Abstract
The seasonal variability in proximate composition and essential elements demonstrates that the habitat and feeding habits of fish species play a vital role in energy transfer.We aimed to ascertain seasonal variability in the biochemical composition (protein, lipids, carbohydrates, ash, and moisture) and the amounts of Na, K, Ca, Mg, Mn, and Zn in the species Nemipterus japonicus, Epinephelus erythrurus, Nematalosa nasus, and Ilisha striatula inhabiting pelagic and demersal zones. We compared the nutritional profile of these fish species and their seasonal importance. The essential elements were detected by flame atomic absorption spectrometry and found in the following order: K > Na > Ca > Mg > Mn > Zn. To determine the proximate composition, we employed a number of methods: the Lowry method for protein analysis, the acid hydrolysis method for fat/lipid analysis, a formula for carbohydrates and moisture, and the incineration method for ash content.
The spring inter-monsoon season showed the highest values for the essential elements in both pelagic and demersal species. However, the pelagic species had the highest biochemical composition levels during the southwest monsoon. The autumn intermonsoon had the lowest bio-profile for the fishes of both regimes.
The summer season, which is not thought to be good for fish consumption, showed the highest biochemical composition levels in the pelagic fish. The nutritional profile of fish flesh can be affected by feeding habits, seasonal variation, and habitat.
Keywords
Seasonal determination, proximate/biochemical composition, essential elements, health risk assessment, distinct marine regimesINTRODUCTION
Feeding habits, habitat characteristics, and the inner physiological rhythm are the key factors that maintain the composition of macro- and micronutrients in organisms. The marine ecosystem is generally segregated into pelagic and demersal zones where physiochemical properties and behavioral adaptations of an organism are of great concern [1]. Sustainable utilization of marine resources can fulfill global seafood needs [2, 3]. According to the FAO, the total fisheries production in 2018 worldwide was 96.4 million tons, to which China contributed 15% and remained on the top, while Pakistan contributed merely 1.037% [4].
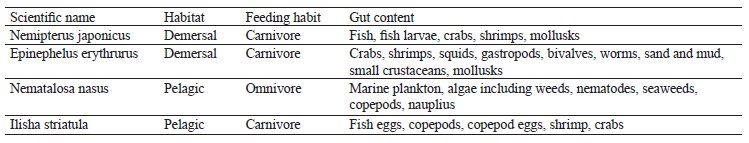
The analysis of fish habitats reveals a great abundance of biological resources and species distribution [5]. Feeding habits also vary according to the habitat. Pelagic fishes show different trends in feeding habits compared to demersal ones because they are found on the upper level of waters and hence depend largely on microorganisms like plankton and small fishes. These microorganisms make the largest biomass in the marine environment and are an important fundamental link within the food chain [6]. On the other hand, demersal fishes dwell near the bottom and mainly feed on fishes, benthic organisms, and zooplankton [7]. Fishes of both pelagic and demersal zones are of great importance in nutritional values, but these values depend exclusively on the specific feeding habit of a fish and its habitat characteristics. Nemipterus japonicus belong to the carnivorous species of the demersal zone, feeding on mollusks, annelids, fish, and fish larvae. Their gut contains crabs, shrimp, and fish juveniles [8]. Epinephelus erythrurus is another group of species with a carnivorous feeding behavior found mostly between 10 and 200 m. Corals are an ideal habitat for these species, while their ideal food includes large invertebrates including crustaceans and fishes close to the substrate [9].
Nematalosa nasus belong to omnivorous species [10–12]. Interestingly, mud and sand account for the bulk of their gut content, and their stomach is modified like a gizzard. Both traits may be due to the bottom-feeding habit of this species [13]. Ilisha striatula, a species with a carnivorous feeding behavior, also has a gizzard-like stomach [14].
Significance of proximate composition and essential elements. Longwe and Kapute stated that essential elements and other nutrients (protein and fats) help to increase a healthy and nutritious food supply [15]. Fish contains vitamins A, D, and B, as well as vital elements. Seafood consumption facilitates the overall nutritional quality of a mixed diet [16]. Nutritional components of fish have many functional benefits for humans, with fish oil proven to be the most essential source of polyunsaturated fatty acid [17, 18]. About 60% of food demand is fulfilled by fish in developing countries [19].
Fish is primarily composed of water (72%), protein (19%), and fats (8%) [20]. Seafood in general and fish in particular contain a large number of metals as they can accumulate these from their environment [21]. A blue economy concept has been initiated in a few countries to utilize marine resources in a better way and to promote sustainable fishing. According to Sari and Muslimah, the blue economy will also ensure food security, environmental sustainability, and economic growth [22]. Gram and Dalgaard reported that along with fulfilling the food demand, preserving fish with all its nutritional values is a global challenge that accounts for 25% spoilage of total production [23]. Although studies on the nutritional values and proximate composition of fish have been done throughout the world, including Pakistan, there is a lack of comparative analysis of fish species with different feeding regimes [24–26]. This analysis could help consumers choose a more nutritional fish as part of their diet.
Protein. Seafood consumption plays an immense role in meeting the protein requirement of the human body. Among seafood, fish attain the top ranking in the aquatic food chain and high-quality protein [27]. Fish contain such macronutrients as water (63–84%), protein (14–26%), and lipids (0.1–17%), as reported by Hui et al. [28]. Due to different habitat and physiological characteristics, the protein content varies at 18–20% in demersal and pelagic fishes, respectively. In addition, fish’s lean muscles are better protein carriers than those of red flesh [28]. The demand for high-quality animal protein, especially seafood, is steadily increasing along with the global population [29]. Protein plays a vital role in strengthening the immune system, body framework, and circulatory system. Consuming fish as a source of protein can prevent protein-calorie malnutrition. Protein also defends the human body against various microbial infections and strengthens the immune system [30, 31]. Our diet consists of various sources of protein, which is a major factor in the nutritional profile [32]. Proteins obtained from animal sources are considered more significant than plant protein due to their balanced combination of amino acids. Although all animal proteins are equally healthy and nutritional for humans, fish protein is easier to digest due to the unique amino acid composition of fish muscles [33, 34]. Habitat differences also play a great role in the structural composition of protein. Fish require less structural support to move compared to land animals, and therefore fish muscles contain less connective tissue, which makes fish more tender and delicious. Furthermore, cold- and warm-blooded variations also show differences in the protein lipids of terrestrial and aquatic animals [32].
Lipids. The meager presence of fats in seafood has increased its market demand. However, the presence of long-chain polyunsaturated fatty acid n-3 in fish muscles increases the nutritious index of fish. Therefore, fish should make up an essential part of the human diet [35]. Co-specific species may have contrasting lipid compositions due to variations in environmental conditions, maturity, and age [36]. Hui et al. observed that pelagic fish store lipids in the head and muscles, while demersal species keep them in their livers and below the skin [28].
Carbohydrates. The role of carbohydrates in fish is essential because their deficiency may cause growth retardation [47]. Moreover, carbohydrate deficiency can limit the function of macronutrients in the fish body. Nevertheless, cultured fish usually have a carbohydraterich diet, which is consequently consumed by humans. Carbohydrates have always been considered an excellent source of human nutrition with great biological importance. In fish, however, the importance of carbohydrates varies over time depending on their ecosystem [38]. Mayer et al. reported a versatile range of marine carbohydrate structures [39]. One of the primary functions of carbohydrates is to provide energy by cellular respiration, which is a fundamental constituent of protoplasm. Carbohydrates participate in energy release and storage [40]. A wide range of marine carbohydrates is used in applied sciences to produce nutrient supplements, cosmetics, and pharmaceuticals. Most importantly, carbohydrates play a biomedical role, providing benefits for human health against viral diseases and hematological effects that reduce the risk of hemorrhage [41].
Ash is inorganic matter that remains after the incineration of the organic content. It promotes the physiological and structural growth of the human body. Ash estimation is important for presenting the total amount of essential elements in fish meat [42, 43].
Moisture is one of the major bio-constituents of seafood. Moisture is around 80% in fresh fish muscles and slightly lower in fattier fishes. The protein structure in fish can hold moisture tightly even under high pressure. However, prolonged storage of frozen or chilled fish may affect the ability of protein to hold moisture in fish meat [44]. Many species, particularly those containing large quantities of lipid fat in the flesh and under the skin, are replaced by water as the lipid energy reserve is depleted [33]. Elemental composition shows inorganic contents in the fish muscles, while proximate composition determines organic contents [45]. All elements are divided into non-essential and essential elements based on their harmful or useful effects on the environment and human health. Essential elements such as Cu, Mn, Ni, Fe, and Zn are useful for aquatic organisms, as well as humans, within permissible limits [46]. A comprehensive study of metal concentrations in the entire ecosystem of Hawks Bay, Karachi defined how biotic and abiotic components are linked in terms of metal sharing [14]. Further, the authors elaborated the vulnerability of the important coastal ecosystem. According to Adewumi et al., increased concentrations of essential elements, which go beyond the permissible limits, cause them to accumulate in the human muscles, while their deficiency causes the failure of various body functions [45]. Such studies on metals in seafood are of great importance today and they are quite common throughout the world [21].
Fish is attaining great importance among healthy foods available on the global market since it contains a good combination of organic and inorganic essential elements and proximate nutrients [47]. Essential elements are responsible for numerous functions of the human body, including various enzymatic activities, as well as anabolic and catabolic functions of cells [48].
Health risk assessment. Metals pose a significant threat to people’s health [49]. Metals are found in the edible tissues of fish species at the top of the aquatic food chain and are absorbed by humans through ingestion [50]. Therefore, one of our aims was to determine the health risks of Mn and Zn accumulations in the edible tissues of fishes from the Pakistan coasts.
STUDY OBJECTS AND METHODS
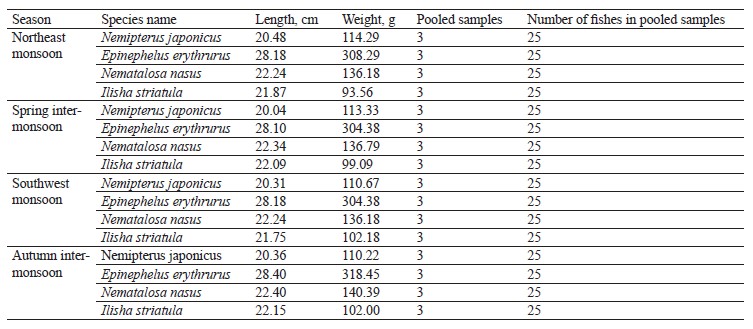
Fish sampling identification and laboratory handling. Two demersal and two pelagic fish species were seasonally (northeast monsoon, spring inter-monsoon, southwest monsoon, and autumn inter-monsoon) collected from the Karachi Fish Harbor. The samples were placed in an ice box and taken to the lab for analysis. Distilled water was poured over the samples to avoid any contamination. The species were identified by using the FAO’s field guide and the Fishbase (Table 1) [51]. The scientific name, habitat, feeding habits, and gut contents were examined in the lab. The weight and length of the samples were measured. The stomach of each sample was removed, and the gut content was analyzed under a binocular microscope.
Essential elements analysis. To determine essential elements, 5 g of fish muscles (wet weight) was dried in an oven at a maintained temperature for 6 h and then homogenized to powder. Then, we shifted the homogenized sample to a beaker, added 5 mL of 65% HNO3, heated the solution at 80–100°C until it became clear, and filtered it through Whatman filter paper. Distilled water was then added gradually to make up 100 mL of the solution. The sample was then transferred to a glass bottle and labeled for further analysis on an Analyst 400 flame atomic absorption spectrometer. The concentrations of elements were expressed as mg/L dry weight for comparison [52]. Na and K were determined by flame emission spectrometry, since their concentrations were beyond the highest standards of selected elements in atomic absorption spectrometry. However, Ca, Mg, Zn, and Mn were determined by flame atomic absorption in the presence of HCL lamps, as they provide precise values even at higher concentrations.
Biochemical composition analysis. Lowry’s method was modified for protein analysis, as described by Esen [53].
The acid hydrolysis method was used for the AOAC lipid analysis [54]. The ash content was measured by incineration in a muffle furnace at 600-700EC for 5–8 h [55]. Moisture was determined as a difference between dry and wet weights [53]. Carbohydrates, %, were calculated by using the following Eq. (1) [52]:
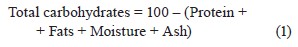
Health risk estimation. The Pakistan Pure Food Laws cover 104 food items and regulate chemicals, heavy metals, as well as purity in raw food [56].
Fish muscle tissues were analyzed to evaluate the risk of Mn and Zn concentrations for human health. The daily intake of these metals from fish consumption was estimated for adults. The estimated daily intake (EDI) depends on metal levels and the amount of fish consumed. The EDI of Mn and Zn was determined using the equation below:
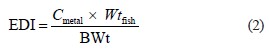
where Cmetal is the concentration of Mn and Zn in fish; Wtfish represents the average daily consumption of fish according to the National Bureau of Statistics (Pakistan) and FAO’s international consumption surveys (5.81 kg/capita/year), which is equal to 15.92 mg/kg/day; BWt is the adult body weight of 70 kg [57]. The estimated weekly intake (EWI) was obtained by multiplying the EDI values by 7. To estimate the human health risk from consuming metal-contaminated fish, the target hazard quotient (THQ) was calculated as per Regional Screening Levels (RSLs) [58]. The THQ is an estimate of the risk level (non-carcinogenic) due to contaminant exposure. It was calculated as follows:
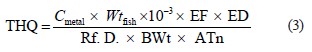
where THQ is the target hazard quotient; Cmetal is the concentration of Mn and Zn in fish, mg/kg; Wtfish is the fish consumption rate, g/day; EF is the exposure frequency, day/year, or the number of exposure events per year of exposure; ED is the exposure duration, year; Rf .D. is the reference dose, mg/kg·day; BWt is the body weight, kg; and ATn is the averaging time, noncarcinogens, day/year. We used the reference doses established by the United States Environmental Agency and the Rik Assessment Information System [58, 59]. The values for Mn and Zn are 1.4×10–1 and 3.0×10–1, respectively [58, 59]. The hazard index (HI) from THQs can be expressed as the sum of hazard quotients:
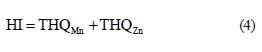
The health protection standard of lifetime risk for HI is 1 [58]. If HI = > 1.0, then the EDI of a particular metal exceeds the reference dose, indicating that there is a potential risk associated with that metal.
Statistical analysis. ANOVA was used to investigate the data throughout the season for both proximate composition and essential elements, except for an autumn inter-monsoon season (p < 0.05).
RESULTS AND DISCUSSION
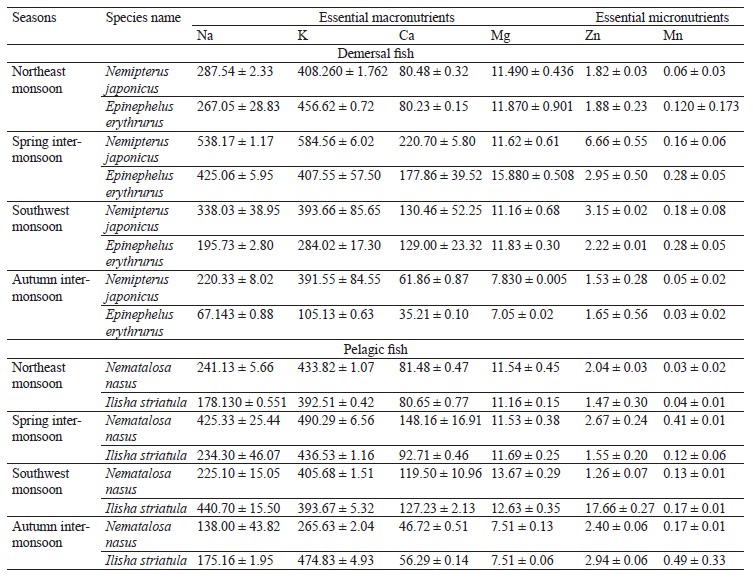
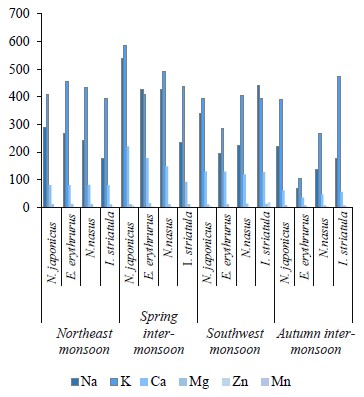
We compared the essential elements and biochemical composition of two pelagic and two demersal fishes to understand their nutritional quality for human dietary demands. Fish habitat, feeding habits, and gut contents are given in Table 1. The average weight, length, and number of the sampled fishes are indicated in Table 2. The micronutrients, such as zinc (Zn) and manganese (Mn), and macronutrients sodium (Na), potassium (K), calcium (Ca), and magnesium (Mg) were extracted seasonally (Table 3). The concentrations of micro- and macroelements in our study show the same trends as in [60, 61], namely K < Na < Ca. The lowest value of K was observed during the autumn inter-monsoon season, while its highest value was found in the spring intermonsoon season.
We found that food availability, season (winter-summer), pollution, and fishing pressures affect the levels of nutrients in the pelagic and demersal fishes. Further, the feeding habits and habitat of a fish can characterize the categorical composition of nutrients in its flesh. Total mineral contents in fish muscles range from 0.6–1.5% in wet tissues [62]. Our study provided extensive seasonal analytic data on the nutritional composition of two pelagic and two demersal fish species sampled from the Karachi fish harbor. The purpose of the seasonal analysis was to observe the trends and fluctuations in the concentrations of essential micro- and macroelements in the fish samples and their proximate composition.
Seasonal variation in essential elements among the four selected fish species was shown in the northeast monsoon, spring inter-monsoon, southwest monsoon, and autumn inter-monsoon seasons respectively. The concentrations of Na, K, and Ca were high in both the pelagic and the demersal fishes. Na had a higher concentration in the demersal fishes than in the pelagic species in our study. Its concentration was also higher than in the study conducted by Nordhagen et al., who determined the same micro- and macroelements [63]. Higher concentrations of Na and K in the demersal fish are due to their diet and gender. Like crustaceans, they are a great source of K, Na, and Ca [62]. The minimum values of Na in the demersal species were lower than its minimum values in the pelagic species. Further, the concentrations of Na in both pelagic species were lower than those reported by Nordhagen et al. and higher than those determined by Lilly et al. [60, 63].
All the species were richer in K than Na, which may be because fish have a high capability of accumulating K. The concentration levels showed the same hierarchy as the one observed by Ersoy and Celik, namely K < Na < Ca < Mg [61]. Ca is found more in the demersal fishes than in the pelagic ones. However, its highest levels were observed in the spring inter-monsoon season, while its lowest values were found in the autumn inter-monsoon season. The Ca values in both the demersal and pelagic fishes were lower than the ones reported by Nordhagen et al. but higher than in the study conducted by Lilly et al. [60, 63]. In this study, Mg was found in low concentrations similarly to Ca [60]. The demersal species in our study showed the highest Mg levels during the spring inter-monsoon season. In the pelagic zone, Mg was only slightly higher in the southwest monsoon season compared to the spring inter-monsoon.
The high concentrations of Na, K, Ca, and Mg in all the four fishes in the spring inter-monsoon are due to the mixing of water and a high availability of food during this season, as well as heavy rainfall. Higher land runoff from different areas results in increasing these elements in a water body in particular seasons [61]. The same levels of metals were found by Ersoy and Celik [61], namely K > Na > Ca > Mg. Stepanova and Lugovaya suggested that high concentrations of Na, K, and Ca in carnivore fish species were due to their diet and feeding on other small fishes and crustaceans, which are the greatest source of these elements [64]. Fish is usually richer in K than Na because seawater is responsible for the morphological alteration in fish.Zn was greater in the pelagic fishes than in the demersal ones. Afandi et al. suggested that it is because pelagic fishes are more adapted to feeding at a higher level in the food chain and they can easily bio-accumulate Zn [65]. In our study, the concentrations of Zn were below the permissible limit of 30 mg/kg established by the Ministry of Agriculture, Fisheries and Food in all the four seasons [66].
Other essential elements such as Mg and Mn varied in the studied species. These micronutrients are necessary only in minute quantities since their high levels in the muscles or tissues can increase metabolic reactions [67]. Excessive concentrations of these essential elements can lead to health problems. For example, excessive consumption of Mn causes hemochromatosis and may cause thalassemia [68].
The spring inter-monsoon is the best season to utilize seafood with high bio-nutrients, while the autumn intermonsoon season showed the lowest trends. Furthermore, various morphological and physiological factors of species, physicochemical factors of water, reproductive cycles, and anthropogenic activities can affect metal accumulation in fish muscles [69]. For example, anthropogenic activities are lower during the winter season, which can contribute to less metal accumulation in fish muscles.
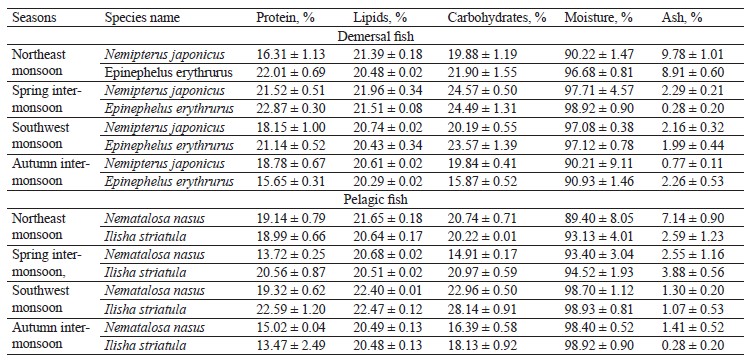
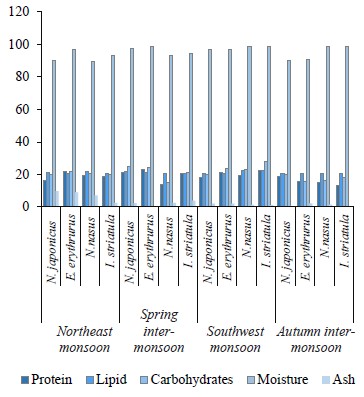
The proximate composition of the fish samples was also investigated seasonally, including the contents of protein, lipids, carbohydrates, ash, and moisture (Table 4). Our results confirmed that fish is a source of both bionutrients and essential elements, but the bio-profile of species helps to understand which fish is good to consume in what season. The protein and lipid contents in the targeted demersal species in our study were higher than those in the study by Nordhagen et al. but similar to those indicated by Nurnadia et al. [63, 69]. The carbohydrates showed higher trends when compared to the study by Nurnadia et al. [69]. Both species showed higher bio-nutrients in the spring inter-monsoon season. Nemipterus japonicus is carnivorous and feeds mostly on crustaceans throughout the year, while Epinephelus erythrurus is a migratory species that consumes more food during the spring [8, 70]. Migration is due to an abundance of nutrients, which could explain the high protein-lipid and carbohydrate trends in the spring intermonsoon season for both demersal species. Further, physiological, ecological, and physicochemical conditions could also be a reason for the fluctuation of bio-nutrients in the fish muscles [71]. The moisture content of the demersal species in our study was high, while the ash content was within the permissible limits, except during the northeast monsoon season [69].
The protein content in the pelagic species under study showed lower trends than in the study Nordhagen et al. and differed slightly from the study by Nurnadia et al. [63, 69]. The lipid content, however, showed similar trends to those in these two studies. The carbohydrate content in our study was higher than the one reported by Nurnadia et al. [69]. Both pelagic zone species, Nematalosa nasus and Ilisha striatula, showed higher bio-nutrient trends in the southwest monsoon season. This is due to a huge share of plankton consumption in their diet [71]. The pelagic species showed the highest levels of protein, lipids, and carbohydrates in the summer due to the abundance of macronutrients in the pelagic zone. Since summer is a euphotic period with more light penetration, it has ideal conditions for the growth of plankton [72].
This could be a reason for the high bio-nutrient content in the summer season. Monsoon causes advection (when warm air moves into a cool region) and upwelling, which generate ocean currents and cause the mixing of nutrients and photo-chemicals [73]. Also, there is a direct link between plankton abundance and high proximate composition values of pelagic species, as well as an indirect relationship between fats and moisture [44]. In our study, the moisture content was higher than in the study conducted by Nurnadia et al., while the ash content was within the permissible limits with some variation [69]. Interestingly, we also found that the carnivorous species showed higher carbohydrate levels than to the omnivorous ones.
Carnivorous species contain a high level of starch or carbohydrates, as observed by Moon [74]. Omnivorous species use carbohydrates as a source of energy, while carnivorous species show less or no use of carbohydrates as an energy source, so the selection of food may also be a cause of fluctuating carbohydrate levels between the species with different feeding habits [37].
Apart from the seasonal differences, we found some other interesting links, particularly a direct and indirect link between the feeding behavior and the proximate composition of the species.
Our study also showed an interesting link between the species with omnivorous and carnivorous feeding behavior. The omnivorous species are rich in lipids due to their feeding on zooplankton, including copepods. Zooplankton stores accumulate lipids in various body parts. This selection of food could be a reason for high lipid levels in N. nasus or omnivorous fish [73].
There is also an indirect link between protein and lipid levels, as well as a direct link between protein and carbohydrate values, which may be due to the changes in reproductive stages and the physiology of the fish body.
The sampled species from the demersal zone showed the highest levels of essential elements throughout the year, namely K > Na > Ca > Mg in the northeast monsoon, spring inter-monsoon, southwest monsoon, and autumn inter-monsoon, respectively (Fig. 1). Potassium and Sodium were the most abundant macronutrients found in both species throughout the study period. The demersal species N. japonicus and E. erythrurus showed the highest values of macro- and micronutrients during the spring inter-monsoon season and the lowest in the autumn inter-monsoon season. Although both N. japonicus and E. erythrurus are demersal species, the contents of Na, K, and Ca were high in N. japonicus. The concentration of K was significant in the spring inter-monsoon and lowest in the autumn inter-monsoon season. This may be due to the seasonal transition of feeding. The proximate composition of both demersal species showed the same trends throughout the year, with the highest values in the spring inter-monsoon and the lowest values in the autumn inter-monsoon (Table 4). Although N. japonicus and E. erythrurus are both demersal and therefore rich in protein, the levels of protein and carbohydrates were higher in E. erythrurus. The fat content was high in N. japonicus. Thus, our results showed a direct link between the protein and carbohydrate levels and an indirect link between the protein and lipid contents. The bio-nutrient profile of the selected species is shown seasonally in Fig. 2.
N. nasus, one of the most ecologically important species in Pakistan, showed the highest contents of macro- and micronutrients between the both pelagic zone species during the spring inter-monsoon season, with exception of Mg, which was highest in the southwest monsoon. I. striatula showed the highest values during the southwest monsoon season, except for K and Zn in comparison of both targeted pelagic zone species. The lowest levels of all essential elements between both pelagic species were observed during the autumn inter-monsoon season, except for K and Mn (Fig. 1). N. nasus showed the highest values of macro- and micronutrients during the spring inter-monsoon, except for Ca. I. striatula showed the highest macronutrient values (Na, Ca, and Mg) during the southwest monsoon, except for K (Fig. 1). N. nasus showed the highest proximate composition values during the southwest monsoon, except for the ash content. I. striatula showed high proximate composition values during the southwest monsoon, while ash was high in the northeast monsoon season. The protein and carbohydrate contents were found higher in I. striatula, while N. nasus showed a higher lipid content throughout the season (Fig. 2).
Risk assessment. Provisional tolerable weekly intake estimates the amount per unit body weight of a likely hazard contaminant in fish that can be consumed over a lifetime without risk of unfavorable health effects. Provisional tolerable weekly intake is meant to emphasize that long-term exposure is substantial for metals that accumulate in the body. Adverse effects for people are observed with many metals in the range of exposure. Provisional tolerable weekly intake should be compared to well-established and internationally accepted tolerance. Provisional tolerable weekly intake is established for metals that do not leave the body instantly and may remain there permanently. The provisional tolerable weekly intake safe level for Zn is 7, but the Joint Expert Committee on Food Additives has not established such a level for Mn [75, 76].
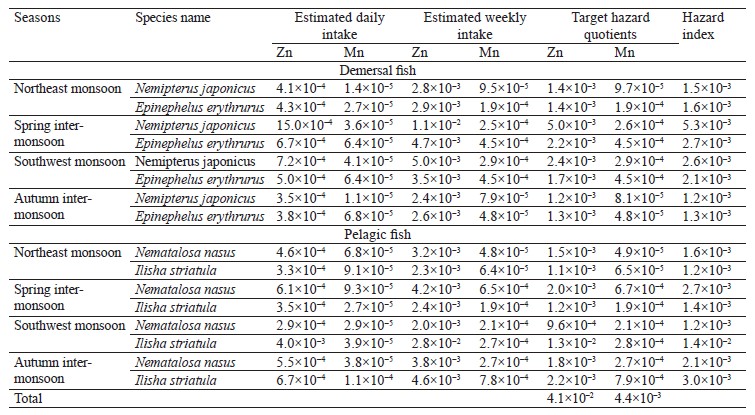
Assumptions are used in risk assessments. The US Environmental Protection Agency’s Regional Screening Levels and the Risk Assessment Information System present methods for estimating the non-cancer risk [58, 59]. The theoretical and estimated lifetime target hazard quotients were calculated for adults exposed to Mn and Zn from the consumption of fish from Pakistan coasts of the Arabian Sea (Table 5).
The hazard index of less than 1 indicates that the estimated exposure is below the USEPA reference dose for the relevant metals for all seasons and both demersal and pelagic fish species. We found that the hazard index value for Mn and Zn was lower than standard 1 for all four fish species, demonstrating that the ingestion of these fishes from Pakistan coasts of the Arabian Sea will not result in overexposure to these contaminants. Thus, they have no adverse effects on the health of consumers.
The estimated daily intake was calculated by taking the weighted average of Zn and Mn in each fish species and multiplying it by the respective consumption rate. The daily intakes of Zn were estimated between 0.000286 and 0.004 mg for adults during all seasons, much lower than the Rf.D. value (0.3 mg/day) [58, 59]. The estimated weekly intakes for adults were between 0.002 and 0.028 mg/kg, respectively. The safe provisional tolerable weekly intake value for Zn is 7 mg per kg of body weight [75]. In our study, the Zn levels were quite below this safe value.
The daily intakes of Mn were calculated between 0.000011 and 0.00011 mg for an adult, which is well below the Rf.D. (0.14 mg/day) [58, 59]. The provisional tolerable weekly intake value for Mn has not been estimated yet [76]. Although there is no specific assessment of Mn, it appears that Mn in fish contact materials does not cause any concern [76].CONCLUSION
Our overall results validated that macronutrients such as K and Na were present in significant quantities in the fish inhabiting both pelagic and demersal zones. The spring inter-monsoon was found to be the season in which essential elements peaked. Therefore, this season can be suitable for fish consumption. Our study also found that the demersal zone showed a good bio-nutrient profile in the spring inter-monsoon season, while the pelagic species were high in bio-nutrients in the summer (southwest monsoon) season.
It cannot be ignored that feeding habits play a vital role in energy and nutrient flows in an ecosystem. Interestingly, the carnivorous fishes accumulated carbohydrates more sufficiently than the omnivorous fishes in our study. We also observed a direct link between protein and carbohydrates and an indirect link with lipids. We hope that our study will help to understand the nutritional dynamics and energy flow in different species and zones.
The risk assessment showed that the two demersal and two pelagic fish species in our study had Zn and Mn levels below the allowable values, and the estimation of non-carcinogenic risk revealed no possible adverse effects on human health.
Contribution
All the authors were equally involved in the research analysis and manuscript writing.
CONFLICTS OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
REFERENCES
- Callaghan CT, Major RE, Lyons MB, Martin JM, Kingsford RT. The effects of local and landscape habitat attributes on bird diversity in urban greenspaces. Ecosphere. 2018;9(7). https://doi.org/10.1002/ecs2.2347
- Tacon AGJ, Metian M. Fish matters: the importance of aquatic foods in human nutrition and global food supply. Reviews in Fisheries Science. 2013;21(1):22–38. https://doi.org/10.1080/10641262.2012.753405
- Abbey L, Glover‐Amengor M, Atikpo MO, Atter A, Toppe J. Nutrient content of fish powder from low value fish and fish byproducts. Food Science and Nutrition. 2017;5(3):374–379. https://doi.org/10.1002/fsn3.402
- The state of world fisheries and aquaculture. Sustainability in action. Rome: FAO; 2020. 244 p. https://doi.org/10.4060/ca9229en
- Hattab T, Lasram FBR, Albouy C, Sammari C, Romdhane MS, Cury P, et al. The use of a predictive habitat model and a fuzzy logic approach for marine management and planning. PLoS One. 2013;8(10). https://doi.org/10.1371/journal.pone.0076430
- Costalago D, Navarro J, Álvarez-Calleja I, Palomera I. Ontogenetic and seasonal changes in the feeding habits and trophic levels of two small pelagic fish species. Marine Ecology Progress Series. 2012;460:169–181. https://doi.org/10.3354/meps09751
- Takahashi M, Iwami T. The summer diet of demersal fish at the South Shetland Islands. Antarctic Science. 1997;9(4):407–413.
- Manojkumar PP. Some aspects on the biology of Nemipterus japonicus (Bloch) from Veraval in Gujarat. Indian Journal of Fisheries. 2004;51(2):185–191.
- Heemstra PC, Golani D. Clarification of the Indo-Pacific groupers (Pisces: Serranidae) in the Mediterranean Sea. Israel Journal of Zoology. 2013;39(4):381–390.
- Pernthaler J, Amann R. Fate of heterotrophic microbes in pelagic habitats: Focus on populations. Microbiology and Molecular Biology Reviews. 2005;69(3):440–461. https://doi.org/10.1128/MMBR.69.3.440-461.2005
- Krishnamurthy K, Jeyaseelan MP. The early life history of fishes from Pichavaram mangrove ecosystem of India. In: Lasker R, Sherman K, editors. The early life history of fish: recent studies. Copenhagen; 1981; pp. 416–423.
- Mukherjee M, Suresh VR, Manna RK, Panda D, Sharma AP, Pati MK. Dietary preference and feeding ecology of Bloch’s gizzard shad, Nematalosa nasus. Journal of Ichthyology. 2016;56(3):373–382. https://doi.org/10.1134/S0032945216030097
- Bapat SV, Bal DV The food of some young clupeids. Proceedings/Indian Academy of Sciences. 1950;32(1):39–58.
- Jan M, Panhwar SK, Zafar FHS. Ecosystem based approach to delineate coastal degradation of Hawks bay, Karachi, Pakistan. Chemosphere. 2022;301. https://doi.org/10.1016/j.chemosphere.2022.134648
- Longwe P, Kapute F. Nutritional composition of smoked and sun dried pond raised Oreochromis karongae (Trewavas, 1941) and Tilapia rendalli (Boulenger, 1896). American Journal of Food and Nutrition. 2016;4(6):157–160.
- Pawar HM, Sonawane SR. Fish muscle protein highest source of energy. International Journal of Biodiversity and Conservation. 2013;5(7):433–435.
- Rafflenbeul W. Fish for a healthy heart. European Journal of Lipid Science and Technology. 2001;103(5):315–317. https://doi.org/10.1002/1438-9312(200105)103:5<315::AID-EJLT315>3.0.CO;2-H
- Saoud IP, Batal M, Ghanawi J, Lebbos N. Seasonal evaluation of nutritional benefits of two fish species in the eastern Mediterranean Sea. International Journal of Food Science and Technology. 2008;43(3):538–542. https://doi.org/10.1111/j.1365-2621.2006.01491.x
- Mishra SP. Significance of fish nutrients for human health. International Journal of Fisheries and Aquatic Research. 2020;5(3):47–49.
- Hantoush AA, Al-Hamadany QH, Al-Hassoon AS, Al-Ibadi HJ. Nutritional value of important commercial fish from Iraqi waters. International Journal of Marine Science. 2015;5(11):1–5.
- Jonathan MP, Muñoz-Sevilla NP, Gongora-Gomez AM, Varela RGL, Sujitha SB, Escobedo-Urias DC, et al. Bioaccumulation of trace metals in farmed pacific oysters Crassostrea gigas from SW Gulf of Califonia Coast, Mexico. Chemosphere. 2017;187:311–319. https://doi.org/10.1016/j.chemosphere.2017.08.098
- Sari DAA, Muslimah S. Blue economy policy for sustainable fisheries in Indonesia. IOP Conference Series: Earth and Environmental Science. 2020;423. https://doi.org/10.1088/1755-1315/423/1/012051
- Gram L, Dalgaard P. Fish spoilage bacteria – problems and solutions. Current Opinion in Biotechnology. 2002;13(3):262–266. https://doi.org/10.1016/S0958-1669(02)00309-9
- Payne SA, Johnson BA, Otto RS. Proximate composition of some northeastern Pacific forage fish species. Fish Oceanography. 1999;8(3):159–177. https://doi.org/10.1046/j.1365-2419.1999.00097.x
- Kumaran R, Ravi V, Gunalan B, Murugan S, Sundramanickam A. Estimation of proximate, amino acids, fatty acids and mineral composition of mullet (Mugil cephalus) of Parangipettai, Southeast Coast of India. Advances in Applied Science Research. 2012;3(4):2015–2019.
- Iqbal R, Naeem M, Masud S, Ishtiaq A. Effect of graded dietary protein levels on body composition parameters of hybrid (Labeo rohita♀ and Catla catla♂) from Pakistan. Sarhad Journal of Agriculture. 2020;36(2):548–558. https://doi.org/10.17582/journal.sja/2020/36.2.548.558
- Ahmed I, Jan K, Fatma S, Dawood MAO. Muscle proximate composition of various food fish species and their nutritional significance: A review. Journal of Animal Physiology and Animal Nutrition. 2022;106(3):690–719. https://doi.org/10.1111/jpn.13711
- Simpson BK. Food biochemistry and food processing. Wiley-Blackwell; 2008. 901 p.
- Adewuyi SA, Phillip BB, Ayinde IA, Akerele D. Analysis of profitability of fish farming in Ogun State, Nigeria. Journal of Human Ecology. 2010;31(3):179–184. https://doi.org/10.1080/09709274.2010.11906313
- Balami S, Sharma A, Karn R. Significance of nutritional value of fish for human health. Malaysian Journal of Halal Research. 2019;2(2):32–34. https://doi.org/10.2478/mjhr-2019-0012
- Chen J, Jayachandran M, Bai W, Xu B. A critical review on the health benefits of fish consumption and its bioactive constituents. Food Chemistry. 2022;369. https://doi.org/10.1016/j.foodchem.2021.130874
- Kristinsson HG, Rasco BA. Fish protein hydrolysates: Production, biochemical, and functional properties. Critical Reviews in Food Science and Nutrition. 2000;40(1):43–81. https://doi.org/10.1080/10408690091189266
- Venugopal V, Shahidi F. Structure and composition of fish muscle. Food Reviews International. 1996;12(2):175–197. https://doi.org/10.1080/87559129609541074
- Yáñez E, Ballester D, Monckeberg F, Heimlich W, Rutman M. Enzymatic fish protein hydrolyzate: Chemical composition, nutritive value and use as a supplement to cereal protein. Journal of Food Science. 1976;41(6):1289–1292. https://doi.org/10.1111/j.1365-2621.1976.tb01154.x
- Khalili Tilami S, Sampels S. Nutritional value of fish: Lipids, proteins, vitamins, and minerals. Reviews in Fisheries Science and Aquaculture. 2018;26(2):243–253. https://doi.org/10.1080/23308249.2017.1399104
- Kiessling A, Pickova J, Johansson L, Asgard T, Storebakken T, Kiessling K-H. Changes in fatty acid composition in muscle and adipose tissue of farmed rainbow trout (Oncorhynchus mykiss) in relation to ration and age. Food Chemistry. 2001;73(3):271–284. https://doi.org/10.1016/S0308-8146(00)00297-1
- Stone DAJ. Dietary carbohydrate utilization by fish. Reviews in Fisheries Science. 2003;11(4):337–369. https://doi.org/10.1080/10641260390260884
- Wilson RP. Utilization of dietary carbohydrate by fish. Aquaculture. 1994;124(1–4):67–80. https://doi.org/10.1016/0044-8486(94)90363-8
- Mayer AMS, Rodríguez AD, Berlinck RGS, Fusetani N. Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology. 2011;153(2):191–222. https://doi.org/10.1016/j.cbpc.2010.08.008
- Shoba SP, Candida XV, Mary TAA, Rajeswari R, Rose MRB. Comparative analysis of biochemical composition of few freshwater and marine fishes. International Journal of Fisheries and Aquatic Studies. 2020;8(3):115–119.
- Kang H-K, Seo CH, Park Y. The effects of marine carbohydrates and glycosylated compounds on human health. International Journal of Molecular Sciences. 2015;16(3):6018–6056. https://doi.org/10.3390/ijms16036018
- Marshall MR. Ash analysis. In: Nielsen SS, editor. Food Analysis. New York: Springer; 2010. pp. 105–115. https://doi.org/10.1007/978-1-4419-1478-1_7
- Olagunju A, Muhammad A, Mada SB, Mohammed A, Mohammed AH, Mahmoud KT. Nutrient composition of Tilapia zilli, Hemisynodontis membranacea, Clupea harengus and Scomber scombrus consumed in Zaria. World Journal of Life Sciences and Medical Research. 2012;2.
- Pal J, Shukla BN, Maurya AK, Verma HO, Pandey G, Amitha. A review on role of fish in human nutrition with special emphasis to essential fatty acid. International Journal of Fisheries and Aquatic Studies. 2018;6(2):427–430.
- Adewumi AA, Adewole HA, Olaleye VF. Proximate and elemental composition of the fillets of some fish species in Osinmo Reservoir, Nigeria. Agriculture and Biology Journal of North America. 2014;5(3):109–117.
- Turkmen M, Turkmen A, Tepe Y, Ateş A, Gökkuş K. Determination of metal contaminations in sea foods from Marmara, Aegean and Mediterranean seas: Twelve fish species. Food Chemistry. 2008;108(2):794–800. https://doi.org/10.1016/j.foodchem.2007.11.025
- Yılmaz AB, Yılmaz L. Influences of sex and seasons on levels of heavy metals in tissues of green tiger shrimp (Penaeus semisulcatus de Hann, 1844). Food Chemistry. 2007;101(4):1664–1669. https://doi.org/10.1016/j.foodchem.2006.04.025
- Al-Fartusie FS, Mohssan SN. Essential trace elements and their vital roles in human body. Indian Journal of Advances in Chemical Science. 2017;5(3):127–136.
- Bat L. The contamination status of heavy metals in fish from the Black Sea, Turkey and potential risks to human health. In: Sezgin M, Bat L, Ürkmez D, Arici E, Öztürk B, editors. Black Sea marine environment: The Turkish shelf. Istanbul: Turkish Marine Research Foundation; 2017. pp. 322–418.
- Bat L, Arici E. Heavy metal levels in fish, molluscs, and crustacea from Turkish seas and potential risk of human health. In: Holban AM, Grumezescu AM, editors. Food quality: Balancing health and disease. A volume in handbook of food bioengineering. Academic Press; 2018. pp. 159–196. https://doi.org/10.1016/B978-0-12-811442-1.00005-5
- Field guide to the commercial marine and brackish water species of Pakistan. Rome: FAO; 1985.
- Zafar FHS, Zahid M, Bat L. Effects of processing on essential and heavy metal composition of popular fish species consumed in the Karachi coast of the Arabian Sea. Carpathian Journal of Food Science and Technology. 2019;11(2):141–151. https://doi.org/10.34302/crpjfst/2019.11.2.11
- Esen A. A simple method for quantitative, semi quantitative, and qualitative assay of protein. Analytical Biochemistry. 1978;89(1):264–273. https://doi.org/10.1016/0003-2697(78)90749-2
- Official methods of analysis. 21st ed. Arlington: AOAC; 2019.
- Hossain MA, Ngo HH, Guo WS, Setiadi T. Adsorption and desorption of copper (II) ions onto garden grass. Bioresource Technology. 2012;121:386–395. https://doi.org/10.1016/j.biortech.2012.06.119
- Food and agricultural import regulations and standards country report. USDA Foreign Agricultural Service; 2022.
- Siddique Z, Rashid U, Saddozai S, Panhwar A, Achakzai WM, Rahim M, et al. Heavy metal content and their health risk assessment in Rastrelliger kanagurta, fish from Gwadar port, Pakistan. Pakistan Journal of Science. 2021;73(3):596–604.
- Regional Screening Levels (RSLs) – Generic Tables [Internet]. [cited 2023 Apr 20]. Available from: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables
- Toxicity values [Internet]. [cited 2023 Apr 20]. Available from: https://rais.ornl.gov/tutorials/toxvals.html
- Lilly TT, Immaculate J, Jamila P. Macro and micronutrients of selected marine fishes in Tuticorin, South East coast of India. International Food Research Journal. 2017;24(1):191–201.
- Ersoy B, Celik M. The essential and toxic elements in tissues of six commercial demersal fish from Eastern Mediterranean Sea. Food and Chemical Toxicology. 2010;48(5):1377–1382. https://doi.org/10.1016/j.fct.2010.03.004
- Soundarapandian P. Mineral composition of edible crab Podophthalmus vigil Fabricius (Crustacea: Decapoda). Arthropods. 2014;3(1):20–26.
- Nordhagen A, Rizwan AA, Aakre I, Moxness Reksten A, Pincus LM, Bøkevoll A, et al. Nutrient composition of demersal, pelagic, and mesopelagic fish species sampled off the coast of Bangladesh and their potential contribution to food and nutrition security – The EAF-Nansen programme. Foods. 2020;9(6). https://doi.org/10.3390/foods9060730
- Stepanova EM, Lugovaya EA. Macro- and microelements in some species of marine life from the Sea of Okhotsk. Foods and Raw Materials. 2021;9(2):302–309. https://doi.org/10.21603/2308-4057-2021-2-302-309
- Afandi I, Talba S, Benhra A, Benbrahim S, Chfiri R, Labonne M, et al. Trace metal distribution in pelagic fish species from the north-west African coast (Morocco). International Aquatic Research. 2018;10:191–205. https://doi.org/10.1007/s40071-018-0192-7
- Monitoring and surveillance of nonradioactive contaminants in the aquatic environment and activities regulating the disposal wastes at sea, of 1993. MAFF; 1995.
- Ako PA, Salihu SO. Studies on some major and trace metals in smoked and oven-dried fish. Journal of Applied Sciences and Environmental Management. 2004;8(2):5–9. https://doi.org/10.4314/jasem.v8i2.17232
- Hovinga ME, Sowers M, Humphrey HE. Environmental exposure and lifestyle predictors of lead, cadmium, PCB, and DDT levels in Great Lakes fish eaters. Archives of Environmental Health: An International Journal. 1993;48(2): 98–104. https://doi.org/10.1080/00039896.1993.9938402
- Nurnadia AA, Azrina A, Amin I. Proximate composition and energetic value of selected marine fish and shellfish from the West coast of Peninsular Malaysia. International Food Research Journal. 2011;18:137–148.
- Waschkewitz R, Wirtz P Annual migration and return to the same site by an individual grouper, Epinephelus alexandrinus (Pisces, Serranidae). Journal of fish Biology. 1990;36(5):781–782. https://doi.org/10.1111/j.1095-8649.1990.tb04332.x
- Love RM. Biochemical dynamics and the quality of fresh and frozen fish. In: Hall GM, editor. Fish processing technology. New York: Springer; 1997. pp. 1–31. https://doi.org/10.1007/978-1-4613-1113-3_1
- Barber RT, Marra J, Bidigare RC, Codispoti LA, Halpern D, Johnson Z, et al. Primary productivity and its regulation in the Arabian Sea during 1995. Deep Sea Research Part II: Topical Studies in Oceanography. 2001;48(6–7):1127–1172. https://doi.org/10.1016/S0967-0645(00)00134-X
- Lee RF, Hagen W, Kattner G. Lipid storage in marine zooplankton. Marine Ecology Progress Series. 2006;307:273–306. https://doi.org/10.3354/meps307273
- Moon TW. Glucose intolerance in teleost fish: fact or fiction? Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2001;129(2–3):243–249. https://doi.org/10.1016/S1096-4959(01)00316-5
- Trace elements in human nutrition and health. Geneva: WHO; 1996. 360 p.
- Council of Europe’s policy statements concerning materials and articles intended to come into contact with foodstuffs. Policy statement concerning metals and alloys. Technical document guidelines on metals and alloys used as food contact materials. Strasbourg: Council of Europe; 2001. 88 p.
- Venugopal V, Sasidharan A. Seafood industry effluents: Environmental hazards, treatment and resource recovery. Journal of Environmental Chemical Engineering. 2021;9(2). https://doi.org/10.1016/j.jece.2020.104758
- Dural M, Göksu MZL, Özak AA. Investigation of heavy metal levels in economically important fish species captured from the Tuzla lagoon. Food Chemistry. 2007;102(1):415–421. https://doi.org/10.1016/j.foodchem.2006.03.001