Аннотация
Introduction. The increasing global consumption of processed meat products has led to certain concerns. For instance, processed meat products are known to contain carcinogen precursor compounds, thus creating the risk of chronic diseases. The present study was performed to estimate the food safety status of processed meat products available in Iran and evaluate the related effective factors.Study objects and methods. 140 samples of seven most popular commercial types of cooked sausages were obtained from four major meat factories (A, B, C and D) in 140 samples were collected from seven most popular commercial types of cooked sausages as follows: beef salami 90%, chicken salami 90%, dry cured sausage 70%, dry cured salami 60%, beef sausages 55%, chicken sausages 55% and Frankfurt sausage 40% (n = 5) from four major meat factories (A, B, C and D) in Tehran. The samples were screened for residual nitrite, ascorbic acid, and nitrosamine contents on days 0, 7, 14, 21, and 28. The results indicated that products from meat factory B had lower residual nitrite content in the samples with high content of meat. Beef salami (90% of meat) and Frankfurt sausage (40% of meat) contained the lowest and highest amounts of residual nitrite on day 0 – 73.99 and 177.42 mg of nitrite per 1 kg of meat, respectively.
Results and discussion. Beef salami contained 90% of meat, chicken salami – 90%, dry cured sausage –70%, dry cured salami – 60%, beef sausages – 55%, chicken sausages – 55%, and Frankfurt sausage – 40% (n = 5). Nitrite reduction rates in sausages with a smaller diameter, e.g. Frankfurt sausage, were significantly lower (P < 0.05), compared to salami samples. The difference can be explained by the shorter cooking time. Nitrosamine formation increased during refrigerated storage; however, it was not significant in all samples. During refrigerated storage, nitrosamine formation depended on the level of added nitrite, the amount of residual nitrite, ascorbic acid, pH, and cooking temperature. Ascorbic acid content decreased significantly (P < 0.05) during refrigerated storage.
Conclusion. The findings demonstrate significant correlation between the meat content, cooking time, nitrite content, and nitrosamine formation.
Ключевые слова
Meat industry, processed meat, meat products safety, carcinogenic agents, preservationВВЕДЕНИЕ
To reduce the adverse effects of red meat and artificial additives on human health, one can reduce the consumption of red meat or processed meat products. However, studying possible ways to reduce the harmful effects might be as effective as limiting consumption levels. Nitrite/nitrate salts are usually added to meat products to guarantee their safety, since these salts inhibit the growth of Clostridium botulinum and prevent heat-resistant spores from producing toxins. Moreover, nitrite/nitrate salts improve the color, flavor, and aroma of the finished product and postpone lipid oxidation processes [1].
In spite of the numerous advantages, an excessive intake of nitrite can produce adverse effects on human health. Nitrite can be transformed to a nitrosating agent (NO+), which reacts with biogenic amines and creates carcinogenic N-nitrosamines. Bacterial and meat enzymes cause decarboxylation of free amino acids, which leads to the formation of biogenic amines. Several intrinsic and extrinsic factors, e.g. salt content, can also affect the biogenic amine formation level [2–4].
In meat and meat products, carcinogenic N-nitrosamines are usually formed under acidic conditions caused by the electrophilic reaction between a nitrosating agent, e.g. nitrite or nitrous acid, and secondary/tertiary amines that result from protein and lipid degradation [5]. The mechanism of nitrosamine formation in meat products has been thoroughly studied. Nitrosamines with generic chemical structure of R2NN = O are produced under certain conditions of low pH, high temperature, and presence of some reducing agents associated with processing and composition of a particular meat product [6]. There are several hurdle technologies that can reduce the concentration of nitrite required to inhibit bacterial growth, e.g. ascorbic/erythorbic (isoascorbic) acid and essential oils, certain processing conditions, or various non-thermal methods [1, 7]. Exposure level of N-nitrosodimethylamine (NDMA) compound via consumption of food and beverages was estimated to be 0.09 and 0.1 μg/day in the Netherlands and Germany, respectively [8, 9]. The level of these compounds in nitrite-preserved meat products varies significantly. It depends on the ingoing volume of nitrite, meat quality, and fat content, as well as on processing, ripening, and storing conditions. There have been many reports on nitrosamines detected in processed meat products [10, 11].
The research objective was to examine the safety of emulsion-type cooked sausages available on the Iran markets by assessing the contents of residual nitrite, ascorbic acid, and nitrosamine. In addition, we also studied the effects of processing conditions and related factors on these compounds to provide information for health professionals and food manufacturers.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Samples. The samples were collected from four major meat factories in Tehran (A, B, C, and D) out of the total of 189 meat factories in Iran. Seven most popular commercial types of cooked sausages were randomly purchased. Seven most popular commercial types of cooked sausages were randomly purchased as follows: beef salami 90%, chicken salami 90%, dry cured sausage 70%, dry cured salami 60%, beef sausages 55%, chicken sausages 55% and Frankfurt sausage 40%.
Five samples (2 kg) from each type were examined on days 0, 7, 14, 21, and 28 of storage. The samples contained beef (15% fat) orchicken (10% fat), water, oil, sodium caseinate, sodium polyphosphate, garlic, salt, wheat flour starch, spices, gluten, natural flavorings (paprika, curcumin, ginger, and cinnamon), ascorbic acid, and sodium nitrite. The samples were immediately transferred to the laboratory and kept refrigerated until tested.
Residual nitrite determination. The nitrite content was evaluated during 28 days on days 0, 7, 14, 21, and 28 using slightly modified calorimetric method of AOAC method no. 973.31 [12]. The samples were cut into pieces and homogenized. An aliquot of about 2.5 g of minced sausage was added to 5 mL of saturated borax and 25 mL of deionized water (> 70°C) in Falcon tubes. After stirring and cooling in room temperature, 1 mL of each Carrez solution (I and II) was added to each sample and adjusted to the volume of 50 mL. Carrez solution I was prepared by dissolving 10.6 g of ferrocyanide in distilled water (100 mL) according to the method introduced by Ramezani et al. [13]. Carrez solution II was also made by mixing 21.9 g of zinc acetate with acetic acid (3 mL) and adjusted to the volume of 100 mL using distilled water. After centrifugation at 4000 rpm for 5 min (HeltichRotorfix 32A), 25 mL of supernatant was transferred to a 100 mL tube. Ten ml of sulfanilamide reagent and 6 mL of dilute HCl were added to the supernatants and kept in the dark for 5 min. Then, 2 mL of naphtyl-ethylenadiamine solution was added to obtain high intensity azo dyes. The samples were kept in the dark for 10 min to achieve complete reactions. Absorbance was measured at 538 nm.
Ascorbic acid determination. To determine the ascorbic acid content, 2 g of minced sausage was mixed with 10 mL of meta-phosphoric acid solution (3%), tertiary butylhydroquinone (TBHQ) (0.1%), and acetic acid (8%). After that, they were centrifuged at 4000 rpm for 10 min. After filtration, 20 μL of supernatant solution was injected to a Cecil CE-4900 high-performance liquid chromatograph coupled to a UV-vis detector (HPLC-UV/VIS, Cambridge, England). The analytical HPLC was equipped with two CE-4100 pumps, vacuum degasser, six port valves (Rheodyne, USA), mixing chamber, multiple solvent delivery unit, and an ODS column (250 mm·4 I.D., 5 μm). The mobile phase consisted of acetonitrile and sodium phosphate buffer solution (50:50). The flow rate was 1.2 mL·min–1 at room temperature [14].
N-nitrosamine determination. The experiment evaluated seven volatile nitrosamines in the popular cooked sausages from four major Iran meat factories. The nitrosamines included N-nitrosodimethylamine (NDMA), N-nitrosodiethylamine (NDEA), N-nitrosomorpholine (NMOR), N-nitrosopyrrolidine (NPYR), N-nitrosopiperidine (NPIP), N-nitrosodi-n-butylamine (NDBA), and N-nitrosodiphenylamine (NDPheA). The presence of nitrosamine was determined and quantified according to the method described in our previous study [13]. The method presupposed using microwaveassisted extraction coupled with dispersive liquid–liquid micro extraction (DLLME) followed by gas chromatography–mass spectrometry (GC-MS).
Statistical analysis. All experiments were carried out in triplicate. One-way ANOVA was performed to determine significant differences. Duncan’s multiple range test was used to define the differences of mean value. Data analysis was performed using SPSS version 21 (SPSS Inc., Chicago, IL, USA); P < 0.05 was considered statistically significant.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
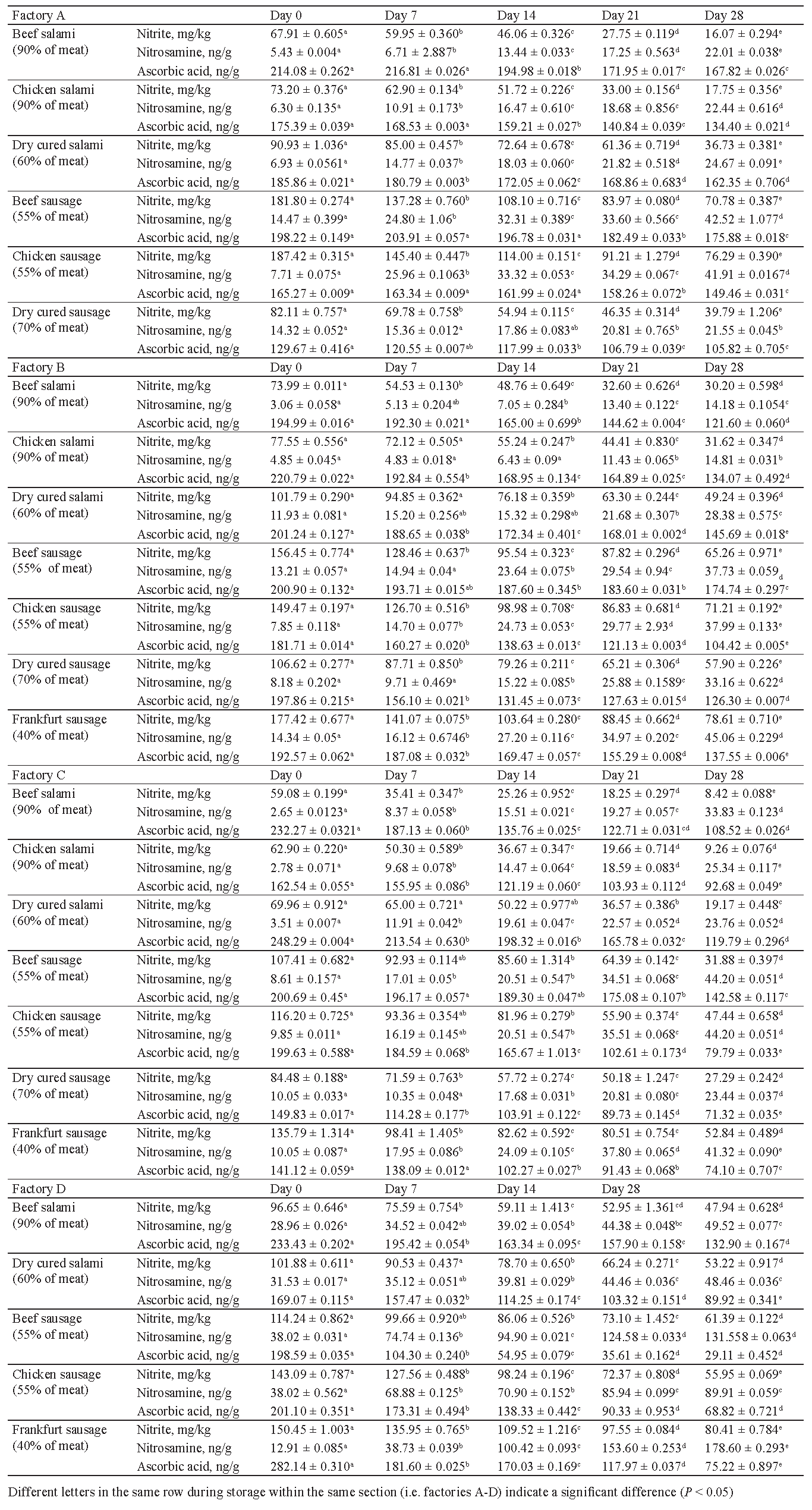
The present research featured samples from seven most popular types of sausages in Iran. The selected sausages differed in the amount of nitrite, ascorbic acid, and nitrosamine. This difference is associated with the variations in concentration of added nitrate and nitrite salts (sodium and potassium), storage conditions, and different pH values [15, 16]. Following the EU legislations, 2006/52/EC directive limited the usage of nitrite to 150 mg per 1 kg of meat. However, the national laws in Iran are more restricting. Iranian provisions allow for maximum 120 mg of nitrite per 1 kg of meat (ppm) in sausages [17]. Table 1 displays the residual nitrite, ascorbic acid, and nitrosamine contents in the meat samples under study during storage.
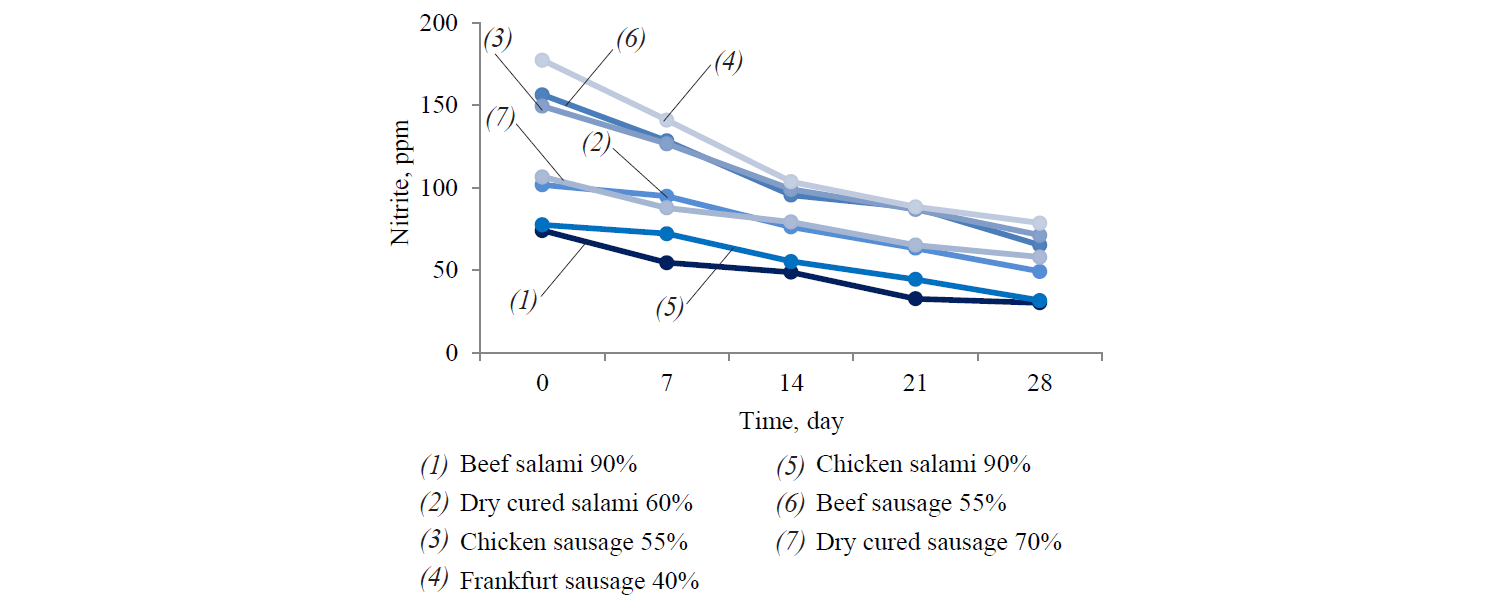
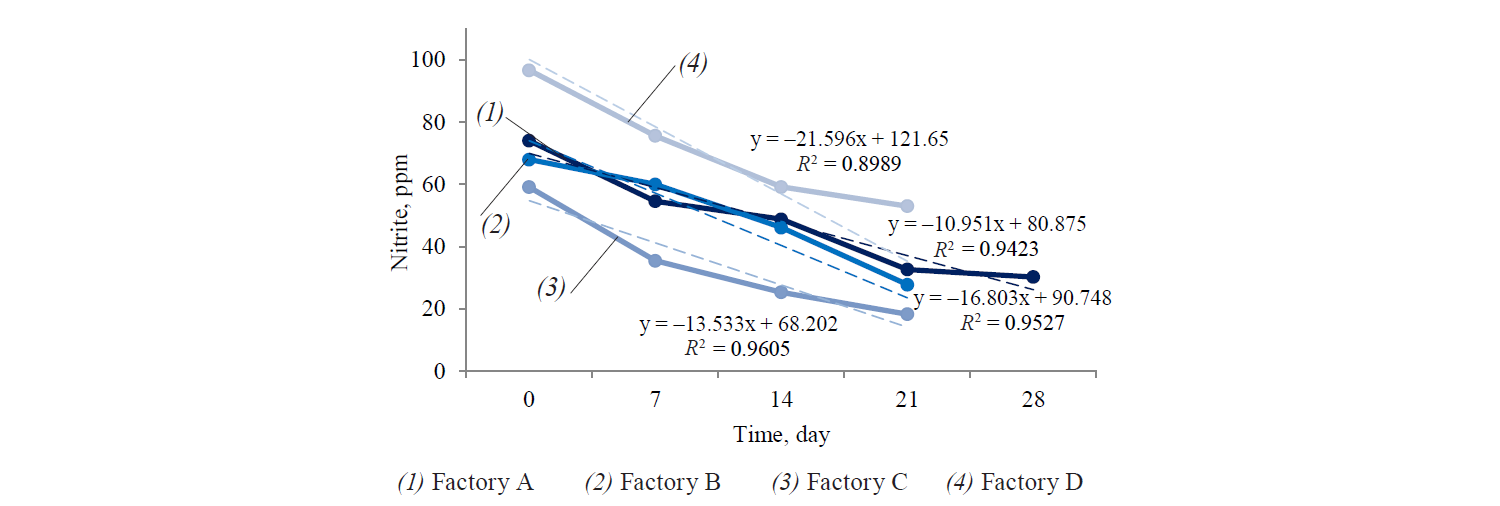
Figure 1 illustrates the results of nitrite content changes (mg/kg meat) in the meat samples obtained from factory B as representative of all the four meat factories.
As it was expected, the amount of nitrite residue in meat products of all four meat factories decreased during 28 days of refrigerated storage. The increase in meat content increased the reduction rate of sodium nitrite in the products. In other words, lower residual nitrite content was detected in high content meat samples. The highest reduction rate of nitrite content was observed in chicken and beef salami (90% of meat). On the first day of refrigerated storage, the Frankfurt sausage (40%) and both beef and chicken sausages (55%) contained 80 μg/kg of nitrite.
The detected amount exceeded the level permitted by Iranian laws as defined by the Institute of Standard and Industrial Research of Iran (ISIRI). The maximum permissible burden of nitrite is 66 and 63 μg/kg for processed meat products with 40% and 55% of meat content, respectively. The concentration of nitrite was higher than the expected levels even after 28 days of storage. However, the residual nitrite content was not exactly equal to the initial added nitrite. First, it was partly degraded by heating process. Second, it decreased when ascorbic acid was applied to the meat product during the heating process to accelerate conversion of nitrite to nitric oxide.
The nitrite content varied due to interaction with heme-containing components, non-heme proteins, and fat tissues, conversion to nitrate, production of such gases as N2, CO2, and NO, and nitrosamines [18]. Therefore, residual nitrite was associated with meat content due to different contents of myoglobin [19]. As a reactive agent, nitrite converts to nitrite oxide and forms nitrite-heme-nitrosomyoglobin complex with myoglobin, thus producing nitrosomyochromogen. The latter is responsible for the characteristic bright pink color of cured meat products [20].
Hence, higher content of myoglobin results in lower nitrite content in the final meat product. The results obtained by statistical analysis indicate that residual nitrite in Frankfurt sausage (40%) was significantly higher (P < 0.05), whereas in beef salami (90%) it was at its lowest. No significant differences were observed in other sausage and salami products (P > 0.05).
Ascorbic acid, or ascorbate, is applied to meat products as an additive with high water solubility for three major reasons. First, the nitrosomyoglobin-forming reduction of nitrite to nitric oxide produces the required color. Second, the antioxidative activity of ascorbic acid slows down oxidation of pigments and lipids, which results in color and flavor stability. Third, residual nitrite decreases due to binding to nitrite in heated samples [21].
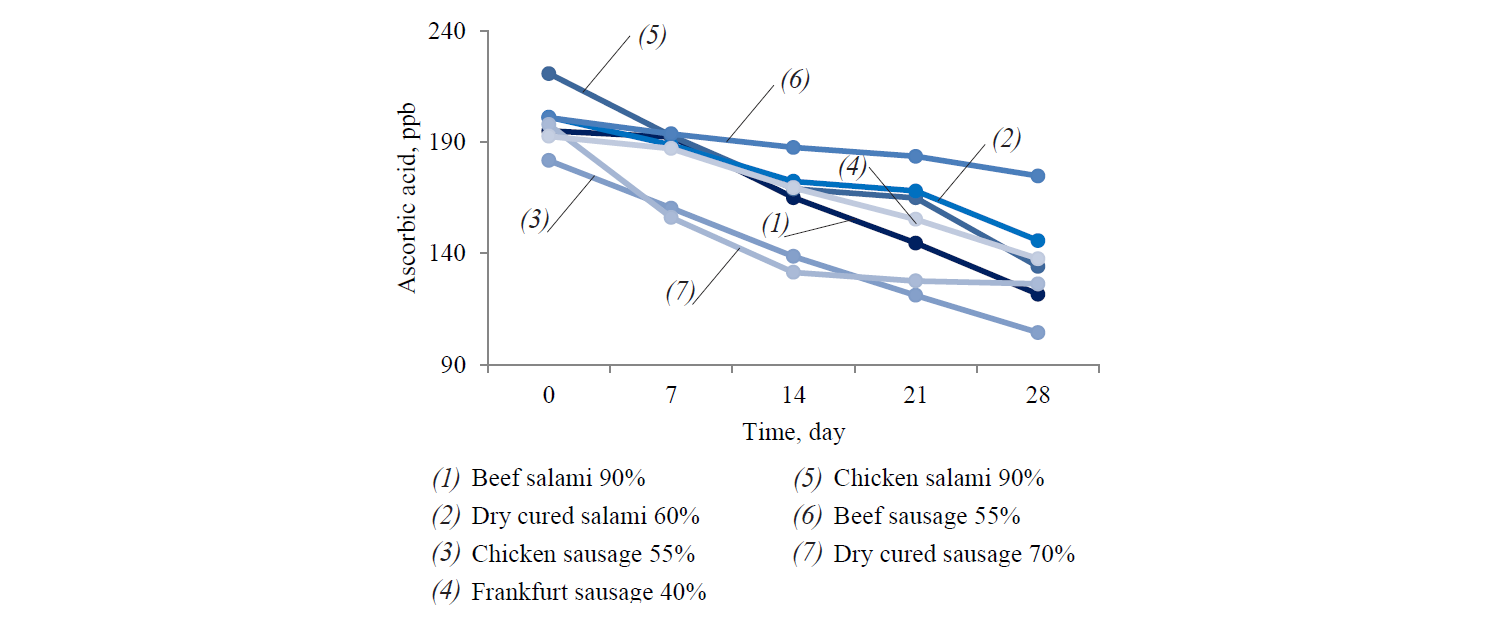
Ascorbate proved effective in nitrosamine inhibition. This quality is associated with rapid reactions of ascorbate with nitric oxide compared to nitrosating agents, e.g. amines. Therefore, nitrosamines are formed when the reaction rate constant of ascorbate is not much larger than amines [16]. Figure 2 and Table 1 demonstrate that ascorbic acid content of samples decreased significantly (P < 0.05) during refrigerated storage.
Meat products with higher meat content exhibited lower ascorbic acid reduction rate, which was due to lower residual nitrite content. Ascorbic acid degradation in meat products could be related to oxygen content, temperature, light, water activity, presence of metal ions, e.g. copper and ferric iron, and storage time [22–24]. Degradation of ascorbic acid is increased in acidic conditions (pH = 3.3–5.5). In the current study, pH values were in the range of 5–6 in all samples. Therefore, ascorbic acid degradation might have been due to the relative acidic condition of meat products and heating process.
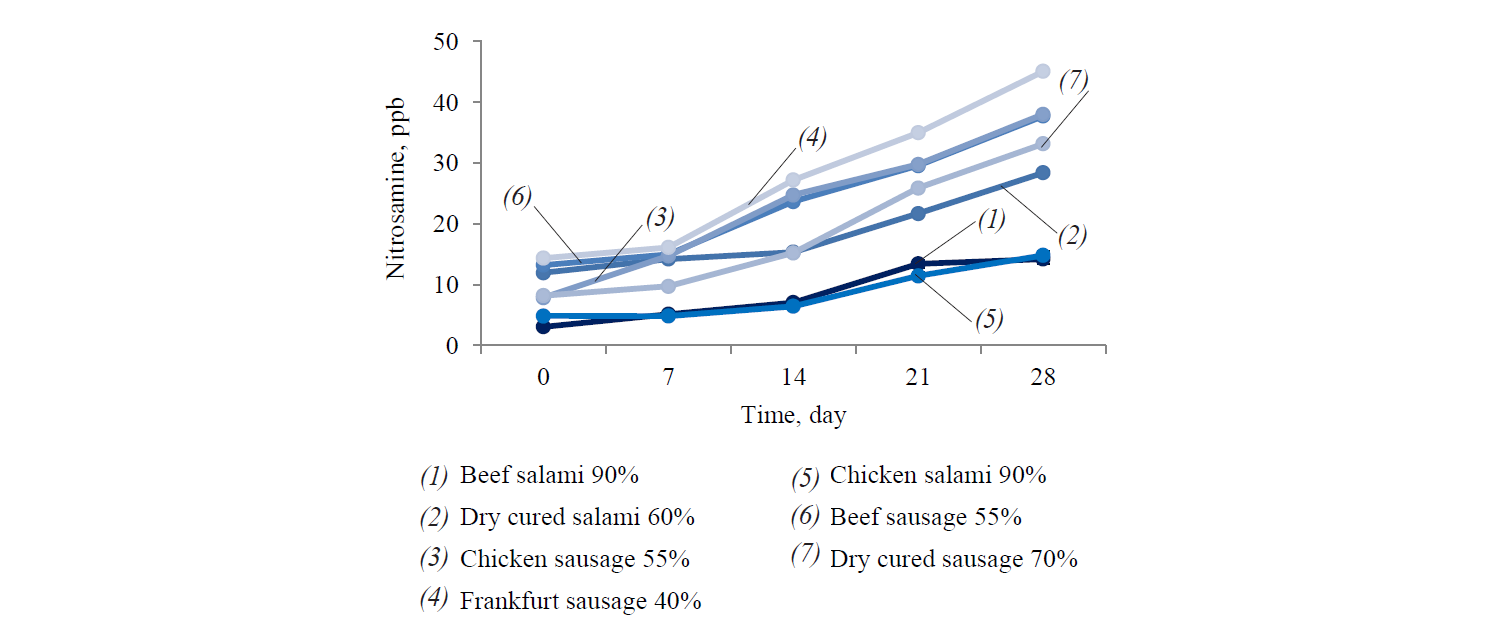
The obtained results showed an increase in nitrosamine content of samples during refrigerated storage. However, it was not significant in all samples (Fig. 3).
The nitrite reduction rates in the sausages with a smaller diameter, e.g. Frankfurt sausage, appeared significantly lower (P < 0.05) than in the samples with a bigger diameter, e.g. salami. This difference can be explained by the longer cooking time for salami (5–6 h) compared to Frankfurt sausages (3–4 h), which is associated with a higher reduction rate of residual nitrite. According to the results, the total volatile nitrosamine level of Iranian meat products with lower meat contents was generally higher than that of samples with high meat contents. The nitrosamine content in meat products was associated with the added nitrite and the residual nitrite [25]. The residual nitrite is a reactive agent that can be reduced by heating treatment or exposure to such meat components as proteins, lipids, and pigments [26, 27].
Figure 4 shows that there was a significant correlation between the amounts of added nitrite and the nitrosamine contents in beef salami (90%) samples from all four meat factories.
In this study, the correlation factor was 0.9, which asserts the effects of added nitrite and also residual nitrite contents on nitrosamine formation in the processed meat product. Therefore, the products with lower meat content showed higher residual nitrite and, consequently, a greater nitrosamine formation. In meat products, presence of nitrosating agents increases the concentration of nitrosamine, whether grouped in NO2 related agents, e.g. N2O4, or grouped in nitrous acid derivatives, e.g. N2O3 and HNO2 [28].
There are several suggested strategies to reduce nitrosamine formation in meat products to improve their healthy status and safety. The present research clarified that the levels of nitrosamines in the samples depended on the amount of residual nitrite, ascorbic acid, pH, and cooking temperature. Higher levels of residual nitrite were detected in the samples with a lower amount of meat, compared to those with a higher amount of meat.
ВЫВОДЫ
In the current study, the residual nitrite, ascorbic acid, and nitrosamine contents of seven most popular Iranian processed meat products, namely sausages with different amounts of meat were evaluated to monitor the safety status of the meat industry. The samples contained various concentrations of nitrite and nitrosamine, which were above the permitted standard level. Several factors were found to affect the residual nitrite and nitrosamine contents, the meat content being a significant variable. Nitrite interacted with hemecontaining components, non-heme proteins, and fat tissues, thus conversed to nitrate and nitrosamine. Therefore, the contents of residual nitrite and, consequently, nitrosamine in products with lower meat content were significantly higher. A longer cooking time also decreased residual nitrite and nitrosamine concentration.
Further research is needed to identify new substances that could replace nitrites, as well as factors that reduce the required amount of nitrite in meat products.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare that there is no conflict of interest regarding the publication of this article.
БЛАГОДАРНОСТИ
We would like to thank National Nutrition and Food Technology Research Institute for the financial support.
ФИНАНСИРОВАНИЕ
This research was financially supported by National Nutrition and Food Technology Research Institute (NNFTRI) of Iran.СПИСОК ЛИТЕРАТУРЫ
- Herrmann SS, Granby K, Duedahl-Olesen L. Formation and mitigation of N-nitrosamines in nitrite preserved cooked sausages. Food Chemistry. 2015;174:516–526. DOI: https://doi.org/10.1016/j.foodchem.2014.11.101.
- Herrmann SS, Duedahl-Olesen L, Christensen T, Olesen PT, Granby K. Dietary exposure to volatile and non-volatile N-nitrosamines from processed meat products in Denmark. Food and Chemical Toxicology. 2015;80:137–143. DOI: https://doi.org/10.1016/j.fct.2015.03.008.
- Vazquez-Torres A, Baumler AJ. Nitrate, nitrite and nitric oxide reductases: from the last universal common ancestor to modern bacterial pathogens. Current Opinion in Microbiology. 2016;29:1–8. DOI: https://doi.org/10.1016/j.mib.2015.09.002.
- Oliveira N, Girao C, Girao C. Impact of nitrosamines on carcinogenesis at the digestive system level. DNA. 2017;3.
- Park J-E, Seo J-E, Lee J-Y, Kwon H. Distribution of seven N-nitrosamines in food. Toxicological Research. 2015;31(3):279–288. DOI: https://doi.org/10.5487/tr.2015.31.3.279.
- De Mey E, De Maere H, Paelinck H, Fraeye I. Volatile N-nitrosamines in meat products: Potential precursors, influence of processing, and mitigation strategies. Critical Reviews in Food Science and Nutrition. 2017;57(13):2909–2923. DOI: https://doi.org/10.1080/10408398.2015.1078769.
- Colle MJ, Richard RP, Colle MC, Loucks WI, Doumit ME. Effects of ascorbic acid and rosemary extract on quality characteristics and sensory perception of extended aged beef. Meat Science. 2016;(112). DOI: https://doi.org/10.1016/j.meatsci.2015.08.091.
- Keszei AP, Schouten LJ, Driessen ALC, Huysentruyt CJR, Keulemans YCA, Goldbohm RA, et al. Vegetable, fruit and nitrate intake in relation to the risk of Barrett’s oesophagus in a large Dutch cohort. British Journal of Nutrition. 2014;111(8):1452–1462. DOI: https://doi.org/10.1017/s0007114513003929.
- Tricker AR, Preussmann R. Carcinogenic N-nitrosamines in the diet – occurrence, formation, mechanisms and carcinogenic potential. Mutation Research/Genetic Toxicology. 1991;259(3–4):277–289. DOI: https://doi.org/10.1016/0165-1218(91)90123-4.
- Zhu QQ, Wang JP, Liu SS, Zhang Y. Determination of four volatile N-nitrosamines in the process of Chinese preserved meat. Proceedings of the 2015 International Conference on Materials, Environmental and Biological Engineering. 2015;10:618–621.
- Cantwell M, Elliott C. Nitrates, nitrites and nitrosamines from processed meat intake and colorectal cancer risk. Journal of Clinical Nutrition & Dietetics. 2017;3(4):27–30. DOI: https://doi.org/10.4172/2472-1921.100062.
- Official methods of analysis of AOAC International, 18th Edition. Washington DC: The Association of Official Analytical Chemists, 1995.
- Ramezani H, Hosseini H, Kamankesh M, Ghasemzadeh-Mohammadi V, Mohammadi A. Rapid determination of nitrosamines in sausage and salami using microwave-assisted extraction and dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry. European Food Research and Technology. 2015;240(2): 441–450. DOI: https://doi.org/10.1007/s00217-014-2343-4.
- Hernandez Y, Lobo MG, Gonzalez M. Determination of vitamin C in tropical fruits: A comparative evaluation of methods. Food Chemistry. 2006;96(4):654–664. DOI: https://doi.org/10.1016/j.foodchem.2005.04.012.
- Zhang HC, Sun CB, Han WJ, Zhang JX, Hou JC. Analysis of the monitoring status of residual nitrite in meat products in China from 2000 to 2011. Meat Science. 2018;136:30–34. DOI: https://doi.org/10.1016/j.meatsci.2017.10.009.
- Waga M, Takeda S, Sakata R. Effect of nitrate on residual nitrite decomposition rate in cooked cured pork. Meat Science. 2017;129:135–139. DOI: https://doi.org/10.1016/j.meatsci.2017.03.002.
- Hosseini H, Ahmadi H, Akhavan H, Ferdowsi R, Khaksar R, Shahraz F, et al. Growth patterns of aerobic mesophilic and psychrotrophic microorganisms, moulds and yeasts in four heated red-meat product groups during storage. Iranian Journal of Nutrition Sciences & Food Technology. 2008;3(2):33–40.
- Ford PC. Reactions of NO and nitrite with heme models and proteins. Inorganic Chemistry. 2010;49(14):6226–6239. DOI: https://doi.org/10.1021/ic902073z.
- Honikel KO. The use and control of nitrate and nitrite for the processing of meat products. Meat Science. 2008; 78(1–2):68–76. DOI: https://doi.org/10.1016/j.meatsci.2007.05.030.
- Vasilopoulos C, De Vuyst L, Leroy F. Shelf-life reduction as an emerging problem in cooked hams underlines the need for improved preservation strategies. Critical Reviews in Food Science and Nutrition. 2015;55(10):1425–1443. DOI: https://doi.org/10.1080/10408398.2012.695413.
- Varvara M, Bozzo G, Celano G, Disanto C, Pagliarone CN, Celano GV. The use of ascorbic acid as a food additive: technical-legal issues. Italian Journal of Food Safety. 2016;5(1). DOI: https://doi.org/10.4081/ijfs.2016.4313.
- Burdurlu HS, Koca N, Karadeniz F. Degradation of vitamin C in citrus juice concentrates during storage. Journal of Food Engineering. 2006;74(2):211–216. DOI: https://doi.org/10.1016/j.jfoodeng.2005.03.026.
- Pogge DJ, Lonergan SM, Hansen SL. Effects of duration of vitamin C supplementation during the finishing period on postmortem protein degradation, tenderness, and meat color of the longissimus muscle of calf-fed steers consuming a 0.31 or 0.59% sulfur diet. Journal of Animal Science. 2015;93(5):2567–2575. DOI: https://doi.org/10.2527/jas. 2014-8798.
- Ruiz BG, et al. An original approach to study ascorbic acid degradation kinetics as a function of temperature (50–90°C) and O2 concentration (0–30%). 12th International Congress on Engineering and Food; 2015. Québec.
- Pegg RB, Shahidi F. Nitrite curing of meat: The N-nitrosamine problem and nitrite alternatives. Wiley-Blackwell; 2008. 280 p.
- Villaverde A, Ventanas J, Estevez M. Nitrite promotes protein carbonylation and Strecker aldehyde formation in experimental fermented sausages: Are both events connected? Meat Science. 2014;98(4):665–672. DOI: https://doi.org/10.1016/j.meatsci.2014.06.017.
- Feng XC, Li CY, Jia X, Gu Y, Lei N, Hackman RM, et al. Influence of sodium nitrite on protein oxidation and nitrosation of sausages subjected to processing and storage. Meat Science. 2016;116:260–267. DOI: https://doi.org/10.1016/j.meatsci.2016.01.017.
- Williams DLH. Nitrosation reactions and the chemistry of nitric oxide. Elsevier; 2004. 280 p. DOI: https://doi.org/10.1016/B978-0-444-51721-0.X5000-2.