Аннотация
A complex of amylases, proteases, and hemicellulases is known to enhance deep conversion of polysaccharides and proteins, especially in the processing of difficult-to-ferment raw materials, such as rye, providing grain wort with soluble carbohydrates, amino acids, and peptides. Grain is also a source of phosphorus, whose bioavailability can be increased by hydrolysing the grain with phytase-containing enzyme preparations. However, their catalytic action during the preparation of grain wort for alcohol production has hardly been studied. This study aimed to investigate the effect of a new complex phytasecontaining enzyme preparation on yeast metabolism and the efficiency of rye wort fermentation. The work was carried out in the Russian Research Institute of Food Biotechnology. The Glucavamorin complex enzyme preparations derived from recombinant strains were the object of our research. The preparations differed in the activity level of the main enzyme, lucoamylase, and minor hemicellulase enzymes, as well as in the presence of phytase. The results confirmed their biocatalytic ability to efficiently hydrolyse polymers of rye grain. An increased content of hemicellulases in Glucavamorin-Xyl improved the rheological properties of rye wort. The greatest effect was achieved with the phytase-containing Glucavamorin-Ply. This preparation improved the phosphorus nutrition of yeast, which increased its biomass by 30% and decreased the level of fermentation by-products by 18–20%. Alcohol yield tended to increase and its strength reached 10.5–10.9% vol. When using a phytase-containing enzyme complex, it was possible to reduce the amount of the main enzyme, glucoamylase, without causing the key fermentation indicators to degrade.Ключевые слова
Rye wort, phytase, enzyme preparations, yeast, ethanol, fermentation, metabolitesВВЕДЕНИЕ
Modern alcohol technologies are based on complex and deep processing of agricultural raw materials aimed at improving production profitability. The effectiveness of biotechnological processing is achieved by developing new biocatalysts of various action and substrate specificity. This ensures deep hydrolysis of high molecular weight polymers of grain, especially rye with its high content of non-starch polysaccharides, gum substances, and mucus.
As shown by many studies, the use of complex enzyme preparations with broad substrate specificity can increase the depth of hydrolysis of grain polymers into ethanol, especially when making concentrated grain wort [1–4]. The complex should contain amylolytic, proteolytic, and hemicellulase enzymes. Amylolytic enzymes play a part in starch conversion: α-amylase in starch dextrinization and liquefaction and glucoamylase in its saccharification). Proteases are beneficial for the generation and metabolism of alcoholic yeast, since their catalytic effect on protein enriches the wort with easily digestible amino acids assimilated by yeast [5]. Hemicellulases (β-glucanases and xylanases), catalysing the hydrolysis of non-starch polysaccharides, decrease the wort viscosity and lead to the formation of additional fermented carbohydrates due to the destruction of grain xylans and glucans. The synergic action of amylolytic, proteolytic and hemicellulase enzymes improve the quality of grain wort and its rheological properties, especially when processing difficult-to-ferment raw materials, such as rye and barley. Improved biocatalytic conversion of grain polymers intensifies alcohol fermentation, increasing the target product (ethanol) yield and decreasing side metabolites formation.
A number of studies into the phytase effect on the processing of sorghum and corn for lager beer production showed a potential possibility of improving nutritional conditions for yeast during the fermentation of raw whole grains [6]. Some researchers noted a positive effect of phytase treatment on the embryo and fibre yield during the dry grinding of yellow dent corn [7]. A study of the phytolytic effect on the quality of wheat bread enriched with bran revealed an increase in the bioavailability of iron contained in it [8]. To make the conversion of phytin-containing raw materials more efficient, microorganism strains with phytase activity were selected and identified [9, 10]. Considerable research was conducted into the use of phytase to improve the digestibility of feed nutrients, including phytate phosphorus [11, 12]. However, there is a lack of studies into the effectiveness of phytolytic enzymes during the preparation of concentrated grain wort for alcohol production, especially from rye.
Currently, extensive studies are underway to obtain enzyme preparations based on recombinant strains of microscopic fungi using genetic engineering and mutagenesis [13–15]. The preparations contain a complex of enzymes with an increased biocatalytic capacity for xylanase, β-glucanase, and cellulase, and they can be used in the alcohol industry [16]. New highly active multienzyme preparations can contribute towards the implementation of innovative technologies for deep conversion of grain into ethanol.
Of particular interest are phytolytic enzymes. Phytase is an enzyme that breaks down phytic acid. Phytic acid in the form of myo-inositol hexaphosphoric acid or phytate (acid salt) is the main form of mineral phosphorus in plant tissues [17]. Cereal grains have a particularly high content of phytic acid [18]. Phosphorus is essential for yeast cells to grow and develop. Under anaerobic conditions, yeast assimilates phosphorus mainly in the initial period of fermentation when its consumption is 80–90% of the maximum content in yeast. Young, actively breeding yeast cells are richer in phosphorus than non-budding old cells. For example, after 6 h of fermentation, yeast cells accumulate 2.15% of phosphorus per dry matter, while this value is only 1% at the end of fermentation. Therefore, when making grain wort, it is important to enrich it with phosphorus to ensure a stable process of yeast generation and alcoholic fermentation.
Cereals are the main source of phosphorus, whose bioavailability can be enhanced by hydrolysis of grain with phytase-containing enzyme preparations. Phytate hydrolysis helps reduce the consumption of enzyme preparations, as it inhibits many enzymes and enables the release of valuable trace elements, such as calcium, magnesium, zinc, etc. [19, 20]. This way, it provides alcoholic yeast with additional nutrition.
Apart from generating the main products of fermentation, namely alcohol and carbon dioxide, yeast cells synthesise metabolites called secondary products or by-products of fermentation. The biosynthesis of by-products is associated with the cell’s regulatory functions. By-products formation depends on the medium composition, the level of nitrogen, carbon, and phosphorus in the medium, the conditions of yeast cultivation and the genetic characteristics of the strain used [3, 4]. One of the ways to improve the efficiency of alcohol production is to create conditions to reduce carbohydrate expenditure for the formation of fermentation by-products through the use of media with a balanced amino acid composition. The amount of ethanol impurities can also be reduced by regulating technological processes in such a way that conditions are provided to promote ethanol synthesis with decreased formation of fermentation by-products [4]. Therefore, complex enzyme preparations contribute to a more rational use of high-molecular components of grain raw materials.
Our aim was to study the effect of a new complex phytase-containing preparation on yeast metabolism and the efficiency of rye wort fermentation.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
This research was conducted at the Department for Biotechnology of Enzyme Preparations, Yeast, Organic Acids and Biologically Active Substances of the Russian Research Institute of Food Biotechnology, a branch of the Federal Research Centre of Nutrition, Biotechnology and Food Safety.
The study objects included rye grain, the Saccharomyces cerevisiae 985T alcohol yeast, and complex enzyme preparations (EP), Glucavamorin Xyl and Glucavamorin Ply. The preparations were obtained in the Laboratory of New Enzyme Producers of the Russian Research Institute of Food Biotechnology from the transformants of the commercial Aspergillus awamori strain [21]. They differed in the activity level of the major enzyme (glucoamylase) and minor enzymes (hemicellulases).
We used the following methods to determine the catalytic activity of the enzyme preparations.
Amylolytic and glucoamylase activity was determined according to State Standard 54330-2011. The method for determining amylolytic activity is based on the quantification of starch hydrolysed by amylolytic enzymes to dextrins of various molecular weight under standard conditions (temperature 30°С; pH 6.0 for bacterial and 4.7 for fungal α-amylase; hydrolysis duration 10 min). The method for determining glucoamylase activity is based on the quantification of glucose formed during starch hydrolysis by glucoamylase under standard conditions (temperature 30°С; pH 4.7; hydrolysis duration 10 min).
β-glucanase activity (β-GcS) was determined according to State Standard 53973-2010. The method is based on the quantitative determination of reducing sugars resulting from β-glucanase action on β-1,4 bonds of β-glucan under standard conditions (temperature 50°С; pH 4.7; hydrolysis duration 10 min).
Cellulase activity was determined according to State Standard 55293-2012 (Enzyme preparations for food industry. Method for determination of cellulase activity. Moscow: Standartinform, 2014). The method is based on the quantitative determination of reducing sugars resulting from cellulase action on the substrate of sodium carboxymethylcellulose (CMC) at 50°C.
Xylanase activity was determined according to State Standard 55302-2012. The method is based on the quantitative determination of reducing sugars resulting from xylanase (exoxylanase) action on β-1,4 bonds of xylan.
Phytase activity was determined according to State Standard 31487-2012. One unit of phytase activity is the amount of enzyme that catalyses the hydrolysis of sodium phytate to form 1 μmol of inorganic phosphate per minute under standard conditions (temperature 37°C; pH 5.5; hydrolysis duration 15 min).
Rye, used as a raw material to make grain wort, was prepared by 'soft' enzymatic cooking at a water ratio of 1:3. At the mash stage, thermostable α-amylase was used for starch dextrinization at the rate of 0.5 unit/g starch. At the saccharification stage, the test samples included complex enzyme preparations, namely Glucavamorin Xyl and Glucavamorin Ply in an amount of 6–10 units/g starch each. The grain wort used as a control sample was made with commercial enzyme preparations (EP) without phytase: Glucomil L-706 as a source of glucoamylase and BrewZyme BGX as a source of xylanase, β-glucanase, and cellulase. The amount of EPs for the biocatalysis of rye wort polymers in the control sample was 10; 0.4; 0.05; and 0.2 unit/g starch for glucoamylase, xylanase, β-glucanase, and cellulase, respectively.
The wort was fermented with the Saccharomyces cerevisiae 985T alcohol yeast, which has thermotolerant and osmophilic properties, by the fermentation samples method. The fermentation was carried out at 35°C for 72 h. The Guidelines for the Technical and Chemical Control of Alcohol Production were followed to determine the biochemical indicators of rye grain, wort concentration, the number of yeast cells, the percentage of budding cells, the content of total and residual reducing carbohydrates (RS, reducing substances), and ethanol concentration and yield [3, 5]. The composition and level of side metabolites formed during fermentation was analysed on an Agilent 6850 gas chromatograph according to State Standard 55792-2013.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
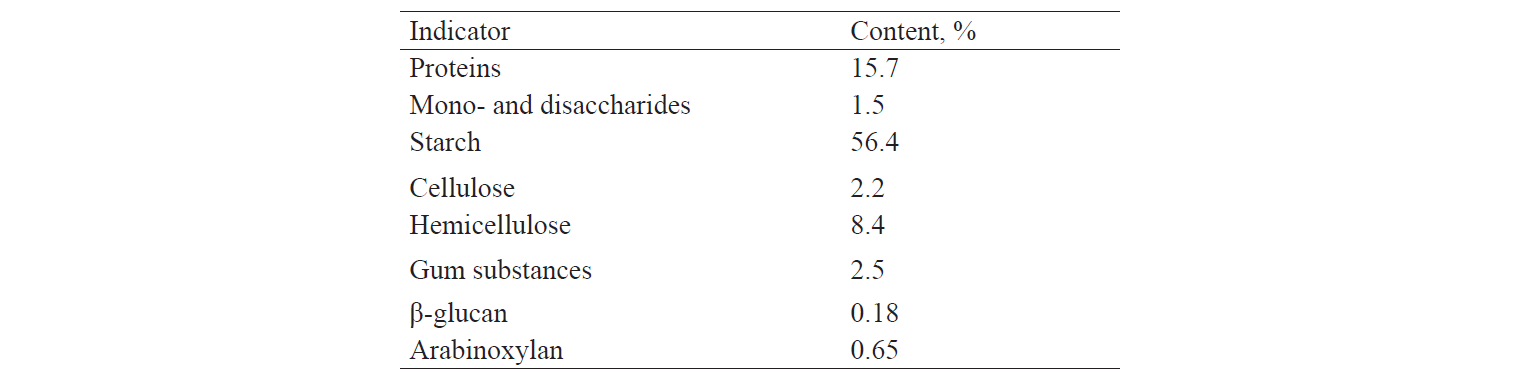
Ground rye grain, whose biochemical composition is given in Table 1, was used as a substrate for fermentation. Rye is known to be a multicomponent substrate characterised by a high content of hemicelluloses and gum substances. The studied rye grain contained 56.4% of starch, 8.4% of hemicellulose, and 2.2% of cellulose. The presence of non-starch polysaccharides complicates the process of preparing concentrated wort that has good rheological properties and contains soluble carbohydrates in a form that is accessible to yeast cells.
Therefore, new enzyme preparations of glucoamylase and hemicellulase action were tried to prepare media ensuring stable yeast generation and alcohol fermentation.
To prepare the grain for fermentation, we used the Glucavamorin complex enzyme preparations based on A. Аwamori recombinant strains and containing xylanase, β-glucanase, and cellulase, along with amylolytic enzymes.
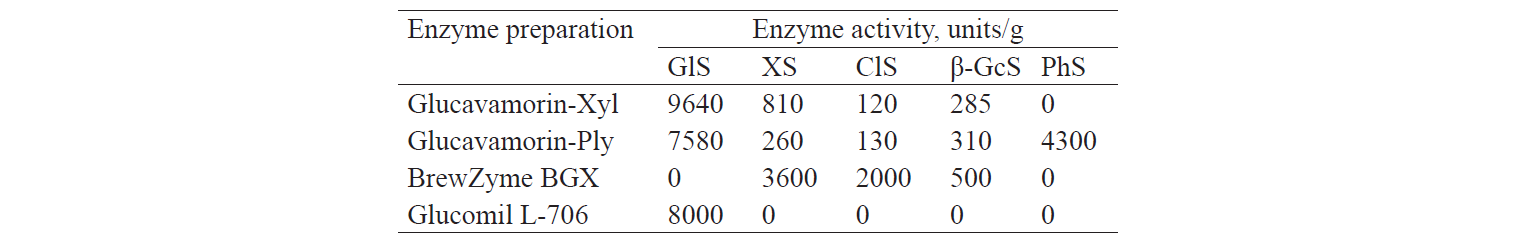
The Glucavamorin enzyme preparations had glucoamylase (GlS), xylanase (XS), β-glucanase (β-GcS), and cellulase (ClS) activities. Of particular interest was the Glucavamorin-Ply preparation, which additionally exhibited a high level of phytase (PhS) activity (4300 units/g). Glucavamorin-Xyl had a higher level of xylanase (810 units/g) and glucoamylase (9640 units/g) activity. The results are in Table 2.
The complex enzyme preparations were used as sources of glucoamylase and concomitant enzymes. Therefore, their amount was based on the glucoamylase quantity of 6–10 units/g starch. The level of concomitant enzymes contained in the complex EPs was also monitored (Table 3).
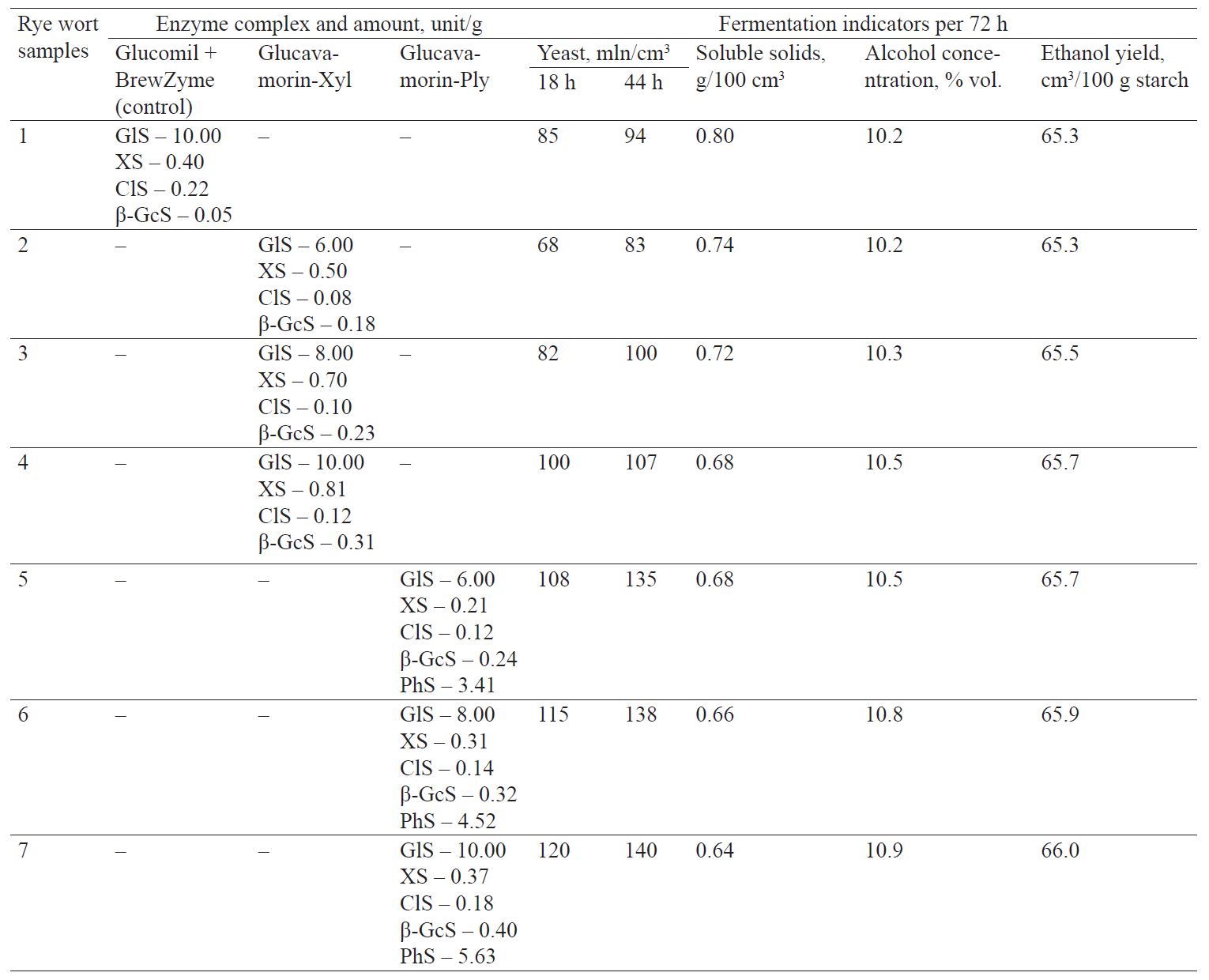
In the control sample (No. 1) we used Glucomil, for starch saccharification, and BrewZyme, for the catalytic hydrolysis of hemicelluloses. Glucavamorin-Xyl was used in test samples No. 2, 3, and 4 in an amount of 6.0, 8.0, and 10.0 units/g starch, respectively, at the stage of saccharification at 58–60°C. Glucavamorin-Ply was used in test samples No. 5, 6, and 7 in an amount of 6.0–10.0 units/g starch.
The studies showed that the concentration of soluble solids (SS) in the rye wort prepared with various amounts of the Glucavamorin complex enzymes was 25.8–26.7%, and the content of reducing carbohydrates was 14.9–15.9%. The highest rates of reducing substances were achieved with the use of Glucavamorin-Ply. Apparently, this was due to a higher β-glucanase activity of the preparation. The catalytic action of β-glucanase contributed to the hydrolysis of grain glucans and the formation of additional reducing carbohydrates (Table 3).
Further studies showed that the quality of the grain wort made with the complex enzyme preparations affected the processes of yeast generation and alcohol fermentation.
The comparative studies into the fermentation of rye wort treated with phytase-free enzyme preparations revealed a higher efficiency of Glucavamorin-Xyl, especially in sample No. 4, where it was used at the maximum amount (10 units/g starch). As seen in Table 4, the yield of ethanol reached 65.7 cm3/100 g starch, exceeding the rate in the control sample (65.3 cm3/100 g starch).
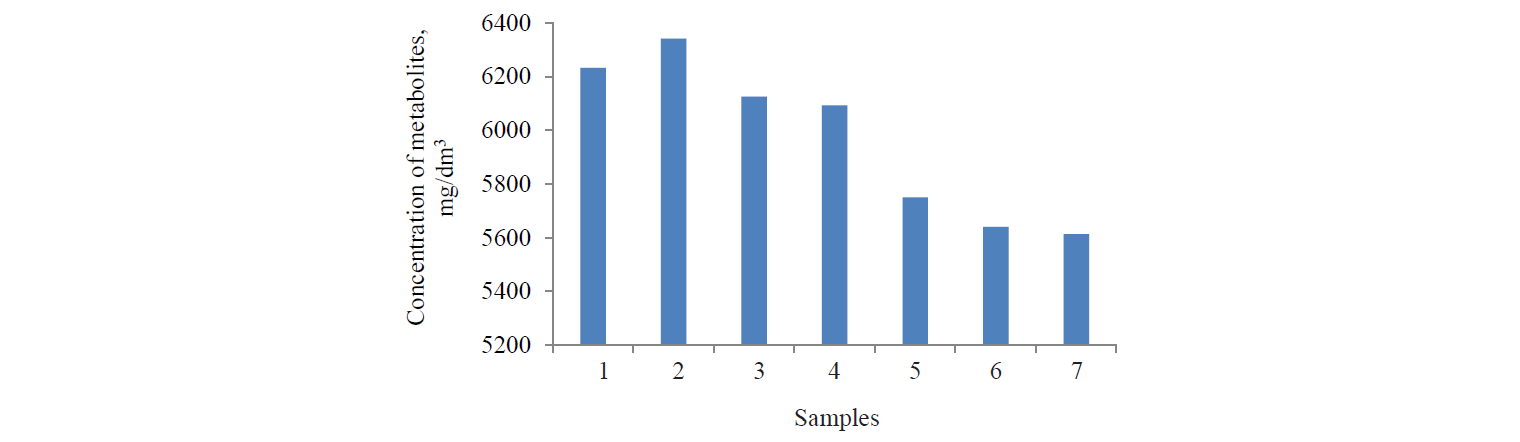
Thus, the above confirmed that the synergic action of the enzymes was determined by their catalytic effect on the grain structural polymers interrelated with each other. We found that to improve the technological parameters of concentrated grain wort, it was necessary to use a complex of hemicellulase enzymes (xylanase, β-glucanase, and cellulase), along with the traditionally used amylases. The effective destruction of non-starch polymers improved the rheological properties of the rye wort, which had a positive effect on the fermentation process. The use of Glucavamorin-Xyl with an increased concentration of hemicellulases contributed to a slight rise in ethanol yield compared to the control sample with the same amount of glucoamylase (10 units/g starch; Table 4).The greatest effect was achieved with Glucavamorin-Ply, which included phytase, apart from a hemicellulase complex. For example, the use of Glucavamorin-Ply in samples No. 5, 6, and 7 in amounts of 6.0; 8.0; and 10.0 units/g starch, respectively, intensified the processes of yeast generation and alcohol fermentation, which increased the ethanol yield to 66.0 cm3/100 g and decreased the concentration level of residual carbohydrates in the mash to 0.64 g/100 cm3 (Fig. 1, Table 4).
Such enzyme complexes contribute to a more rational use of grain components, reduce the wort viscosity, enrich the wort with nutrients, increase the physiological and fermentation activity of yeast and, as a result, accelerate the processes of yeast generation and alcohol fermentation.
The phytolytic action of the preparation had a positive effect on yeast generation and led to a higher concentration of yeast cells compared to the control (I) and those samples where a phytase-free glucoamylase EP was used (Table 2, samples No. 2–4). There was a tendency towards an increase in ethanolyield to 65.7–66.0 cm3/100 g starch, with alcohol concentration of 10.5–10.9% vol., even though the amount of glucoamylase was reduced by 20–40% (from 10.0 to 6.0–8.0 units/g starch) in samples No. 2 and 4.
By catalysing the hydrolysis of phytic acid in the raw material, phytase contained in Glucavamorin-Ply appeared to release additional mineral phosphorus, assimilated by alcohol yeast. This improved the growth, activity and productivity of yeast cells.
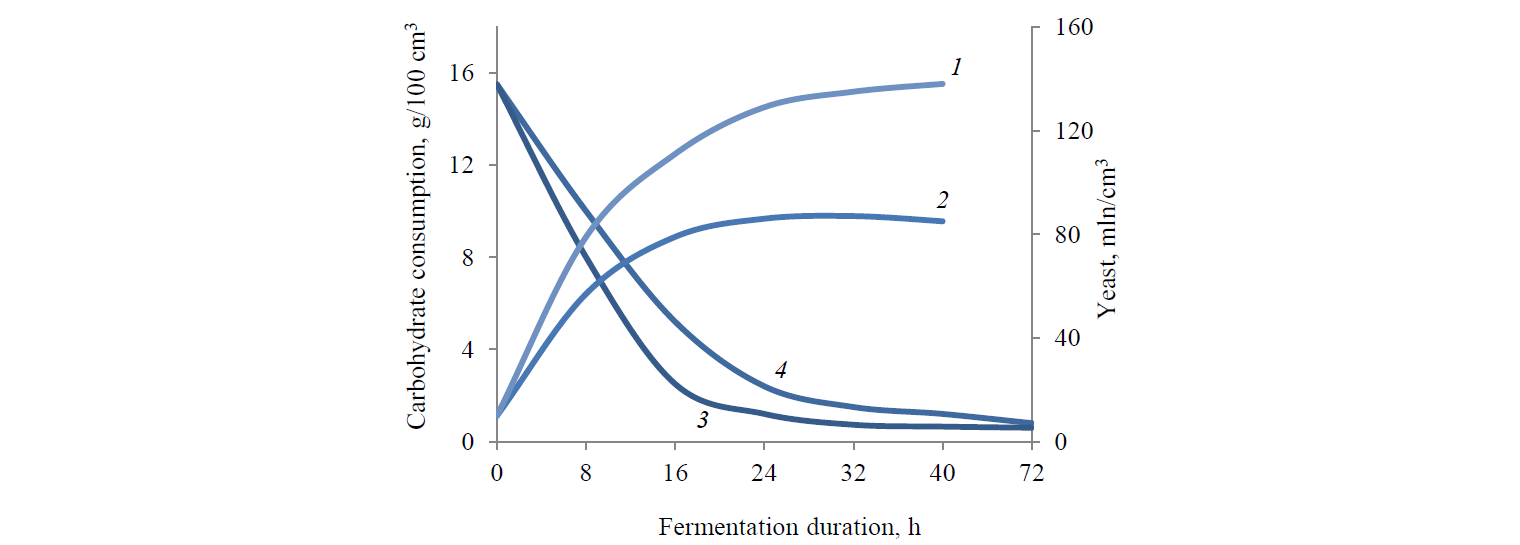
We studied Saccharomyces cerevisiae 985T yeast cultured under anaerobic conditions on rye media with enzyme preparations that differed in the content of phytolytic enzymes. The study showed that Glucavamorin-Ply contributed to increased physiological activity of yeast cells (Fig. 1).
As seen in Fig. 1, the presence of phosphorus in the medium led to intensified yeast development, especially in the lag phase (first 18–24 h), accelerated carbohydrate consumption, and increased concentration of yeast cells (1.4–1.5 times). The fermentation process was more complete, with the minimal amount of residual carbohydrates (0.64 g/100 cm3) and the maximum ethanol yield (Table 4).
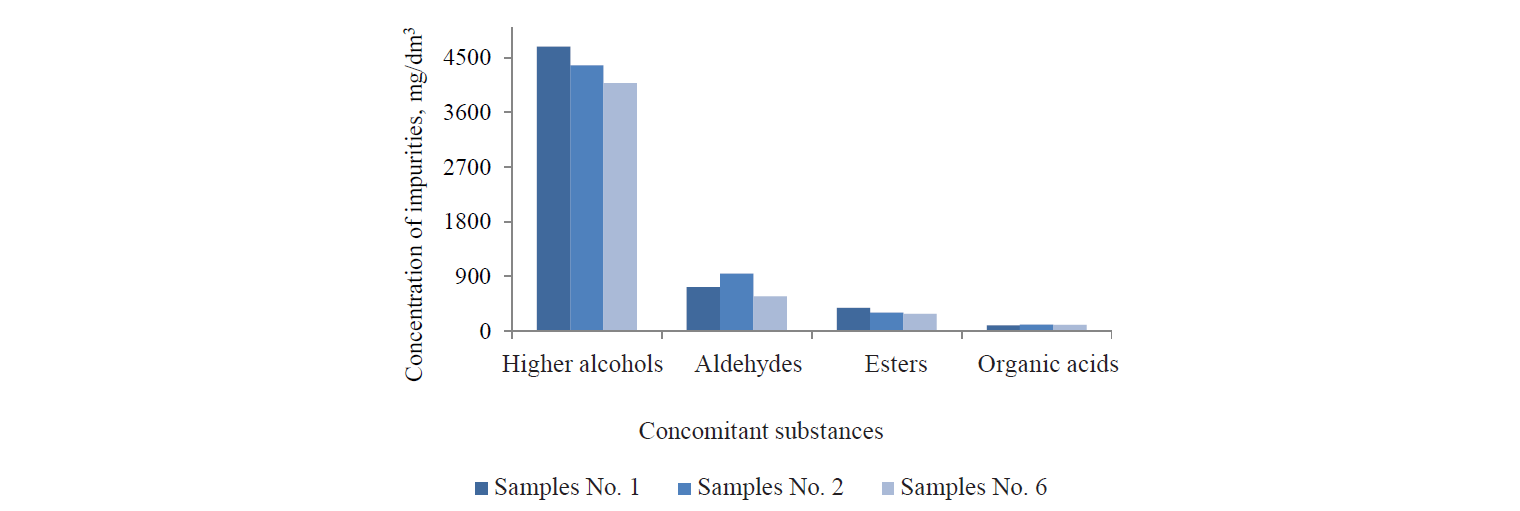
The analysis of the phytolytic effect of the enzyme preparations on the metabolism of yeast cells showed that Glucavamorin-Ply contributed to an 18–20% decrease in the formation of side metabolites accompanying ethanol synthesis, thereby improving the quality of the target product (Fig. 2).
We compared the metabolites synthesised during the fermentation of rye wort made with the glucoamylase enzyme preparations. We found that the phytasecontaining Glucavamorin-Ply (sample No. 4) lowered the content of volatile substances by the end of fermentation, compared to the control (sample No. 1) and the sample with the phytase-free Glucavamorin-Xyl. It did so by reducing the synthesis of major impurities: higher alcohols, aldehydes, and esters (Fig. 3). This improved the organoleptic and analytical indicators of the final product, i.e. ethanol.
ВЫВОДЫ
The study showed that the use of the Glucavamorin complex enzyme preparations, derived from Aspergillus awamori recombinant strains, at the stage of preparing rye wort for fermentation enhanced the efficiency of yeast generation and alcohol fermentation. The increased content of minor hemicellulase enzymes in Glucavamorin-Xyl improved the rheological properties of the rye wort and had a positive effect on the fermentation process.
The catalytic effect of the phytase-containing Glucavamorin-Ply enzyme preparation improved the phosphorus nutrition of yeast. This intensified yeast generation, increased the concentration of yeast cells in the rye wort by 30%, reduced the level of side metabolites by 18–20%, and enhanced ethanol yield. The study revealed that using a phytase-containing enzyme complex made it possible to reduce the amount of the main enzyme, glucoamylase, from 10.0 to 6.0–8.0 units/g starch without causing the key fermentation indicators to degrade.
Thus, the study confirmed that the synergic effect of enzymes with different substrate specificity on the polymers of grain raw materials enhanced the efficiency of their conversion when fermenting rye wort.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare no conflict of interest.
БЛАГОДАРНОСТИ
The study was financed with a federal subsidy within the framework of the Programme for Basic Scientific Research of the State Academies of Sciences (Subject No. 0529-2019-0066).
СПИСОК ЛИТЕРАТУРЫ
- Turshatov M.V., Ledenev V.P., Kononenko V.V., et al. The modern technology of spirit producing. Manufacture of alcohol liqueur and vodka products, 2011, no. 1, pp. 28–29. (In Russ.).
- Turshatov M.V., Ledenev V.P., Kononenko V.V., et al. Techno-Economic Aspects of the Production of Alcohol from Recyclable Materials Generated by Integrated Processing of Wheat. Manufacture of alcohol liqueur and vodka products, 2015, no. 1, pp. 33–35. (In Russ.).
- Abramova I.M. Processing Wheat Raw Materials to Produce High-Quality Alcohol. Manufacture of alcohol liqueur and vodka products, 2012, no. 1, pp. 4–6. (In Russ.).
- Rimareva L.V., Overchenko M.B., Ignatova N.I., et al. Influence of ferment complexes on metabolism of alcoholic yeast and accumulation of ions of nonorganic nature in concentrated grain wort. Vestnik of the Russian agricultural sciences, 2016, no. 3, pp. 28–31. (In Russ.).
- Velikoretskaya I.A., Sereda A.S., Kostyleva E.V., et al. Ehffektivnostʹ novogo fermentnogo preparata kisloy proteazy na osnove rekombinantnogo shtamma Penicillium canescens pri sbrazhivanii pshenichnogo susla [The effectiveness of a new acid protease enzyme preparation based on the recombinant strain of Penicillium canescens in the fermentation of wheat wort]. Acta Naturae (Russian edition), 2016, no. S2, pp. 237. (In Russ.).
- Kruger J., Oelofse A., Taylor J., and Taylor J.R.N. Potential for improvement in yeast nutrition in raw whole grain sorghum and maize lager brewing and bioethanol production through grain genetic modification and phytase treatment. Journal of the Institute of Brewing, 2012, vol. 118, no. 1, pp. 70–75. DOI: https://doi.org/10.1002/jib.16.
- Khullar E., Shetty J.K., Rausch K.D., Tumbleson M.E., and Singh V. Use of Phytases in Ethanol Production from E-Mill Corn Processing. Cereal Chemistry, 2011, vol. 88, no. 3, pp. 223–227. DOI: https://doi.org/10.1094/CCHEM-04-10-0058.
- Sanz-Penella J.M., Laparra J.M., Sanz Y., and Haros M. Influence of Added Enzymes and Bran Particle Size on Bread Quality and Iron Availability. Cereal Chemistry, 2012, vol. 89, no. 5, pp. 223–229. DOI: https://doi.org/10.1094/CCHEM-09-11-0109.
- Nuobariene L., Arneborg N., and Hansen A.S. Phytase Active Yeasts Isolated From Bakery Sourdoughs. 9th Baltic Conference On Food Science And Technology “Food For Consumer Well-Being” “Foodbalt 2014”, Latvia, 2014, pp. 223–227.
- Mittal A., Singh G., Goyal V., Yadav A., and Aggarwal N.K. Production of Phytase by Acido-Thermophilic Strain of Klebsiella sp. Db-3fj711774.1 Using Orange Peel Flour Under Submerged Fermentation. Innovative Romanian Food Biotechnology, 2012, no. 10, pp. 18–27.
- Lenkova T.N., Egorova T.A., Sysoeva I.G., and Krivopishina L.V. Otechestvennaya fitaza [Russian phytase]. Ptitsevodstvo [Poultry farming], 2015, no. 10, pp. 2–6. (In Russ.).
- Kulova F.M. Effect of the enzymatic preparation fitasa in diets with different mineral content on zootechnical calves’ indexes. Proceedings of Gorsky State Agrarian University, 2016, vol. 53, no. 1, pp. 71–76. (In Russ.).
- Rozhkova A.M., Semenova M.V., Rubtsova E.A., et al. Creation of a heterologous gene expression system on the basis of Aspergillus awamori recombinant strain. Applied Biochemistry and Microbiology, 2011, vol. 47, no. 3, pp. 279–287. DOI: https://doi.org/10.1134/S0003683811030124.
- Sinitsyn A.P., Rubtsova E.A., Shashkov I.A., et al. Preparation and properties of new biocatalysts for the degradation of nonstarch plant polysaccharides. Catalysis in Industry, 2017, vol. 17, no. 4, pp. 331–338. (In Russ.). DOI: https://doi.org/10.18412/1816-0387-2017-4-331-338.
- Vinetsky Y.P., Rozhkova A.M., Semenova M.V., et al. Increase in glucoamylase productivity of Aspergillus awamori strain by combination of radiation mutagenesis and plasmid transformation methods. Applied Biochemistry and Microbiology, 2010, vol. 46, no. 6, pp. 633–640. DOI: https://doi.org/10.1134/S0003683810060128.
- Sereda A.S., Ignatov N.I., Overchenko M.B., et al. Research of hydrolytic ability of the complex fermental preparations received on the basis of highly effective recombinant stamms Aspergillus awamori, in relation to polysaccharides of grain raw materials. Storage and processing of farm products, 2011, no. 3, pp. 54–56. (In Russ.).
- Benesova K., Belakova S., Mikulikova R., and Svoboda Z. Survey of the Analytical Methods for the Phytic Acid Determination. Kvasny Prumysl, 2013, vol. 59, no. 5, pp. 127–133. DOI: https://doi.org/10.18832/kp2013013.
- Mikulski D. and Klosowski G. Phytic acid concentration in selected raw materials and analysis of its hydrolysis rate with the use of microbial phytases during the mashing process. Journal of the Institute of Brewing, 2015, vol. 121, no. 2, pp. 213–218. DOI: https://doi.org/10.1002/jib.221.
- Zhulʹkov A.Yu, Vitol I.S., and Karpilenko G.P. Rolʹ zernovoy fitazy pri proizvodstve i sbrazhivanii rzhanogo susla. Chastʹ I. Issledovanie fitaznogo kompleksa rzhi [The role of grain phytase in the production and fermentation of rye wort. Part I. A study of the rye phytase complex]. Storage and processing of farm products, 2009, no. 5, pp. 50–55. (In Russ.).
- Zhulʹkov A.Yu, Vitol I.S., and Karpilenko G.P. Rolʹ zernovoy fitazy pri proizvodstve i sbrazhivanii rzhanogo susla. Chastʹ II. Sposoby i rezhimy polucheniya susla [The role of grain phytase in the production and fermentation of rye wort. Part II. Ways and modes of making wort]. Storage and processing of farm products, 2009, no. 6, pp. 48–50. (In Russ.).
- Rimareva L.V., Tsurikova N.V., Kostyleva E.V., and Sereda A.S. Rekombinantnyy shtamm mitselialʹnogo griba Aspergillus awamori – produtsent kompleksa fermentov glyukoamilazy i ksilanazy [The recombinant strain of the mycelial fungus Aspergillus awamori as a producer of the glucoamylase and xylanase enzyme complex]. Patent RF, no. 2457246, 2012.