Аннотация
Introduction. Medicinal herbs are susceptible to microbial contamination which can have profound effects on the consumer’s health. Our study aimed to evaluate microbial contamination of common medicinal herbs in Ahvaz.Study objects and methods. We collected 80 samples of traditional and industrial medicinal plants from the supply market, namely valeriana, fennel, licorice, and shirazi thyme. The reference method was used to determine microbial indices such as the total count of microorganisms, yeast and mold, Bacillus cereus, coliforms, and Escherichia coli.
Results and discussion. We found that the total microbial count, yeast and mold, B. cereus, and coliform contamination accounted for 45, 77, 55, and 55% of the total samples, respectively, exceeding the allowed limits. There was a significant difference between the industrial and traditional samples in fungal and coliform contamination, with the traditional samples being more highly contaminated. However, no significant difference was observed between them in total count and B. cereus contamination. E. coli contamination was detected in 31.2% of the samples, mostly in traditional. Total microbial count and yeast and mold contamination were highest among valeriana plants. Fennel showed the highest B. cereus and coliform contamination. The lowest contamination was observed in licorice.
Conclusion. The results showed that a considerable percentage of the medicinal herbs under study were contaminated at levels exceeding the standard limits. Plants could be contaminated during harvesting, processing or storage. Finally, different species of plants have different antimicrobial activities that affect their microbial contamination.
Ключевые слова
Microbiology, microbial contamination, quality control, medicinal herbs, total microbial countВВЕДЕНИЕ
Medicinal plants become contaminated by a variety of sources such as heavy metals, insect larvae and seed, different bacteria, and fungi [1]. Heat and humidity of the environment, long-time drying, irrigation with contaminated water, and lack of farmer training may result in considerable microbial contaminations and reduce the quality of plants. Moreover, microbial contamination of plants may take place during unhealthy collection, cleaning, storage, transportation, and packaging. Contact of herbal products with external factors such as plastic, glass, and other materials may lead to cross contamination.
Medicinal plants can be contaminated by a wide range of microorganisms, such as fungi, yeasts, protozoa, and viruses, most of which are transferred from soil [2, 3]. Total microbial count is an important factor in determining the health status or probable detection of a contamination source [4]. Yeast and mold are the most common contaminants of medicinal herbs. Various species of molds and yeasts that proliferate on food stuff secrete metabolic toxic materials such as mycotoxins, which are harmful for humans and animals [5]. The WHO (World Health Organization) has a large amount of data in this direction [6].
Coliforms (Escherichia, Enterobacter, and Klebsiella) from the Enterobacteriaceae family inhabit human and animal intestines. Most of them are not pathogenic, although some E. coli strains could be highly pathogenic and cause food poisoning [7]. According to Iran’s national standard, coliform contamination in most dried vegetables should not exceed the maximum level of 1000 CFU/g, while the presence of E. coli is not allowed. Bacillus cereus is widely distributed in the environment and some its strains are harmful for human health and can cause food poisoning. This bacterium secretes enterotoxin, hemolysin, and lecithinase C which are responsible for disease [9]. Dried vegetables contamination by Bacillus cereus should not exceed the maximum level of 100 CFU/g.
Valeriana (Nardostachys jatamansi L.) from the Valerianceae family is known for its anticonvulsant, sedative, anti-asthmatic, and cardiotonic properties [9]. Fennel (Foeniculum vulgare L.) from the Umbelliferae family has culinary and medicinal properties (anti-inflammatory, anti-spasmodic, diuretic, laxative, analgesic, antioxidant, and woundhealing) [10]. Licorice (Glycyrrhiza glabra L.) growing in Mediterranean countries, Central Asia, and Europe has a wide range of pharmacological effects such as antioxidant activity, liver protection, and regulation of the immune system [11]. Shirazi thyme (Zataria multiflora Boiss L.) is used in the south of Asia as tea or spice and in traditional medicine as a gastrointestinal disinfectant, diuretic, or an antiinflammatory remedy [12].
Contaminants such as microorganisms, heavy metals, and pesticides affect the quality and the efficacy of herbal products. Since it is impossible to remove all contaminants, precautionary measures should be taken to prevent or limit contamination [2, 3]. Therefore, our study aimed to show the effect of these contaminations on consumer’s health.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
Collection and preparation of samples. For this study, samples were randomly collected from medicinal herb retailers and drugstores of Ahvaz (Iran) from December 2017 for 6 months. A total of 80 samples were used: 40 traditional (10 samples for each traditional herb) and 40 industrial herbs from different companies (19 shirazi thyme, 6 fennel, 8 valeriana, and 7 licorice samples). The amounts of industrial samples were not equal due to their insufficient availability.
Total microbial count. Total microbial count was performed as described by Standard No.5272, Iran. Different dilutions of medicinal herbs were prepared and cultured on Plate count agar (PCA, Merck, Germany). Triplicate plates for each dilution were cultured and incubated for 72 h at 30°C. Then, the average of counted colonies was measured taking into account the dilution coefficient.
Mold and yeast count. Fungal count was performed according to Standard No.10899, Iran. Different dilutions of medicinal herbs were inoculated on Sabouraud dextrose agar (SDA, Merck, Germany) in triplicates and incubated at 25°C for 5 days. Then, the average number of molds and yeasts per gram of herb was estimated.
Bacillus cereus detection. To detect and count B. cereus (Standard No. 2324, Iran), dilutions of medicinal herbs were prepared and cultured in triplicate on Mannitol-egg yolk-polymyxin (MYP) agar (Merck, Germany) at 30°C for 48 h. The agar contained an egg yolk emulsion and polymyxin B sulfate (Shijiazhuang Pharma, China). Large and pink colonies (lack of manitol fermentation) with a sedimentary halo (lecithinase producer) were counted as probable B. cereus. To confirm the suspected colonies, a hemolysis test was performed on Blood agar (Merck, Germany).
Coliform detection and enumeration. Coliform detection and enumeration were performed according to Standard No. 9263, Iran. Different dilutions of medicinal herbs were inoculated (pour plate and twolayer culture) in triplicate on Crystal violet neutral red bile lactose (VRBL, Merck, Germany) agar and incubated at 37°C for 24 h. Typical red purple colonies were confirmed on Brilliant green bile lactose (BGBL) broth (Merck, Germany) contained in Durham tubes at two temperatures (37 and 44°C) for 24 h.
Escherichia coli detection. Following coliform detection, positive BGBL tubes (gas production) were inoculated into peptone water and incubated at 44°C for 48 h. Gas production in BGBL and production of indole in peptone water were recorded for presence of E. coli.
Statistical analysis. Analysis of data was performed using SPSS statistical software. The significance of the results was evaluated by McNemar nonparametric test with significance level of P < 0.05.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
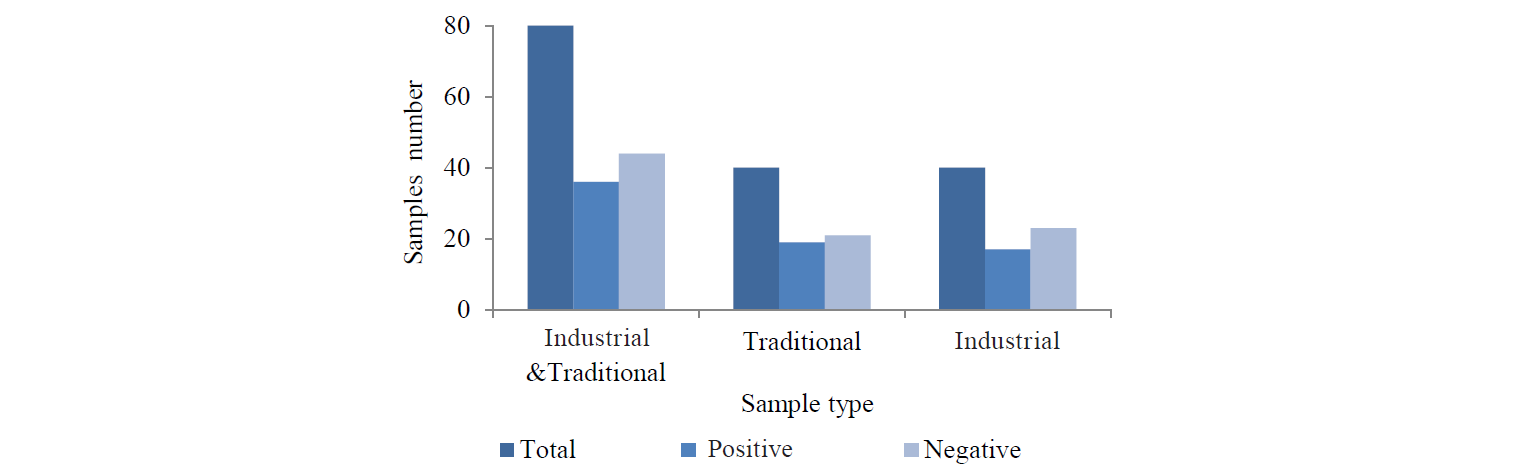
Total microbial contamination. According to the results of total microbial count (Fig. 1), 45% of the total herbs (36 samples out of 80) showed contamination over the limit (105 CFU/g). Of them, 19 (48%) and 17 (43%) were traditional and industrial, respectively. The microbial load in the samples with contamination over the limit varied from 5.03 ± 0.03 to 6.25 ± 0.03 log CFU/g. This ranged from 5.14 ± 0.01 to 6.25 ± 0.03 in the traditional samples and from 5.03 ± 0.3 to 6 ± 0.05 log CFU/g in the industrial samples. However, there was no significant difference between the total microbial contamination in the traditional and industrial samples (P > 0.05). Among the studied herbs, valeriana (Nardostachys jatamansi L.) and licorice (Glycyrrhiza glabra L.) showed the highest and the lowest contamination ‒ 56 and 18%, respectively. Also, the total microbial contamination of shirazi thyme (Zataria multiflora Boiss L.) and fennel (Foeniculum vulgare L.) was over the limit in 55% and 44% of the samples, respectively.
Fungal contamination. The results of fungal contamination are presented in Fig. 2. As we can see, 61 (77.5%) out of 80 samples had mold and yeast contamination over the limit (103 CFU/g). They comprised 34 (85%) traditional and 27 (67.5%) industrial samples. The fungal load in the samples with contamination over the limit varied from 3.02 ± 0.00 to 4.78 ± 0.06 log CFU/g. This reached from 3.02 ± 0.00 to 4.78 ± 0.06 in the traditional samples and from 3.04 ± 0.01 to 4.60 ± 0.01 log CFU/g in the industrial samples. It should be mentioned that the traditional samples were significantly contaminated with mold and yeast (P < 0.05). Meanwhile, valeriana (100%) and licorice (47%) showed the highest and the lowest contamination, respectively. Also, 79% and 75% of shirazi thyme and fennel, respectively, showed over the limit fungal contamination.
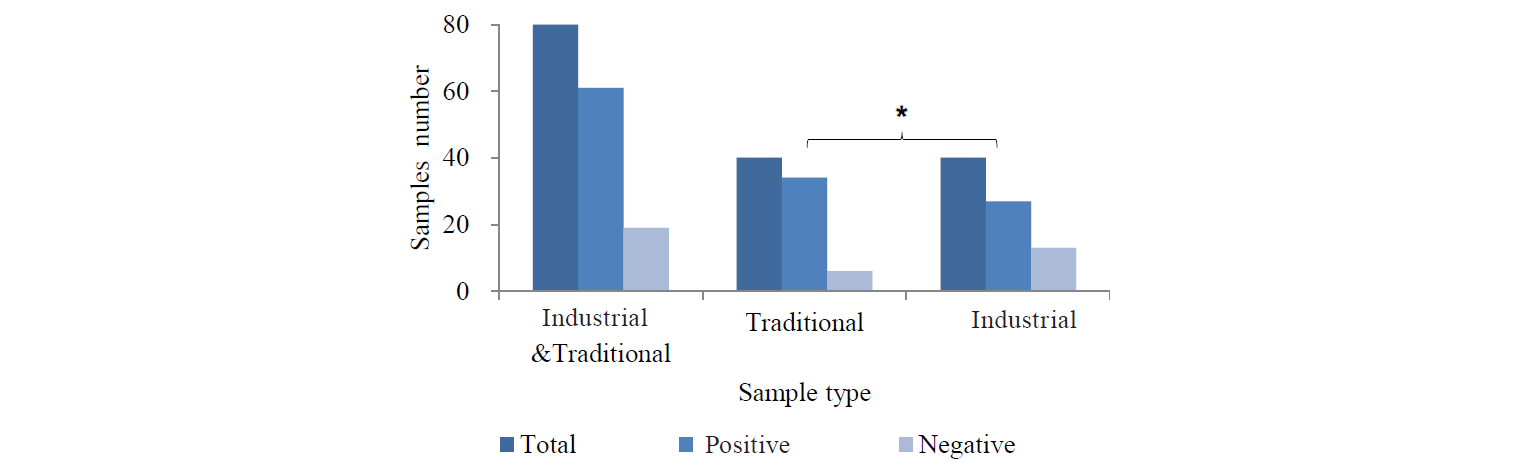
Bacillus cereus contamination. B. cereus contamination in 44 samples (55%) of the total herbs exceeded the limit (103 CFU/g) (Fig. 3), including 23 (57.5%) traditional and 21 (52.5%) industrial herbs. Over the limit B. cereus contamination varied from 2.03 ± 0.03 to 3.84 ± 0.06 log CFU/g. In the traditional samples, it ranged from 2.03 ± 0.03 to 3 ± 0.06 log CFU/g and in the industrial samples, from 2.03 ± 0.03 to 3.84 ± 0.06 log CFU/g. Our results showed that there was no significant difference between the traditional and industrial samples in B. cereus contamination (P > 0.05). The contamination in fennel (94%) and valeriana (66%) was significantly higher (P < 0.05). Shirazi thyme (41%) and licorice (29%) showed a lower level of B. cereus contamination.
Coliform contamination. Coliform contamination was found over the limit (103 CFU/g) in 44 samples (55%) of the total herbs. Among these samples, 29 (72.5%) and 15 (37.5%) were from traditional and industrial herbs, respectively (Fig. 4). Over the limit coliform contamination ranged from 3.01 to 4.16 ± 0.03 log CFU/g, namely from 3.01 to 4.16 ± 0.03 in the traditional samples and from 3.03 ± 0.02 to 4.15 ± 0.03 log CFU/g in the industrial samples. According to the results, the traditional samples showed a significantly higher coliform contamination than the industrial samples (P < 0.05). Fennel (81%) and valeriana (66%) revealed a very high level of contamination (P < 0.01), while the lowest level was recorded in licorice (41%) and shirazi thyme (41%).
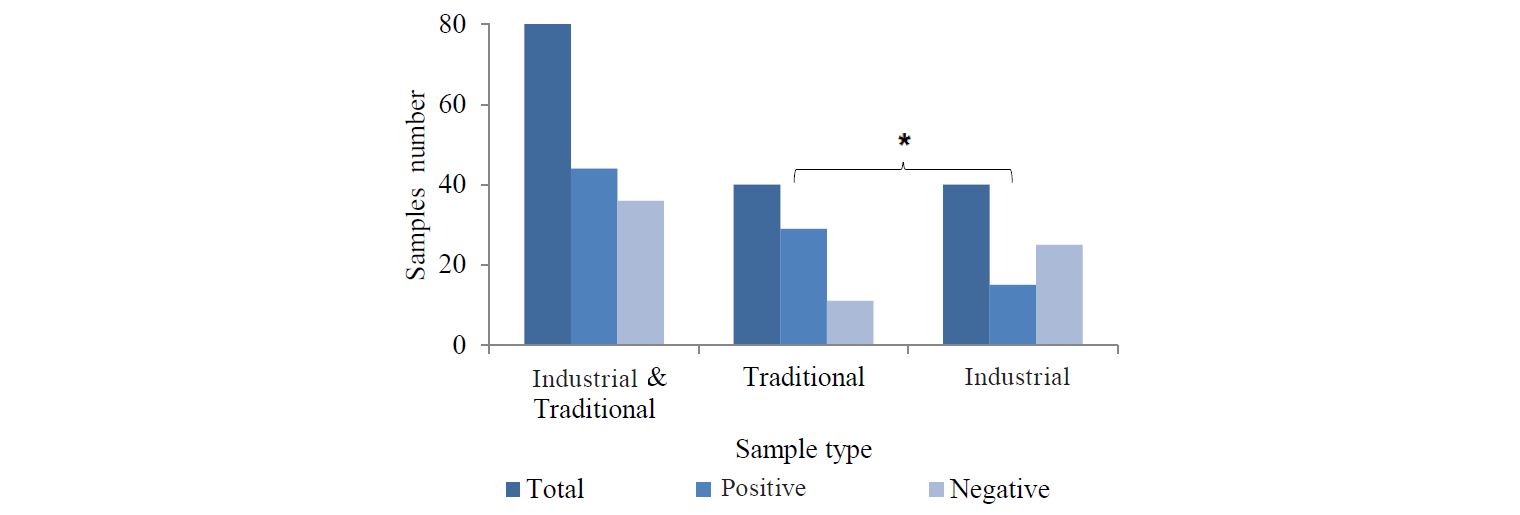
Escherichia coli contamination. There should be no E. coli contamination in dried vegetables [8]. According to our results, 25 samples (31.2%) of the total herbs showed E. coli contamination. Of them, 19 (47.5%) and 6 (15%) were from traditional and industrial herbs, respectively (Fig. 5). E. coli contamination in the traditional samples was significantly higher than in the industrial samples (P < 0.05). The contamination in fennel (43.7%) and valeriana (33.3%) was significantly higher (P < 0.05) than that in licorice (23.5%) and shirazi thyme (27.5%).
One of the important aspects is contamination of medicinal herbs by different types of harmful factors such as microbes, heavy metals, as well as radioactive and chemical materials [2]. Our results, in many cases, indicated high contamination of the herbs under study with different microbial agents. These contaminations could occur during different stages of cultivation, extraction, drying, packing or distribution [2, 3, 13]. In our study, the microbial contamination level in the traditional samples was higher than that in the industrial samples. This result could be due to different production and packaging conditions.
Researchers have reported that the differences in technological level and preparation, supply and production of medicinal herbs could affect their contamination level [13–15]. Worldwide, a high level of contamination has been reported in a variety of medicinal herbs. For example, Banerjee et al., in a study of 154 dried plants collected from shops in India, showed that the total microbial count was over the limit in 51% of the samples, and 97% of them had mold contamination [16].
Moreover, Abba et al., in a study of powdered medicinal plants in Nigeria, reported that 87% of them had high microbial contamination [17]. Their contamination level was significantly higher than that in our study (45%), which could be due to environmental factors, soil or inappropriate packing conditions. Some studies in different locations showed that many of the investigated medicinal herbs were contaminated with various fungi [5, 16, 18, 19]. Alwakeel, in a study on 32 samples of various medicinal plants in Saudi Arabia, showed Bacillus cereus as the most common microbial contaminant [20]. Martins et al. found the same result in more than 90% of the studied medicinal plants in Portugal [21].
In our study, fennel and valeriana showed the highest and licorice showed the lowest levels of contamination with B. cereus and coliform. In a study of the antimicrobial activity of Turkish spices, fennel showed a lower antibacterial effect on B. cereus [22]. Moreover, Lang et al. in Austria reported that fennel had a lower inhibitory effect on coliform than licorice [23]. It seems that the antimicrobial properties of medicinal herbs could also explain the differences in their contamination levels. In the previous studies, high microbial contaminations were reported in valeriana [19, 24]. We found that valeriana had the highest contamination level. This result can be due to the fact that most of its active medicinal ingredients are in the root of the plant, which is in direct contact with soil, so more microorganisms can be transmitted to it [19]. In our study, licorice showed a considerably lower contamination level than other plants. It could be due to differences in plant production, its active constituents, and distribution processes [3, 25]. In addition, most studies have shown that licorice had higher antimicrobial and antifungal activities than other plants, especially against B. cereus and E. coli [22, 35–38].
ВЫВОДЫ
In our study, we tested 80 samples of traditional and industrial herbs, such as valeriana, fennel, licorice, and shirazi thyme, for microbial contamination. The results of the experiment showed that microbial indices in considerable percentage of the samples exceeded the standard limit. Valeriana had the highest total microbial count and yeast/mold contamination, fennel ‒ B. cereus and coliforms, while licorice was not massively contaminated.
We also revealed that fungal, coliform, and E. coli contamination in traditional herbs was considerably higher than that in industrial samples. However, there was no significant difference between them in total microbial count and B. cereus contamination.
Thus, our results demonstrated the importance of monitoring medicinal plants contamination to control the quality of herbal products.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare no conflict of interest.
БЛАГОДАРНОСТИ
This study was financially supported by Ahvaz Jundishapur University of Medical Sciences and extracted from the thesis of Ms Sara Shamsaei (B-9605).
СПИСОК ЛИТЕРАТУРЫ
- Chan K. Some aspects of toxic contaminants in herbal medicines. Chemosphere. 2003;52(9):1361–1371. DOI: https://doi.org/10.1016/S0045-6535(03)00471-5.
- Kosalec I, Cvek J, Tomic S. Contaminants of medicinal herbs and herbal products. Archives of Industrial Hygiene and Toxicology. 2009;60(4):485–501. DOI: https://doi.org/10.2478/10004-1254-60-2009-2005.
- Kneifel W, Czech E, Kopp B. Microbial contamination of medicinal plants – A review. Planta Medica. 2002;68(1): 5–15. DOI: https://doi.org/10.1055/s-2002-20060.
- Khan RS, Grigor J, Winger R, Win A. Functional food product development – Opportunities and challenges for food manufacturers. Trends in Food Science and Technology. 2013;30(1):27–37. DOI: https://doi.org/10.1016/j.tifs.2012.11.004.
- Hashem M, Alamri S. Contamination of common spices in Saudi Arabia markets with potential mycotoxin-producing fungi. Saudi Journal of Biological Sciences. 2010;17(2):167–175. DOI: https://doi.org/10.1016/j.sjbs.2010.02.011.
- WHO Monographs on Medicinal Plants Commonly Used in the Newly Independent States (NIS). Geneva: World Health Organization; 2010. 450 p.
- Lee LH, Wu M, Peri A, Chu T. Method evaluations for Escherichia coli and coliforms detection in northern New Jersey water bodies. Journal of BioSciences. 2014;3(1):40–45. DOI: https://doi.org/10.5176/2251-3140_3.1.49.
- Xu ZB, Xie JH, Liu JY, Ji LL, Soteyome T, Peters BM, et al. Whole-genome resequencing of Bacillus cereus and expression of genes functioning in sodium chloride stress. Microbial Pathogenesis. 2017;104:248–253. DOI: https://doi.org/10.1016/j.micpath.2017.01.040.
- Purnima, Meenakshi B, Preeti K. A review article on phytochemistry and pharmacological profiles of Nardostachys jatamansi DC-medicinal herb. Journal of Pharmacognosy and Phytochemistry. 2015;3(5):102–106.
- Badgujar SB, Patel VV, Bandivdekar AH. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed Research International. 2014. DOI: https://doi.org/10.1155/2014/842674.
- Parvaiz M, Hussain K, Khalid S, Hussnain N, Iram N, Hussain Z, et al. A review: medicinal importance of Glycyrrhiza glabra L. (Fabaceae Family). Global Journal of Pharmacology. 2014;8(1):8–13. DOI: https://doi.org/10.5829/idosi.gjp.2014.8.1.81179.
- Saleem M, Nazli R, Afza N, Sami A, Ali MS. Biological significance of essential oil of Zataria multiflora boiss. Natural Product Research. 2004;18(6):493–497. DOI: https://doi.org/10.1080/14786410310001608064.
- Ghisleni DD, Bragaa MD, Kikuchia IS, Brasoveanu M, Nemtanu MR, Dua K, et al. The microbial quality aspects and decontamination approaches for the herbal medicinal plants and products: An in-depth review. Current Pharmaceutical Design. 2016;22(27):4264–4287. DOI: https://doi.org/10.2174/1381612822666160623070829.
- Sahil K, Sudeep B, Akanksha M. Standardization of medicinal plant materials. International Journal of Research in Ayurveda and Pharmacy. 2011;2(4):1100–1109.
- Schweiggert U, Carle R, Schieber A. Conventional and alternative processes for spice production – a review. Trends in Food Science and Technology. 2007;18(5):260–268. DOI: https://doi.org/10.1016/j.tifs.2007.01.005.
- Banerjee M, Sarkar PK. Microbiological quality of some retail spices in India. Food Research International. 2003;36(5):469–474. DOI: https://doi.org/10.1016/S0963-9969(02)00194-1.
- Abba D, Inabo HI, Yakubu SE, Olonitola OS. Contamination of herbal medicinal products marketed in Kaduna metropolis with selected pathogenic bacteria. African Journal of Traditional Complementary and Alternative Medicines. 2009;6(1):70–77. DOI: https://doi.org/10.4314/ajtcam.v6i1.57076.
- Abou Donia MA. Microbiological quality and aflatoxinogenesis of Egyptian spices and medicinal plants. Global Veterinaria. 2008;2(4):175–181.
- Vali Asill R, Azizi M, Bahreini M, Arouie H. The survey of microbial quality of the dry sample, extract and brewing of some medicinal plants. Notulae Scientica Biologicae. 2014;6(4):478–482. DOI: https://doi.org/10.1583/nsb649286.
- Alwakeel S. Microbial and heavy metals contamination of herbal medicines. Research Journal of Microbiology. 2008;3(12):683–691. DOI: https://doi.org/10.3923/jm.2008.683.691.
- Martins HM, Martins ML, Dias MI, Bernardo F. Evaluation of microbiological quality of medicinal plants used in natural infusions. International Journal of Food Microbiology. 2001;68(1–2):149–153. DOI: https://doi.org/10.1016/S0168-1605(01)00480-9.
- Sagdic O, Ozcan M. Antibacterial activity of Turkish spice hydrosols. Food Control. 2003;14(3):141–143. DOI: https://doi.org/10.1016/S0956-7135(02)00057-9.
- Lang GR, Buchbauer G. A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. A review. Flavour and Fragrance Journal. 2012;27(1):13–39. DOI: https://doi.org/10.1002/ffj.2082.
- Weightman RM. Heavy metal and microbial contamination of valerian (Valeriana officinalis L.) roots gown in soil treated with sewage sludge. Journal of Herbs, Spices & Medicinal Plants. 2007;12(3):77–88. DOI: https://doi.org/10.1300/J044v12n03_06.
- Fatima A, Gupta VK, Luqman S, Negi AS, Kumar JK, Shanker K, et al. Antifungal activity of Glycyrrhiza glabra extracts and its active constituent glabridin. Phytotherapy Research. 2009;23(8):1190–1193. DOI: https://doi.org/10.1002/ptr.2726.
- Nitalikar MM, Munde KC, Dhore BV, Shikalgar SN. Studies of antibacterial activities of Glycyrrhiza glabra root extract. International Journal of PharmTech Research. 2010;2(1):899–901.
- Walter C, Shinwari ZK, Afzal I, Malik RN. Antibacterial activity in herbal products used in Pakistan. Pakistan Journal of Botany. 2011;43:155–162.
- Ates DA, Erdogrul OT. Antimicrobial activities of various medicinal and commercial plant extracts. Turkish Journal of Biology. 2003;27(3):157–162.
- WHO guidelines on safety monitoring of herbal medicines in pharmacovigilance systems. Geneva: World Health Organization; 2004. 82 p.