Аннотация
As a good source of natural anti-oxidants, oyster mushroom flour can be incorporated in dairy products. However, very few scientific publications provide formulations for dairy products fortified with oyster mushroom flour. This research featured the physicochemical and antioxidant properties of oyster mushroom flour pretreated with 0.5% citric acid solution. Three samples of mozzarella cheese were incorporated with 1, 2, and 3% oyster mushroom flour and tested for physicochemical properties, total phenolic content, total flavonoid content, and 2, 2-diphenyl-1-picrylhydrazyl (DPPH). The mushroom flour had 11.09 ± 0.88% moisture content, 20.70 ± 0.74% protein, 3.25 ± 0.13% ash, 7.43 ± 0.35% crude fiber, 3.31 ± 0.51% fat, and 54.20 ± 0.81% carbohydrate. The DPPH was 87.00 ± 0.15 mg GAE/g DM, the total phenolic content was 2.09 ± 0.68 mg GAE/g DM, and the total flavonoid content was 1.67 ± 0.27 mg QE/g DM. The texture and water holding capacity of the mozzarella cheese samples fortified with oyster mushroom flour decreased as the proportion of mushroom flour increased. The color (L* lightness, b* redness, and a* yellowness) was significantly lower than in the control (cheese without oyster mushroom flour). The test samples contained significantly (p ≤ 0.05) higher amount of DPPH and phenolic compounds than the control. The sensory attributes were assessed by 30 semi-trained panelists, who gave the highest score to the sample fortified with 1% oyster mushroom flour. As a natural antioxidant, oyster mushroom flour proved to be an excellent component for functional cheese products.Ключевые слова
Mozzarella cheese, mushroom, drying, textural properties, antioxidant, sensory analysisВВЕДЕНИЕ
The food industry produces bio-functional foods supplemented with plant ingredients to meet consumer’s high demand for healthy lifestyle [1]. Novel cheese products are an important part of this strategy because cheese is extremely rich in bio-active compounds. Natural cheeses can be fortified with both dairy and non-dairy ingredients, e.g., mushrooms, wheat fiber, vegetables, meat, egg protein, fruit juices and pulp, oats, nuts, etc. [2].
Mushrooms are classified as healthy food because they are low in fat but rich in proteins and dietary fibers [3]. They are often included in highly nutritive soups or mixed with herbs as a strengthening agent. Mushrooms are also a good source of energy: 454 g of fresh mushrooms provide 120 kcal. They are rich in iron, copper, calcium, potassium, vitamin D, folic acid, zing, etc., which makes it possible to use them as capsules or extracts [4].
In fact, researchers compare mushrooms with milk and non-vegetarian foods such as eggs and meat [5]. Most edible mushrooms belong to the Agaricaceae family of the basidiomycetes class [6]. This group includes about 14 000 different species of mushrooms, of which at least 1450 species are edible and 25 species are included in daily diets worldwide [7].
Unfortunately, mushrooms have a very short shelf life, which can be extended by various processing methods, e.g., freezing, drying, canning, sterilization, pickling, etc. As a result, the market value of mushrooms is rising every year due to their monetary potential, nutritional constituents, and the growing demand for a healthy life style.
Cheese is a fermented milk-based food product obtained by milk coagulation and draining. It is a consolidated curd of milk solids in which milk fat is entrapped by coagulated casein [8]. Cheese is one of the oldest fermented foods in human history. Nowadays, it exists in thousands of varieties. This diversity is a result of multiple factors, one of which is the type of milk (cow, buffalo, goat, sheep, etc.).
Mozzarella cheese gained a worldwide popularity as a salad ingredient or as a ready-to-eat meal. Mozzarella cheese has a wide application in the food industry due to its nutritional value and diversity [9]. For instance, mozzarella cheese contains a lot of calcium, which contributes to weight loss, as well as protects from breast cancer and metabolic disorders associated with cardiovascular diseases [10]. Mozzarella cheese is a rich source of proteins, short-chain fatty-acids, vitamins, e.g., riboflavin, thiamin, vitamin B12, etc., and minerals, e.g., calcium and phosphorus [11]. Milk is an important source of such macro-nutrients as fat, proteins, and sugar (lactose), as well as micro-nutrients, represented mostly by various minerals [12]. The textural properties of cheese depend on the composition and pretreatment of milk, fortification of cheese, etc.
Various studies report better textural properties and nutritional functionality of processed cheese by fortification with spice extracts, vegetable oil, and oat flour [13–15]. However, very few publications feature mozzarella cheese fortified with mushroom powder. The present work aims at utilizing oyster mushroom powder as a source of additional nutrients for functional cheese. We tested the effect of different concentrations of mushroom flour on the textural and nutritional properties of mozzarella cheese.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Collecting the raw materials. The instant fullcream milk powder was purchased from the local market in the vicinity of the Hajee Mohammad Danesh Science and Technology University (Bangladesh). The oyster mushrooms (Pleurotus ostreatus L.) came from the Horticulture Center in the city of Dinajpur (Bangladesh). The chemicals and the starter culture were purchased from Chr. Hansen (Hoersholm, Denmark). The experiments were conducted on the premises of the Department of Food Engineering and Technology, Hajee Mohammad Danesh Science and Technology University.
Preparing the mushroom flour. Fresh oyster mushrooms were rinsed under running water and sliced into 3-mm pieces. After that, we soaked 250 g of mushroom in 1 L of 0.2% citric acid solution for 30 min. Subsequently, the slices were spread over plastic trays and allowed draining. Then, they were dried in a cabinet dryer at 60°C with hot air at a flow rate of 0.305 m/s. The dry mass was grounded into fine flour in a CM/L-7360065 blender (Japan). The resulting oyster mushroom flour was sieved and packed in airtight highdensity polyethylene (HDPE) bags. The flour remained in desiccators at room temperature until further use.
Compositional analysis of mushroom flour. The contents of moisture, ash, protein, fat, and fiber were determined as recommended by the Association of Official Analytical Chemists for mushroom flour [16]. The carbohydrate content was expressed by subtracting the resulting protein, fat, ash, moisture, and fiber from 100.
Preparing sample extracts from mushroom flour. The extraction of all samples followed the procedure described in [17]. We mixed 5 g of sample with 50 mL of 80% methanol at a solid to liquid ratio of 1:10, extracted it at room temperature, and kept it in a hot plate, shaking it for 3 h. The procedure was followed by centrifugation using an MF 300 General Centrifuge at 4000 rpm for 15 min. The supernatant extraction was filtered using Whatman No. 1 filter paper. The remaining residue was extracted again in the same procedure to collect the supernatant. This procedure was repeated twice. The supernatants were combined and made up to 30 mL by adding methanol. All the samples underwent the same procedure. The supernatants served as extract to determine the total phenolic content, the total flavonoid content, and the antioxidant properties. All the tests were done in triplicates in immediate succession, and the samples were stored at 5°C until further use.
Antioxidant analysis via DPPH assay. The antioxidant profile was investigated using the 1,1-diphenyl- 2-picrylhydrazyl (DPPH) radical as a free radical scavenging model. The free radical scavenging ability was measured using a modified version of the DPPH assay described by Islam et al. [18]. We combined 100 μL of extract and 1.9 mL of 0.30 mM of DPPH solution in a test tube. The resulting mix was stored at room temperature for 30 min. A spectrophotometer (UV/VIS, UV1800) was used to measure the absorbance at 520 nm. Methanol served as control. The antioxidant activity, %, was calculated as a percentage suppression of the DPPH radical by the following Eq. (1):
![]()
where As is the absorbance of the sample and DPPH solution and Ao is the absorbance of the control.
Determining the total phenolic content. The total phenolic content of each sample was evaluated using the Folin-Ciocalteu assay, which was modified slightly from the standard described by Kabir et al. [17]. After mixing 0.5 mL of the sample extract with 0.5 Folin-Ciocalteu solutions, we added 1 mL of sodium bicarbonate (7.5% solution) to the mix, which was diluted with distilled water to obtain 10 mL of solution. The mix was vortexed for a few seconds. The solutions were centrifuged for 10 min at 4000 rpm after spending 35 min at room temperature in the dark. After that, the absorbance was detected using a spectrophotometer (UV–VIS, UV-1800) at 725 nm. The standard curve was calibrated with gallic acid (0–200 L M). The results were presented as milligrams of Gallic acid equivalent per gram of dry solids (mg GAE/g DM).
Determining the total flavonoid content. The colorimetric approach reported by Islam et al., with a few adjustments, helped to determine the total flavonoid content [18]. We mixed 1 mL of the extracts with 4 mL of distilled water and 0.3 mL of a 5% NaNO2 solution in 15-mL falcon tubes. The tubes were then allowed standing for 5 min before adding 0.3 mL of 10% AlCl3 to the mix and left to stand for 1 min. After that, 2 mL of 1 M NaOH and 2.4 mL of distilled water were poured together and mixed thoroughly. The tubes were maintained in a dark place at room temperature for 15 min following 10 min of centrifugation at 4000 rpm. The absorbance was measured at 510 nm against a blank made in the same way but with methanol instead of water. A standard curve of quercetin was used to quantify the total flavonoid content. From a quercetin standard curve, the total flavonoid content was determined, and the results were represented as milligrams of quercetin equivalent per gram of dry sample (mg QE/g DM).
Preparing mozzarella cheese with mushroom flour. Four mozzarella cheese samples were prepared by the method described by Shams et al.: control cheese (without mushroom flour), cheese with 1% mushroom flour, cheese with 2% mushroom flour, and cheese with 3% mushroom flour [19]. Table 1 illustrates the formulations.
Physical properties analysis of mozzarella cheese. We defined the physical properties of the mozzarella cheese fortified with mushroom flour. Its textural profile included such parameters as hardness, adhesiveness, cohesiveness, resilience, chewiness, and springiness. The analysis involved a penetration test using a texture analyzer equipped with a 2-kg load cell. The viscosity was calculated after mixing the sample at 4°C for 60 s using a viscometer (Brookfield, USA). The samples were analyzed using a number 2 spindle at 12 rpm. The resulting viscosity was recorded in centipoise (Cp).
Guzman et al. developed a centrifuge technique to assess the water holding capacity of cheese [20]. We centrifuged 5 g of cheese (C) for 30 min at 1250×g. The precipitant was weighed after removing the ejected whey (WE). The water holding capacity (WHC, %) was determined as follows:
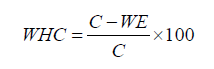
The syneresis of the mozzarella cheese was assessed through the curd drainage test at room temperature based on a modified version of the procedure developed by Harwalkar et al. and Mahomud et al. [21, 22]. We cut the cheese in a beaker with a steel knife and transferred it into a funnel fitted with a stainless-steel screen. After that, the whey was drained into a graduated cylinder. The syneresis, %, percentage was calculated as the weight of the expelled water divided by the weight of the initial sample multiplied by 100:
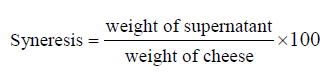
The color of the mozzarella cheese was analyzed using a BIOBASE Colorimeter (BC-110/200, China). The color attribute was determined by the L*, a*, and b* system, where L* indicated lightness (100 – white, 0 – black), a* denoted redness (+)/greenness (–), and b* indicated yellowness (+)/blueness (–) [23].
Sensory evaluation of mozzarella cheese. The sensory properties of the mozzarella cheese were valued according to the hedonic rating test as recommended by Roessler et al. [24]. Randomly coded samples were presented to 30 semi-trained panelists, who were asked to rate the color, flavor, texture, taste, mouthfeel, and overall acceptability on a 9-point scale, where 1 = dislike extremely, 2 = dislike very much, 3 = dislike moderately, 4 = dislike slightly, 5 = neither like nor dislike, 6 = like slightly, 7 = like moderately, 8 = like very much, 9 = like extremely.
Statistical analysis. All experiments were done in triplicates, and the results were provided as the mean ± SD. Statistical software (SPSS for Windows Version 26.0) was used to calculate all the experimental data. The Duncan’s Multiple Range Test was employed to determine the significant differences between the means of textural, sensory, and antioxidant properties of the mozzarella cheese at p ≤ 0.05.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
Table 2 shows the proximate composition of the oyster mushroom flour. In this experiment, the moisture content of the mushroom flour equaled 11.09%. This value was lower than the value reported by Das et al., which was 12.33% [25]. This value was consistent with 11% moisture content of the mushroom flour. The amount of ash in the mushroom flour was 3.25%. This result was also lower than the 5.54% reported by Prodhan et al. [26]. In the present study, the protein content of the oyster mushroom flour was determined as 20.70%. This value was similar to the 20.0% reported by Charles et al. [27]. As for the protein content, Maray et al. and Farooq et al. obtained higher results: 21.9 and 28.69%, respectively [28, 29].
In our research, the fat content of the mushroom flour proved to be 3.31%, which was similar to the findings reported by Ozturk et al., who detected 3.05% of fat in mushroom flour [30]. We determined 7.43% of crude fiber, which approximated the value of 7.90% published by Salehi et al. [31]. However, our result for crude fiber was higher than the 5.6% reported by Gonzalez et al. [32]. The carbohydrate of the mushroom flour appeared to be 54.20%, which exceeded the result published by Painuli et al., i.e., 52.74% [33].
In this research, the experimental value of the mushroom flour was lower than that obtained by Maray et al., who reported 58.76% [28]. Finally, the proximate composition analysis of the mushroom flour revealed much protein and little ash. Proteins are essential for life: every cell in the body is made up of protein. The basic structure of protein is an amino acid chain. A proteinrich diet helps human body repair and replace cells. Protein is especially important for children, adolescents, and pregnant women.
Antioxidant properties of mushroom flour. The antioxidant properties of the mushroom flour were examined by extracting the oyster mushroom flour with an 80% methanol solution. The total phenolic content of the mushroom flour was estimated by the Folin- Ciocalteu method (Table 3). The methanol extracts obtained from the mushroom flour had the total phenolic content of 2.09 mgGAE/g DM. A similar total phenolic content was reported by Rashidi et al. as 2.21 mg/g [34]. Zalewska et al. obtained a greater total phenolic content of mushroom flour of 9.48 mg/g [35]. Radzki et al. claimed that hot-air drying can significantly reduce the total phenolic compounds in mushrooms [36].
As for the total flavonoid content in this research, it was 1.67 mg/g, which approximated the 1.16 mg/g obtained by Mutukwa et al. [37]. However, Nguyen et al. reported a much higher value of 3.28 mg/g [38].
The effect of heat on the cell wall released the cell bound flavonoids, which affected the total flavonoid content [39]. According to the DPPH radical scavenging assay, the DPPH of the mushroom flour was 75.96% in this experiment. Much lower DPPH values were reported as 57% by Chirinang et al. and 58.13% by Reis et al., while Tsai et al. obtained an even greater DPPH of 77.37% [40–42]. The difference in DPPH might be attributed to different factors, e.g., extraction methods, species peculiarities, blanching conditions, etc. Nevertheless, oyster mushrooms could serve as a prospective source of natural antioxidants.
Physical properties of mozzarella cheese fortified with oyster mushroom flour. Table 4 summarizes the physical properties of mozzarella cheese. The textural properties of the mozzarella cheese were determined as 0.37 ± 0.02 kg for the control sample without mushroom flour and 0.42 ± 0.05 kg for the cheese sample with 3% mushroom flour. The texture of S1 was the lowest and that of S4 was the highest. The texture increased slightly together with the mushroom flour amount. The hardness correlated with the texture of cheese according to Ozturk et al. [30].
The viscosity of mozzarella cheese ranged from 2.317 ± 5.56 to 2.479 ± 9.53 Cp. The experimental values of the control sample and the one with 1% mushroom flour were significantly similar (p ≤ 0.05) whereas those of the samples with 1 and 3% mushroom flour were significantly different. The viscosity increased moderately with the incorporation of mushroom flour.
The water holding capacity of the mozzarella cheese increased slightly with increasing in an oyster mushroom amount (Table 4). The test values were significantly similar (p ≤ 0.05) in all the samples. The water holding capacity increased slightly with the oyster mushroom flour proportion.
The physical values of mozzarella cheese varied significantly (p ≤ 0.05) in the control, the sample with 1% mushroom flour, and the sample with 3% mushroom flour. The syneresis was at its highest in the sample with 3% mushroom flour and equaled 2.50 ± 0.10% compared to the control sample, which was 2.03 ± 0.06%. The syneresis of the control mozzarella cheese was lower than that of the other samples. This property increased together with the percentage of the oyster mushroom flour.
The color characteristics of the cheese samples are presented in Table 4. The test values of L* were significantly similar (p ≤ 0.05) in all the samples. However, the a* and b* values were significantly different (p ≤ 0.05) in all the samples. The control and the sample with 1% mushroom flour showed higher L*, a*, and b* values. The extra mushroom flour affected the color of the cheese, which decreased slightly in the sample with 2% and the sample with 3% mushroom flour. The semi-dark color of the experimental cheese might have been caused by the enzymatic browning during the mushroom flour processing (Fig. 1). Enzymatic browning is known to deteriorate the color because ambient oxygen oxidates the mono-phenolic substances and polyphenol oxidase in mushroom matter [43].
Antioxidant properties of mozzarella cheese fortified with oyster mushroom flour. Figure 2 illustrates the total phenolic compounds of the mozzarella cheese fortified with various amounts of oyster mushroom flour. The total phenolic content varied from 0.16 to 1.08 mg GAE/g DM. The experimental values were significantly different (p ≤ 0.05) in every sample. The highest total phenolic content was found in the sample with 3% of mushroom flour. The results were expressed as milligram of gallic acid equivalents per gram (mg GAE/g DM) of mozzarella cheese. Phenolic compounds are important for fruit because they have antioxidant properties to prevent the breakdown of hydro peroxides into free radicals or inactivate lipid free radicals [44].
The DPPH of the mozzarella cheese ranged from 9.97 to 30.25 mg GAE/g DM (Fig. 3). The DPPH was the highest in the sample with 3% mushroom flour and the lowest in the control. The DPPH of the mozzarella cheese increased gradually as the percentage of the mushroom flour increased. The results for the control and the test samples were significantly different. The values were significantly different (p ≤ 0.05) in all the samples. Higher DPPH levels indicated a higher antioxidant activity. On the other hand, lower DPPH values indicated a lower antioxidant activity [45]. The total flavonoid content was measured three times for each sample, but the values were so low that they could be neglected. The absence of flavonoids in the fortified mozzarella cheese might be attributed to the very small amount of total flavonoids detected in the oyster mushroom flour.
Sensory evaluation of mozzarella cheese fortified with mushroom flour. Table 5 demonstrates the effect of mushroom flour on the properties of mozzarella cheese. The sample with 1% mushroom flour was well accepted by the panelists due to its superior color, flavor, texture, taste, and mouthfeel compared to those of other mozzarella cheese. The sample with 1% oyster mushroom flour increased the hedonic acceptability of the mozzarella cheese because it had a more appealing appearance and texture. The samples with 2 and 3% of mushroom flour received a lower score because of their texture, flavor, and color. Therefore, oyster mushroom flour can be fortified with skim milk powder to produce mozzarella cheese with good sensory attributes.
ВЫВОДЫ
The research revealed the physicochemical and antioxidant properties of dehydrated oyster mushroom flour, as well as the effect of its different proportions on the textural, nutritional, antioxidant, and sensory properties of mozzarella cheese. The total antioxidant activity was 75.96 ± 3.00%, the total phenolic content was 2.09 ± 0.68 mg GAE/g DM, and the total flavonoid content was 1.67 ± 0.27 mg QE/g DM. The antioxidant properties and the total phenolic content of the experimental mozzarella cheese fortified with oyster mushroom flour were superior compared to the control. The best sensory evaluation score belonged to the sample fortified with 1% oyster mushroom flour. This amount can be recommended for functional mozzarella cheese since this percentage had no negative effect on the sensory properties of the mozzarella cheese. The samples with 2 and 3% mushroom flour demonstrated better antioxidant properties and a greater phenolic content, but the extra percentage of mushroom flour reduced the consumer acceptability. The new functional cheese proved safe and beneficial for health. Oyster mushroom flour possesses properties that can be used in the food industry. The obtained results reinforce the importance of investments in the development of innovative products with oyster mushroom flour. Economically, these attempts may result in gains for both agricultural practice and the food industry.Вклад авторов
The authors were equally involved in writing the manuscript and are equally responsible for plagiarism.КОНФЛИКТ ИНТЕРЕСОВ
The authors declare that there is no conflict of interests.БЛАГОДАРНОСТИ
The authors gratefully acknowledge the National Science and Technology (NST) fellowship under the Ministry of Science and Technology (MoST), Bangladesh, for research grants (Funding No. ES 390; year 2021–2022).ФИНАНСИРОВАНИЕ
This work was financially supported by the National Science and Technology (NST) fellowship under the Ministry of Science and Technology (MoST), Bangladesh (grant No. ES 390; year 2021–2022).СПИСОК ЛИТЕРАТУРЫ
- Farahat ESA, Mohamed AG, El-Loly MM, Gafour WAMS. Innovative vegetables-processed cheese: I. Physicochemical, rheological and sensory characteristics. Food Bioscience. 2021;42. https://doi.org/10.1016/j.fbio.2021.101128
- Rafiq SM, Ghosh BC. Effect of potato incorporation on the physico-chemical, textural and sensory properties of processed cheese. Journal of Food Measurement and Characterization. 2017;11(2):776–780. https://doi.org/10.1007/s11694-016-9448-3
- Manzi P, Aguzzi A, Pizzoferrato L. Nutritional value of mushrooms widely consumed in Italy. Food Chemistry. 2001;73(3):321–325. https://doi.org/10.1016/S0308-8146(00)00304-6
- Alam SM, Raza MS. Importance of mushrooms. Industry and Economy. 2001;1:125–133.
- Sharma A, Singh S, Kuiry BM, Himanshu, Singh K, Shivani Cultivation and processing of edible mushrooms. International Journal of Agricultural Sciences. 2021;17(1):745–750.
- Srivastava A, Attri BL, Verma S. Development and evaluation of instant soup premix using oyster mushroom powder. Mushroom Research. 2019;28(1):65–69. https://doi.org/10.36036/MR.28.1.2019.91960
- Miles PG, Chang S-T. Mushrooms. Cultivation, nutritional value, medicinal effect, and environmental impact. Boca Raton: CRC Press; 2004. 480 p. https://doi.org/10.1201/9780203492086
- Adams MR, Moss MO. Food Microbiology. Second Edition. London: Royal Society of Chemistry; 2002. 479 p.
- Jana AH, Tagalpallewar GP. Functional properties of Mozzarella cheese for its end use application. Journal of Food Science and Technology. 2017;54(12):3766–3778. https://doi.org/10.1007/s13197-017-2886-z
- Huma N, Rafiq S, Sameen A, Pasha I, Khan MI. Antioxidant potential of buffalo and cow milk Cheddar cheeses to tackle human colon adenocarcinoma (Caco-2) cells. Asian-Australasian Journal of Animal Sciences. 2018;31(2):287–292. https://doi.org/10.5713/ajas.17.0031
- Lopez-Exposito I, Amigo L, Recio I. A mini-review on health and nutritional aspects of cheese with a focus on bioactive peptides. Dairy Science and Technology. 2012;92(5):419–438. https://doi.org/10.1007/s13594-012-0066-5
- Altun SK, Aydemir ME. Determination of some minerals and heavy metal levels in Urfa cheese and cow's milk. Food and Health. 2021;7(3):185–193.
- Krumov K, Ivanov G, Slavchev A, Nenov N. Improving the processed cheese quality by the addition of natural spice extracts. Advance Journal of Food Science and Technology. 2010;2:335–339.
- Shabani J, Sarfarazi M, Mirzaei H, Jafari SM. Influence of the sunflower oil content, cooking temperature and cooking time on the physical and sensory properties of spreadable cheese analogues based on UF white-brined cheese. International Journal of Dairy Technology. 2016;69(4):576–584. https://doi.org/10.1111/1471-0307.12305
- Hamdy SM, Hassan MG, Ahmed RB, Abdelmontaleb HS. Impact of oat flour on some chemical, physicochemical and microstructure of processed cheese. Journal of Food Processing and Preservation. 2021;45(9). https://doi.org/10.1111/jfpp.15761
- Official Methods of Analysis of the Association of Official Analytical Chemist. 17th edition. Washington: AOAC; 2000.
- Kabir MR, Hasan MM, Islam MR, Haque AR, Hasan SMK. Formulation of yogurt with banana peel extracts to enhance storability and bioactive properties. Journal of Food Processing and Preservation. 2021;45(3). https://doi.org/10.1111/jfpp.15191
- Islam MR, Haque AR, Kabir MR, Hasan MM, Khushe KJ, Hasan SMK. Fruit by-products: The potential natural sources of antioxidants and α-glucosidase inhibitors. Journal of Food Science and Technology. 2021;58(5):1715–1726. https://doi.org/10.1007/s13197-020-04681-2
- Shams EA, Yousef NS, El-Shazly H. Chemical, sensory and rheological evaluation of Karish cheese made by oyster mushroom mycelium. Journal of Food and Dairy Sciences. 2020;11(7):187–193.
- Guzman-González M, Morais F, Ramos M, Amigo L. Influence of skimmed milk concentrate replacement by dry dairy products in a low fat set-type yoghurt model system. I: Use of whey protein concentrates, milk protein concentrates and skimmed milk powder. Journal of the Science of Food and Agriculture. 1999;79(8):1117–1122. https://doi.org/10.1002/(SICI)1097-0010(199906)79:8<1117::AID-JSFA335>3.0.CO;2-F
- Harwalkar VR, Kalab M. Comparison of centrifugation and drainage methods. Milchwissenschaft. 1983;38(9):517–522.
- Mahomud MS, Haque MA, Akhter N, Asaduzzaman M. Effect of milk pH at heating on protein complex formation and ultimate gel properties of free-fat yoghurt. Journal of Food Science and Technology. 2021;58(5):1969–1978. https://doi.org/10.1007/s13197-020-04708-8
- Jha SN. Colour measurements and modeling. In: Jha SN, editor. Nondestructive evaluation of food quality. Theory and practice. Heidelberg: Springer Berlin; 2010. pp. 17–40. https://doi.org/10.1007/978-3-642-15796-7_2
- Roessler EB, Pangborn RM, Sidel JL, Stone H. Expanded statistical tables for estimating significance in paired-preference, paired-difference, duo-trio and triangle tests. Journal of Food Science. 1978;43(3):940–943. https://doi.org/10.1111/j.1365-2621.1978.tb02458.x
- Das R, Sarker M, Lata MB, Islam MA, Al Faik MA, Sarkar S. Physicochemical properties and sensory evaluation of sponge cake supplemented with hot air and freeze dried oyster mushroom (Pleurotus sajor-caju). World Journal of Engineering and Technology. 2020;8(4):665–674. https://doi.org/10.4236/wjet.2020.84047
- Prodhan UK, Linkon KMMR, Al-Amin MF, Alam MJ. Development and quality evaluation of mushroom (Pleurotus sajor-caju) enriched biscuits. Emirates Journal of Food and Agriculture. 2015;27(7):542–547.
- Charles M, Kyambadde D, Namugumya B. Effect of pretreatments and drying methods on chemical composition and sensory evaluation of oyster mushroom (Pleurotus ostreatus) powder and soup. Journal of Food Processing and Preservation. 2014;38(1):457–465. https://doi.org/10.1111/j.1745-4549.2012.00794.x
- Maray ARM, Mostafa MK, El-Fakhrany AE-DMA. Effect of pretreatments and drying methods on physico-chemical, sensory characteristics and nutritional value of oyster mushroom. Journal of Food Processing and Preservation. 2017;42(1). https://doi.org/10.1111/jfpp.13352
- Farooq M, Rakha A, Hassan JU, Solangi IA, Shakoor A, Bakhtiar M, et al. Physicochemical and nutritional characterization of mushroom powder enriched muffins. Journal of Innovative Sciences. 2021;7(1):110–120. https://doi.org/10.17582/journal.jis/2021/7.1.110.120
- Öztürk M, Güncü BG. Effect of brine calcium concentration on the surface solubilization and texture of fresh perline Mozzarella cheese. Turkish Journal of Agriculture – Food Science and Technology. 2021;9(4):650–654. https://doi.org/10.24925/turjaf.v9i4.650-654.3764
- Salehi F. Quality, physicochemical, and textural properties of dairy products containing fruits and vegetables: A review. Food Science and Nutrition. 2021;9(8):4666–4686. https://doi.org/10.1002/fsn3.2430
- Gonzalez A, Nobre C, Simões LS, Cruz M, Loredo A, Rodríguez-Jasso RM, et al. Evaluation of functional and nutritional potential of a protein concentrate from Pleurotus ostreatus mushroom. Food Chemistry. 2021;346. https://doi.org/10.1016/j.foodchem.2020.128884
- Painuli S, Semwal P, Egbuna C. Mushroom: Nutraceutical, mineral, proximate constituents and bioactive component. In: Egbuna C, Dable Tupas G, editors. Functional foods and nutraceuticals. Bioactive components, formulations and innovations. Cham: Springer; 2020. pp. 307–336. https://doi.org/10.1007/978-3-030-42319-3_17
- Rashidi ANM, Yang TA. Nutritional and antioxidant values of oyster mushroom (P. sajor-caju) cultivated on rubber sawdust. International Journal on Advanced Science, Engineering and Information Technology. 2016;6(2):161–164. https://doi.org/10.18517/ijaseit.6.2.610
- Zalewska M, Marcinkowska-Lesiak M, Onopiuk A. Physicochemical properties of white button mushrooms (Agaricus bisporus) as affected by coating. Journal of Food Processing and Preservation. 2017;42(2). https://doi.org/10.1111/jfpp.13419
- Radzki W, Sławinska A, Jabłonska-Rys E, Gustaw W. Antioxidant capacity and polyphenolics content in dried wild growing edible mushrooms. International Journal of Medicinal Mushrooms. 2014;16(1):65–75. https://doi.org/10.1615/IntJMedMushr.v16.i1.60
- Mutukwa IB, Hall III CA, Cihacek L, Lee CW. Evaluation of drying method and pretreatment effects on the nutritional and antioxidant properties of oyster mushroom (Pleurotus ostreatus). Journal of Food Processing and Preservation. 2019;43(4). https://doi.org/10.1111/jfpp.13910
- Nguyen TH, Nagasaka R, Ohshima T. Effects of ex-traction solvents, cooking procedures and storage conditions on the contents of ergothioneine and phenolic compounds and anti-oxidative capacity of the cultivated mushroom Flammulina velutipes. International Journal of Food Science and Technology. 2012;47(6):1193–1205. https://doi.org/10.1111/j.1365-2621.2012.02959.x
- Choi Y, Lee SM, Chun J, Lee HB, Lee J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chemistry. 2006;99(2):381–387. https://doi.org/10.1016/j.foodchem.2005.08.004
- Chirinang P, Intarapichet K-O. Amino acids and antioxidant properties of the oyster mushrooms, Pleurotus ostreatus and Pleurotus sajor-caju. Science Asia. 2009;35:326–331. https://doi.org/10.2306/scienceasia1513-1874.2009.35.326
- Reis FS, Barros L, Martins A, Ferreira ICFR. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food and Chemical Toxicology. 2012;50(2):191–197. https://doi.org/10.1016/j.fct.2011.10.056
- Tsai S-Y, Wu T-P, Huang S-J, Mau J-L. Antioxidant properties of ethanolic extracts from culinary-medicinal button mushroom Agaricus bisporus (J. Lange) Imbach (Agaricomycetideae) harvested at different stages of maturity. International Journal of Medicinal Mushrooms. 2008;10(2):127–137. https://doi.org/10.1615/IntJMedMushr.v10.i2.30
- Garcia MM, Paula VB, Olloqui ND, García DF, Combarros-Fuertes P, Estevinho LM, et al. Effect of different cooking methods on the total phenolic content, antioxidant activity and sensory properties of wild Boletus edulis mushroom. International Journal of Gastronomy and Food Science. 2021;26. https://doi.org/10.1016/j.ijgfs.2021.100416
- Yeriikaya O, Akan E, Bayram OY, Karaman AD, Kinik O. The impact of medicinal and aromatic plant addition on antioxidant, total phenolic, antimicrobial activities, and microbiological quality of Mozzarella cheese. International Food Research Journal. 2021;28(3):508–516. https://doi.org/10.47836/ifrj.28.3.10
- Cateni F, Gargano ML, Procida G, Venturella G, Cirlincione F, Ferraro V. Mycochemicals in wild and cultivated mushrooms: Nutrition and health. Phytochemistry Reviews. 2022;21(2):339–383. https://doi.org/10.1007/s11101-021-09748-2

