indians, for instance, use this plant as a sudorific, laxative, stimulant, and galactagogue. The infusion of H. suaveolens is used for infections of the uterus and its leaf juice is taken for colic and stomach ache.
The extract from the roots of H. suaveolens is good for cleansing blood and also for women’s diseases. In some parts of Asia, it is taken as a medicinal tea and in South America, as an essential oil and food. The flowering stems are edible and can also be used as a spice [7]. Therefore, we aimed to determine the phytochemicals and bacterial growth restriction potency of H. suaveolens extracts on some pathogenic bacteria.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Plant sample collection. Hyptis suaveolens L. leaves were harvested from the plant’s natural habitat in Uzairue, Edo State, Nigeria. The leaves were confirmed by Dr. Odoligie of the Department of Biological Science, Edo State University. The plant voucher No. LH 1116 was retained in the university herbarium.
Processing of leaf samples. The leaf samples of H. suaveolens were rinsed in distilled water and spread on concrete floor for three weeks to dry at room temperature of 27 ± 2°C. Then, it was pulverized into smooth powder using a mechanical grinder. In this process, 100 g of ground leaves were added to 250 mL of ethanol and 250 mL of water. The mixture was steeped for 24 h and then filtered to separate the liquid from the solid material. The filtrate from the ethanol extract was concentrated in vacuo, while the aqueous extract was evaporated in a water bath at 55°C. The semi-solid ethanol and aqueous extracts were kept in a sterile glass bottle for use.
Collection of test bacteria species. Five clinical bacteria isolates, namely Streptococcus pneumoniae, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus, were obtained from the Microbiology Department of the Federal University of Technology, Akure. The American type cultures included P. aeruginosa ATCC 27853, S. aureus ATCC 25923, K. pneumoniae ATCC 49619, and E. coli ATCC 35218. The bacterial isolates were purified by sub-culturing on nutrient agar and incubated for 24 h at 37°C. The purified bacteria isolates were Gramstained for cell morphology and identified as either Gram-positive or Gram-negative. The pure isolates were characterized and identified to species level by performing oxidase, catalase, hydrolysis of starch, indole, motility, triple sugar ions, coagulase, nitrate reduction, and Voges-Proskauer tests [8]. Before use, the isolates were grown in peptone water for 18 h. The turbid cultures were diluted with sterile distilled water to obtain the McFarland’s standard of 107 columnforming units per milliliter (CFU/mL).
Extract inhibition test. The extracts inhibition test was performed on the test bacteria species by the agar well diffusion method. For this, 107 CFU/mL of the test bacteria was streaked on the surface of the Mueller- Hinton agar with a sterile swab stick and left for 2 h for the seeded bacteria to establish in the culture medium. Wells were created on the agar plates with a sterile cork borer with a diameter of 4 mm. Each of the wells was leveled up with 0.05 mL of the extract. The plates were left at room temperature for 2 h for the extract to diffuse and for the bacteria to establish in the culture medium before incubating for 24 h at 37°C. Bacterial inhibition was measured and the degree of sensitivity was recorded.
Minimum inhibitory concentration determination. The extract was evaluated by using the broth dilution technique. This involved adding 8 mL of Mueller-Hinton broth to test tubes, then adding 1 mL of the extract and 1 mL of a 24-h broth culture of the test bacteria. The tubes were then incubated at 37°C for 24 h and observed for bacterial growth. The minimum inhibitory concentration of the extract was determined as the lowest concentration at which no bacterial growth was observed.
Minimum bactericidal concentration determination. To determine the minimum bactericidal concentration of the extract, the tubes with the extract in the minimum inhibitory concentration without visible bacterial growth were shaken and 1 mL was obtained and pure-plated with the plate count agar. The plates were incubated for 24 h at 37°C. The plate without bacterial growth was taken as the minimum bactericidal concentration of the extract.
Qualitative phytochemical analysis of H. suaveolens. Chemical methods. The methods of Harbone and Williams and Trease and Evans were adopted to qualitatively determine the phytochemical components in the plant extracts, specifically tannins, alkaloids, flavonoids, cardiac glycosides (keller-killani test), saponins, phlobatannins, steroids, and terpenoids [9, 10].
Quantitative phytochemical analysis of H. suaveolens. Total phenolic compounds determination. A solution was prepared by adding 100 mg of the extract into 100 mL of distilled water. Then, 1 mL of this solution was taken with a pipette and added to a glass test tube. Next, 0.5 mL of 2 N Folin-Ciocalteu reagent and 1.5 mL of 20% Na2CO3 solutions were mixed with it and the volume was made up to 10 mL with distilled water. The mixture was left to stand for 2 h after being properly shaken, and the absorbance was read on a spectrophotometer at 765 nm. The obtained data were then used to estimate the phenol content by comparing it to the standard curves generated from different diluted concentrations of garlic acid.
Total flavonoids determination. The method was based on the formation of a flavonoids-aluminum complex with a maximum absorptivity at 415 nm. For this, 5 mL of the extract was mixed with 100 μL of 20% aluminum trichloride in methanol. After 40 min, the absorbance of the mixture was read at 415 nm. Blank samples were prepared from 100 mL of the plant extract and then mixed with a drop of acetic acid and 5 mL of methanol. The absorbance of 0.5 mg/mL of standard rutin in methanol was measured. The tests were performed in triplicate.
Total alkaloids determination. To determine total alkaloids, 5 g of the extract was mixed with 200 mL of 10% acetic acid prepared with ethanol in a 250-mL glass beaker. The mixture was covered and left to stand for 4 h. The solution was filtered and concentrated in a water bath to obtain one-quarter of the original volume. Then, the solution was mixed with concentrated ammonium hydroxide added drop-wise and left to settle for precipitate to form. The formed precipitate was harvested, washed with dilute ammonium hydroxide, and filtered. The residue, which is alkaloids, was dried and weighed.
Tannin determination. The extract concentrate was mixed with distilled water to a solution and boiled for an hour. The Folin-Denis reagent and а sodium carbonate solution were added to the boiled solution for color development. Using a spectrophotometer, absorbance was determined at 750 nm. The concentration of tannic acid was then calculated based on the tannic acid standard.
Saponins determination. In a reflux condenser containing pure acetone, saponins were extracted for 2 h. Then, they were exhaustively extracted for 2 h in a Soxhlet apparatus containing methanol. The methanol was evaporated and the extract was weighed. The remaining sample content was calculated as the percentage of saponins.
Steroids determination. One gram of the plant extracts was dissociated in a few drops of acetic acid. The mixture was warmed gently and cooled down under a running tap water. After that, it was mixed with a drop of concentrated sulphuric acid at the side of the test tube. Green color in that reaction indicated the presence of steroids [11].
Glycoside determination. The Keller-Killan test was used to determine cardiac glycosides. For this, 5 mL of the extract was mixed with 2 mL of glacial acetic acid incorporated with a drop of a ferric chloride solution. It was then underlaid with 1 mL of concentrated H2SO4. The appearance of a brown ring at the interface was an indication of deoxy-sugar, a characteristic of cardenolides. Below the brown ring might appear a violet ring and a green ring in the acetic acid layer in a graduate process throughout the thin layer.
In vitro antioxidants determination. Ferric reducing antioxidant potential of extracts. The ferric reducing antioxidant potential of the extracts was determined using the criterion described by Buricova and Reblova [12]. An extract of 0.1 g was added to water (20 mL), properly shaken to dissolve, and allowed settling. After filtering, 2.5 mL of the filtered extract was obtained and mixed with 2.5 mL of phosphate buffer (pH 6.6) and potassium ferrocyanide. It was incubated at 50°C and then mixed with 10% trichloroacetic acid, 5 mL of distilled water, and 1 mL of 0.1% ferric chloride. The absorbance of the samples and the standard was read using a spectrophotometer at 700 μm. All the measurements were performed in duplicate.
Free radical scavenging. An extract sample of 20 mL was mixed with 0.5 mL of 1 mM 1,1-diphenyl 1-2 picrylhydrazyl (0.05 mg/mL), dispersed in cuvettes, and kept for 20 min. Using a spectrophotometer, the absorbance of the mixed solution was measured at 520 μm. The absorbance was considered as mg of L-ascorbic acid/1 g dry weight of the plant substance. The calculation was used when the plant’s extracts were replaced with a freshly prepared solution of ascorbic acid in deionized water (concentration from 0 to 1.6– 100 mg/mL) [13]. The DPPH free radicals percentage was determined using the equation below. The experiment was performed in triplicate.
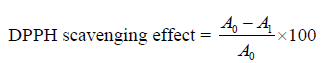
where A0 was the absorbance of the control and A1 was the absorbance in the presence of the extract or standard.
Hydroxyl radical scavenging. This test was based on the method of Halliwell et al. [14]. The hydroxyl radical scavenging assay of the plant’s extracts was determined using iron-ascobate-EDTA-H20 which reacted with deoxyribose to form thiobarbituic acid reactive substances. This reaction produced a pink chromagen at low pH when heated with trichloroacetic acid. The reaction mixture contained 4 mM deoxyribose, 0.3 mM ferric chloride, 0.2 mM EDTA, 0.2 mM ascorbic acid, 2 mM H20, and extracts in different concentrations. After incubation at 37°C for 20 min, 0.4 mL of trichloroacetic acid (5%) and 0.4 mL of trichloroacetic acid (1%) were added to the mixture and boiled for 20 min. Using a spectrophotometer at 532 μm, the intensity of the pink chromogen was measured against a blank sample. The hydroxyl radical scavenging potency of the plant’s extracts was determined as inhibitions of deoxyribose degeneration and calculated with the equation below. Ascorbic acid served as the positive control and tests were performed in triplicate.
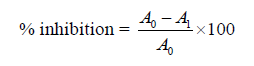
where A0 was the absorbance of the control and A1 was the positive control.
Determination of proximate and mineral contents. The method of the Association of Official Analytical Chemists was used to determine the proximate and mineral contents of the leaves samples [15].
Statistical analysis. The results were expressed as mean ± standard deviation (m ± SD). The obtained data were subjected to a one-way analysis of variance (ANOVA). The least significant difference (LSD) was performed for the pairwise mean comparisons. To determine the considerable treatment amount at a 95% level of confidence, variance was considered statistically significant at (p < 0.5).
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
The color, consistency, odor, and the time of extraction were observed and recorded. The physical characteristics of the extracts are shown in Table 1.
Antibacterial effects. The antibacterial potentials of the ethanol and water extracts of Hyptis suaveolens L. were tested on five clinical bacteria isolates (Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumoniae, Escherichia coli, and Streptococcus pneumoniae) and American type cultures (P. aeruginosa ATCC 27853, S. aureus ATCC 25923, K. pneumoniae ATCC 49619, and E. coli ATCC 35218). The size of inhibition zones around the wells was used to calculate antimicrobial activity. The plant’s extracts from both solvents had inhibitory effects on the tested bacteria (Table 2). The ethanol extract was most active against E. coli ATCC 35218 with an inhibition zone of 32 mm, followed by K. pneumoniae ATCC 49619 with an inhibition zone of 31 mm, and P. aeruginosa with an inhibition zone of 10 mm. The aqueous extract had the strongest inhibitory effect on S. aureus ATCC 25923 with an inhibition zone of 25 mm, followed by E. coli ATCC 35218 with an inhibition zone of 20 mm, and P. aeruginosa ATCC 27853 with an inhibition zone of 8 mm. K. pneumoniae and P. aeruginosa were not inhibited by the aqueous extract at the tested concentrations. Their resistance to the aqueous extract could be down to the low quantity of phytochemicals screened. However, these two species are among the Gram-negative bacteria commonly detected to form resistance to multiple drugs. This is explained by their built-in ability to find new ways of resistance due to a plasmid gene they possess. In addition, their outer membrane contains phospholipids that bound to the inner leaflet of the membrane and lipopolysaccharides that bound to the outer leaflet, which causes endo-toxic shock [16]. Yang et al. reported that any alteration in the outer membrane, like changes in hydrophobic properties or mutations in purines, can make them resistant, as well as other factors [17]. However, we found that the type cultures were more inhibited than the clinical isolates.
The minimum inhibitory concentrations of the ethanol extract on the tested bacteria species ranged from 50 to 200 mg/mL. The bacteria inhibited with small zones of inhibition were suppressed with high minimum inhibitory concentrations of 150–200 mg/mL, while those inhibited with larger zones were suppressed with minimum inhibitory concentrations of 50–100 mg/mL. Meanwhile, the minimum bactericidal concentrations of this extract were effective on the tested bacteria with concentrations of 50–100 mg/mL. The concentration of 50 mg/mL suppressed S. aureus ATCC 25923, K. pneumoniae ATCC 49619, E. coli ATCC 35218, and the clinical S. aureus species.
Our research suggests that the extract of H. suaveolens may be applied as a controlling antibacterial agent against the selected pathogens of P. aeruginosa, S. aureus, K. pneumoniae, E. coli, S. pneumoniae, and several other bacterial pathogens that are threatening human health. Almost all of these species have formed resistance to the existing antibiotics known for their cure and prevention. Therefore, medicinal plants rich in biological compounds, such as H. suaveolens, can be used to suppress and reliably inhibit these bacteria. Edeoga et al. studied the antibacterial activity of H. suaveolens leaves against P. aeruginosa, S. aureus, K. pneumoniae, E. coli, and S. pneumoniae [18]. We found some similarities and differences in the inhibition of these bacteria, which could be due to the age of the plants, climate change, environmental conditions, and the mode of extraction. Mishra et al. and Jin et al. reported that climate change and environmental stress could have an impact on gene expression and life cycle patterns in plants, as well as their phytochemical composition [6, 19]. Another factor could be the variability in secondary active metabolites produced in different plant parts, as well as their quality and quantity as they age.
Quantity and quality of phytochemicals. The qualitative phytochemical examination showed that both the water and the ethanol extracts yielded many secondary metabolites (Table 3). Alkaloids, phenol, flavonoids, glycosides, steroids, phlobatannins, saponins, and terpenoids were qualitatively and quantitatively present in the ethanol extract. They were also present in the water extract, except for saponins. These screened phytochemicals, though serving as protection for the plants under certain conditions, are essentially phytoconstituents of pharmacological potential useful in the health care system.
However, ethanol extracted these chemicals more than water. These chemicals were thought to be responsible for various degrees of inhibition observed on the tested bacteria species. The ethanol extract produced higher inhibition zones than the aqueous extract due to a higher content of phytochemicals. The presence of some of these chemicals in H. suaveolens has been qualitatively and quantitatively reported by Odusina and Oretuga [20]. Useful chemicals, such as α -phellandrene, α-copanene, 4-terpineol, β-pinene, β-elenene, γ-terpinene, and several others, have also been reported in H. suaveolens [21].
It has been shown that when solvents like ethanol and water are used for plant extraction, the extracted components are able to exhibit inhibitory effects on microorganisms [22]. In our study, the leaf ethanol extract of H. suaveolens showed a maximum degree of inhibition against the bacteria species with varied diameters.
In vitro antioxidants. Table 4 represents the in vitro antioxidant assay of the ethanol and aqueous extracts of H. suaveolens leaves. The free radical scavenging activity in the aqueous and ethanol extracts was 1.44 ± 0.50 and 1.57 ± 1.40 mg of ascorbic acid/1 g dry leaves, respectively. The ferric reduction in the aqueous and ethanol extracts was 1.19 ± 0.40 and 1.69 ± 0.18 mg of ascorbic acid/1 g dry leaves, respectively. For hydroxyl scavenging, it was 65.00 ± 0.90 and 0.43 ± 0.50 mg of ascorbic acid/1 g dry leaves for the aqueous and ethanol extracts, respectively. The leaves possessed appreciable antioxidant properties. Since they contained large amounts of bioactive compounds, they could be screened for more medical-related indices to further justify their value in therapy. Although the body produces several of the antioxidants it uses, exogenous antioxidants found in diets are also very important for health maintenance. These free radical scavengers interact with free radicals and neutralize them to avert cellular injury [23].
The reducing power was stronger in the ethanol extract, which could be related to the hydrogen-denoting capacity of the free radical scavenged by the DPPH assay. The leaves of H. suaveolens could therefore be important in reducing the harmful effects caused by free radicals and in managing health issues such as Parkinson’s disease, the aging process, dementia, and cancer [22]. The ferric-reducing activity of the ethanol leaf extract further strengthened the correlation of the observed free radical scavenging activity. However, in addition to the extract’s suitability in managing certain diseases, as well as the correlation between the DPPH and FRAP values, it can also act as a free electron and engage with free radicals’ conversion to militate a higher product that could break the radical chain reaction and possibly prevent cardiovascular diseases.
Nutritional contents. The proximate composition of the leaf extracts under study included reasonable crude fiber, crude protein, ash, lipid, carbohydrate, as well as chemical elements as sodium, calcium, nitrogen, phosphorus, potassium, and magnesium (Tables 5 and 6). The leaves’ valuable nutritional contents are useful for maintaining body cells and other health benefits. The essential mineral elements present in the leaves are highly important in human nutrition due to their role in health care. These minerals help repair physiological damages, which makes them vital elements as food supplements for a healthy immune system. For instance, Umedum et al. reported that copper is helpful in the elimination of free radicals and in many other physiological processes [24]. According to Rai et al., iron is used as an active site of many redox enzymes associated with cellular respiration, as well as oxidation and reduction in animals [25]. Edeoga et al. reported on fiber’s physiological effect on the gastrointestinal ability to lower the rate of tracolonic pressure, which is useful in diverticular disease [26]. The authors also found that it has a biochemical effect on the absorption and re-absorption of bile acids, cholesterol, and dietary fats.
Despite the fact that several plants have been screened for their phytochemicals and antimicrobial activities, the majority of these plants are not edible. Those compounds which are biologically active and edible are important mostly in medicinal plants used in traditional medicine to avoid the danger of poisoning among local users of urban and rural areas. H. suaveolens will be used by many people since it is an edible plant with leaves rich in alkaloids with medical implications, as well as other health-benefiting metabolites [27].
ВЫВОДЫ
The antibacterial activity of the Hyptis suaveolens L. extracts was due to the bioactive compounds present in the plant. This confirms and supports previous observations, suggesting that the extracts of H. suaveolens may be useful as a control agent against certain bacterial pathogens. The extracts also exhibited good phytochemical and antioxidant activities. It should be noted that the ethanol extract had better antibacterial, phytochemical and antioxidant properties compared to the water one. Further research is needed to identify, isolate, and characterize those compounds which could contribute to a new medicine that is readily available, affordable, and effective in treating infectious diseases.
Вклад авторов
Fred Coolborn Akharaiyi conceived the research idea, as well as designed and wrote the first draft. Chioma Bertha Ehis-Eriakha and Peter Taiwo Olagbe- mide reviewed the literature, while Faith Hukwu Igbudu analyzed the data. All the authors read and approved the final draft of the manuscript before submitting it for publication.КОНФЛИКТ ИНТЕРЕСОВ
The authors declare that there is no conflict of interest.СПИСОК ЛИТЕРАТУРЫ
- Wang C-H, Hsieh Y-H, Powers ZM, Kao C-Y. Defeating antibiotics-resistant bacteria: Exploring alternative therapies for a post-antibiotic era. International Journal of Molecular Sciences. 2020;21(3). https://doi.org/10.3390/ijms21031061
- Nwobodo DC, Ugwu MC, Anie CO, Al-Quqaili MTS, Ikem JC, Chigozie UV, et al. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. Journal of Clinical Laboratory Analysis. 2022;36(9). https://doi.org/10.1002/jcla.24655
- Anand T, Gokulakrishnan K. Phytochemical analysis of Hybanthus enneaspermus using UV, FT-IR and GC-MS. IOSR Journal of Pharmacy. 2012;2(3):520–524.
- Nieto G. How medicinal plants are useful when added to foods. Medicines. 2020;7(9). https://doi.org/10.3390/medicines7090058
- Kibe MN, Konyole S, Nguka G, Oloo MO, Kathure D, Wangari PM. The role of phytochemicals in prevention and control of chronic diseases. International Journal of Current Research. 2017;9(12):62540–62543.
- Mishra P, Sohrab S, Mishra SK. A review on the phytochemical properties of Hyptis suaveolens (L.) Poit. Future Journal of Pharmaceutical Sciences. 2021;7(1). https://doi.org/10.1185/s43094-021-00219-1
- Sharma PP, Roy RK, Anurag, Gupta D, Sharma VK. Hyptis suaveolens (L.) poit: A phyto-pharmacological review. International Journal of Chemical and Pharmaceutical Sciences. 2013;4(1).
- Holt JG. Bergey’s manual of determinative bacteriology, 9th edn. Baltimore: Lippincott Williams and Wilkins; 1994. 787 p.
- Harbone JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55(6):481–504. https://doi.org/10.1016/S0031-9422(00)00235-1
- Trease GE, Evans WC. Pharmacognosy 13th edn. London, Philadelphia: Baillière Tindall; 1989. 832 p.
- Satheesh KB, Suchetha KN, Vadisha SB, Sharmila KP, Mahesh PB. Preliminary phytochemical screening of various extracts of Punica granatum peel, whole fruit and seeds. Nitte University Journal of Health Science. 2012;2(4):34–38. https://doi.org/10.1055/s-0040-1703609
- Buricova L, Reblova Z. Czech medicinal plants as possible sources of antioxidants. Czech Journal of Food Sciences. 2008;26(2):132–138.
- Dorman HJD, Bachmayer O, Kosar M, Hiltunen R. Antioxidant properties of aqueous extracts from selected Laminiaceae species grown in Turkey. Journal of Agricultural and Food Chemistry. 2004;52(4):762–770. https://doi.org/10.1021/jf034908v
- Halliwell B, Gutteridge JMC, Aruoma OI. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Analytical Biochemistry. 1987;165(1):215–219. https://doi.org/10.1016/0003-2697(87)90222-3
- Official Method of Analysis of the AOAC, 13 edn. Washington: The Association of Official Analytical Chemists; 1980. 1038 p.
- Jubeh B, Breijyeh Z, Karaman R. Resistance of Gram-positive bacteria of current antibacterial agents and overcoming approaches. Molecules. 2020;25(12). https://doi.org/10.3390/molecules25122888
- Exner M, Bhathacharya S, Christiansen B, Gebel J, Goroncy-Bermes P, Hartemann P, et al. Antibiotic resistance: What is so special about multidrug-resistant Gram-negative bacteria? GMS Hygiene and Infection Control. 2017;12. https://doi.org/10.3205/dgkh000290
- Edeoga HO, Omosun G, Uche LC. Chemical composition of Hyptis suaveolens and Ocimum gratissimum hybrids from Nigeria. African Journal of Biotechnology. 2006;5(10):892–895.
- Dai J, Mumper RJ. Mumker. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313–7352. https://doi.org/10.3390/molecules15107313
- Poonkodi K, Karthika J, Tamilselvi V, Anitha R, Vasanthamani S. Chemical composition of essential oils of Hyptis suaveolens (L) POIT and its in vitro anticancer activity. Journal of Pharmacy Research. 2017;11(5):410–413.
- Akharaiyi FC, Boboye B, Akhambang VO. Adetuyi FC. Phytochemical and antioxidant effect of Spathodea campanulata leaf extracts. International Journal of Biochemistry Research & Review. 2015;7(3):148–159. https://doi.org/10.9734/IJBCRR/2015/16371
- Pizzino G, Irrena N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative stress: Harms and benefits for human health. Oxidative Medicine and Cellular Longevity. 2017;2017. https://doi.org/10.1155/2017/8416763
- Umedum NL, Udeozo IP, Muoneme O, Okoye N, Iloamaeke I. Proximate analysis and mineral content of three commonly used seasonings in Nigeria. IOSR Journal of Environmental Science, Toxicology and Food Technology. 2013;5(1):11–14.
- Rai I, Bachheti RK, Joshi A, Pandey DP. Physicochemical properties and elemental analysis of some non-cultivated seed oild collected from Garhwal region, Uttarkland (India). International Journal of ChemTech Research. 2013;5(1):232–236.
- Edeoga HO, Gomina A. Nutritional values of some nonconventional leafy vegetables of Nigeria. Journal of Economic and Taxonomic Botany. 2000;24:7–13.
- Ulhe SK, Narkhede SD. Histological and phytochemical studies on aromatic plant, Hyptis suaveolens (L.) of family Lamiaceae (MS) India. Science Research Reporter. 2013;3(1):44–48.
- Roy A. A review on the alkaloids: an important therapeutic compound from plants. International Journal of Plant Biotechnology. 2017;3(2):1–9.

