Аннотация
Essential oils are known to be a natural preservative due to their antimicrobial and antioxidant properties. The aim of this study was to evaluate an effect of thyme and cumin essential oils (EOs) in combination with air packaging and vacuum packaging on the shelf life of burgers from surimi and chicken meat. The study was conducted at 2°C for 27 days. We tested four groups of samples: (a) burgers in air package, (b) burgers with cumin and thyme EOs in air packaging, (c) burgers in vacuum packaging, and (d) burgers with cumin and thyme EOs in vacuum packaging. The greatest effect (P < 0.001) on the chemical and microbiological characteristics of the novel burgers displayed burgers with EOs of thyme and cumin packaged under vacuum. It can be explained by synergistic effect, which made it possible to extend the shelf life of the burgers. These results allowed us to suggest that surimi could be used as a basic ingredient in burgers production.Ключевые слова
Burgers, surimi, cumin essential oil, thyme essential oil, air packaging, vacuum packagingВВЕДЕНИЕ
Fish meat is an ideal source of animal protein which has a high nutritional value. Nowadays, consumers are interested in healthy food [1]. Nevertheless, convenience food, including burgers, has remained common all over the world [2]. Ready-to-cook fish products is becoming popular among consumers due to their high nutritional value and short time of cooking [2]. Still, to preserve its quality, fish meat should be processed properly [3].
In recent years, changing socioeconomic factors, namely, an increase in the number of employed women, have led to an increased demand for convenience products. Therefore, some efforts have been made to extend the shelf life of ready-to-eat food [4, 5].
Surimi, stabilised myofibrillar proteins of fish muscle, can be made of both sea-water and fresh-water fish. To obtain surimi, fish fillet is minced, washed by water, and stabilised by blending with cryoprotectants. A cryoprotectant mix, containing sugar, sorbitol, and phosphates, is added to the minced fish [6]. Surimi is an important ingredient for food production in many countries due to its technological properties [6].
Currently, there are a number of ways to control the growth of pathogenic microorganisms in food products. One of the ways is the use of essential oils (EOs). EOs are aromatic oily extracts obtained from different parts of plants, such as flowers, leaves, wood, bark, roots, seeds, or peel, which exhibit bactericidal or bacteriostatic properties [7]. EOs are considered as natural preservatives for raw or mildly processed food [8]. EOs have a wide spectrum of antimicrobial properties. As an antimicrobial agent, EOs destroy both the lipid bi-layer of cell membranes and enzyme systems as well as inactivate the genetic material of bacteria [9].
EOs display their antimicrobial action against pathogenic microorganisms, including gram-positive and gram-negative, as well as mold and parasites [10–14]. In addition, EOs are reported to have antioxidant properties [15–17]. Natural antioxidants have an advantage over artificial ones because of their high content in phenolic compounds as well as other active components which can effectively inhibit oxidative reactions [17, 18].
Cumin (Cuminum cyminum L.) is a flowering plant in the family Apiaceae. Its seeds have been commonly used for centuries as a spice [19]. Thyme (Zataria Multiflora Boiss.) is an aromatic perennial evergreen herb beloning to the family Labiateae and used in cooking [20]. In addition, there is data on the successful use of thyme EO as an antimicrobial agent in chicken meat patties [21].
The aim of this work was to find a way to prolong the characteristics and shelf life of novel burgers made of chicken meat and surimi, as well as to investigate chemical and microbiological changes in the burgers stored at 2°C for 27 days.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Preparation of minced chicken meat and surimi. Fresh chicken and silver carp (Hypophthalmichthys molitrix L.) were purchased from a local market in Ahvaz, Khuzestan Province, Iran. The chicken was minced and then kept at –18°C until used. Fresh fish was transported on ice into a laboratory, washed, beheaded, gutted, and filleted. The fillet obtained was thoroughly washed, put through a meat mincer with 4 mm diameter holes (EG-1200-EBS, Jahan Ava, Iran) for 2 min.
The minced fish was washed with a triple volume of water (4°C) and stirred for 10 min. The washed minced fish was filtered through two layers of cheesecloth and then subsequently dewatered by using a manual juicer extractor. Washing was performed three times. The third washing was carried out with 0.5% NaCl (Merck, Germany) solution. A ratio of the minced fish to NaCl was 1:3 (w/w).
After dewatering, the minced fish was mixed with cryoprotectants, i.e. sucrose 3% (Merck, Germany) and sorbitol 3% (Merck, Germany), for 60 s and frozen using a blast freezer. The surimi obtained was kept at –18°C until used.
Preparation of combined burgers and treatments. Before burgers preparation, frozen surimi and minced chicken meat were put in a refrigerator (at 4°C) at night. Meat for burgers was prepared from surimi (63%) and minced chicken meat (37%).
The meat was then blended with toasted flour, 8.2%; wheat flour, 2%; soy flour, 3%; sunflower oil, 1%; freshly grated onion, 7%; garlic powder, 1%; sodium chloride, 1%; white pepper, 0.5%; lemon juice, 1%; and sodium tri-polyphosphate, 0.3% (Merck, Germany).
All the ingredients in combination with 125 mg/L of nisin (Sigma Aldrich, England) were ground through a blender with a 5 mm plate (Gosonic, Turkey) for 4–5 min. Nisin solution, which was added to avoid the growth of Clostridium botulinum, was prepared by dissolving a required amount of nisin powder in sterilised 0.02N HCl solution. Burgers (25 g in weight, 50–60 mm in diameter, and 1 cm in thickness) were formed by a burger-maker according to [22].
RSM (response surface methodology) was used to optimise the formulation. The results were analysed using Design Expert 6.0.2 software, and each of the dependent variables in the form of a quadratic regression model was presented as follows:
where β0, βi, βii and βij are regression coefficients, and Xi and Xj are coded independent variables. The formula was selected based on the results of the sensory evaluation of the burgers that were stored at 2°C before testing. The test was performed with the help of RSM software.
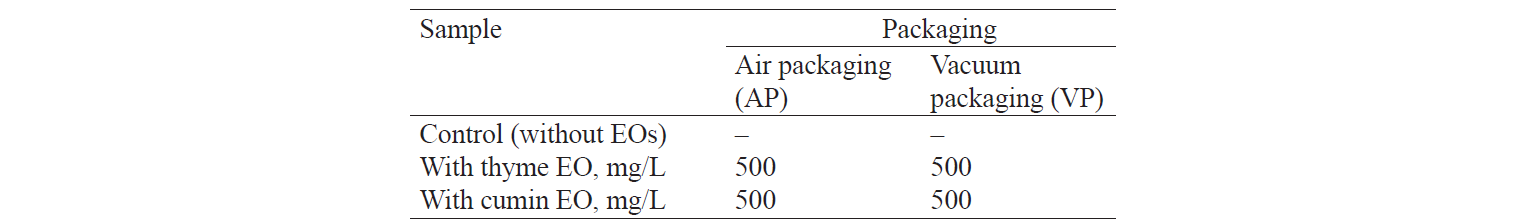
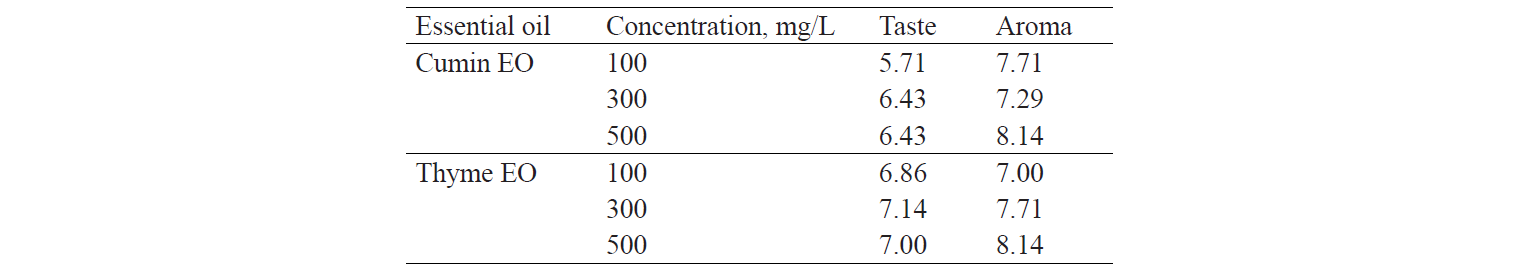
As control samples were used burgers made without essential oils. They were objected to analyses of proximate composition and cooking characteristics. The control samples included burgers with 100, 300, and 500 mg/L of both cumin essential oil (Barij Essen, Iran) and thyme essential oil (Barij essence, Iran). Based on the sensory evaluation results, an optimal concentration for each of the EOs was selected.
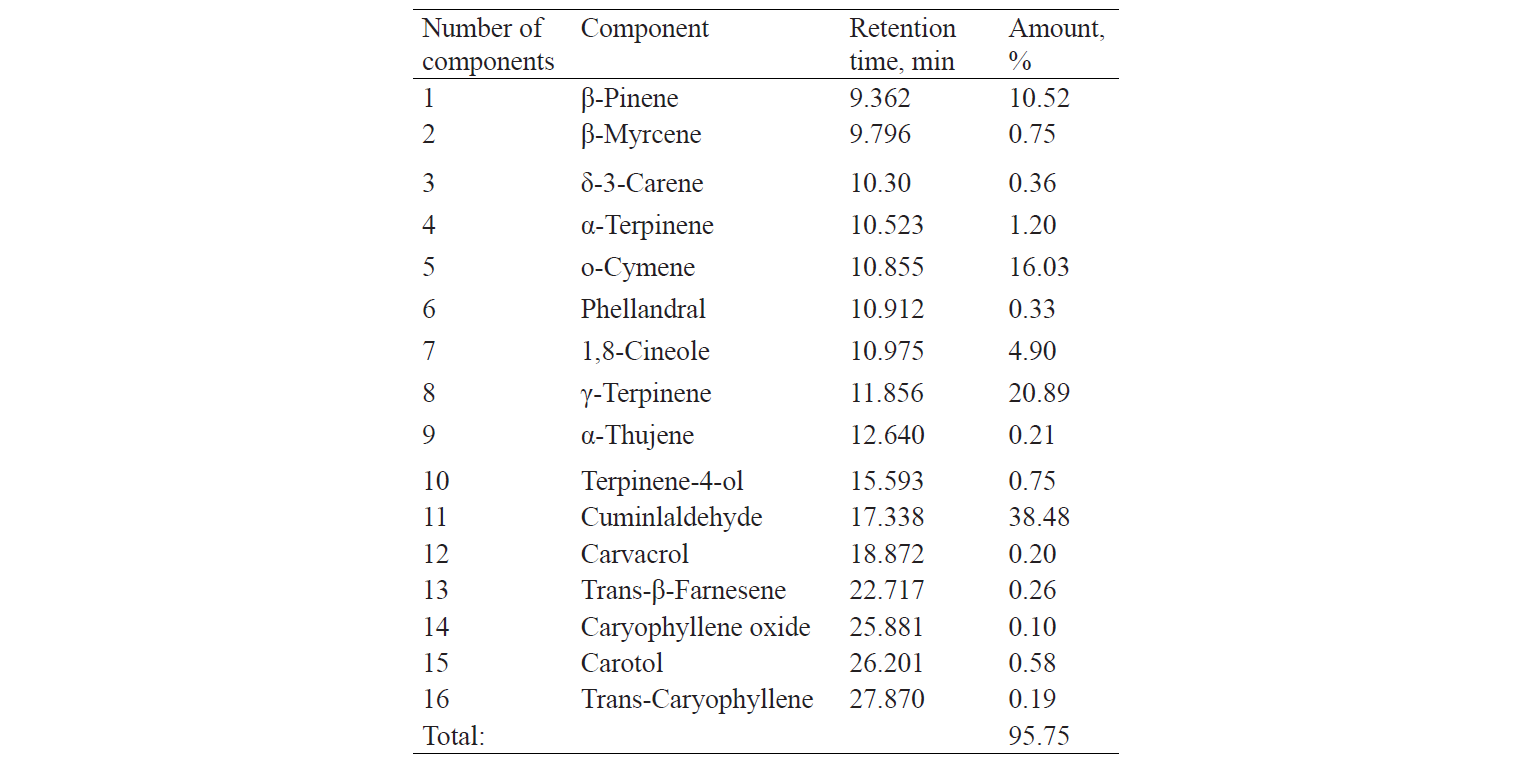
Tables 1 and 2 demonstrate results of the composition analysis of thyme and cumin EOs. The analysis was carried out by Barij Essence Company (Iran) by means of gas chromatography-mass spectrometry (GC-MS).
The burgers were subdivided into two groups. One group was packaged in high density polyethylene (HDPE) bags under vacuum, and another group – in bags (aerobically), six burgers in each bag. Each group included control, thyme EO and cumin EO samples. The packaged burger samples were stored at 2°C for 27 days. Microbiological and chemical evaluation of three different batches was carried out on day 0, 3, 6, 9, 12, 15, 18, 21, 24, and 27 of storage.
Sensory analysis. Sensory evaluation was performed by a panel of seven experienced (laboratory-trained) judges. To optimise the fish burger formulation, the panellists were asked to evaluate taste, colour, aroma, and overall quality of burgers on a nine-point scale. The scale points were: excellent, 9; very good, 8; good, 7; acceptable, 5–6; unacceptable, 1–4 [23].
Proximate composition. Protein, moisture, ash and fat contents were measured by AOAC method [24].
Cooking characteristics. The thickness and diameter of raw burgers were estimated at room temperature. The burgers were fried in sunflower oil at 170°C for 5 min until an inner temperature of 72°C was reached [25]. Cooking yield, shrinkage and moisture retention were determined by the following equation:
Microbiological analyses. Twenty five grams of burger sample was added into 225 mL of sterile peptone water and blended using a Stomacher lab blender (Interscience Bag Mixer, China) for 1 min. Homogenates of various concentrations were prepared for the microbial test. Cultured Plate Count Agar (PCA) (Merck, Darmstadt, Germany) was incubated at 7°C for 10 days for psychrotrophic bacteria count and at 30°C for 48 h for total viable count (TVC) [26]. Lactic acid bacteria (LAB) were determined on de Man Rogosa Sharpe Agar (MRS) (Q Lab, Canada) incubated at 30°C for 72 h [27]. Sulfite-reducing clostridia were grown on Sulphite Polymyxin Sulfadiazine Agar (Merck, Darmstadt, Germany) [28] incubated at 30°C for 48 h in a plastic anaerobic AnaeroGen sachet (Anaerobic gas pack A, Merck, Darmstadt, Germany). All microbiological analyses were performed in triplicate, and results were expressed as logarithm colony forming unit (log CFU)/g sample.
Mold and yeast were counted on Yeast Extract Agar (Merck, Darmstadt, Germany) incubated at 25°C for 5 days [29]. The experiment was performed in duplicate.
Chemical analysis. pH value was determined using a digital pH meter on the first homogenised concentration of samples (Sartorius, USA) [30]. Total volatile base nitrogen (TVB-N) content was quantified by the method of Malle and Poumeyrol [31], while thiobarbituric acid (TBA) amount was calculated by the method of Tsironi et al. [32].
Peroxide value (PV) was determined according to the method described by AOAC [33]. All chemical analyses were performed in triplicate.
Statistical Analysis. Statistical analysis was carried out with the help of SPSS 19 (SPSS, 2010) software and one-way variance. Results were expressed as mean values and standard deviation (S.D.). Analysis of variance (ANOVA) data were subjected to determining significant differences (P < 0.05).
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
Sensory analysis. Average scores of sensory characteristics were evaluated using RSM method. The results of the analysis are shown in Table 4. The optimal burger formulation was selected, which contained 63% of surimi and 37% of minced chicken meat. Also, based on average scores of sensory evaluation, a concentration of 500 mg/L for each EO was selected as optimal (Table 5).
Proximate analysis. Proximate composition was performed in burgers made without EOs before storage. Samples had moisture of 70.40% and contained 19.98% of protein, 4.27% carbohydrate, 3.35% fat, and 2.0% ash. Our results are in good agreement with those obtained by Vanitha et al. [34].
Cooking characteristics. The cooking characteristics of samples with no EOs were determined before storage. Cooking yield, shrinkage, and moisture retention were found to be 94.73, 10.19, and 80.98%, respectively. These data are in accordance with those of Heydari et al., who measured cooking properties in camel burgers during freezer storage [25].
Microbiological analysis. Analysis of variance showed that both packaging and EOs used had a significant effect on the microbial characteristics of burgers (P < 0.001).
Figure 1a demonstrates changes in TVC of the burgers under study during storage. Results indicate a significant effect (P < 0.001) of storage time, EOs addition and packaging conditions on TVC. The maximum TVC value obtained (107 CFU/g) was acceptable for fresh and frozen fish [35].
The initial (day 0) TVC of burgers in air packaging, with and without the EOs, was 4.05–4.38 log CFU/g. For burgers in vacuum packaging, with or without cumin/thyme EO, these values were 4.46–4.82 log CFU/g. These results are consistent with those obtained by Cózar et al. for fish burgers (4 log CFU/g) and indicate a good burger quality [36]. Eventually, by day 27, TVC was 8.39–8.78 and 6.13–6.74 log CFU/g in air packaged and in vacuum packaged burgers, respectively.
As one can see in Fig. 1a, burgers with thyme EO in vacuum packaging demonstrated the least microbial growth, which indicates inhibitory properties of thyme EO. Similar results were found in an edible film containing 0.10% of oregano and 0.15% of thyme EO in fresh chicken sausages [17, 21].
Initial counts of psychrotrophic bacteria in samples in air and vacuum packaging were 4.34–4.76 log CFU/g, which reached 7.04–8.79 log CFU/g by day 27 of storage (Fig. 1b).
Kilinc et al. observed an increase in TVC and psychrotrophic bacteria count in sardine patties from 2.50 and 2.60 log CFU/g to 6.72 and 6.98 log CFU/g on day 7 of storage [37]. According to Pavelková et al., the initial TVC value in control chicken breast was 4.72 log CFU/g, while after 18 days of storage at 4 ± 0.5°C, it was 3.68 and 4.05 log CFU/g for samples with oregano and thyme EOs in vacuum packaging [38].
In our research, thyme EO acted as a synergist to vacuum packaging, combinations of air packaging + cumin EO and air packaging + thyme EO were less effective in inhibiting microbial growth. Soni et al. also reported lower psychrophilic bacteria counts in chicken patties containing 0.10% of oregano and 0.15% of thyme Eos [21]. Similar results were obtained by Sharma et al. in fresh chicken sausages during storage [17].
This inhibitory effect was also apparently due to large amounts of phenolic substances and flavonoids in thyme and cumin EOs.
Initially, a lacto acid bacteria (LAB) amount was 3.16 log CFU/g. By the end of the storage, it was recorded to be 7.47–7.98 for burgers in air packaging and 4.15–4.40 log CFU/g for those in vacuum packaging (Fig. 1c). In [39], the initial LAB concentration in control minced goat meat was 2.75 log CFU/g, which increased to 6 log CFU/g by the end of vacuum storage at 4°C. Also, Fratianni et al. reported that thyme essential oil decreased total viable bacteria count and lactic acid bacterial growth in chicken breast; total microbial content reduced down to 50% compared to the control samples [40].
In the work of Pavelková et al, the LAB count in a control chicken breast fillet was within the range from 4.31 (day 3) to 2.62 log CFU/g (day 15), while the best result was observed in the vacuum packing + thyme EO group (the highest count was 4.29 log CFU/g, on day 3, and the lowest count was 1.43 log CFU/g, on day 6) [38]. The authors found that addition of 0.20% (v/w) of thyme EO and storage of samples in vacuum allowed shelf life of the chicken breast fillet to be extended.
Clearly, it can be concluded that vacuum packaging inhibits LAB growth. Of the samples examined in this study, the vacuum packaging + thyme EO sample had the maximum impact on the LAB growth. LAB are one of the main components of meat product microflora that decreases pH of meat product through carbohydrate fermentation [41].
We found that, due to the antibacterial properties of cumin and thyme EOs, the shelf life of burgers with the EOs in vacuum packaging increased. The cause of that can be the presence of phenolic compounds such as thymol and carvacrol in thyme and cuminaldehyde in cumin.
In this study, initial mold and yeast counts were approximately 2 log CFU/g and reached 6.49–6.95 and 2.03–3.08 log CFU/g in samples stored in air and vacuum packaging, respectively (Fig. 1d). Lower mold and yeast counts in test samples compared to control indicates the presence of EOs antifungal constituents in meat products [42].
As for sulfite-reducing clostridia, they were not detected in any of the samples throughout the storage peroid.
Chemical analysis. Figure 2a demonstrates a significant decrease in pH values of control and treated samples during storage (P < 0.001). The initial pH value in burger samples was 6.41. By day 27, their pH values were 4.34–4.53 for all samples in air packaging and 4.71–4.98 for all samples in vacuum packaging. This decrease can be due to a reduced oxygen content as a result aerobic microflora growth and CO2 production. Another cause of the pH decrease can be sugar contained in the burgers, which is utilised as a cryoprotectant.
According to Bingol and Ergun, pH diminishes by the end of storage [43]. They also reported that the pH of meat is influenced by various factors however the major one is lactic acid bacteria growth resulted from lactic acid production. Similar results were also obtained by Soni et al. in regard to chicken patties stored at refrigerator temperature [21].
Total volatile base nitrogen (TVB-N) content is often used as an index to determine a degree of meat decomposition. As one can see in Figure 2b, TVB-N values of burgers increased significantly during storage (P < 0.001). TVN concentration was determined to be between 5 and 25 mg N/100g [44].
TVB-N content was the highest (P < 0.001) in samples in air packaging, which indicates that air packaging alone, even without EOs, can significantly increase TVB-N formation. Erkan investigated TVB-N in vacuum-packaged filleted hot smoked rainbow trout [45]. By day 27 of storage at 2°C, the TVB-N content increased to 33.82 and 24.16 mg/100 g flesh in untreated and treated with thyme EO samples, respectively. Also, Eskandari et al. reported that a TVB-N value in fish samples treated with black cumin remained below its acceptable limit by day 27 [46].
According to hygienic standards, the TVB-N acceptable limit in fish muscle is 20 mg/100 g. Thus, the results of this study demonstrated that TVB-N values in vacuum packaged samples with thyme and cumin EOs were below the limit during storage.
Fat oxidation is the main cause of fish putrefaction; an increasing amount of thiobarbituric acid (TBA) and peroxide leads to rancidity. A steady increase in TBA in burgers was observed during 27 days of storage (Fig. 2c). Vacuum packaging effectively protected the burgers from zero days, keeping TBA scores lower than 1 mg MDA/kg during the storage period. EOs in the combination with vacuum packaging displayed a positive effect on the inhibition of oxidation. Köse et al. found that a TBA level in surimi was acceptable up to day 15, while a TVB-N concentration reached 38.2 mg/100 g by day 13, which exceed the limit of acceptability [5].
Karabagias et al. reported that thyme did not protect lamb meat in air packaging from oxidation, at least not within its normal shelf life [47]. This finding is in contrast to the results of Botsoglou et al. who observed a three-fold reduction in a degree of lipid oxidation in turkey in air packaging [48].
According to Liu et al., TBA increased from 0.16 mg/kg (day 0) to 0.42 mg/kg (day 35) in samples stored at –1°C. In [45], the initial TBA index value for hot smoked rainbow trout fillets was 0.77 mg MDA/kg and reached 1.5 mg MDA/kg by day 27. The lower production of TBA in vacuum packing + thyme samples can contribute to the antioxidant properties of thyme oil. Soni et al. noticed lower TBA values in chicken patties containing 0.10% of oregano and 0.015% of thyme EOs. Jayawardana et al. suggested that a cause of the reduction of TBA values could be polyphenols present in EOs [49].
In our research, TBA values did not exceed the acceptable limit in all samples. Similar results were obtained by Eskandari et al. in fish treated with black cumin [46]. Therefore, TBA cannot be used as a reliable quality index for burgers. TBA of 2–4 mg MDA/kg indicates a good quality of fish. TBA values in this study were lower than 1 mg MDA/kg in all treatments throughout the storage period. It was apparently due to a relatively low fat content in fish (surimi).
We revealed that the antioxidant properties of cumin and thyme EOs prolonged significantly the burger shelf life. Sarıçoban and Yilmaz also confirmed the antioxidant effect of cumin and thyme on TBA, which is due to the antioxidant activity of phenolic compounds contained in different parts of plants [44]. The main compounds of cumin are gammaterpinene, 2-methyl-3-phenyl-propanal, myrtenal, and glucopyranosides [44].
Figure 2d demonstrates an effect of packaging and thyme and cumin EOs on a PV value in the burgers under study. The initial PV value was 0.16–0.18 meq/kg of lipid in all the burgers, while, by day 27, it reached 5.82–8.75 and 1.11–2.35 meq/kg of lipid in samples in air and vacuum packaging, respectively (P < 0.001).
In this study, PV was increasing up to day 21 of storage in all samples and then, by day 27, decreased. At the end of the storage time, PV in all vacuumpackaged samples did not reach the acceptable limit (5 meq/kg). Similar findings were obtained by Çoban and Keleştemur in catfish burger treated with thyme [50]. Such findings are an evidence of EOs inhibitory effect on microorganisms which cause burger spoilage. The reduction of PV after day 21 can be due to hydroperoxide degradation. The decay of hydroperoxides results in the formation of degradation products [51]. The reduction in PV in samples with cumin EO can be due to cumin aldehyde, which prevents lipid peroxidation [52].
ВЫВОДЫ
We found that the shelf life of the novel burgers from surimi and minced chicken meat could be extended by using essential oils and vacuum packaging. According to the results of the microbiological analysis, the shelf life of the burgers was as follows: 9 days for burgers in air packaging, 12 days for burgers with cumin and thyme EOs in air packaging, 18 days for burgers in vacuum packaging, and 21 days for burgers with cumin and thyme EOs in vacuum packaging.
The shelf life for vacuum-packed burgers treated with thyme and cumin EOs was established as 18 days at 2°C, in compared to that for untreated burgers, which was 6 days. In addition, vacuum packaging alone was found to maintain burger freshness during 15 days.
Thus, burger shelf life was extended by 9 days for the combination of thyme/cumin EO + air packaging, 15 days for vacuum-packaged samples, and 18 days for the combination of thyme/cumin EO + vacuum packaging. Overall, the combined use of vacuum packaging and thyme/cumin EO demonstrated their synergistic effect on the shelf life of the novel burgers. These results allowed us to suggest that surimi could be successfully used as an alternative ingredient to minced meat in burgers production.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare no conflict of interest.
БЛАГОДАРНОСТИ
This article was written based on the Ph.D. thesis of Dr. Azadeh Rashidimehr. The authors wish to express their gratitude to the research council of the Shahid Chamran University of Ahvaz and to Mrs. P. Esfahani, for her kind technical assistance in the food hygiene laboratory.
ФИНАНСИРОВАНИЕ
This study was financially supported by the Shahid Chamran University of Ahvaz (Grant No.: 97/3/02/26247).
СПИСОК ЛИТЕРАТУРЫ
- Rodgers S. Value adding with functional meals. Food Service Technology. 2004;4(4):149–158. DOI: https://doi.org/10.1111/j.1471-5740.2004.00101.x.
- Ahlgren M. Gustafsson I-B, Hall G. Attitudes and beliefs directed towards ready-meal consumption. Food Service Technology. 2004;4(4):159–169. DOI: https://doi.org/10.1111/j.1471-5740.2004.00102.x.
- Bainy EM, Lenzi EK, Corazza ML, Lenzi MK. Mathematical modeling of fish burger baking using fractional calculus. Thermal Science. 2017;21(1):41–50. DOI: https://doi.org/10.2298/TSCI160422241B.
- Bochi VC, Weber J, Ribeiro CP, Victorio AD, Emanuelli T. Fishburgers with silver catfish (Rhamdia quelen) filleting residue. Bioresource Technology. 2008;99(18):8844–8849. DOI: https://doi.org/10.1016/j.biortech.2008.04.075.
- Kose S, Boran M, Boran G. Storage properties of refrigerated whiting mince after mincing by three different methods. Food Chemistry. 2006;99(1):129–135. DOI: https://doi.org/10.1016/j.foodchem.2005.06.047.
- Dey SS, Dora KC. Effect of sodium lactate as cryostabilizer on physic-chemical attributes of croaker (Johnius gangeticus) muscle protein. Journal of Food Science and Technology. 2010;47(4):432–436. DOI: https://doi.org/10.1007/s13197-010-0071-8.
- Burt S. Essential oils: their antibacterial properties and potential applications in foods – a review. International Journal of Food Microbiology. 2004;94(3):223–253. DOI: https://doi.org/10.1016/j.ijfoodmicro.2004.03.022.
- Nychas GJE. Natural antimicrobials from plants. In: Gould GW, editor. New Methods of Food Preservation. Boston: Springer-Science+Business Media; 1995. pp. 58–89. DOI: https://doi.org/10.1007/978-1-4615-2105-1_4.
- Solomakos N, Govaris A, Koidis P, Botsoglou N. The antimicrobial effect of thyme essential oil, nisin and their combination against Escherichia coli O157:H7 in minced beef during refrigerated storage. Meat Science. 2008;80(2):159–166. DOI: https://doi.org/10.1016/j.meatsci.2007.11.014.
- Frangos L, Pyrgotou N, Giatrakou V, Ntzimani A, Savvaidis IN. Combined effects of salting, oregano oil and vacuumpackaging on the shelf-life of refrigerated trout fillets. Food Microbiology. 2010;27(1):115–121. DOI: https://doi.org/10.1016/j.fm.2009.09.002.
- Giarratana F, Muscolino D, Ziino G, Lo Presti V, Rao R, Chiofalo V, et al. Activity of Catmint (Nepeta cataria) essential oil against Anisakis larvae. Tropical Biomedicine. 2017;34(1):22–31.
- Giarratana F, Muscolino D, Beninati C, Ziino G, Giuffrida A, Panebianco A. Activity of R(+) limonene on the maximum growth rate of fish spoilage organisms and related effects on shelf-life prolongation of fresh gilthead sea bream fillets. International Journal of Food Microbiology. 2016;237:109–113. DOI: https://doi.org/10.1016/j.ijfoodmicro.2016.08.023.
- Busatta C, Vidal RS, Popiolski AS, Mossi AJ, Dariva C, Rodrigues MRA, et al. Application of Origanum majorana L. essential oil as an antimicrobial agent in sausage. Food Microbiology. 2008;25(1):207–211. DOI: https://doi.org/10.1016/j.fm.2007.07.003.
- Marotta SM, Giarratana F, Parco A, Neri D, Ziino G, Giuffrida A, et al. Evaluation of the antibacterial activity of bergamot essential oils on different Listeria monocytogenes strains. Italian Journal of Food Safety. 2016;5(4):210–213. DOI: https://doi.org/10.4081/ijfs.2016.6176.
- Daniel AP, Veeck APL, Klein B, Ferreira LF, da Cunha MA, Parodi TV, et al. Using the Essential Oil of Aloysia triphylla (L’Her.) Britton to Sedate Silver Catfish (Rhamdia quelen) during Transport Improved the Chemical and Sensory Qualities of the Fish during Storage in Ice. Journal of Food Science. 2014;79(6):S1205–S1211. DOI: https://doi.org/10.1111/1750-3841.12463.
- Fernandes RPP, Trindade MA, Lorenzo JM, Munekata PES, de Melo MP. Effects of oregano extract on oxidative, microbiological and sensory stability of sheep burgers packed in modified atmosphere. Food Control. 2016;63:65–75. DOI: https://doi.org/10.1016/j.foodcont.2015.11.027.
- Sharma H, Mendiratta SK, Agarwal RK, Kumar S, Soni A. Evaluation of anti-oxidant and anti-microbial activity of various essential oils in fresh chicken sausages. Journal of Food Science and Technology. 2017;54(2):279–292. DOI: https://doi.org/10.1007/s13197-016-2461-z.
- Lorenzo JM, Vargas FC, Strozzi I, Pateiro M, Furtado MM, Sant’Ana AS, et al. Influence of pitanga leaf extracts on lipid and protein oxidation of pork burger during shelf-life. Food Research International. 2018;114:47–54. DOI: https://doi.org/10.1016/j.foodres.2018.07.046.
- Li X-M, Tian S-L, Pang Z-C, Shi J-Y, Feng Z-S, Zhang Y-M. Extraction of Cuminum cyminum essential oil by combination technology of organic solvent with low boiling point and steam distillation. Food Chemistry. 2009;115(3):1114–1119. DOI: https://doi.org/10.1016/j.foodchem.2008.12.091.
- Baranauskiene R, Venskutonis PR, Viskelis P, Dambrauskiene E. Influence of nitrogen fertilizers on the yield and composition of thyme (Thymus vulgaris). Journal of Agricultural and Food Chemistry. 2003;51(26):7751–7758. DOI: https://doi.org/10.1021/jf0303316.
- Soni A, Gurunathan K, Mendiratta SK, Talukder S, Jaiswal RK, Sharma H. Effect of essential oils incorporated edible film on quality and storage stability of chicken patties at refrigeration temperature (4±1°C). Journal of Food Science and Technology-Mysore. 2018;55(9):3538–3546. DOI: https://doi.org/10.1007/s13197-018-3279-7.
- Corbo MR, Speranza B, Filippone A, Granatiero S, Conte A, Sinigaglia M, et al. Study on the synergic effect of natural compounds on the microbial quality decay of packed fish hamburger. International Journal of Food Microbiology. 2008;127(3):261–267. DOI: https://doi.org/10.1016/j.ijfoodmicro.2008.07.014.
- Spinelli S, Conte A, Lecce L, Incoronato AL, Del Nobile MA. Microencapsulated Propolis to Enhance the Antioxidant Properties of Fresh Fish Burgers. Journal of Food Process Engineering. 2015;38(6):527–535. DOI: https://doi.org/10.1111/jfpe.12183.
- Official Methods of Analysis of Association of Analytical Chemists International, 17th Edition. Gaithersburg: The Association of Official Analytical Chemists; 2000. pp. 20877–2417.
- Heydari F, Varidi MJ, Varidi M, Mohebbi M. Study on quality characteristics of camel burger and evaluating its stability during frozen storage. Journal of Food Measurement and Characterization. 2016;10(1):148–155. DOI: https://doi.org/10.1007/s11694-015-9288-6.
- Zamuz S, Lopez-Pedrouso M, Barba FJ, Lorenzo JM, Dominguez H, Franco D. Application of hull, bur and leaf chestnut extracts on the shelf-life of beef patties stored under MAP: Evaluation of their impact on physicochemical properties, lipid oxidation, antioxidant, and antimicrobial potential. Food Research International. 2018;112:263–273. DOI: https://doi.org/10.1016/j.foodres.2018.06.053.
- Doulgeraki AI, Paramithiotis S, Kagkli DM, Nychas G-JE. Lactic acid bacteria population dynamics during minced beef storage under aerobic or modified atmosphere packaging conditions. Food Microbiology. 2010;27(8):1028–1034. DOI: https://doi.org/10.1016/j.fm.2010.07.004.
- Selani MM, Shirado GAN, Margiotta GB, Saldana E, Spada FP, Piedade SMS, et al. Effects of pineapple byproduct and canola oil as fat replacers on physicochemical and sensory qualities of low-fat beef burger. Meat Science. 2016;112:69–76. DOI: https://doi.org/10.1016/j.meatsci.2015.10.020.
- Petrou S, Tsiraki M, Giatrakou V, Savvaidis IN. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. International Journal of Food Microbiology. 2012;156(3):264–271. DOI: https://doi.org/10.1016/j.ijfoodmicro.2012.04.002.
- Namir M, Siliha H, Ramadan MF. Fiber pectin from tomato pomace: characteristics, functional properties and application in low-fat beef burger. Journal of Food Measurement and Characterization. 2015;9(3):305–312. DOI: https://doi.org/10.1007/s11694-015-9236-5.
- Malle P, Poumeyrol M. A New Chemical Criterion for the Quality-Control of Fish: Trimethylamine/Total Volatile Basic Nitrogen (%). Journal of Food Protection. 1989;52(6):419–423. DOI: https://doi.org/10.4315/0362-028x-52.6.419.
- Tsironi T, Dermesonlouoglou E, Giannakourou M, Taoukis P. Shelf life modelling of frozen shrimp at variable temperature conditions. LWT – Food Science and Technology. 2009;42(2):664–671. DOI: https://doi.org/10.1016/j.lwt.2008.07.010.
- Official methods of analysis of AOAC International, 18th Edition. Gaithersburg: The Association of Official Analytical Chemists, 2010.
- Vanitha M, Dhanapal K, Reddy GVS. Quality changes in fish burger from Catla (Catla Catla) during refrigerated storage. Journal of Food Science and Technology-Mysore. 2015;52(3):1766–1771. DOI: https://doi.org/10.1007/s13197-013-1161-1.
- Microorganisms in foods. Vol. 2. Sampling for Microbiological Analysis: Principles and Scientific Applications. Oxford: Blackwell Scientific; 1986. 293 p.
- Cozar A, Rubio N, Vergara H. Combined effect of the spice and the packaging method on lamb burgers shelf-life made with high value cuts. Cyta – Journal of Food. 2018;16(1):544–552. DOI: https://doi.org/10.1080/19476337.2018.1431310.
- Kilinc B, Cakli S, Tolasa S. Quality changes of sardine (Sardina pilchardus) patties during refrigerated storage. Journal of Food Quality. 2008;31(3):366–381. DOI: https://doi.org/10.1111/j.1745-4557.2008.00205.x.
- Pavelkova A, Kacaniova M, Horska E, Rovna K, Hleba L, Petrova J. The effect of vacuum packaging, EDTA, oregano and thyme oils on the microbiological quality of chicken’s breast. Anaerobe. 2014;29:128–133. DOI: https://doi.org/10.1016/j.anaerobe.2013.09.002.
- Babji Y, Murthy TRK. Effect of inoculation of mesophilic lactic acid bacteria on microbial and sensory changes of minced goat meat during storage under vacuum and subsequent aerobic storage. Meat Science. 2000;54(2):197–202. DOI: https://doi.org/10.1016/S0309-1740(99)00101-1.
- Fratianni F, De Martino L, Melone A, De Feo V, Coppola R, Nazzaro F. Preservation of Chicken Breast Meat Treated with Thyme and Balm Essential Oils. Journal of Food Science. 2010;75(8):M528–M535. DOI: https://doi.org/10.1111/j.1750-3841.2010.01791.x.
- Egan AF. Lactic-acid bacteria of meat and meat-products. Antonie Van Leeuwenhoek. Journal of Microbiology. 1983;49(3):327–336. DOI: https://doi.org/10.1007/BF00399507.
- Rao A, Zhang YQ, Muend S, Rao R. Mechanism of Antifungal Activity of Terpenoid Phenols Resembles Calcium Stress and Inhibition of the TOR Pathway. Antimicrobial Agents and Chemotherapy. 2010;54(12):5062–5069. DOI: https://doi.org/10.1128/AAC.01050-10.
- Bingol EB, Ergun O. Effects of modified atmosphere packaging (MAP) on the microbiological quality and shelf life of ostrich meat. Meat Science. 2011;88(4):774–785. DOI: https://doi.org/10.1016/j.meatsci.2011.03.013.
- Saricoban C, Yilmaz MT. Effect of thyme/cumin essential oils and butylated hydroxyl anisole/butylated hydroxyl toluene on physicochemical properties and oxidative/microbial stability of chicken patties. Poultry Science. 2014;93(2):456–463. DOI: https://doi.org/10.3382/ps.2013-03477.
- Erkan N. The Effect of Thyme and Garlic Oil on the Preservation of Vacuum-Packaged Hot Smoked Rainbow Trout (Oncorhynchus mykiss). Food and Bioprocess Technology. 2012;5(4):1246–1254. DOI: https://doi.org/10.1007/s11947-010-0412-7.
- Eskandari S, Hosseini H, Gholamzadeh M, Khaneghah AM, Hosseini E. The Effects of Black Cumin, Black Caraway Extracts and Their Combination on Shelf Life Extension of Silver Carp (Hypophthalmichthys Molitrix) during Refrigerated Storage. Journal of Food Safety. 2015;35(2):154–160. DOI: https://doi.org/10.1111/jfs.12155.
- Karabagias I, Badeka A, Kontominas MG. Shelf life extension of lamb meat using thyme or oregano essential oils and modified atmosphere packaging. Meat Science. 2011;88(1):109–116. DOI: https://doi.org/10.1016/j.meatsci.2010.12.010.
- Botsoglou NA, Grigoropoulou SH, Botsoglou E, Govaris A, Papageorgiou, G., The effects of dietary oregano essential oil and α-tocopheryl acetate on lipid oxidation in raw and cooked Turkey during refrigerated storage. Meat Science. 2003;65(3):1193–1200. DOI: https://doi.org/10.1016/S0309-1740(03)00029-9.
- Jayawardana BC, Liyanage R, Lalantha N, Iddamalgoda S, Weththasinghe P. Antioxidant and antimicrobial activity of drumstick (Moringa oleifera) leaves in herbal chicken sausages. LWT – Food Science and Technology.2015;64(2):1204–1208. DOI: https://doi.org/10.1016/j.lwt.2015.07.028.
- Coban OE, Kelestemur GT. Qualitative improvement of catfish burger using Zataria multiflora Boiss. essential oil. Journal of Food Measurement and Characterization. 2017;11(2):530–537. DOI: https://doi.org/10.1007/s11694-016-9420-2.
- Chaijan M, Benjakul S, Visessanguan W, Faustman C. Changes of lipids in sardine (Sardinella gibbosa) muscle during iced storage. Food Chemistry. 2006;99(1):83–91. DOI: https://doi.org/10.1016/j.foodchem.2005.07.022.
- Ehsani A, Jasour MS, Hashemi M, Mehryar L, Khodayari M. Zataria multiflora Boiss essential oil and sodium acetate: how they affect shelf life of vacuum-packaged trout burgers. International Journal of Food Science and Technology. 2014;49(4):1055–1062. DOI: https://doi.org/10.1111/ijfs.12400.