Аннотация
Negative physiological and biochemical effects of chronic and subchronic doses of benzoates and sorbates may pose a certain risk to human health. Identifying new biomarkers responsible for the body’s response to these compounds could provide significant details in determining the mechanism of their toxicity. To assess comparatively physiological, cytological, cytogenetic, and biochemical parameters in onion roots cells we used an Allium test. The roots were previously treated with sorbic and benzoic acids. The study recorded the dose-dependent toxic effect of these preservatives on the root mass growth. The EC50 values obtained for benzoic and sorbic acids (10 mg/L and 110 mg/L respectively) were significantly lower than the regulated concentrations prescribed by the standards for their content in certain types of food products. With an increase in concentrations of these acids, the mitotic index of meristematic cells decreased in experimental groups compared to control groups. The data obtained confirmed the necessity of estimating the mitotic index when choosing onion for the Allium test. The necessity resulted from the fact that low proliferative activity could cause false positive results. Sorbic and benzoic acids in concentrations below the corresponding EC50 increased the frequency of chromosomal aberrations in apical meristematic cells of the roots compared to control. Thus, benzoic and sorbic acids had reliable mitodepressive and genotoxic effects on the dividing cells of onion roots. The study explored the dynamics of lipid oxidation biomarker accumulation (malon dialdehyde, MDA) after exposure to benzoic and sorbic acids. The toxic effect of benzoic acid appeared not to be associated with oxidative damage to root cell lipids, whereas sorbic acid in concentrations from 20 to 200 mg/L resulted in a multiple increase in MDA concentration in the test samples compared to control. At the same time, lipid peroxidation showed a higher level of sensitivity compared to other indicators of this test. Further, the data obtained on the toxic influence of sorbic and benzoic acids can be used in express methods to assess food and ecological security of these acids.Ключевые слова
Food preservatives, Allium cepa, biotesting, lipid peroxidation toxicity, cytogenetic analysis, biomarkersВВЕДЕНИЕ
Food preservation has remained a problem throughout the human history. It is caused by the activity of environmental microorganisms and enzymatic reactions in the products during their production and storage [1, 2]. About a third of the population in the developed countries are estimated to suffer from diseases transmitted through food especially falsified [3]. Food safety is directly related to the development of chemicals that prevent or slow down the spoilage of these products.
Sorbic and benzoic acids, as well as their salts, are known to be widely used as food preservatives. Their production is steadily increasing. These acids are contained in some fruits, berries, dairy products. Sorbic acid is an unsaturated fatty acid and is used only as a preservative in food, animal feed, tobacco, cosmetics and pharmaceuticals. It is metabolized like normal fatty acids, so this acid was assumed to have no side effects. Benzoic acid is a synthetic additive, used as a preservative and antioxidant. It is excreted by the human body through the kidneys.
There are numerous data on the health safety of these compounds in regulated food products. Recently, however, there are more discussions on the necessity to develop scientific approaches to studying mechanisms of their toxicity [4, 5]. The interest in this problem is due to by the detected adverse effects of the chronic and subchronic benzoate and sorbate intake by both animals and humans. Thus, adding benzoic acid to pig feed increased the liver enzymes activity and changed the blood formula negatively, eventually damaging the liver and spleen, respectively [6].
In vitro studies of human erythrocytes demonstrated that sodium benzoate reduced the level of key metabolic enzymes of amino acids (aspartate and alanine aminotransferase) and alkaline phosphatase significantly [7]. There is strong evidence that attention deficit and hyperactivity syndrome in children and anxiety conditions in rats could be associated with high doses of sodium benzoate [8–9]. Other researchers showed that sodium benzoate caused numerous negative physiological and biochemical changes in mice and rats. The changes included reducing the mass of reproductive organs and embryos and the level of sex hormones in mice [10]. As for human blood cell culture, sorbic acid demonstrated the inhibitory effect on biochemical reactions in the activated immune response [11].
However, the mechanism of toxicity for these preservatives is still unclear. In addition, creating a new algorithm for assessing food safety is debated a lot. The algorithm especially concerns foods containing several food additives because of their potential additive and synergetic effect of toxicity [12]. It is yet to be found out if the food preservatives may exert increased activity in people with specific diseases or genetic defects. To rise up to the challenge, it is necessary to go beyond standard toxicity tests to identify molecular biological protection mechanisms and to identify biomarkers responsible for the body reaction to the effects of chemical compounds. All the more so, as modern methodology and instrumentation system are able to tackle these complex problems. New approaches should not only monitor and evaluate toxic effects, but also result in the adequate test systems for modelling detoxification and metabolism of food preservatives in the human body. It is important to develop new model systems. They should be simple to execute, cheap, and able to simulate the reactions of the human body, both on the physiological and molecular levels, with the maximum available accuracy.
In this aspect, the special interest is given to the work on a comprehensive assessment of biomarkers of neurotoxicity and antioxidant enzymes activity in daphnia under the influence of food sweetener sucralose. It is due to the evidence that Gammarus zadachi and Daphnia magna crustaceans exposed to this sweetener altered their swimming behavior [13]. The tests were carried out on these organisms to compare the activity of acetylcholinesterase (AChE), lipid peroxidation enzymes, and the ability to absorb oxygen radicals (ORAC assay) in them. The authors observed the stimulating effect of sucralose on the activity of AChE and lipid peroxidation, but not on the antioxidant capacity (ORAC). In humans, an increased AChE activity was also associated with neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and restless legs syndrome. It is important to note that the data obtained in this work are consistent with those in other experimental studies on human cell cultures and vertebrates. However, plant test systems are also of interest, in particular Allium cepa L. onion roots (Allium test).
Traditionally, the Allium test has been used as a bioindicator in numerous studies on toxicity, cytotoxicity, and genotoxicity of various chemical compounds. It is recommended by WHO experts as a standard for the cytogenetic monitoring of the environment. Recently, it has been increasingly used to assess the genotoxic potential of medicinal plants, food additives, and even ionizing radiation [14–17]. The Allium test was an excellent eukaryot model in vivo. It was one of the few direct methods for measuring damages in biological systems after exposure to various toxicants and mutagenes. Its main advantages include the following characteristics. First, the apical meristematic root cells can show constant mitotic division. Second, the roots may incubate directly with the object being tested. Third, these cells have large chromosomes, which allows a comprehensive analysis of DNA damage. In addition, the test indicators were shown to be more sensitive than the models on microorganisms, cell cultures, and even animals [15, 18].
Allium cepa was also presented as an effective test object in studying the reaction of plant cell biomarkers to chemical toxicants of different nature. It is known that chemical pollutants can induce the formation of active forms of oxygen. In its turn, oxygen can activate enzymes of peroxidation and result in damaging various biological molecules, including lipids. Thus, it was found that herbicide glyphosate and copper salts significantly increased lipid peroxidation in plant cells [19, 20]. In our opinion, the Allium test can help significantly expand our knowledge of the mechanisms of damage to biological systems of eukaryotes, including the damage after exposure to sorbic and benzoic acids. Moreover, no information was found on an effect of these preservatives on physiological and biochemical parameters in the meristematic cells of onion roots. The aim of the research was to compare changes in the mass growth, activity of lipid peroxidation enzymes, cytological and cytogenetic parameters of Allium cepa roots after treatment with sorbic and benzoic acids.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
In the research we used such preservatives as sorbic acid (Alfa Aesar by Thermo Fisher Scientific) and benzoic acid (Alfa Aesar by Thermo Fisher Scientific). Allium cepa onion bulbs (Stuttgarter sort) of the same size (2.5–3 cm in diameter) and mass (5–7 g) were selected as a test organism. Dry scales were removed from the bulbs before incubation. Preliminarily, the germination was conducted in 15 mL test tubes with bottled water for 2 days in the dark at 25°С.
The bulbs with roots over 1 cm long were selected for further studies. Before treatment with benzoic and sorbic acid solutions, the average mass of the roots was determined in a separate group of the control bulbs. Then the bulbs were transferred to the solutions of these acids in the bottled water and incubated for 2 or 3 days depending on the purpose of the experiment. After the incubation, the roots were cut off, dried with filter paper, and weighed [21]. The EC50 value was determined by the concentration of the preservative, which retarded the root mass growth by 50% compared to control, considering the average mass of the roots before treatment with acids. For cytogenetic analysis, the apical meristematic cells of the roots were stained with acetoorcein (1 g of orcein dye was diluted in 50 mL of 45% CH3COOH). The roots were placed in a 70% solution of ethyl alcohol for the long-term storage. Next, instant squash preparations were obtained, the analysis of which was carried out with the help of a light microscope Axioskop 40, Zeiss.
The lipid oxidation level was determined by the concentration of malon dialdehyde (MDA) in the onion roots [22]. The sample weight of approximately 0.25 g to the fourth decimal place was measured in a 15 mL test tube. Then 1 mL of trichloroacetic acid solution (Merck, Germany), concentration of 200 g/dm3, was added. The mixture was thoroughly stirred with a glass stick. Then the stick was washed with 3 mL of the same solution of trichloroacetic acid. The tubes were tightly corked and centrifuged at 1000 g and 4°C for 15 min. One milliliter of supernatant was transferred to a clean 15 mL test tube. Four milliliters of thiobarbituric acid solution (0.5 g of thiobarbituric acid (Diaem, Russia)) was added to 100 mL of trichloroacetic acid solution (200 g/dm3). The test tubes were closed and placed in a water bath at 95°С for 30 min. Then the test tubes were pulled out and cooled in an ice bath. The cooled solutions were centrifuged at 1000 g and 20°C for 10 min.
The spectrophotometric detection was performed with the obtained solutions at 600 and 532 nm. The MDA content was calculated according to the formula:
where ABS532 is the absorption value at 532 nm;
ABS600 is the absorption value at 600 nm;
K is the dilution factor;
Ke is the molar coefficient of extinction;
l is a beam path length, cm;
mwt is the weight of the sample, g.
The statistical processing of the results was carried out in Microsoft Excel and Statistica programs (v. 12). In the paper, the analysis of average values by Student’s criterion with Fisher’s angular transformation was used for comparative estimation of percentages.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
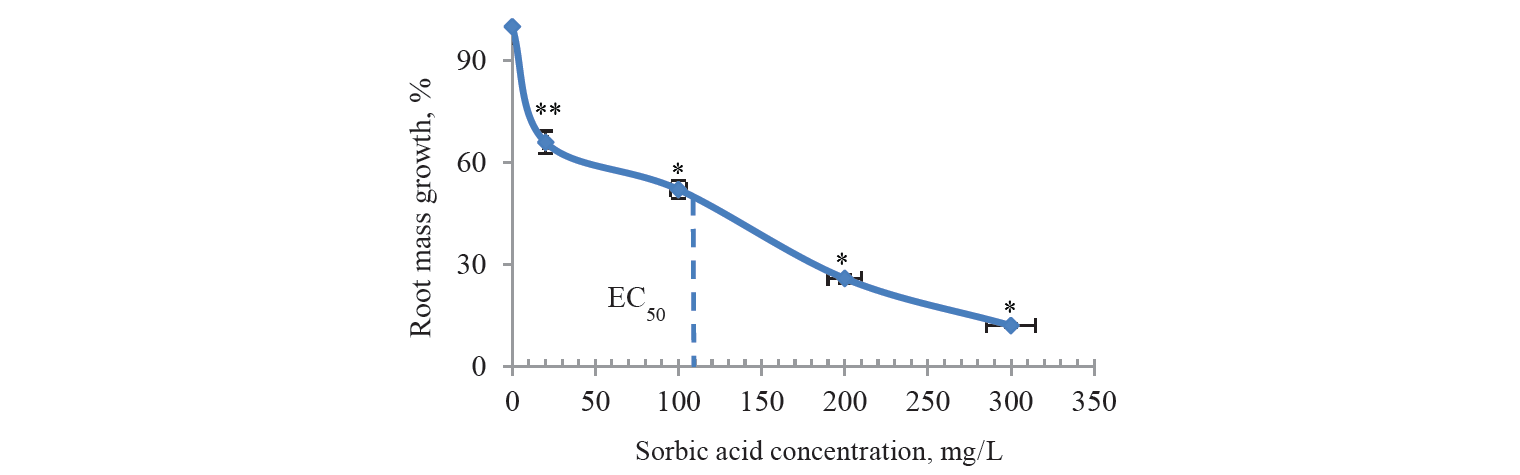
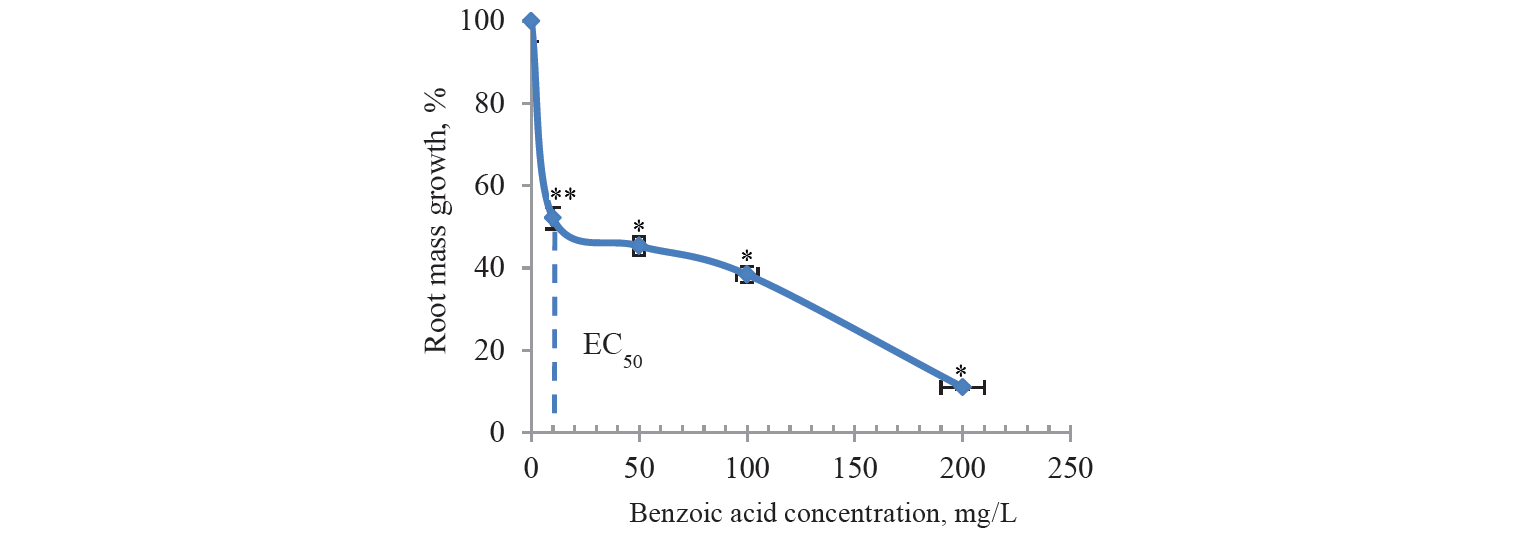
The macroscopic parameters were studied and comparatively evaluated, particularly, for the levels of mass growth in the onion roots after treatment with benzoic and sorbic acid solutions. According to the literature review, the macroscopic parameters appeared more sensitive in comparison with the cytological and cytogenic parameters [23]. This conclusion seemed logical because these parameters reflected the final effect of all disorders in the plant cells. In this work, when calculating the growth of root mass, the average weight of roots was subtracted both in control and experimental samples before their treatment with preservatives solutions. Thus, the EC50 overstatement error was eliminated in these samples. In the preliminary experiments the solutions of preservatives were used with the concentrations not exceeding the permissible levels for some food products, namely, 1 g/L and 2 g/L. Death of the roots was observed after 2 days of incubation. Therefore, we reduced the range of acid concentrations significantly. As a result, the root growth and dose-dependent toxic effects were observed during the same incubation period (Figs. 1 and 2). The roots in the samples remained white and unchanged in shape throughout the incubation. However, there were statistically significant differences between the control and test samples, namely, when treated with benzoic acid at concentrations of 0.01 (P < 0.1); 0.05 (P < 0.05); 0.1 (P < 0.05) and 0.2 g/L (P < 0.05) and with sorbic acid at concentrations of 0.02 (P < 0.1); 0.1 (P < 0.05); 0.2 (P < 0.05) and 0.3 g/L (P < 0.05). EC50 was 10 mg/L for benzoic acid and 110 mg/L for sorbic acid. Thus, these values differed significantly from the domestic regulatory norms on the content of these food additives in certain types of food.
As far as we know, this is the first study in which EC50 values were identified for these preservatives in the Allium test. At the same time, cyanobacteria with EC50 from 9 mg/L were the most sensitive to benzoic acid in similar studies using different organisms living in water when treated for 14 days. In molluscs, fish and amphibians, EC50/LC50 values were determined within 100–1291 mg/L for 24–96 h [24]. The results of these studies confirmed the high sensitivity of the macroscopic parameters in the Allium test.
However, the studies on the toxicity of benzoic and sorbic acids using the Allium test focused mainly on the microscopic indicators reflecting the peculiarities of cell division and chromosomal aberrations occurring in its process, and EC50 was not determined. At the same time, the tested concentrations of preservatives were usually much higher than the EC50 values we found. So the exposure of the roots to the preservative solutions in these studies usually did not exceed several hours [25, 26]. We believe that such experimental conditions are suitable only for acute toxicity testing. They are totally unacceptable for the study of subchronic and chronic consequences of negative effects, especially at the biochemical level. The last aspect should be considered the most interesting in the case of food preservatives. Therefore we believe that the Allium test scheme previously proposed for environmental monitoring did not lose its relevance for studying the toxic effects of these preservatives. The Allium test included a comparative analysis of macro- and microindicators at concentrations of toxicants within their EC50 [23].
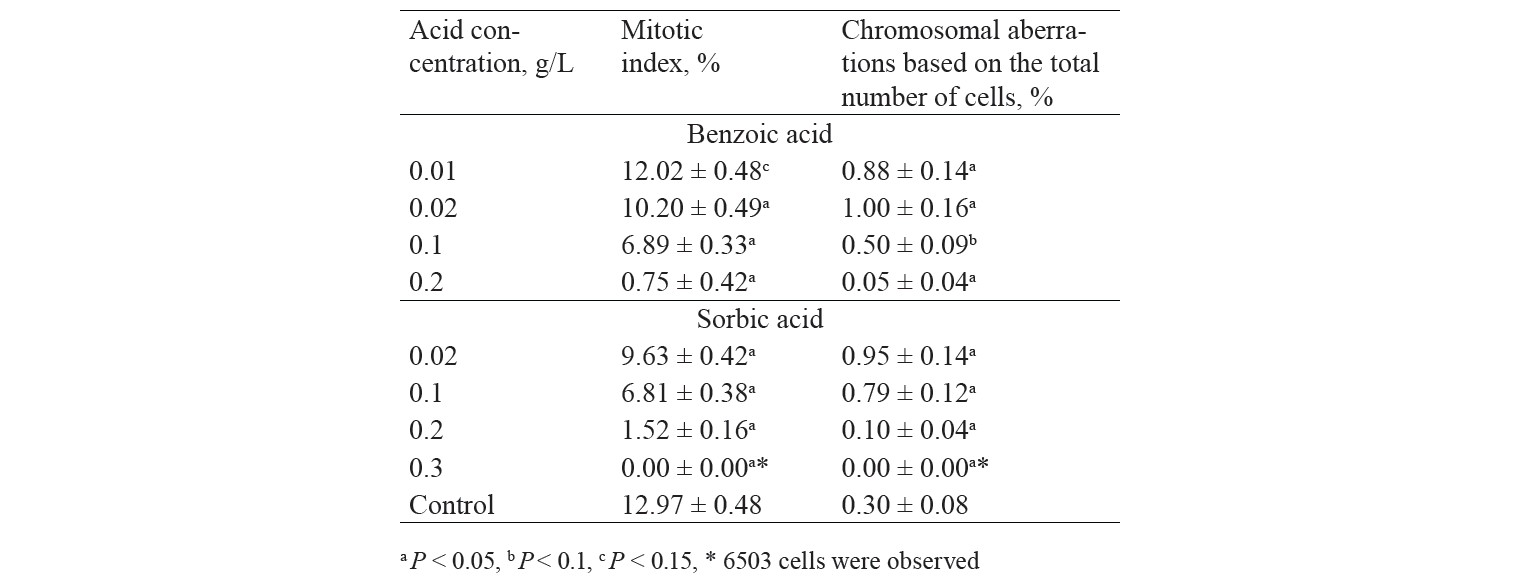
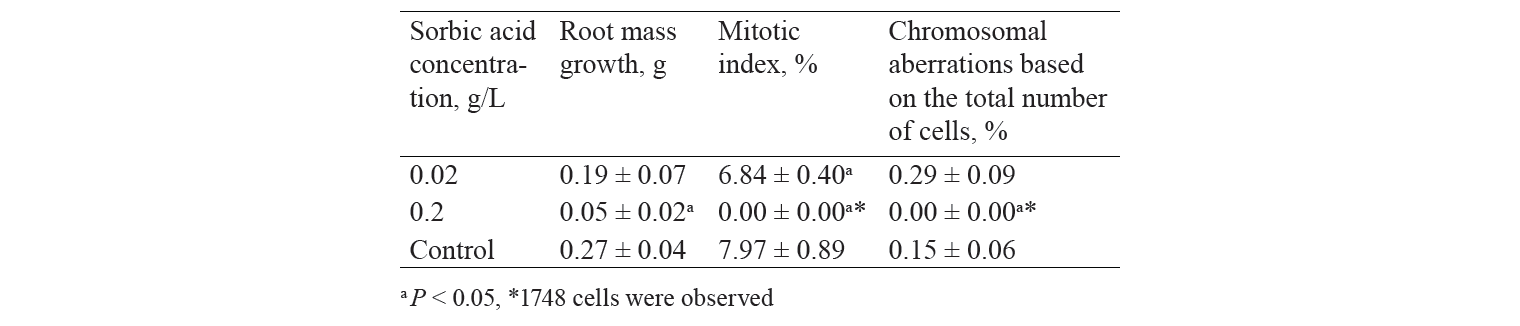
The mitotic index is one of the microindicators in the Allium test which is used as an indicator of the level of cell proliferation. It is known that the dose-dependent deviation of the mitotic index in the experimental samples compared to the control values, both increasing and decreasing, indicates cytotoxicity of the tested chemical. In our previous study, the mitotic index of cells of their meristem was decreasing significantly with an increase in concentrations of preservatives (Table 1). In testing highly toxic doses of sorbic acid (from 1 to 2 g/L), the mitotic index decreased only slightly when the concentration of this acid increased [26]. This data confirmed the previous assumption that there may be difficulties in interpreting the research results due to the high concentration of preservative.
The cytogenetic analysis was carried out on the squash preparations of the apical meristematic cells of onion roots obtained in the previous study. The analysis determined the accumulation dynamics of chromosomal aberrations when the concentrations of sorbic and benzoic acids were increased. According to Table 1, when acid concentrations increased, the proportion of mitosis pathologies also increased, peaked, and then decreased. It is noteworthy that the highest percentage of chromosomal aberrations coincided with acid concentrations close to the corresponding EC50 of these preservatives. The drop in chromosomal aberrations at high concentrations of acids is probably associated with a significant decrease in the number of divisible cells in the meristematic cells of roots.
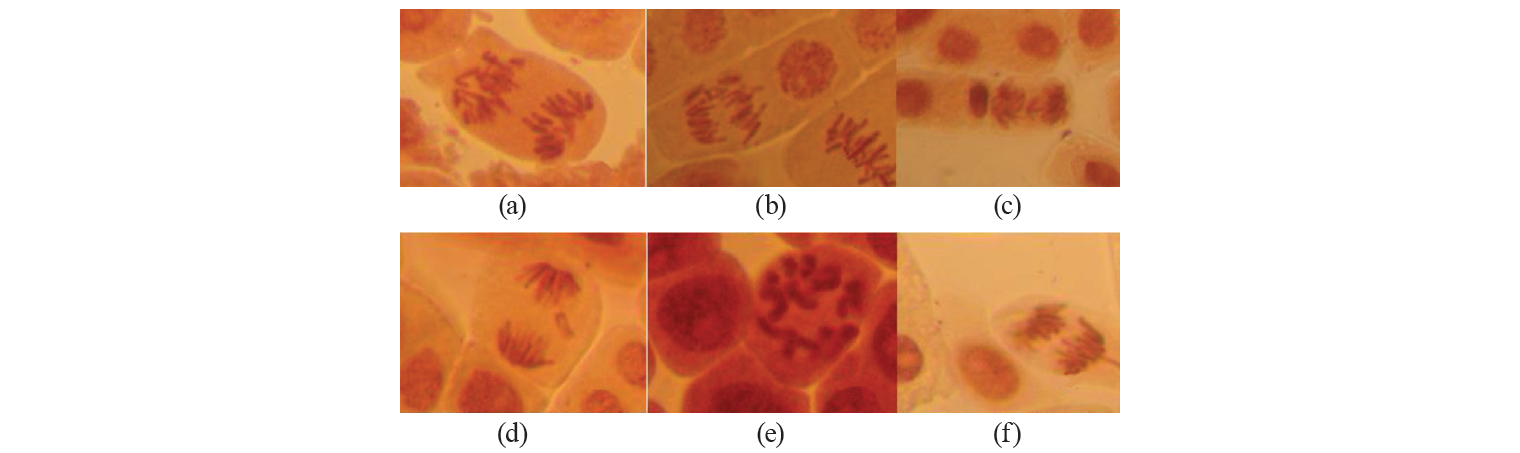
The types of major chromosomal aberrations detected in the experiment are shown in Fig. 3. The analysis of the data allows us to conclude that stickness of chromosomes in metaphase and chromosomes with laggard in anaphase make the main contribution to the spectrum of chromosomal aberrations. These anomalies account respectively for aberrations ranging from 23.8% to 70% (for sticky metaphase) and from 13.6% to 45.2% (for chromosome with laggard). Also, there were the following aberrations of the mitosis process detected in micropreparations: C-mitosis, multiple fragmentation of chromosomes, change in the spatial orientation of chromosomes at the metaphase stage in cells. The least observed anomalies included bridges and fragments (about 2%, depending on the concentration of the tested substances).
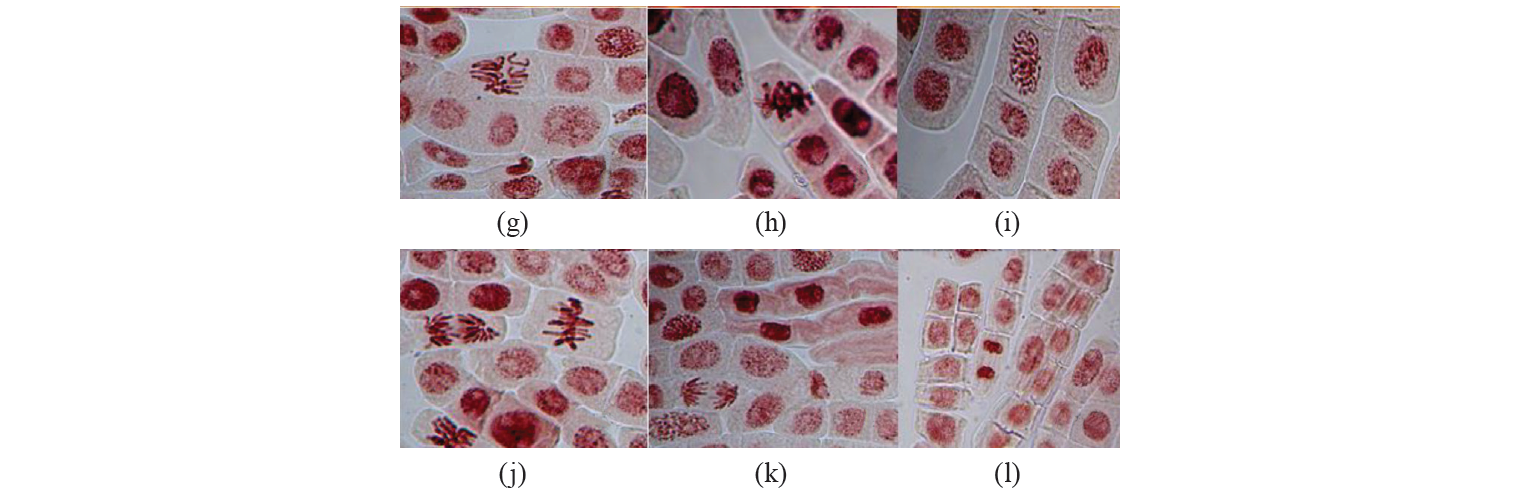
It seems remarkable to consider the whole spectrum of aberrations. The most numerous anomalies found while analyzing biomaterial can be due to the effects of mitotic spindle disorder and changes in the surface of chromosomes. The aberrations occur in this group probably due to the influence of the tested substance on the proteins regulating the work of the mitotic spindle in the cell [27]. On the other hand, bridges, fragments, and micronuclei are associated with clastogenic aberrations (arising from the fracture of the chromosome and anomalies of the further molecular genetic processes, unequal translocation or inversion of the chromosome segments). In the study [28], the analysis of genotoxicity of sodium benzoate (in concentrations from 20 to 100 mg/kg) discovered the prevalence of aberrations related to mitotic spindle disorders and changes in the surface of chromosomes. These are the main types of agglutination and C mitosis disorders. The clastogenic effect of the factor was not recorded at all for this indicator.
On the other hand, studies with high concentrations of sodium benzoate exposed a much wider spectrum of chromosomal aberrations. The aberrations included agglutination and fragmentation of chromosomes, their reduction, the formation of binuclear cells, chromosomal bridges and other disorders [29]. According to data [30], treating cells with sorbic acid resulted in the chromosomal aberrations associated with mitotic spindle disorder. Clastogenic aberrations were not detected. Similar data were obtained in the study of the effects of sorbic acid on the formation of micronuclei in cells [31].
This study recorded reliable mitodepressive and genotoxic effects at very low concentrations of preservatives (10 and 20 mg/L for benzoic and sorbic acids, respectively). It is important to note that the data obtained were consistent with the results on genotoxicity of these acids and their salts for human and animal cell culture. The results were published in a number of papers, describing the exposure to both low and high doses of these preservatives. Thus benzoic acid caused sister chromatid exchange, chromosomal aberrations, and micronuclei formation in human lymphocyte cells [32]. Other researchers demonstrated the genotoxic effect of sodium sorbate on Chinese hamster cells, as well as clastogenic, mutagenic and cytotoxic effects of sodium benzoate on the cell culture of human lymphocytes [29, 33].
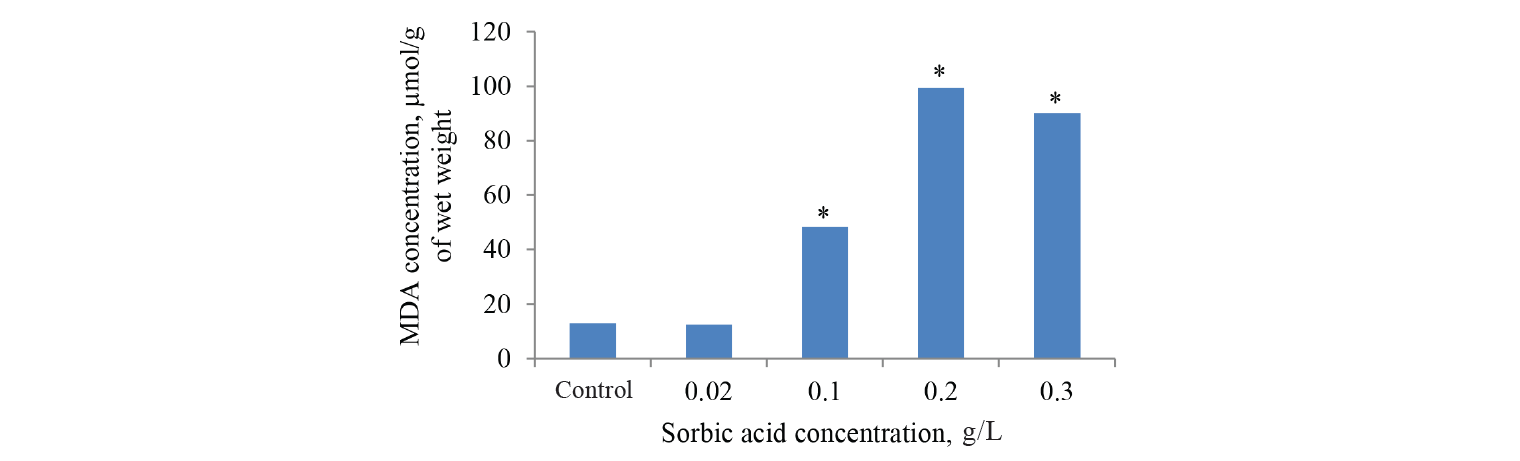
MDA concentration is commonly used as an indicator of lipid peroxidation when the tissues are exposed to chemical toxicants. MDA was measured in the onion roots of the control and experimental groups obtained in our previous study. In the experimental groups of onion roots this biomarker analysis showed a significant dose-dependent increase in the MDA levels (by 760%) compared to the control samples. At the same time benzoic acid did not have a significant effect on the process (Figs. 4 and 5). Since sorbic acid can be subjected to partial hydrolysis, MDA was measured in a solution of sorbic acid (0.2 g/L) after 4 days of incubation, but its content exceeded only by 57% compared to the values in the control samples of onion roots.
Since the biomarkers of oxidative stress usually showed a two-phase response rather than a linear response, we expanded the range of sorbic acid dilutions and increased the period of incubation with acids up to 3 days to identify the dynamics of MDA biosynthesis. Indeed, this pattern of the two-phase response was confirmed again [13]. With an increase in sorbic acid concentration, the level of this biomarker increased evenly at first, reached its peak, and then dropped dose-dependent until it reached MDA value in the control samples (Fig. 6). Like in the previous study, the maximum concentration of MDA was recorded for sorbic acid at 200 mg/L. The concentration was above that in the control group by almost 2000%, i.e. lipid oxidation level also increased with exposure time.
As far as we know, these are the first experiments to study the dynamics of lipid oxidation biomarker generation in the Allium test after exposure to benzoic and sorbic acids. Both acids reduced root growth and the mitotic index of apical meristematic cells. However, these negative phenomena were accompanied by the simultaneous increase in MDA only in the case of sorbic acid. These results are consistent with the data on the treatment of wheat seeds with benzoic acid [34]. This study did not detect any change in lipid peroxidation activity different from control when treated with low concentrations of this preservative.
In the case of benzoic acid, its toxic effects were probably not associated with oxidative damage to lipids. In addition, the study showed the protective reaction in the plant cell to benzoic acid in concentrations of 1 to 10 mM. The reaction was accompanied by an increased activity of glutamate and malate dehydrogenase, enzymes activating catabolic and metabolic processes [35]. In the current study, both the MDA level and root mass growth increased with an increase in the concentration of sorbic acid from 20 to 200 mg/L (Figs. 5 and 6). Thus, there was a clear correlation between the physiological index and MDA, the latter being even more sensitive.
According to the literature, the meristematic cell mitotic index in the control samples when using theAllium test is both close to our result (12.97 ± 0.48) and well below it [20, 36, 37]. This indicator could change depending on the quality of the batch of onions, its variety, and storage conditions. However, the question remained whether there was a dependence between the initial level of the mitotic index and the ability of root cells to fully respond to the effects of toxicants.
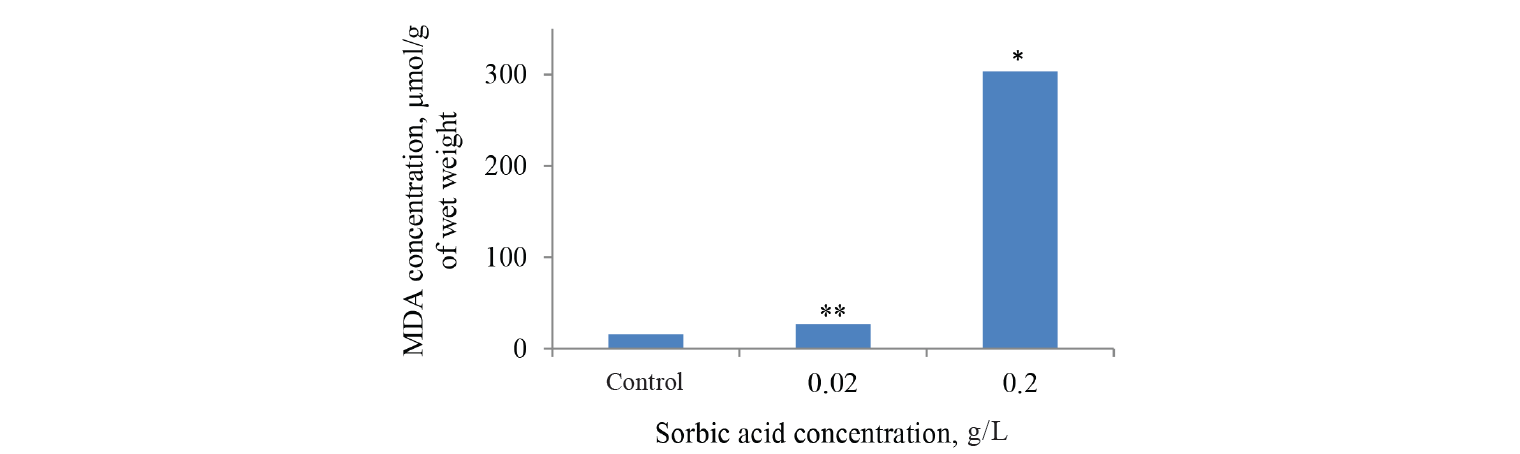
To this end, another study was conducted to examine the toxic effect of sorbic acid solutions at low and high concentration on onions with a small part of meristematic dividing cells in control. In this study, the miotic index in the control samples (Table 2) was 40% lower than that obtained in the previous study (Table 1). The conditions of the Allium test in the previous and the current study did not differ. The comparative analysis showed the following negative trends in the results. First, the roots in the experimental groups became soft and acquired a yellowish hue after 2 days of incubation with the acid. Second, there was no gain in the root mass compared to control (Table 2) when treated with a high-concentration acid solution (0.2 g/L), whereas in the previous study the gain was 25%, and roots did not change the color (Fig. 1). Similar negative changes were recorded at the biochemical level in MDA measurement (Fig. 7). The lipid oxidation activity in these samples compared to the previous study was significantly higher both in the test and control samples.
According to the obtained results, it seems advisable to select batches of bulbs before the Allium test. This selection is necessary as the low values of the mitotic index may result in false positive results, both in terms of EC50 estimates and biochemical indicators.
ВЫВОДЫ
The results of this study showed that sorbic and benzoic acids caused toxic effects in the roots of Allium cepa. These preservatives affected the physiological, biochemical, cytological, and genetic characteristics of the plant system. Treating onion roots with these acids in concentrations of 1 and 2 g/L, which are acceptable for some food products, was so highly toxic as to lead to their death. When concentrations of these acids decreased, EC50 limits for benzoic and sorbic acids were shown to be 10–20 and 20–100 mg/L, respectively. These concentrations of preservative solutions induced a 50% retardation in root growth, a significant decrease in the mitotic index, especially in the case of sorbic acid, and almost a triple increase in chromosomal disorders.
Thus, these preservatives at very low concentrations gave a chronic and subchronic toxic effect. Based on the conducted studies, it is necessary to use the concentration of food preservatives within their detected EC50 values to assess these toxicity indicators in the Allium test. If these conditions are met, it is possible to simulate the processes of detoxification and metabolism for these compounds, both at the cellular level and the whole organism.
Therefore, it can help gain a better understanding of the biological actions of these agents. Indeed, the negative effects found under these conditions for sorbic acid, but not benzoic acid, were correlated with the lipid oxidation biomarker. In this regard, we believe that the study of this biomarker can provide valuable information for monitoring and predicting early effects of sorbic acid on animal and human cells. Yet, it is probably necessary to study the role of catabolic processes to determine the molecular mechanisms of activation of enzymes with benzoic acid [35].
КОНФЛИКТ ИНТЕРЕСОВ
The authors state that there is no conflict of interest.
ФИНАНСИРОВАНИЕ
The materials were prepared as part of the government assignment to V.M. Gorbatov Federal Scientific Center for Food Systems.СПИСОК ЛИТЕРАТУРЫ
- Petrov AN, Galstyan AG, Radaeva IA, Turovskaya SN, Illarionova EE, Semipyatniy VK, et al. Indicators of quality of canned milk: Russian and international priorities. Foods and Raw Materials. 2017;5(2):151–161. DOI: https://doi.org/10.21179/2308-4057-2017-2-151-161.
- Strizhko M, Kuznetsova A, Galstyan A, Semipyatniy V, Petrov A, Prosekov A. Development of osmotically active compositions for milk-based products with intermediate humidity. Bulletin of the International Dairy Federation. 2014;35–40.
- Petrov AN, Khanferyan RA, Galstyan AG. Current aspects of counteraction of foodstuff’s falsification. Problems of Nutrition. 2016;85(5):86–92. (In Russ.)
- Prosekov AYu. Fundamentalʹnye osnovy tekhnologii produktov pitaniya [Fundamentals of food technology]. Kemerovo: Kemerovo State University; 2019. 498 p. (In Russ.).
- Prosekov AYu, Ivanova SA. Food security: The challenge of the present. Geoforum. 2018;91:73–77. DOI: https://doi.org/10.1016/j.geoforum.2018.02.030.
- Shu Y, Yu B, He J, Yu J, Zheng P, Yuan ZC, et al. Excess of dietary benzoic acid supplementation leads to growth retardation, hematological abnormality and organ injury of piglets. Livestock Science. 2016;190:94–103.
- Monanu MO, Uwakwe AA, Onwubiko D. In vitro effects of sodium benzoate on the activities of aspartate and alanine amino transferases, and alkaline phosphatase from human erythrocytes of different genotypes. Biokemistri. 2005;17(1):33–38.
- Lok KYW, Chan RSM, Lee VWY, Leung PW, Leung C, Leung J, et al. Food additives and behavior in 8-to 9-yearold children in Hong Kong: A randomized, double-blind, placebo-controlled trial. Journal of Developmental and Behavioral Pediatrics. 2013;34(9):642–650. DOI: https://doi.org/10.1097/DBP.0000000000000005.
- Noorafshan A, Erfanizadeh M, Karbalay-Doust S. Sodium benzoate, a food preservative, induces anxiety and motor impairment in rats. Neurosciences. 2014;19(1):24–28.
- Shahmohammadi M, Javadi M, Nassiri-Asl M. An overview on the effects of sodium benzoate as a preservative in food products. Biotechnology and Health Sciences. 2016;3(3). DOI: https://doi.org/10.17795/bhs-35084.
- Winkler C, Frick B, Schroecksnadel K, Schennach H, Fuchs D. Food preservatives sodium sulfite and sorbic acid suppress mitogen-stimulated peripheral blood mononuclear cells. Food and Chemical Toxicology. 2006;44(12):2003–2007. DOI: https://doi.org/10.1016/j.fct.2006.06.019.
- Blaauboer BJ, Boobis AR, Bradford B, Cockburn A, Constable A, Daneshian M, et al. Considering new methodologies in strategies for safety assessment of foods and food ingredients. Food and Chemical Toxicology. 2016;91:19–35. DOI: https://doi.org/10.1016/j.fct.2016.02.019.
- Wiklund AKE, Adolfsson-Erici M, Liewenborg B, Gorokhova E. Sucralose induces biochemical responses in daphnia magna. PLOS ONE. 2014;9(4).
- Camparoto ML, Teixeira RD, Mantovani MS, Vicentini PVE. Effects of Maytenus ilicifolia Mart. and Bauhinia candicans Benth infusions on onion root-tip and rat bone-marrow cells. Genetics and Molecular Biology. 2002;25(1):85–89. DOI: https://doi.org/10.1590/s1415-47572002000100016.
- Renjana PK, Thoppil JE. Toxicological evaluation of root methanolic extract of Strobilanthes heyneanus nees using Allium test. International Journal of Pharmaceutical Sciences and Drug Research. 2013;5(3):125–128.
- Samoilov AV, Suraeva NM, Zaitseva MV, Kurbanova MN, Stolbova VV. Сomparative assessment of artificial sweeteners toxicity via express biotest. Health Risk Analysis. 2019;(2):83–90. (In Russ.). DOI: https://doi.org/10.21668/health.risk/2019.2.09.eng.
- Saghirzadeh M, Gharaati MR, Mohammadi S, Ghiassi-Nejad M. Evaluation of DNA damage in the root cells of Allium cepa seeds growing in soil of high background radiation areas of Ramsar – Iran. Journal of Environmental Radioactivity. 2008;99(10):1698–1702. DOI: https://doi.org/10.1016/j.jenvrad.2008.03.013.
- Hara RV, Marin-Morales MA. In vitro and in vivo investigation of the genotoxic potential of waters from rivers under the influence of a petroleum refinery (Sao Paulo State – Brazil). Chemosphere. 2017;174:321–330. DOI: https://doi.org/10.1016/j.chemosphere.2017.01.142.
- Meng Q, Zou J, Zou J, Jiang W, Liu D. Effect of Сu2+ concentration on growth, antioxidant enzyme activity and malondialdehyde content in garlic (Allium sativum L.). Acta Biologica Cracoviensia. Series Botanica. 2007;49(1):95–101.
- Cavusoglu K, Yalcin E, Turkmen Z, Yapar K, Cicek F. Investigation of toxic effects of the glyphosate on Allium cepa. Tarim Bilimleri Dergisi-Journal of Agricultural Sciences. 2011;17(2):131–142.
- Kurbanova MN, Suraeva NM, Rachkova VP, Samoylov AV. Comparative study of indicators of toxic activity in the Allium-test. Agrarian Bulletin of the Urals. 2018;171(4):25–30. (In Russ.).
- Zhang H, Jiang Y, He Z, Ma M. Cadmium accumulation and oxidative burst ingarlic (Allium sativum). Journal of Plant Physiology. 2005;162(9):977–984. DOI: https://doi.org/10.1016/j.jplph.2004.10.001.
- Fiskesjo G. The Allium test as a standard in environmental monitoring. Hereditas. 1985;102(1):99–112. DOI: https://doi.org/10.1111/j.1601-5223.1985.tb00471.x.
- Wibbertmann A, Kielhorn J, Könnecker G, Mangelsdorf I, Melber C. Benzoic acid and sodium benzoate. IPCS Concise International Chemical Assessment Documents; 2000; Geneva. Geneva: World Health Organization; 2000. pp. 49.
- Olufunsho A, da Silva JAT, Akintonwa A. Mitodepressive effect of four food additives using the Allium cepa assay. The African Journal of Plant Science and Biotechnology. 2010;4(1):114–117.
- Pandey H, Kumar V, Roy BK. Assessment of genotoxicity of some common food preservatives using Allium cepa L. as a test plant. Toxicology Reports. 2014;1:300–308. DOI: https://doi.org/10.1016/j.toxrep.2014.06.002.
- Shahin SA, Elamoodi KH. Induction of numerical chromosomal-aberrations during DNA-synthesis using the fungicides nimrod and rubigan-4 in root-tips of Vicia faba L. Mutation Research. 1991;261(3):169–176. DOI: https://doi.org/10.1016/0165-1218(91)90064-s.
- Türkoglu S. Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2007;626(1–2):4–14. DOI: https://doi.org/10.1016/j.mrgentox.2006.07.006.
- Zengin N, Yuzbasioglu D, Unal F, Yilmaz S, Aksoy H. The evaluation of the genotoxicity of two food preservatives: Sodium benzoate and potassium benzoate. Food and Chemical Toxicology. 2011;49(4):763–769. DOI: https://doi.org/10.1016/j.fct.2010.11.040.
- Banerjee TS, Giri AK. Effects of sorbic acid and sorbic acid-nitrite in vivo on bone marrow chromosomes of mice. Toxicology Letters. 1986;31(2):101–106. DOI: https://doi.org/10.1016/0378-4274(86)90002-0.
- Jung R, Cojocel C, Muller W, Bottger D, Luck E. Evaluation of the genotoxic potential of sorbic acid and potassium sorbate. Food and Chemical Toxicology. 1992;30(1):1–7. DOI: https://doi.org/10.1016/0278-6915(92)90130-d.
- Yilmaz S, Unal F, Yuzbasioglu D. The in vitro genotoxicity of benzoic acid in human peripheral blood lymphocytes. Cytotechnology. 2009;60(1–3):55–61. DOI: https://doi.org/10.1007/s10616-009-9214-z.
- Hasegawa MM, Nishi Y, Ohkawa Y, Inui N. Effects of sorbic acid and its salts on chromosome-aberrations, sister chromatid exchanges and gene-mutations in cultured chinese-hamster cells. Food and Chemical Toxicology. 1984;22(7):501–507. DOI: https://doi.org/10.1016/0278-6915(84)90219-9.
- Yadav K, Singh NB. Effects of benzoic acid and cadmium toxicity on wheat seedlings. Chilean Journal of Agricultural Research. 2013;73(2):168–174. DOI: https://doi.org/10.4067/S0718-58392013000200013.
- Chrikishvili D, Sadunishvili T, Zaalishvili G. Benzoic acid transformation via conjugation with peptides and final fate of conjugates in higher plants. Ecotoxicology and Environmental Safety. 2006;64(3):390–399. DOI: https://doi.org/10.1016/j.ecoenv.2005.04.009.
- Onyemaobi OI, Williams GO, Adekoya KO. Сytogenetic effects of two food preservatives, sodium metabisulphite and sodium benzoate on the root tips of Allium cepa linn. Ife Journal of Science. 2012;14(1):155–165.
- Iwalokun BA, Oyenuga AO, Saibu GM. Ayorinde J. Analyses of cytotoxic and genotoxic potentials of Loranthus micranthus using the Allium cepa test. Current Research Journal of Biological Sciences. 2011;3(5):459–467.