Abstract
Fresh fermented milk products have a limited shelf life that can be extended by vacuum freeze-drying. Cryoprotectants are used to increase the survival of lactic acid microorganisms during freeze-drying. The most effective cryoprotectants are those of natural origin. Literature offers little information on the cryoprotective effects of fruit and vegetable purees. Therefore, we aimed to evaluate the effectiveness of fruit and vegetable purees in increasing the survival of lactic acid microorganisms during the freeze-drying and storage of fermented milk products.We studied bioyogurt samples containing pumpkin, fig, and banana purees. Rational modes of freezing and freeze-drying were established on the basis of thermal analysis. The cryoscopic temperature was determined by differential scanning calorimetry. The proportion of frozen moisture was calculated using the Nagaoka formula. Standard methods were employed to evaluate the sensory characteristics of bioyogurts and determine their protein, fat, and non-fat milk solids contents, as well as titratable acidity and microbiological indicators.
The addition of pumpkin puree increased the cryoscopic temperature and reduced the freeze-drying stage and the total drying time by 13 h, depending on the amount of puree. However, the addition of sweet fig and banana purees decreased the cryoscopic temperature and increased the freeze-drying stage and the total drying time by 0.5–1.5 and 1.5–3 h, respectively. Based on the sensory evaluation of the freeze-dried bioyogurts, we selected the formulations with 15% of pumpkin and fig purees and 10% of banana puree. We found that the freeze-dried bioyogurts with puree had higher counts of lactic acid bacteria compared to the control. In the freeze-dried samples, the counts were higher at a storage temperature of 4 ± 2°C than at 20 ± 2°C.
Pumpkin puree provided the best survival of lactic acid microorganisms during freeze-drying and storage.
Keywords
Bioyogurt, vacuum freeze-drying, cryoprotectants, pumpkin puree, fig puree, banana puree, cryoscopic temperature, proportion of frozen moisture, lactic acid microorganisms, shelf lifeINTRODUCTION
Fermented milk products are widely used around the world due to their nutritional value and health benefits. These include yogurt, curdled milk, baked milk, kefir, cottage cheese, and other products. Their positive effect is associated with lactic acid and probiotic microorganisms. These microorganisms normalize the gastrointestinal tract and the lipid profile, enhance immunity, treat allergies, prevent intestinal cancer, maintain normal cholesterol levels, and have many other effects [1–5].
Lactic acid and probiotic bacteria cells in fresh fermented milk products gradually decrease in number, which has a negative effect on their quality. Vacuum freeze-drying is a promising way to preserve the quality of fermented milk products with a high content of viable cells of lactic acid microorganisms [6].
The freeze-drying technology expands the uses of fermented milk products. For example, freeze-dried yoghurt or curdled milk can be used as instant foods, drinks or sauces, in ready-made cereals or in the confectionery and bakery products. Their reduced weight and volume account for lower packaging, handling, and transportation costs. In addition, freeze-dried products can be stored at ambient temperature for a long time. This is convenient for their transportation to the northern regions or to the affected regions during natural disasters [7–9].
Freeze-drying involves removing frozen moisture by the ice-vapor phase transition, which reduces damage to cellular structures [10]. Previous studies have shown that some strains of probiotic lactic acid microorganisms tolerate freeze-drying better than others. This is associated with their size and composition of the cell wall and membrane [10]. During heat treatment and storage of freeze-dried yogurt, the viability of probiotic microorganisms can be affected by oxygen content, high temperature, low pH, water activity, and higher concentrations of solutes [11].
Using cryoprotectants is an efficient way to increase the survival of microorganisms during freezing or freeze-drying [12, 13]. Carbohydrates are known to have a protective effect on freeze-dried probiotics. They include trehalose, sucrose, lactose, fructooligosaccharides, galactooligosaccharides, and inulin [14–19]. Some proteins and antioxidants can also have a protective effect, including skim milk, soy protein, ascorbic acid, and L-cysteine [20]. Some salts, e.g., phosphates, can also act as cryoprotectants [21]. According to Shu et al., the best effect can be achieved by using a combination of several cryoprotectants [20].
In this regard, it is important to study the influence of natural fruit and vegetable purees on the viability of microorganisms in fermented milk products during freeze-drying and further storage. Fruit and vegetable purees contain mono- and disaccharides, dietary fiber, mineral salts, antioxidants, and other substances with cryoprotective properties. We aimed to evaluate the effectiveness of some vegetable and fruit purees as cryoprotectants during the freezing and vacuum freeze-drying of bioyogurts. We also measured their effect on the viability of lactic acid, including probiotic, microorganisms during storage.
STUDY OBJECTS AND METHODS
We studied freshly prepared and freeze-dried bioyogurts produced with starter cultures based on the following new strains of lactic acid bacteria with technological and functional properties: Streptococcus salivarius thermophilus (strain HST-20), Lactobacillus delbrueskii subsp. bulgaricus (strain HLB-8), and Lactobacillus acidophilus (strain HLA-41). Purees from pumpkin (a source of pectins), as well as figs and banana (sources of inulin), were used as cryoprotectants. The puree samples were purchased from OptTorg Company, Russia. Their chemical composition is shown in Table 1.
Bioyogurt preparation. Bioyogurts were produced from reconstituted whole milk powder using the traditional yogurt technology. The test samples (with puree) were produced with the above-mentioned starter of new strains of lactic acid bacteria. The samples were obtained thermostatically by ripening at 37 ± 1°C. After reaching the required titratable acidity (88 ± 5°Т), they were cooled to 4 ± 2°С. The puree amounts were based on literature analysis, preliminary experiments, and mass fractions of solids in the final product. Each type of puree was added to the bioyogurt in the amount of 10, 15, and 20% and mixed until a homogeneous consistency.
The control bioyogurt was produced with a typical starter culture without adding fruit or vegetable purees. The control and test bioyogurts were then analyzed for their physicochemical, microbiological, and sensory parameters. Next, we determined the cryoscopic temperature and plotted the dependence of frozen moisture on temperature.
Thermal analysis. Thermal analysis was used to study the behavior of the bioyogurts during freezing and to determine optimal freezing and drying temperatures. The cryoscopic temperature of the bioyogurts was determined by differential scanning calorimetry (DSC) from +12 to –50°C on a Q20 unit (TA Instruments, USA) at an Ar flow of 50 mL/min.
The proportion of frozen water was calculated using Raoult’s law to substantiate the Nagaoka formula, which describes the process of freezing water as:
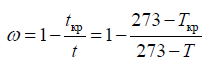
where ω is the proportion of frozen water; tкр is the cryoscopic temperature, t is the current temperature, °C; Ткр and Т are the cryoscopic and current temperatures, K.
Based on the results, graphs were plotted showing the relation between frozen moisture and decreasing temperature. These data can be used to decide on the modes and terms of product storage.
Freezing. The bioyogurt samples were frozen in a freezer at –40°С with intensive air circulation at about 10 m/s to obtain a fine-grained structure. This mode is widely used in industrial freezers. Besides, 95% of moisture crystallizes at –40°С, which is sufficient for high-quality freeze-drying. Next, the trays with the frozen product were placed in an SVP-0.36 laboratory vacuum freeze-drying unit [22].
Vacuum freeze-drying. Vacuum freeze-drying was carried out at the temperatures that ensured the removal of 85% of frozen moisture by the ice-vapor phase transition. This proportion of frozen moisture was based on our previous experiments and the recommendations of domestic and foreign researchers [23, 24]. The choice of the freeze-drying temperature was based on the thermal DSC analysis. The final drying temperature was 38–40°С. The total freeze-drying process lasted from 9 to 14.5 h, depending on the sample. The freeze-drying was considered to be completed when the final moisture in the freeze-dried samples was under 4%. The final moisture was determined by five repetitions.
Bioyogurt quality indicators. The quality indicators of the freeze-dried bioyogurt samples were determined by standard physicochemical, microbiological, and sensory analyses according to State Standard 3624-92 for titratable acidity, State Standard 3626-73 for moisture, and State Standard 30305.4-95 for solubility.
Storage of the samples. The freeze-dried bioyogurts were stored in vacuum light- and gas-tight packaging under two temperature conditions: at 4 ± 2°С in a refrigerator (control) and at 20 ± 2°С in a thermostat. The samples were stored for 12 months.
Sensory evaluation. Freshly prepared and freeze-dried bioyogurts were evaluated for color, taste, smell, consistency, texture, and general acceptance. Each indicator was evaluated on a five-point scale. The freeze-dried bioyogurts were preliminarily rehydrated with water at room temperature to the initial liquid state, with the required mass fraction of solids. The control was a rehydrated freeze-dried bioyogurt produced with a typical starter without fruit or vegetable purees.
The samples selected by the panelists were stored for further use.
Counting of lactic acid microorganisms. Counts of lactic acid microorganisms were determined in the bioyogurt samples before and after vacuum freeze-drying, as well as during storage after 3, 6, 9, and 12 months. This was done by their inoculation and cultivation in sterile skim milk, followed by the estimation of the most probable number of cells. The dishes were incubated anaerobically, while the cultures in sterile milk in the test tubes were incubated under aerobic conditions at 37°C for 5 days according to State Standard 33951.
RESULTS AND DISCUSSION
First, we performed thermal analysis to determine the cryoscopic temperature and the proportion of frozen moisture as the temperature decreased (Fig. 1).
As shown in Fig. 1, the addition of pumpkin puree increased the cryoscopic temperature, whereas the addition of fig or banana puree decreased this indicator. This was due to a higher content of mono- and disaccharides in figs and bananas compared to pumpkin. Their presence in the product, with other parameters being equal, is known to lower the freezing temperature [25].
Based on the differential scanning calorimetry (DSC), we plotted the dependences of frozen moisture on freezing temperature for each type of bioyogurt (Figs. 2–4).
As can be seen in Fig. 2, 85% of moisture in the control bioyogurt (without puree) turned into ice at –16°С. In the samples with 10, 15, and 20% of pumpkin puree, this proportion of frozen moisture formed at –14, –13, and –12°С, respectively.
As shown by Fig. 3, the addition of banana puree to bioyogurt decreased the proportion of frozen moisture at the same temperatures. Particularly, at –16°С, the control bioyogurt (without puree) had 85% of frozen moisture, while the sample with 10% of banana puree, 83%. The required proportion of 85% formed at a lower temperature of –18°С. The samples with 15 and 20% of banana puree had 85% of frozen moisture at –19 and –20 °С, respectively.
The bioyogurts with fig puree showed the same trend as the samples with banana puree (Fig. 4). The temperature at which 85% of moisture turned into ice decreased to –16.5 and –17.5°С in the samples with 10 and 20% of fig puree, respectively.
Based on the cryoscopic temperature and the proportion of frozen moisture, the bioyogurt samples were freeze-dried to 3.5–4.0% of moisture (Table 2).
As we can see, the addition of 10% of pumpkin puree only slightly changed the freeze-drying temperature of the bioyogurt. Yet, larger amounts of 15 and 20% increased the freeze-drying temperature by 3 and 4°С, respectively. We also found that the total freeze-drying time decreased from 12 h (control) to 11 and 9 h (bioyogurts with 10 and 20% of pumpkin puree, respectively).
Adding 10% of banana puree hardly changed the freeze-drying temperature, decreasing it by only 2–3°С. Yet, larger amounts of 15 and 20% lowered the temperature to –19.0 ± 0.5 and –20.0 ± 0.5°С, respectively, and therefore increased the total drying time by 1.5–3 h. Similar data were obtained for the bioyogurts with fig puree.
According to the results, introducing banana, fig, or pumpkin puree into the samples of bioyogurt did not lead to significant changes in the freeze-drying temperatures at which 85% of moisture was removed by the ice-vapor phase transition. This means that these products can be freeze-dried in the same machine with technical characteristics common for industrial machines. An increase in the freeze-drying time with a decrease in the freeze-drying temperature is a pattern that has been reported by other researchers as well [22, 26].
Next, we evaluated the quality indicators of rehydrated freeze-dried bioyogurts. Their physicochemical parameters are shown in Fig. 5.
According to the physicochemical parameters, the addition of purees led to some changes in the chemical composition of the bioyogurts. In particular, all the test samples had a lower content of protein compared to the control. On average, it amounted to 5–10% in the samples with the minimum amount of puree (10%) and 13–16% in the samples with the maximum amount of puree (20%).
The bioyogurts with banana and fig purees had a lower fat content of 2.6%, which is due to low fat in the purees. The opposite trend was observed in the samples with pumpkin puree. Since pumpkin puree contains more fat than the other purees, the samples with 10 and 20% of pumpkin puree had a fat content of 3.5 and 3.8%, respectively.
The mass fraction of non-fat milk solids was within the normal range in all the samples. In particular, it was 10.2–10.5% in the samples with pumpkin puree and 11.1–11.4% in the samples with banana and fig purees. All the test samples of freeze-dried bioyogurts had decreasing values of titratable acidity, which is due to the presence of various acids in the purees.
The physicochemical and microbiological parameters of all the samples were in line with the regulatory requirements for this type of product. For this reason, we proceeded with only sensory evaluation.
The results of the sensory evaluation of the rehydrated freeze-dried bioyogurts are presented in Figs. 6–8.
The sensory evaluation of the bioyogurts showed that adding 10% of pumpkin or fig purees led to slight changes in sensory indicators. In particular, the panelists noted light color shades and weak aromatic notes characteristic of the added purees. Yet, the bioyogurts with 10% of banana puree acquired a pronounced banana taste and aroma.
Consistency was not affected by adding 10% of any of the studied purees. Adding 15% of puree caused significant changes in the sensory characteristics of the bioyogurts. The samples had a more pronounced smell, taste, and color characteristic of the added purees. The panelists also noted a thicker consistency of these samples.
The bioyogurts with 20% of puree had almost no smell or taste of yogurt, acquiring the smell and taste of the added puree. These samples had the thickest consistency compared to the other bioyogurts.
Based on the sensory evaluation, we selected the bioyogurts with 15% of pumpkin and fig purees, as well as the bioyogurt with 10% of banana puree, for further research.
The count of lactic acid microorganisms is one of the regulated microbiological indicators of fermented milk products. The counts for the freshly prepared and rehydrated freeze-dried bioyogurts are presented in Table 3.
We found that all the rehydrated freeze-dried bioyogurts had slightly lower counts of lactic acid microorganisms than the freshly prepared samples. However, their counts remained above the required level of at least 1.0×107 CFU in 1 g/cm3. Notably, the rehydrated bioyogurts with puree had a greater count of lactic acid microorganisms. This may be due to the presence of mono- and disaccharides, as well as soluble dietary fiber, in the puree.
Physically, purees contain substances that penetrate the cells and those that do not. There is a displacement hypothesis that explains the protective effect of penetrating cryoprotectants. According to this hypothesis, when ice crystals form in the object of freezing, cryoprotectants displace concentrations of inorganic ions (salt effect) from the hydration shell of proteins and from the membranes without directly interacting with them. There is also a replacement hypothesis that claims that some penetrating cryoprotectants are able to replace water molecules associated with the polar part of membrane phospholipids, thus preventing the salt effect. Non-penetrating cryoprotectants (e.g., sucrose, fructose, dextran, etc.) “colligatively” displace salts from near-membrane water layers. As a result, during ice formation, salts are concentrated outside the cells, which reduces their damaging effect. Also, non-penetrating cryoprotectants enhance the effect of penetrating cryoprotectants [13].
Our data were consistent with the results of other studies on the freeze-drying of yoghurts. For example, Venir et al. studied the freezing and freeze-drying of low-fat yogurt and yogurt with 10% of sucrose and 10% of blueberries [27]. After freezing, the counts of lactobacilli and streptococci in these yoghurts decreased by 0.7 and 1.7 log CFU/g, respectively, while after freeze-drying, by 1.5 and 2.3 log CFU/g, respectively. The authors suggested that the yogurt with sucrose and blueberries retained more viable microorganisms due to the cryoprotective effect of sucrose.
The selected test and control samples of freeze-dried bioyogurts were put into storage for 12 months at 4 ± 2 and 20 ± 2°С. The results of studies during storage are presented in Figs. 9 and 10.
The numbers of cells of lactic acid microorganisms during storage indicate their high viability in freeze-dried bioyogurts based on new starter cultures.
Their safety was found to be affected by the storage temperature and the type of puree used.
After 12 months of storage at 4 ± 2 and 20 ± 2°С, the smallest count of lactic acid microorganisms was detected in the control sample. Throughout this period, the bioyogurts with puree had higher counts at both storage temperatures than the control. In addition, they met all the requirements established for bioyogurts.
All the samples of freeze-dried bioyogurts had a higher count of lactic acid microorganisms at a storage temperature of 4 ± 2°С. We found that pumpkin puree contributed to a better survival of lactic acid microorganisms during freeze-drying and storage at both temperatures compared to the fruit purees.
Our data were consistent with the results of studies on freeze-drying and storage of fermented milk products supervised by Professor I.A. Radaeva at the All-Russian Research Institute of the Dairy Industry (VNIMI) in the 1970–1980s. In those studies, after 15 months of storage at 4°C, the number of surviving lactic acid bacteria in sweet and fruit yogurt decreased 10 times compared to freshly prepared yogurts. The shelf life of fruit yoghurts stored at 20°С was 12 months and at 1–4°C, 18 months. Such a long shelf life made these products suitable for astronauts [28]. Some researchers noted slight changes in the number of viable Streptococcus thermophilus and Lactobacillus bulgaricus in dried yogurt stored under vacuum at 4°C or under nitrogen for 9 months [29].
Saarela et al. reported that freeze-dried microorganisms Lactobacillus and Bifidobacterium survived better at low temperatures than at room temperature [30].
Araújo et al. studied the protective effect of tropical fruit by-products on Lactobacillus paracasei L-10, Lactobacillus casei L-26, and Lactobacillus acidophilus LA-05 during freeze-drying and storage [31]. They found that the by-products of acerola, cashew, and guava had a protective effect and increased the stability of probiotic lactobacilli during freeze-drying and during 90 days of storage at 4.0 ± 0.5 and 25.0 ± 0.5°C. The authors attributed the protective effect to the contents of monosaccharides, phenolic compounds, and dietary fiber in the by-products.
Our study showed that pumpkin, fig, and banana purees have cryoprotective effects during freezing and freeze-drying of fermented milk products due to the presence of mono- and disaccharides, proteins, antioxidants, and dietary fiber in them.
CONCLUSION
Our study showed the effectiveness of introducing pumpkin, fig, and banana purees into bioyogurt as cryoprotectants during freeze-drying. These purees can also enrich bioyogurts with prebiotics (mono- and disaccharides, dietary fiber, and antioxidants) and increase the viability of lactic acid microorganisms. Monoand disaccharides, minerals, and antioxidants contained in puree reduce the level of cryodamage of lactic acid microorganisms during freezing, contributing to their better survival and preservation after freezedrying.
The addition of puree to bioyogurts affects their thermophysical characteristics. We found that adding 15% of pumpkin puree allows for raising the freezedrying temperature from –16 to –13°С, with all the other parameters being equal. This can decrease specific energy consumption during freeze-drying and reduce its time. All the studied bioyogurts with puree can be freeze-dried under vacuum using domestic industrial freeze-drying units.
Adding puree to bioyogurts can also significantly improve their sensory profile and expand the range of freeze-dried fermented milk products of this type.
Contribution
I.S. Krasnova developed a general idea of using plant-based puree as a cryoprotectant during freezing and freeze-drying based on literature analysis, devised a plan of research, produced bioyogurt samples, evaluated their quality, analyzed the results, and formulated conclusions. V.I. Ganina developed research methodology, determined the counts of lactic acid microorganisms, analyzed the results, and formulated conclusions. G.V. Semenov performed thermal analysis and summarized the data on thermal analysis, freezing, and vacuum freeze-drying, analyzed the results, and formulated conclusions.CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.REFERENCES
- Savaiano DA, Hutkins RW. Yogurt, cultured fermented milk, and health: A systematic review. Nutrition Reviews. 2020;79(5):599–614. https://doi.org/10.1093/nutrit/nuaa013
- García-Burgos M, Moreno-Fernandez J, Alférez MJM, Díaz-Castro J, López-Aliaga I. New perspectives in fermented dairy products and their health relevance. Journal of Functional Foods. 2020;72. https://doi.org/10.1016/j.jff.2020.104059
- Putta S, Yarla NS, Lakkappa DB, Imandi SB, Malla RR, Chaitanya AK, et al. Probiotics: Supplements, food, pharmaceutical industry. In: Grumezescu AM, Holban AM, editors. Therapeutic, probiotic, and unconventional foods. Academic Press; 2018. pp. 15–25. https://doi.org/10.1016/B978-0-12-814625-5.00002-9
- Baskar N, Varadharajan S, Rameshbabu M, Ayyasamy S, Velusamy S. Development of plantbased yogurt. Foods and Raw Materials. 2022;10(2):274–282. https://doi.org/10.21603/2308-4057-2022-2-537
- Statsenko ES, Litvinenko OV, Kodirova GA, Kubankova GV, Korneva NYu, Pokotilo OV. Fermented milk beverages fortified with soy protein. Food Processing: Techniques and Technology. 2021;51(4):784–794. (In Russ.). https://doi.org/10.21603/2074-9414-2021-4-784-794
- Brushani A, Anandharamakrishnan C. Freeze Drying. In: Anandharamakrishnan C, editor. Handbook of drying for dairy products. Wiley-Blackwell; 2017. pp. 95–122.
- Titov EI, Krasnova IS, Ganina VI, Semenova EG. Freeze-dried food in the diet of temporary residents of the Far North. Food Processing: Techniques and Technology. 2021;51(1):170–178. https://doi.org/10.21603/2074-9414-2021-1-170-178
- Prosekov AYu, Ivanova SA. Providing food security in the existing tendencies of population growth and political and economic instability in the world. Food and Raw Materials. 2016;4(2):201–211. https://doi.org/10.21179/2308-4057-2016-2-201-211
- Artyukhova SI, Kozlova OV, Tolstoguzova TT. Developing freeze-dried bioproducts for the Russian military in the Arctic. Foods and Raw Materials. 2019;7(1):202–209. https://doi.org/10.21603/2308-4057-2019-1-202-209
- Ermis E. A review of drying methods for improving the quality of probiotic powders and characterization. Drying Technology. 2022;40(11):2199–2216. https://doi.org/10.1080/07373937.2021.1950169
- Terpou A, Papadaki A, Lappa IK, Kachrimanidou V, Bosnea LA, Kopsahelis N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients. 2019;11(7). https://doi.org/10.3390/nu11071591
- Kriger OV, Noskova SYu. Properties of lactic acid microorganisms: long-term preservation methods. Food Processing: Techniques and Technology. 2018;48(4):30–38. (In Russ.). https://doi.org/10.21603/2074-9414-2018-4-30-38
- Puchkov EO. Biogenic management of ice formation. Nature. 2017;(2):27–37. (In Russ.).
- Stefanello RF, Machado AAR, Cavalheiro CP, Santos MLB, Nabeshima EH, Copetti MV, et al. Trehalose as a cryoprotectant in freeze-dried wheat sourdough production. LWT. 2018;89:510–517. https://doi.org/10.1016/j.lwt.2017.11.011
- Tari A, Handayani C, Hartati S. The characteristics of synbiotic yoghurt freeze-drying supplemented by purple sweet potato (study on sucrose concentration as cryoprotectant). Proceedings of the International Conference on Applied Science and Engineering (ICASE 2018); 2018; Sukoharjo. Atlantis Press; 2018. p. 45–47. https://doi.org/10.2991/icase-18.2018.12
- Basholli-Salihu M, Kryeziu TL, Nebija D, Salar-Behzadi S, Viernstein H, Mueller M. Prebiotics as excipients for enhancement of stability and functionality of Bifidobacterium longum ssp. infantis with potential application as symbiotics in food and pharmaceuticals. Pharmazie. 2019;74(6):326–333. https://doi.org/10.1691/ph.2019.9007
- Savedboworn W, Teawsomboonkit K, Surichay S, Riansa-Ngawong W, Rittisak S, Charoen R, et al. Impact of protectants on the storage stability of freeze-dried probiotic Lactobacillus plantarum. Food Science and Biotechnology. 2018;28(3):795–805. https://doi.org/10.1007/s10068-018-0523-x
- Thakkar U, Preetha R, Nithyalakshmi V. Evaluation of viability of Lactobacillus bulgaricus in symbiotic microcapsules: before and after freeze-drying. International Food Research Journal. 2018;25(4):1642–1646.
- Oluwatosin SO, Tai SL, Fagan-Endres MA. Sucrose, maltodextrin and inulin efficacy as cryoprotectant, preservative and prebiotic – towards a freeze dried Lactobacillus plantarum topical probiotic. Biotechnology Reports. 2022;33. https://doi.org/10.1016/j.btre.2021.e00696
- Shu G, Zhang B, Hui Y, Chen H, Wan H. Optimization of cryoprotectants for Streptococcus thermophilus during freeze-drying using Box-Behnken experimental design of response surface methodology. Emirates Journal of Food and Agriculture. 2017;29(4):256–263. https://doi.org/10.9755/ejfa.2016-07-960
- Bhattacharya S. Cryoprotectants and their usage in cryopreservation process. In: Bozkurt Y, editor. Cryopreservation biotechnology in biomedical and biological sciences. IntechOpen; 2018. pp. 7–19. https://doi.org/10.5772/intechopen.80477
- Semenov GV, Krasnova IS. Freeze-drying. Moscow: DeLi; 2021. 326 p. (In Russ.).
- Semenov GV, Krasnova IS, Petkov II. The choice of parameters for vacuum freeze-drying of dry heat-sensitive material with predetermined properties. Journal of International Academy of Refrigeration. 2017;(1):18–24. (In Russ.). https://doi.org/10.21047/1606-4313-2017-16-1-18-24
- Nowak D, Jakubczyk E. The freeze-drying of foods – The characteristic of the process course and the effect of its parameters on the physical properties of food materials. Foods. 2020;9(10). https://doi.org/10.3390/foods9101488
- Simon AD. Colloidal chemistry: a general course. Moscow: Krasand; 2019. 342 p. (In Russ.).
- Assegehegn G, Brito-de la Fuente E, Franco JM, Gallegos C. Freeze-drying: A relevant unit operation in the manufacture of foods, nutritional products, and pharmaceuticals. Advances in Food and Nutrition Research. 2020;93:1–58. https://doi.org/10.1016/bs.afnr.2020.04.001
- Venir E, Del Torre M, Stecchini ML, Maltini E, Di Nardo P. Preparation of freeze-dried yoghurt as a space food. Journal of Food Engineering. 2007;80(2):402–407. https://doi.org/10.1016/j.jfoodeng.2006.02.030
- Radaeva IA, Rossikhina GA, Usacheva VA, Poyarkova GS, Shulʹkina SP. Biological value and stability of fermented milk products for astronauts during storage. Space Biology and Aerospace Medicine. 1982;16(2):23–26. (In Russ.).
- Nikolov NM, Vitanov TK. Effect of storage conditions on quality and microflora activity of freeze-dried yoghurt. Second Congress for Microbiology: Proceedings of the Second Congress for Microbiology; 1969; Sofia. Sofia; 1969. p. 95–97.
- Saarela M, Virkajärvi I, Alakomi H-L, Sigvart-Mattila P, Mättö J. Stability and functionality of freeze-dried probiotic Bifidobacterium cell during storage in juice milk. International Dairy Journal. 2006;16(12):1477–1482. https://doi.org/10.1016/j.idairyj.2005.12.007
- Araújo CM, Sampaio KB, Menezes FNDD, da Cruz Almeida ÉT, dos Santos Lima Ma, Viera VB, et al. Protective effects of tropical fruit processing coproducts on probiotic Lactobacillus strains during freeze-drying and storage. Microorganisms. 2020;8(1). https://doi.org/10.3390/microorganisms8010096

