Аннотация
Fish contamination by heavy metals, even at low levels, has an adverse effect on human health. Mercury (Hg), cadmium (Cd), and lead (Pb) are the most common heavy metals that contaminate sea foods. Rainbow trout is a fish species which is widely cultured in fresh water regions, e.g. in Yasuj, southwest of Iran. Heavy metal contamination was measured in three different culture areas (A, B, and C), with three different feed pellets used in Yasuj farms (I, II, and III). The sampling was conducted during February-April 2018 and the metals were measured using cold vapour atomic absorption with a Perkin Elmer 4100. The mean values of Hg, Cd, and Pb levels in the muscular tissue of the samples were 0.022, 0.105, and 1.07 mg/kg, respectively. Concentrations of Hg and Cd in edible tissues of rainbow trout were lower than the permitted values set by the WHO, the FDA, and the EC. The samples fed on mixture pellets III showed a significantly higher Hg content and a lower concentration of Cd in the muscle tissue compared to those given feed mixtures I and II (P < 0.05). Pearson correlation tests revealed significant correlations between the Cd and Pb concentrations and the weight of the fish samples (r = –0.519, r = –0.580). The lowest Cd concentration (0.076 mg/kg–1) was found in site A located close to the spring and not polluted by sewage from urban or rural areas. The study showed a correlation between the concentration of heavy metals in the fish samples and their weight, the degree of pollution, and the feeding mixture used in the farms.Ключевые слова
Rainbow trout, heavy metals, mercury, cadmium, leadВВЕДЕНИЕ
Heavy metals in contaminated food crops, even at low concentrations, produce deleterious effects on human health [1]. Metals pollution naturally occurs in the environment; however, human activities including mining and other industries have particular effects on the ecosystem, as well as the aquatic environment [2–4]. Despite the progress in sewage effluent technologies, water contamination is still a threat in many developing countries due to sewage discharge [5]. Heavy metals have an impact on aquatic ecosystems and eventually enter the human’s food chain [6]. Rainbow trout (Oncorhynchus mykiss L.), a native fish of North America, is known as one of the most valuable members of the Pacific trout that became the main freshwater fish species farmed in Iran [7, 8]. The first farm of this fish in Iran was established in 1959. Its production increased from 599 tons in 1978 to 140 000 tons in 2016, making Iran one of the worldleading producers of this salmon [9].
Mercury, cadmium, and lead are known as toxicants associated with fish consumption [10]. They are listed as sixth most dangerous contaminants by the International Program of Chemical Safety (IPSC) [11]. Lead poisoning can affect various systems of the body including renal, haematological, cardiovascular, gastrointestinal, and reproductive systems [12]. Renal exposure to cadmium results in its deposition in proximal tubular cells and causes renal failure due to decreased glomerular filtration rates. Also, skeletal system anomalies occur due to the secondary effects of renal dysfunction and accumulation of lead in bones [13]. Methyl mercury exposure through the consumption of contaminated fish in prenatal period leads to serious abnormalities such as cerebral palsy, mental backwardness, neurological disorders, and infant mortality [14]. Fish, which is an important aquatic component of the human food chain, has been a subject of investigation with regard to metal pollution [15]. Therefore, numerous reports have described metal residues in types of fish species [16–20]. The accumulation of heavy metals in fish tissues is influenced by a number of factors such as feeding habits, nourishment sources, habitat, age, and size [21–23]. In this study, concentrations of heavy metals (Hg, Cd, and Pb) were assessed in fish feed mixtures and edible tissues of rainbow trout farmed in three different culture areas.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
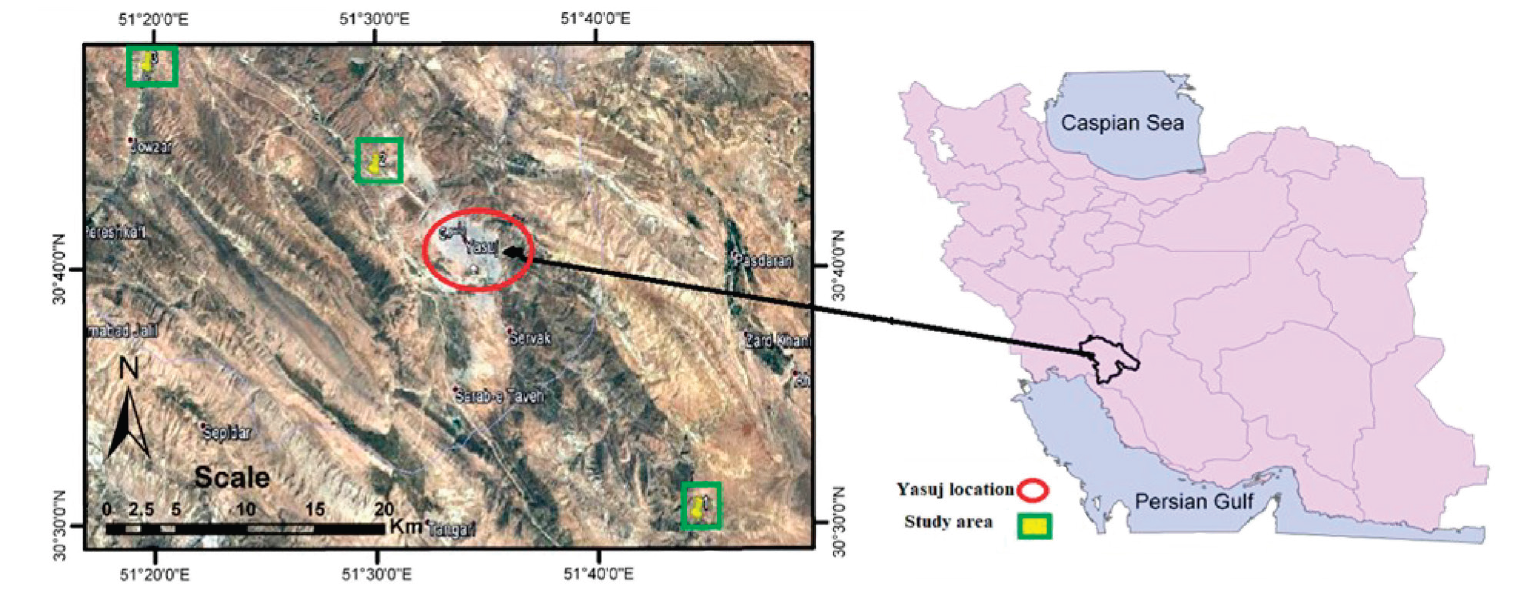
Study area and sample collection. Between February and April 2018, rainbow trout (Oncorhynchus mykiss L.) samples were collected from six farms (five fishes from each farm) in three different culture areas in Yasuj, southwest of Iran (Fig. 1), the third leading producer of this trout in the world [9]. The culture areas were located very close to the spring (site А: 30.502935, 51.743184), downstream of cities and villages (site B: 30.710630, 51.514926), and downstream of a rural area (site C: 30.789658, 51.329715). Their choice was determined by the level of contamination probability. The farms used a raceway farming system, with water supplied by the spring. All the farms practiced manual feeding with commercial pellets two times a day. The fish were collected randomly from two farms in each area (10 fishes in each site). The experiments were approved by the Animal Care and Use Committee of Yasuj University of Medical Sciences (YUMS) in compliance with the ‘Guidelines for the Care and Use of Animals’. At first, the samples’ biological parameters were recorded including wet body weight and total length. Then, they were washed, preserved in ice-boxes, and transported to the Food Chemical Laboratory at YUMS for heavy metal (Hg, Cd, and Pb) determination. The fish were filleted, placed in polyethylene bags, and kept at −20°C prior to analysis.
Four pellets of three types (I, II, and III) of commercially manufactured feed mixtures were frequently applied in the aquaculture trout farms during the fish sampling (12 pellets) and studied to determine the content of heavy metals.
Analytical procedures. Both feeds and fillets were oven-dried at 105°C for 1 h and then cooled. To measure the level of heavy metals, the samples (dry weight) were digested in a mixture of 6 mL concentrated HNO3 (super pure quality; Romil Ltd., Cambridge, UK) and 2 mL H2O2 (supra pure quality; Merck, Darmstadt, Germany) in a microwave digestion system (MARSXpress, CEM). When cooled to room temperature, the digested sample solutions were filtered and adjusted to 50 mL with ultrapure water. The levels of Hg, Cd, and Pb content were determined using cold vapour atomic absorption with a Perkin Elmer 4100 (FIMS 400 Perkin Elmer Inc., USA). The blank samples were also processed to avoid possible contamination during the analysis [22].
Human health risk assessment. The estimated daily intake (mg/kg bw/day) (EDI) of heavy metals was measured to evaluate the daily/weekly intake of heavy metals by the human body through the consumption of fish [24]. The daily intake of metals in adults was calculated as:
Where EF and ED are the exposure frequency (365 days/year) and the exposure duration (60 years), respectively; FIR is the fish ingestion rate (25.2 g/day for Iran); CF is the conversion factor to convert fresh weight to dry weight (0.208); CM is the metal concentration in the fish tissue (μg/g dry weight); WAB is the average body weight for adults (65 kg for Iran); and TA is the average exposure time for non-carcinogens (EF·ED) [4].
The percentage of provisional tolerable weekly intake (PTWI) and target hazard quotient (THQ) were calculated for each heavy metal by the following equations:
where PTWI, EWI, and RfD are provisional tolerable weekly intake (mg/kg bw/week), estimated weekly intake (mg/kg bw/week), and oral reference doses (mg/kg/day), respectively.
When the THQ is less than one, the risk of noncarcinogenic toxic effects for exposed consumer populations is presumed to be low. When it is greater than or equal to one, it is considered as a concern for consumer populations, indicating potential health risks [25].
Biomagnification factor. The biomagnification factor (BMF) is the ratio between the concentration of an element in fish and the concentration of this element in its diet. The BMF was calculated by the following equation: [26]:
where Cfish is a heavy metal concentration in fish edible tissues and Cfeed is a metal concentration in trout commercial pellets.
Statistical analysis. Statistical analysis was performed using the SPSS Statistics 19.0 software package. The mean and standard deviation (mean ± SD) levels of metal concentrations were reported for different areas and foods. The differences between heavy metal levels in edible tissues of fish from different farms and in different commercial foods were tested by the one-way analysis of variance (ANOVA), followed by Duncan’s post hoc test. The Pearson correlation test was used to check for significant relationships between metal concentrations, length, and net weight of fish. P < 0.05 was considered as the level of significance.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
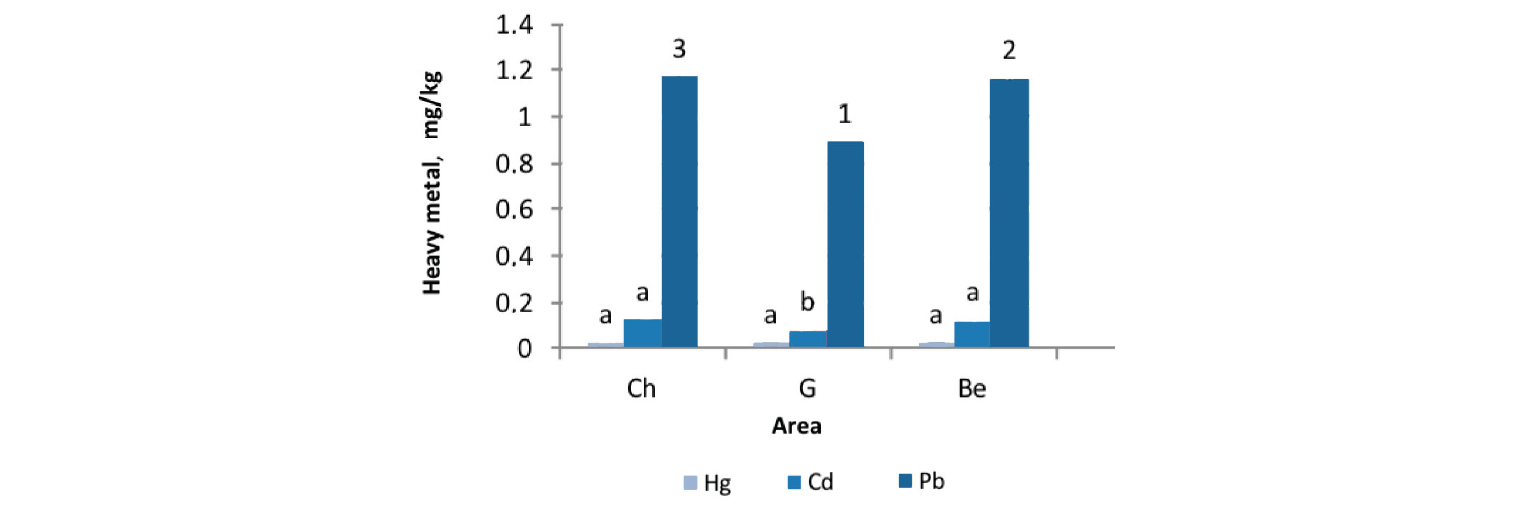
Heavy metal concentration in rainbow trout. Concentrations of heavy metals in edible tissues of rainbow trout farmed in three different areas are shown in Fig. 2.
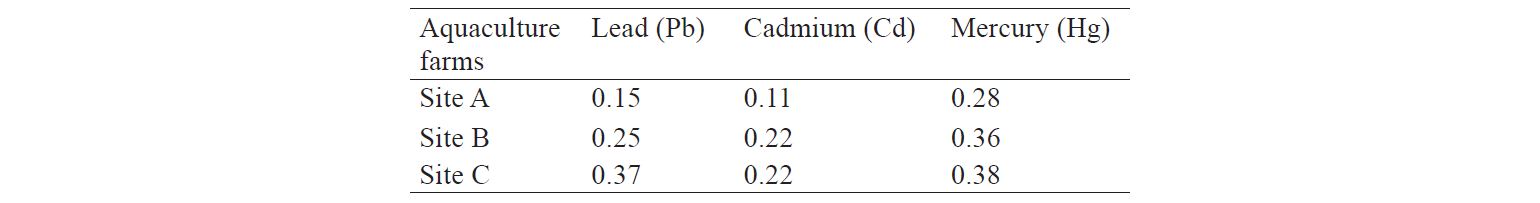
The mean ± SD levels of Hg in the muscle tissues of rainbow trout farmed in sites A, B, and C were 0.021 ± 0.0027, 0.023 ± 0.0026, and 0.024 ± 0.0027 mg/kg–1, respectively. The lower mercury level in site A, compared to the other two sites, had no significant difference (P > 0.05). In addition, the mean concentration of Cd in sites A, B, and C were 0.076, 0.119, and 0.120 mg/kg–1, respectively. The lowest Cd concentration was found in edible tissues of the fishes farmed in locations close to the spring, not polluted by sewage from urban or rural areas. The highest Pb concentration was detected in site B (1.171 mg/kg–1), followed by sites C and A (0.893 mg/kg–1) (P < 0.05). The fishes cultured in sites B and C had significantly higher contents of cadmium and lead, compared to those farmed in site A (P < 0.05). The results indicated that concentrations of Hg and Cd were below the permitted values determined by [27–29]. However, the level of Pb in the muscle tissue of farmed trout exceeded the value set by the WHO [30] (Table 1).
Below the levels established by the WHO, the FDA, and the EC were heavy metal concentrations in different fish species studied in Turkey, in the Barents Sea commercial fish, the fish from Lake Chini in Malaysia, wild fresh water fish from the Khersan river in Iran, rainbow trout and freshwater fish species from Lake Pamvotis in Greece [11, 31–35]. In addition, Bat et al. reported that the Cd, Hg, and Pb concentrations in Cyprinus carpio from the Karasu Stream, Sinop and in four fish species from Sarikum Lake were within certified values allowed to consumers [36, 37].
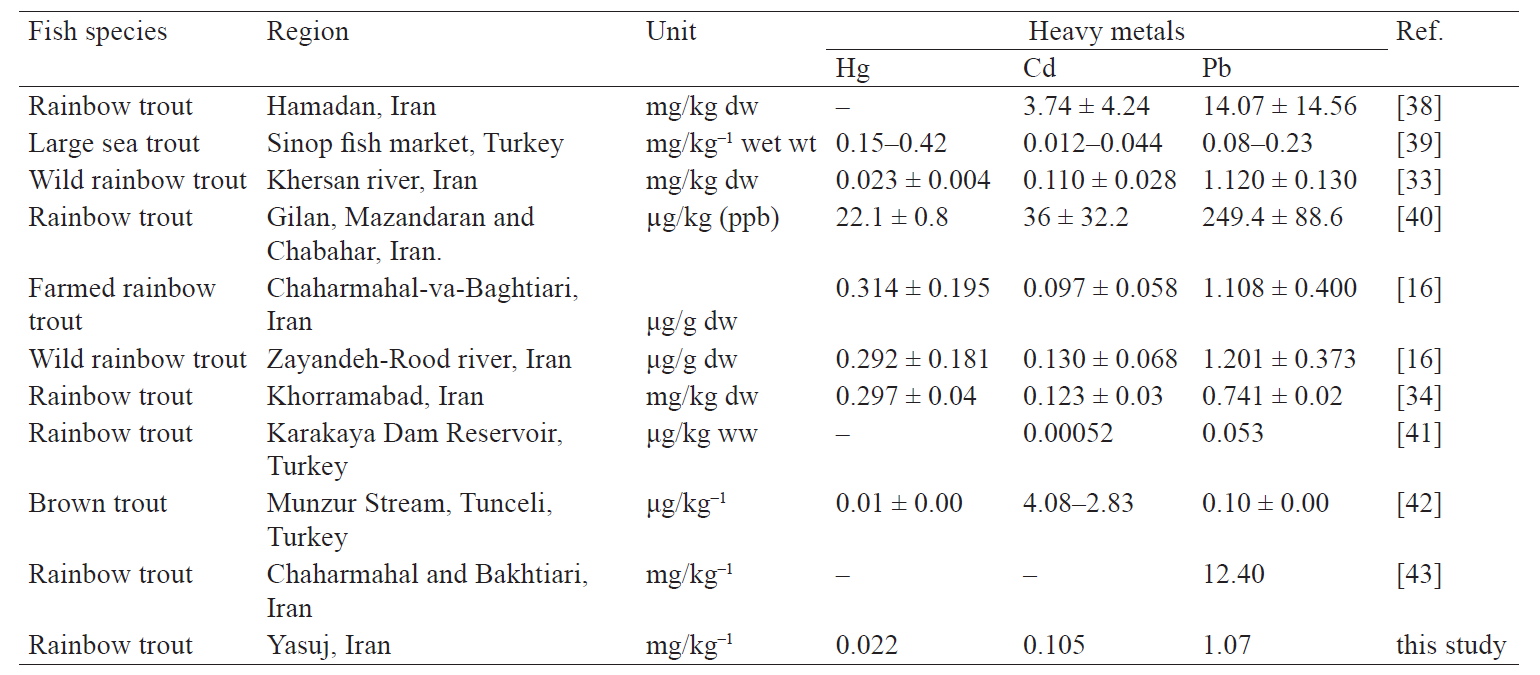
The Pb content was higher than the WHO allowed level in the fish cultured along the river’s upstream in Ghadirabad, Pakistan, wild fresh water fish from the Khersan river in Iran, farmed and wild rainbow trout in the Zayandeh Rood river in Iran, and fresh water fish in North Mexico (4298 mg/kg) [16, 19, 20, 33]. These data support the findings in our study. Table 2 demonstrates the comparison of heavy metal levels in the muscle tissue of rainbow trout from different locations reported in literature.
However, lower Hg, Cd, and Pb levels in site A, compared to the other sites, indicated an important role of water supply in trout aquaculture with regard to heavy metals accumulation in fish tissues. The release of industrial wastewater and pollutants caused by human activities increased the lead content in Liza fish from the Karun River in Iran and from the coast of Cochin in India [44, 45]. It was also reported that wild carps in the downstream areas of the Ravi and the Indus rivers in Pakistan showed a high contamination by heavy metals [46, 47].
Emara et al. observed significant differences in the Cd and Pb accumulation in the muscle tissue of fish in two distinct farms using different water sources [48]. Based on health standards, the concentration of heavy metals such as lead was higher in Mugil cephalus and Trachurus mesiteraneus in the Gulf of Iskenderun [49]. Wagner and Boman reported a greater amount of calcium and iron in the contaminated areas, compared to non-industrial zones [41]. High levels of cadmium and nickel were recorded in fish from Kuetsjarvi Lake (Russia) due to the contamination of surrounding regions and the proximity of smelting plants. A lower concentration of heavy metals in fish was detected in the areas away from factories and contaminating sources [52]. Power plants can reduce water acidity which causes an increase in the water solubility of lead and cadmium, resulting in high accumulation of the metals in aquatic organisms [11].
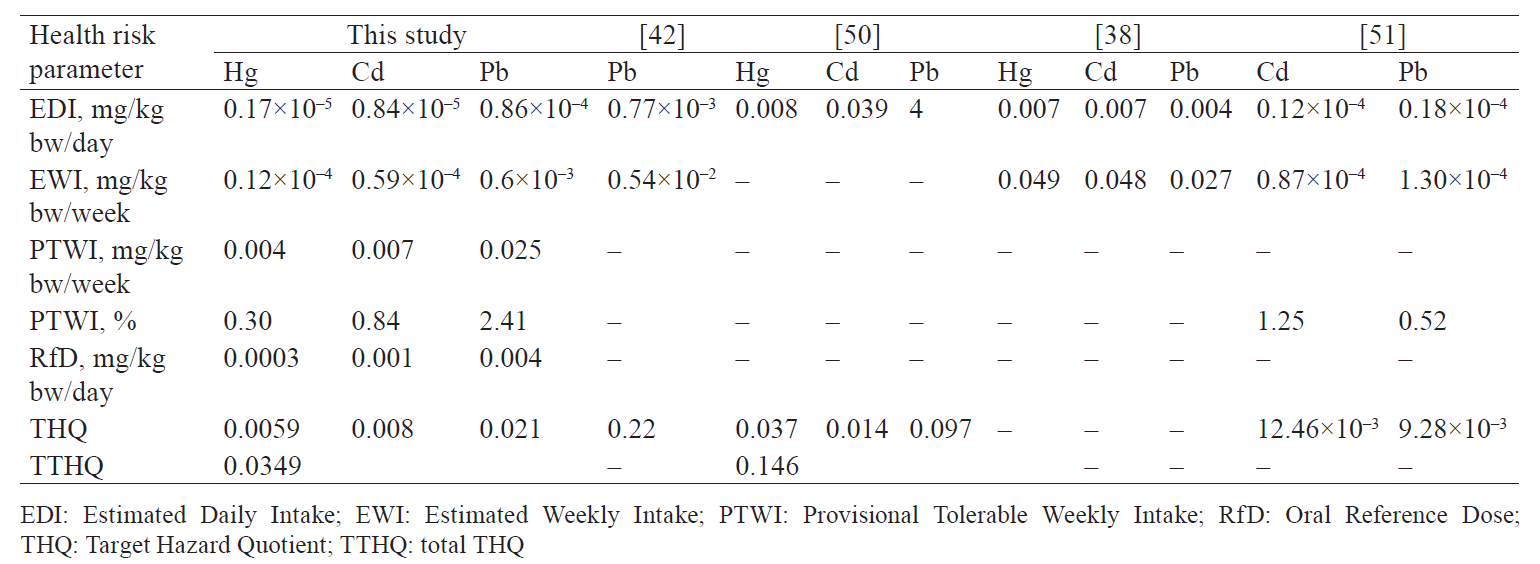
Health risk assessment. Several parameters widely used to assess human health risks include the estimated daily intake (EDI), the estimated weekly intake (EWI), the target hazard quotient (THQ), and the provisional tolerable weekly intake (PTWI). Health risk assessment values including the EDI, the EWI, the THQ, and the PTWI with regard to farmed rainbow trout are presented in Table 3. These parameters showed the following pattern in all the samples under study: Pb > Cd > Hg. We found that the EDI, the EWI, the PTWI, and the THQ obtained here were far below the recommended amounts, compared to the set values (RfD and PTWI) or other studies (Table 3). The THQ values were lower than one for all three studied metals. The findings indicated that the consumption of farmed rainbow trout in the study area does not pose a potential non-carcinogenic risk to human health.
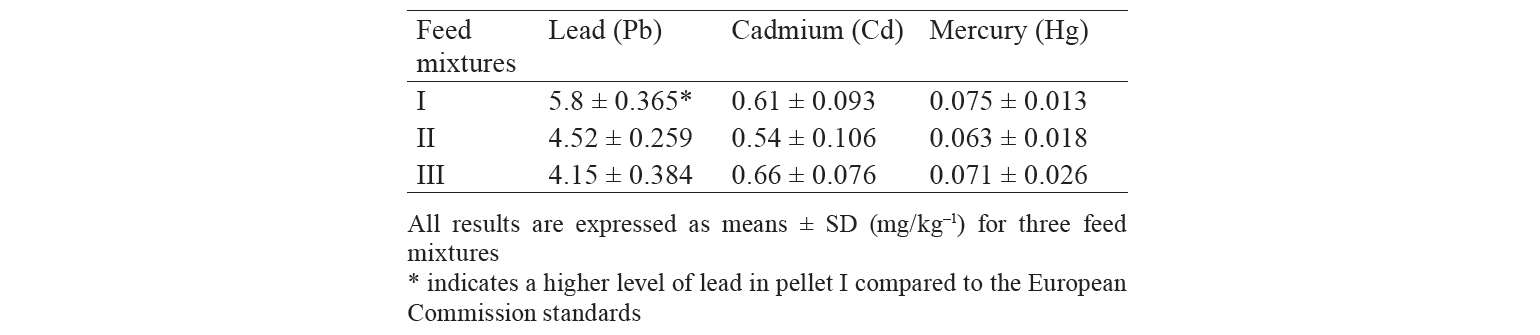
Feeds and heavy metal concentrations. The mean ± SD contents of heavy metals in the commercial feed mixtures (I, II, and III) used in the rainbow trout farms of this region are shown in Table 4. The results indicated some differences in commercial feeds based on the content of heavy metals. Food I contained higher levels of Pb (5.8 mg/kg–1) compared to the European Commission Regulations (EC) [53]. Using different raw materials in fish pellets resulted in some alteration of heavy metal contents in fish feed mixtures.
The mean concentrations of Hg, Cd, and Pb in muscle tissues of rainbow trout with regard to the type of food consumed in each of the farms are presented in Fig. 3.
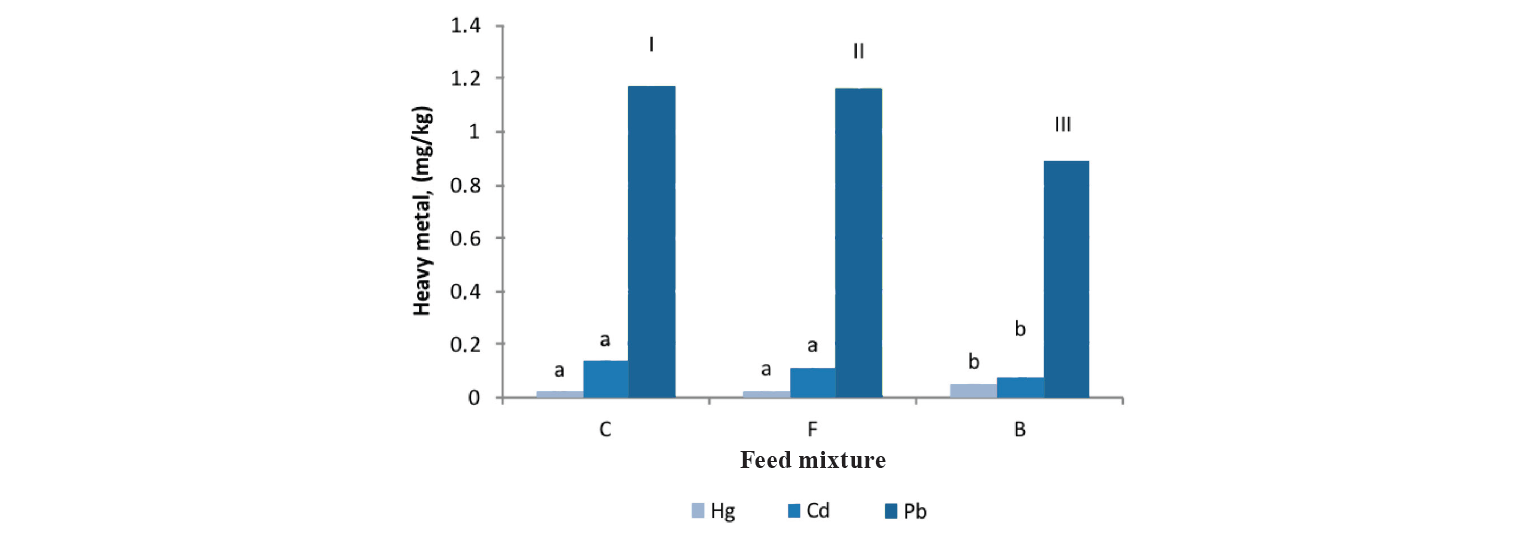
We observed that various commercial mixtures could influence the accumulation of heavy metals in farmed rainbow trout. The concentrations of Hg, Cd, and Pb in the fishes that consumed pellet II were 0.05, 0.076, and 0.893 mg/kg–1, respectively. Using pellet III resulted in a higher content of mercury and a lower concentration of cadmium, compared to the fishes fed on mixtures I and II (P < 0.05). On the whole, we concluded that the accumulation of heavy metals in fish was mainly influenced by water, food, and sediment. However, the accumulation of these elements in water and food is due to various factors including ecology, metabolism, pollution of slope water, food, and sediment, as well as other factors such as solubility, temperature, and interaction of various parameters [54, 55].
In our study, however, food intake had a significant effect on the concentration of heavy metals in rainbow trout muscle tissue: the consumption of pellet III resulted in a marked increase in mercury and a considerable reduction in cadmium and lead. Researchers have reported that there is a large association between the concentration of heavy metals in fish and its nutritional habits [22]. Mixture I had higher levels of Pb (5.8 mg/kg–1) compared to the values established by the European Commission Regulations (EC) [53]. Using different raw materials to prepare feed pellets resulted in some alteration of heavy metal contents in the commercial food mixtures. The accumulation of heavy metals in fish depends on food habits, reproductive status, size, and sex [21, 56]. Deep sediments contain large amounts of heavy metals. Compared to the epipelagic organisms, benthos occupying the deepest layers of water is the largest source of heavy metals [22].
Biomagnification factor. The BMF values for all the metals were under 1 (Table 5). Actually, the concentrations of the examined metals in the commercial pellets used in this region were higher than those in the rainbow trout tissues. As Table 5 demonstrates, the BMF values for the three metals had the following pattern: Hg > Pb > Cd (Table 5). The BMF was applied to show the capability of a contaminant to bioaccumulate. When a metal BMF is less than one, it indicates that no biomagnification occurred in the biosystem [26]. The current study showed that the BMF values were lower than one for all the metals (Table 5), suggesting that these metal contaminants were not biomagnified by rainbow trout from the diet. This finding was similar to the result obtained by Varol et al. [57]. Nevertheless, biomagnification implies inadequate information on the real threat from heavy metals in an aquatic food chain [58].
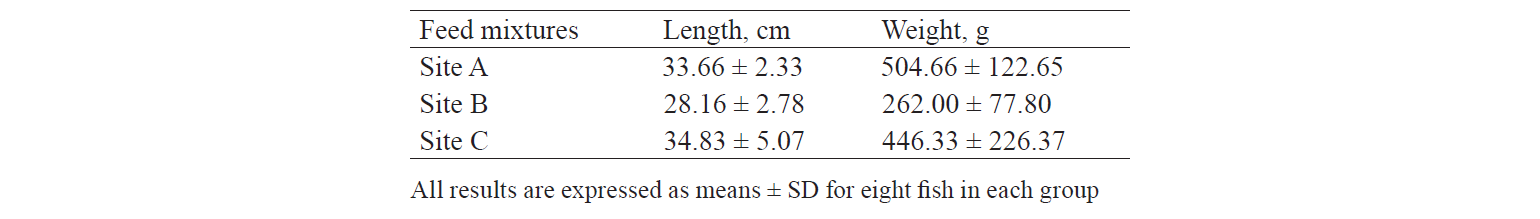
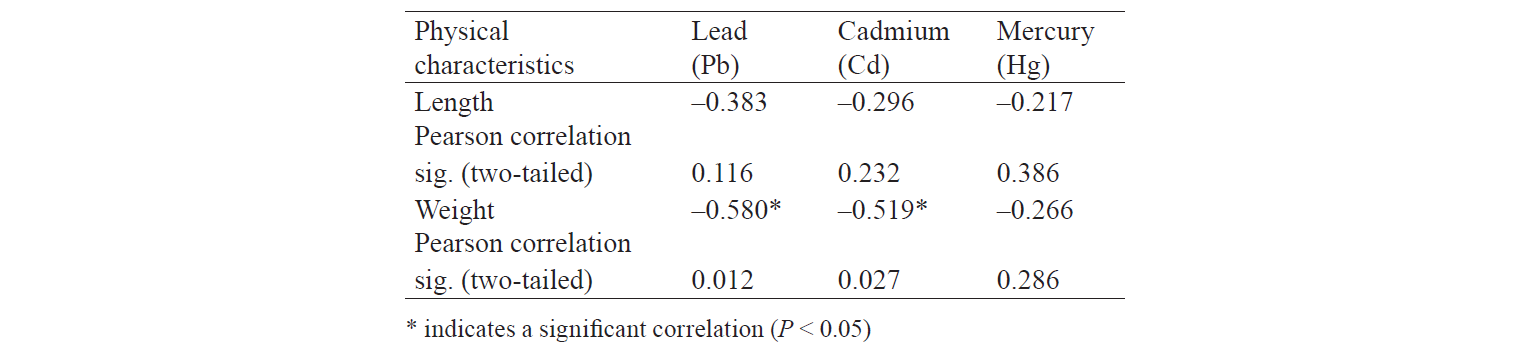
Physical characteristics and heavy metal levels. Table 6 shows the biometric characteristics of the sampled rainbow trouts. The relationship between metal concentrations and fish size (length and weight) is shown in Table 7. Increasing the weight and length of farmed trouts generally reduced the concentration of the three heavy metals. The Pearson correlation test revealed significant negative associations between the cadmium and lead concentrations and the weight of the fish samples (r = –0.519 and r = –0.580, respectively).
Few studies have focused on the relationship between physical characteristics and heavy metal accumulation. To the best of our knowledge, this is the first study on the relationship between the length and weight of farmed rainbow trout and the concentration of mercury, lead, and cadmium. Most of the authors have found a negative relationship between heavy metal accumulation and the size of fish (length and weight). They suggested that metabolic activity is one of the most important factors affecting the accumulation of heavy metals in marine fish [59]. Higher metabolic activity in juvenile fish leads to a higher accumulation of heavy metals [60, 61].
The correlation between the spectroscopic parameters and the concentration of heavy metals has been reported to be negative in various species of fish [61]. Heavy metal content in fish after a certain age remains almost constant [62]. In contrast to our results, Widianarko et al. found that in sturgeon species in the Caspian Sea, a higher accumulation of heavy metals with an increase in age, length, and weight of fish [63]. In general, there is a consensus that metals in living organisms are detoxified and depleted by a special mechanism, which is significantly dependent on the metabolism in the particular weight [64, 65]. Therefore, the negative relationship between heavy metal concentrations and the size of fish does not necessarily mean that a certain amount of metals will accumulate in the body at the beginning of the growth, and no more metals will be subsequently absorbed [66]. It has also been suggested that the absorption of metals in lowcontaminated water sources is more affected by nutrition [67]. In other words, a significant reduction in the amount of heavy metals in organs at the maturity stage is due to a decrease in the daily fish diet with age [68]. The fish at the highest nutritional level are expected to have the highest accumulation of heavy metals [69, 70].
ВЫВОДЫ
According to the results of the study, the levels of mercury, cadmium, and lead in the muscle tissue of rainbow trout farmed in Yasuj were found to be below the permitted values. The findings showed that the health risk assessment parameters (EDI, EWI, THQ) were far below the recommended values. This indicated that the consumption of farmed rainbow trout in the study area did not have any adverse effect on human health caused by heavy metal contamination.
However, the release of urban and rural wastewater and pollutants from human activities into the rivers leads to increased levels of lead and cadmium in the fish farmed in the downstream fields of the countryside and cities. Moreover, the application of various commercial pellets containing different levels of heavy metals can affect the accumulation of these metals in farmed trout.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare that there is no conflict of interest.
БЛАГОДАРНОСТИ
We would like to thank Mr. E. Sharifpoor for his technical assistance.
СПИСОК ЛИТЕРАТУРЫ
- Ibrahim F, Halttunen T, Tahvonen R, Salminen S. Probiotic bacteria as potential detoxification tools: assessing their heavy metal binding isotherms. Canadian Journal of Microbiology. 2006;52(9):877–885. DOI: https://doi.org/10.1139/w06-043.
- Sapkota A, Sapkota AR, Kucharski M, Burke J, McKenzie S, Walker P, et al. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environment International. 2008;34(8):1215–1226. DOI: https://doi.org/10.1016/j.envint.2008.04.009.
- Gu Y-G, Lin Q, Wang X-H, Du F-Y, Yu Z-L, Huang H-H. Heavy metal concentrations in wild fishes captured from the South China Sea and associated health risks. Marine Pollution Bulletin. 2015;96(1–2):508–512. DOI: https://doi.org/10.1016/j.marpolbul.2015.04.022.
- Saha N, Mollah MZI, Alam MF, Rahman MS. Seasonal investigation of heavy metals in marine fishes captured from the Bay of Bengal and the implications for human health risk assessment. Food Control. 2016;70:110–118. DOI: https://doi.org/10.1016/j.foodcont.2016.05.040.
- Chen M, Zeng GM, Zhang JC, Xu P, Chen AW, Lu LH. Global Landscape of Total Organic Carbon, Nitrogen and Phosphorus in Lake Water. Scientific Reports. 2015;5:7. DOI: https://doi.org/10.1038/srep15043.
- Saei-Dehkordi SS, Fallah AA. Determination of copper, lead, cadmium and zinc content in commercially valuable fish species from the Persian Gulf using derivative potentiometric stripping analysis. Microchemical Journal. 2011;98(1):156–162. DOI: https://doi.org/10.1016/j.microc.2011.01.001.
- Fornshell G. Rainbow trout – Challenges and solutions. Reviews in Fisheries Science. 2002;10(3–4):545–557. DOI: https://doi.org/10.1080/20026491051785.
- Kalbassi MR, Abdollahzadeh E, Salari-Joo H. A review on aquaculture development in Iran. Ecopersia. 2013;1(2): 159–178.
- Ebadzadeh MM. Agriculture. Economic aspects – Iran. Tehran: Ministry of Agriculture, Deputy Director of Planning and Economics; 2016. pp. 117–127.
- Castro-Gonzalez MI, Mendez-Armenta M. Heavy metals: Implications associated to fish consumption. Environmental Toxicology and Pharmacology. 2008;26(3):263–271. DOI: https://doi.org/10.1016/j.etap.2008.06.001.
- Demirak A, Yilmaz F, Tuna AL, Ozdemir N. Heavy metals in water, sediment and tissues of Leuciscus cephalus from a stream in southwestern Turkey. Chemosphere. 2006;63(9):1451–1458. DOI: https://doi.org/10.1016/j.chemosphere.2005.09.033.
- Piper D, Restrepo JFC. Lead and Cadmium: Priorities for action from UNEP’s perspective for addressing risks posed by these two heavy metals. 16th International Conference on Heavy Metals in the Environment (ICHMET); 2012; Rome. Rome: EDP Sciences; 2013. DOI: https://doi.org/10.1051/e3sconf/20130130004.
- Cadmium dietary exposure in the European population. EFSA Journal. 2012;10(1):2551. DOI: https://doi.org/10.2903/j.efsa.2012.2551.
- Benefice E, Luna-Monrroy S, Lopez-Rodriguez R. Fishing activity, health characteristics and mercury exposure of Amerindian women living alongside the Beni River (Amazonian Bolivia). International Journal of Hygiene and Environmental Health. 2010;213(6):458–464. DOI: https://doi.org/10.1016/j.ijheh.2010.08.010.
- Burger J, Jeitner C, Gochfeld M. Locational Differences in Mercury and Selenium Levels in 19 Species of Saltwater Fish from New Jersey. Journal of Toxicology and Environmental Health-Part A: Current Issues. 2011;74(13): 863–874. DOI: https://doi.org/10.1080/15287394.2011.570231.
- Fallah AA, Saei-Dehkordi SS, Nematollahi A, Jafari T. Comparative study of heavy metal and trace element accumulation in edible tissues of farmed and wild rainbow trout (Oncorhynchus mykiss) using ICP-OES technique. Microchemical Journal. 2011;98(2):275–279. DOI: https://doi.org/10.1016/j.microc.2011.02.007.
- Hosseini M, Nabavi SMB, Nabavi SN, Pour NA. Heavy metals (Cd, Co, Cu, Ni, Pb, Fe, and Hg) content in four fish commonly consumed in Iran: risk assessment for the consumers. Environmental Monitoring and Assessment. 2015;187(5). DOI: https://doi.org/10.1007/s10661-015-4464-z.
- Moradi S, Nowzari H, Farhadian M. Assessment of cadmium and lead in the water and trout fish (Salmo trutta) of Zayandehroud River, a case study of Zarinshahr rice farms, Isfahan. Iranian Journal of Fisheries Sciences. 2017;16(1):188–199.
- Chatta AM, Khan MN, Mirza ZS, Ali A. Heavy metal (cadmium, lead, and chromium) contamination in farmed fish: a potential risk for consumers’ health. Turkish Journal of Zoology. 2016;40(2):248–256. DOI: https://doi.org/10.3906/zoo-1506-1.
- Nevarez M, Leal LO, Moreno M. Estimation of Seasonal Risk Caused by the Intake of Lead, Mercury and Cadmium through Freshwater Fish Consumption from Urban Water Reservoirs in Arid Areas of Northern Mexico. International Journal of Environmental Research and Public Health. 2015;12(2):1803–1816. DOI: https://doi.org/10.3390/ijerph120201803.
- Turkmen M, Ciminli C. Determination of metals in fish and mussel species by inductively coupled plasma-atomic emission spectrometry. Food Chemistry. 2007;103(2):670–675. DOI: https://doi.org/10.1016/j.foodchem.2006.07.054.
- Monikh FA, Safahieh A, Savari A, Doraghi A. Heavy metal concentration in sediment, benthic, benthopelagic, and pelagic fish species from Musa Estuary (Persian Gulf). Environmental Monitoring and Assessment. 2013;185(1): 215–222. DOI: https://doi.org/10.1007/s10661-012-2545-9.
- Navarro MC, Perez-Sirvent C, Martinez-Sanchez MJ, Vidal J, Marimon J. Lead, cadmium and arsenic bloavallability in the abandoned mine site of Cabezo Rajao (Murcia, SE Spain). Chemosphere. 2006;63(3):484–489. DOI: https://doi.org/10.1016/j.chemosphere.2005.08.017.
- Jiang DS, Hu ZZ, Liu F, Zhang RF, Duo B, Fu JJ, et al. Heavy metals levels in fish from aquaculture farms and risk assessment in Lhasa, Tibetan Autonomous Region of China. Ecotoxicology. 2014;23(4):577–583. DOI: https://doi.org/10.1007/s10646-014-1229-3.
- Barnes DG, Dourson M, Dourson M, Preuss P, Barnes DG, Bellin J, et al. Reference dose (RFD): description and use in health risk assessments. Regulatory Toxicology and Pharmacology. 1988;8(4):471–486. DOI: https://doi.org/10.1016/0273-2300(88)90047-5.
- Kelly BC, Ikonomou MG, Higgs DA, Oakes J, Dubetz C. Mercury and other trace elements in farmed and wild salmon from British Columbia, Canada. Environmental Toxicology and Chemistry. 2008;27(6):1361–1370. https://doi.org/10.1897/07-527.1.
- Evaluation of certain food additives and contaminants. World Health Organization technical report series; 2011;(966). 109 p.
- Leonard B. Fish and Fishery Products: Hazards and Controls Guidance. DIANE Publishing; 2011. 468 p.
- Commission regulation (EC) No 1881/2006 of 19 December 2006 Setting maximum levels for certain contaminants in foodstuffs [Internet]. [cited 2019 April 10]. Available from: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1881&from=EN.
- Evaluation of certain food additive and contaminants (Section 5.2 Lead). World Health Organization technical report series; 2011(960). pp. 162–177.
- Zhilin AY, Plotitsyna NF, Lapteva AM, editors. Heavy Metals in Commercial Fish from the Barents Sea (Winter 2011). 16th International Conference on Heavy Metals in the Environment (ICHMET); 2012; Rome. Rome: EDP Sciences; 2013. DOI: https://doi.org/10.1051/e3sconf/20130111008.
- Ahmad A, Shuhaimi-Othman M. Heavy Metal Concentrations in Sediments and Fishes from Lake Chini, Pahang, Malaysia. Journal of Biological Sciences. 2010;10(2):93–100. DOI: https://doi.org/10.3923/jbs.2010.93.100.
- Majlesi M, Khazaei Y, Berizi E, Sharifpour E. The Concentration of Mercury, Cadmium and Lead in Muscular Tissue of Fishes in Khersan River. International Journal of Nutrition Sciences. 2017;2(3):2–9 .
- Mortazavi A, Hatamikia M, Bahmani M, Hassanzadazar H. Heavy Metals (Mercury, Lead and Cadmium) Determination in 17 Species of Fish Marketed in Khorramabad City, West of Iran. Journal of Chemical Health Risks. 2016;6(1).
- Papagiannis I, Kagalou I, Leonardos J, Petridis D, Kalfakakou V. Copper and zinc in four freshwater fish species from Lake Pamvotis (Greece). Environment International. 2004;30(3):357–362. DOI: https://doi.org/10.1016/j.envint.2003.08.002.
- Bat L, Şahin F, Öztekin A, Arici E, Yardim Ö. Assessment of Cd, Hg, Pb, Cu and Zn amounts in muscles of Cyprinus carpio from Karasu Stream, Sinop. Current Agriculture Research Journal. 2019;7(2). DOI: https://doi.org/10.12944/CARJ.7.2.05.
- Bat L, Yardım Ö, Özteki̇n A, Şahi̇n. F. Bioaccumulation of Metals in Fish from Sarikum Lake. Aquatic Science and Technology. 2019;7(1):1–7. DOI: https://doi.org/10.5296/ast.v7i1.13456.
- Reyahi-Khoram M, Setayesh-Shiri F, Cheraghi M. Study of the heavy metals (Cd and Pb) content in the tissues of rainbow trouts from Hamedan coldwater fish farms. Iranian Journal of Fisheries Sciences. 2016;15(2):858–869.
- Bat L, Oztekin A, Yardim O. Metal levels in large sea trout from Sinop fish market. Fresenius Environmental Bulletin. 2018;27(12):8505–8508.
- Salaramoli J, Salamat N, Razavilar V, Najafpour Sh, Aliesfahani T. A Quantitative Analysis of Lead, Mercury and Cadmium Intake by Three Commercial Aquatics, Hypophthalmichthys Molitrix, Onchorhynchus Mykiss (Walbaum) and Fenneropenaeus Indicus. World Applied Sciences Journal. 2012;16(4):583–588.
- Wagner A, Boman J. Biomonitoring of trace elements in muscle and liver tissue of freshwater fish. Spectrochimica Acta Part B: Atomic Spectroscopy. 2003;58(12):2215–2226. DOI: https://doi.org/10.1016/j.sab.2003.05.003.
- Can E, Yabanli M, Kehayias G, Aksu O, Kocabas M, Demir V, et al. Determination of Bioaccumulation of Heavy Metals and Selenium in Tissues of Brown Trout Salmo trutta macrostigma (Dumeril, 1858) from Munzur Stream, Tunceli, Turkey. Bulletin of Environmental Contamination and Toxicology. 2012;89(6):1186–1189. DOI: https://doi.org/10.1007/s00128-012-0824-3.
- Solgi E, Beigzadeh-Shahraki F. Accumulation and Human Health Risk of Heavy Metals in Cultured Rainbow Trout (Oncorhynchus mykiss) Form Different Fish Farms of Eight Cities of Chaharmahal and Bakhtiari Province, Iran. Thalassas. 2019;35(1):305–317. DOI: https://doi.org/10.1007/s41208-019-0123-4.
- Mohammadi M, Sary AA, Khodadadi M. Accumulation variations of selected heavy metals in Barbus xanthopterus in Karoon and Dez rivers of Khuzestan, Iran. Iranian Journal of Fisheries Sciences. 2012;11(2):372–382.
- Sivaperumal P, Sankar TV, Nair PGV. Heavy metal concentrations in fish, shellfish and fish products from internal markets of India vis-a-vis international standards. Food Chemistry. 2007;102(3):612–620. DOI: https://doi.org/10.1016/j.foodchem.2006.05.041.
- Shakir HA, Qazi JI, Chaudhry AS. Assessing Human Health Risk of Metal Accumulations in a wild carp fish from Selected Sites of a River Loaded with Municipal and Industrial Wastes. International Journal of Environmental Research. 2015;9(2):545–552.
- Jabeen F, Chaudhry AS. Monitoring trace metals in different tissues of Cyprinus carpio from the Indus River in Pakistan. Environmental Monitoring and Assessment. 2010;170(1–4):645–656. DOI: https://doi.org/10.1007/s10661-009-1263-4.
- Emara MM, Farag RS, Dawah AMA, Fathi M. Assessment of heavy metals concentration in water and edible Nile Tilapia (Oreochromis niloticus) from two fish farms irrigated with different water sources, Egypt. International Journal of Environment. 2015;4(1):108–115.
- Yilmaz AB. Levels of heavy metals (Fe, Cu, Ni, Cr, Pb, and Zn) in tissue of Mugil cephalus and Trachurus mediterraneus from Iskenderun Bay, Turkey. Environmental Research. 2003;92(3):277–281. DOI: https://doi.org/10.1016/s0013-9351(02)00082-8.
- Majlesi M, Pashangeh S, Salehi SO, Berizi E. Human Health Risks from Heavy Metals in Fish of a Fresh Water River in Iran. International Journal of Nutrition Sciences 2018;3(3):2–8.
- Miri M, Akbari E, Amrane A, Jafari SJ, Eslami H, Hoseinzadeh E, et al. Health risk assessment of heavy metal intake due to fish consumption in the Sistan region, Iran. Environmental Monitoring and Assessment. 2017;189(11). DOI: https://doi.org/10.1007/s10661-017-6286-7.
- Yilmaz F, Ozdemir N, Demirak A, Tuna AL. Heavy metal levels in two fish species Leuciscus cephalus and Lepomis gibbosus. Food Chemistry. 2007;100(2):830–835. DOI: https://doi.org/10.1016/j.foodchem.2005.09.020.
- Commission regulation (EU) 582/2016 of 15 April 2016, amending Regulation (EC) No 333/2007 as regards the analysis of inorganic arsenic, lead and polycyclic aromatic hydrocarbons and certain performance criteria for analysis. Euratom. 2016;3–6.
- Canli M, Furness RW. Mercury and cadmium uptake from seawater and from food by the Norway lobster Nephrops norvegicus. Environmental Toxicology and Chemistry. 1995;14(5):819–828. DOI: https://doi.org/10.1002/etc.5620140512.
- Geyer HJ, Scheunert I, Bruggemann R, Steinberg C, Korte F, Kettrup A. QSAR for organic-chemical bioconcentration in Daphnia, algae, and mussels. Science of the Total Environment. 1991;109:387–394. DOI: https://doi.org/10.1016/0048-9697(91)90193-i.
- Canli M, Atli G. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environmental Pollution. 2003;121(1):121–136. DOI: https://doi.org/10.1016/s0269-7491(02)00194-x.
- Varol M, Kaya GK, Alp A. Heavy metal and arsenic concentrations in rainbow trout (Oncorhynchus mykiss) farmed in a dam reservoir on the Firat (Euphrates) River: Risk-based consumption advisories. Science of the Total Environment. 2017;599:1288–1296. DOI: https://doi.org/10.1016/j.scitotenv.2017.05.052.
- Liu HL, Li LQ, Yin CQ, Shan BQ. Fraction distribution and risk assessment of heavy metals in sediments of Moshui Lake. Journal of Environmental Sciences. 2008;20(4):390–397. DOI: https://doi.org/10.1016/s1001-0742(08)62069-0.
- Rainbow PS. Trace metal concentrations in aquatic invertebrates: why and so what? Environmental Pollution. 2002;120(3):497–507. DOI: https://doi.org/10.1016/s0269-7491(02)00238-5.
- Nussey G, van Vuren JHJ, du Preez HH. Bioaccumulation of chromium, manganese, nickel and lead in the tissues of the moggel, Labeo umbratus (Cyprinidae), from Witbank Dam, Mpumalanga. Water Sa. 2000;26(2):269–284.
- Widianarko B, Van Gestel CAM, Verweij RA, Van Straalen NM. Associations between trace metals in sediment, water, and guppy, Poecilia reticulata (Peters), from urban streams of Semarang, Indonesia. Ecotoxicology and Environmental Safety. 2000;46(1):101–107. DOI: https://doi.org/10.1006/eesa.1999.1879.
- Douben PE. Lead and cadmium in stone loach (Noemacheilus barbatulus L.) from three rivers in Derbyshire. Ecotoxicology and Environmental Safety. 1989;18(1):35–58.
- Poorbagher H, Hosseini SV, Hosseini SM, Aflaki F, Regenstein JM. Metal accumulation in Caspian sturgeons with different feeding niches, condition factor, body size and age. Microchemical Journal. 2017;132:43–48. DOI: https://doi.org/10.1016/j.microc.2017.01.003.
- Fagerstrom T. Body-weight, metabolic-rate, and trace substance turnover in animals. Oecologia. 1977;29(2):99–104. DOI: https://doi.org/10.1007/bf00345790.
- Newman MC, Doubet DK. Size-dependence of mercury(ii) accumulation kinetics in the mosquitofish, Gambusia affinis (Baird and Girard). Archives of Environmental Contamination and Toxicology. 1989;18(6):819–825. DOI: https://doi.org/10.1007/bf01160295.
- Farkas A, Salanki J, Specziar A. Age- and size-specific patterns of heavy metals in the organs of freshwater fish Abramis brama L. populating a low-contaminated site. Water Research. 2003;37(5):959–964. DOI: https://doi.org/10.1016/s0043-1354(02)00447-5.
- Hellawell JM. Biological indicators of freshwater pollution and environmental management. Springer Netherlands; 2012. 546 p.
- Marmulla G, Rosch R. Maximum daily ration of juvenile fish fed on living natural zooplankton. Journal of Fish Biology. 1990;36(6):789–801. DOI: https://doi.org/10.1111/j.1095-8649.1990.tb05628.x.
- Bustamante P, Bocher P, Cherel Y, Miramand P, Caurant F. Distribution of trace elements in the tissues of benthic and pelagic fish from the Kerguelen Islands. Science of the Total Environment. 2003;313(1–3):25–39. DOI: https://doi.org/10.1016/S0048-9697(03)00265-1.
- Yi YJ, Wang ZY, Zhang K, Yu GA, Duan XH. Sediment pollution and its effect on fish through food chain in the Yangtze River. International Journal of Sediment Research. 2008;23(4):338–347. DOI: https://doi.org/10.1016/s1001-6279(09)60005-6.