Аннотация
The aim of this study was to analyse the effect ferrous gluconate and ferrous lactate on the rheological behaviour of dough from a high extraction rate. For fortification of wheat flour, we used iron ions in a divalent form in amounts of 3, 4, and 5 mg/100 g. To record the rheological characteriscics of the fortified wheat flour dough, Farinograph, Amilograph, Falling Number, Rheofermentometer, and Thermo Haake Mars dynamic rheometer were applied. The Farinograph did not show significant changes in the water absortion values in the samples with ferrous salts. As for dough development time and dough stability, small amounts of ferrous additives increased and large amounts decreased those parameters. The effect was more significant in the samples with ions from gluconate form than from lactate salt. The Amylograph recorded an increased peak viscosity with an increasing ferrous salt quantity. That was the case for both ferrous salt forms. The increased was in a similar way for both types of ferrous salt forms used. The total CO2 volume production and the retention coefficient obtained with the help of the Rheofermentometer device increased in the dough samples with 3 and 4 mg of iron/100 g. However, the addition of 5 mg of iron decreased those indicarors. The decrease was more significant for iron ions from ferrous ferrous gluconate than from ferrous lactate. The fundamental rheological properties of the dough were analysed by using a frequency sweep and oscillatory temperature sweep test. Ferrous lactate and ferrous gluconate influenced both the fundamental and empirical rheological properrties og the dough in similar way.Ключевые слова
Wheat flour, ferrous lactate, ferrous gluconate, rheological propertiesВВЕДЕНИЕ
Iron is a vital element for the humans, hence iron deficiency can seriously affect human’s health [1, 2]. 60% of the world’s population was estimated to be deficient in iron, while 33%, 30% and 15% are deficient in zinc, iodine, and selenium, respectively. Such a status is known as ‘hidden hunger’, due to diet that is poor in essential micronutrients [3, 4]. Iron deficiency has reached epidemic levels in numerous developing countries and affects people of all ages worldwide [5, 6]. The functional iron pool consists of such structural components in heme proteins as hemoglobin, myoglobin, and cytochromes [6, 7]. In addition, iron plays a part in nearly all redox reactions, and it is a vital component in several enzymes [3].
Iron aids the distribution of oxygen to the body, keeps the immune system strong, and helps the body to produce energy. Iron deficiency is caused by an insufficient iron intake, a poor absorption of iron or both. Iron deficiency exerts an adverse effect on mental and motor function, work productivity, immunity, cognitive development, and the quality of life in general [3, 5, 8, 9].
In 2012 WHO (World Health Organization) implemented a plan on maternal, infant, and young child nutrition to achieve a 50% reduction of anaemia by 2025 [10]. The food industry has initiated the use of iron into consumer products such as bread, breakfast cereals, biscuits, and energy bars. The food vehicles recommended to be fortified with iron, apart from staple foods, seasonings (i.e. table salt, soy sauce, fish sauce, broth, and curry powder) have been assayed owing to their extensive use in the various target populations [10]. The fortifying ingredients should however be used in the recommended amounts to prevent the risk of excessive consumption. Blanco-Rojo, Vaquero, and Hurrell [10, 11] reported that iron was the most difficult micronutrient to produce fortified foods. Many of the compounds used as iron fortificants caused unacceptable colour and flavour changes in foods.
Cereal is the main source of food for humans, especially in developing countries where it takes half of the calorie intake. The most appropriate iron compounds recommended by the WHO to fortify cereals are ferrous sulfate, ferrous fumarate, ferric pyrophosphate, and electrolytic iron [10, 12]. Most nutrients are present in the outer layers of wheat and are lost during the milling process. Wheat flour is the raw material in the manufacturing of many foods: bread and bakery products, confectionery products, snacks, and biscuits. In Romania, wheat flour has been the only food item widely used for iron fortification at the national level [13]. An alternative source of this element for the treatment of iron deficiency can be iron fortified bread [3].
Besides flour, bread and other fortified products also contain a number of various ingredients and food additives. Iron can interact with these, which can cause taste, odour and colour changes, enchance the toxicity of added food additives, or decrease the vitamin and mineral content in the products. The rheological properties of fortified dough, among other parameters, change [14, 15] during the technological process.
For successful fortification programme, it is important that the combination of the fortificant and the food item to be easily accepted by the comsumer [16, 17]. This requirement includes not only sensory properties of the fortified food but also economic viability and efficacy (bioavailability) [17, 18]. The interactions between the iron fortificant, the food vehicle and the consumer acceptance can be the subject of a further investigation and a multidisciplinary approach [10].
Iron in other products of plant origin is non-heme, and its disadvantage is to interact with substances in foods that inhibit its absorption such as tannins, phytates, and polyphenols. Therefore, iron has a low bioavailability [19]. The most significant enhancer of iron bioavailability is ascorbic acid, which both reduces and chelates iron, rendering it soluble and availability for absorption in the gut [6, 20].
Fortification of wheat flour with iron is technically more difficult than that with other nutrients because iron is a pro-oxidant and therefore promotes lipid oxidation. Hence, the ideal iron compound for fortification of food should be one that ensures high iron bioavailability and does not affect the nutritional value or sensory properties of food [21–23]. Therefore, that waa the reason why we chose ferrous lactate and ferrous gluconate as an iron source. Theese froous salts ensure a high bioalvalabilty [24, 25], so they are widely reccommened as iron source for food products. In this paper we analysed an effect of fortification of wheat flour from a high extraction rate with iron ions in a divalent form from ferrous lactate and ferrous gluconate in amounts of 3, 4 and 5 mg/100 g on the rheological behaviour of the flour To our knowledge no such complex study on empirical (mixing, pasting, and fermentation) and fundamental rheological behaviour of gough was made using this type of iron ions.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
The wheat flour used in this study was provided by S.C. Dizing S.R.L. (Brusturi. Neam, Romania). The following characteristics of wheat flour were analysed according to Romanian or international standard methods: moisture (ICC 110/1), ash content (ICC 104/1), protein content (ICC 105/2), gluten deformation index (SR 90:2007), wet gluten (ICC 106/1), and Falling Number (ICC 107/1). The analytical characteristics for the wheat flour analysed were the following: 1.25 g/100 g for ash content, 12.8% for moisture, 14.3% for protein, 35% for wet gluten, 3 mm for gluten deformation index, and 262 s for Falling Number. Ferrous gluconate (Fe(C6H11O7)2·2H2O) and ferrous lactate (Fe (CH3CH(OH)COO)2·2H2O) were provided by Jost Chemical (Belgium). The ferrous salts were added in suce a way to achieve the iron ion concentration in wheat flour of 3 mg/100 g, 4 mg/100 g, and 5 mg/100 g.
The empirical rheological tests during mixing, pasting, and fermentation processes of wheat flour dough with and without iron ions addition were carried out using Farinograph, Amilograph, Falling Number, and Rheofermentometer devices.
Empirical rheological properties of the dough during mixing were evaluated by using a Farinograph device (Brabender, Duigsburg, Germany, 300 g capacity) according to ICC method 115/1. We analysed water absorption (WA, %), dough stability (ST, min), dough development time (DT, min), and degree of softening (DS, min) at 10 min.
Viscometric rheological properties of the dough were analysed with the help of a Falling Number device (Perten Instruments AB, Sweden) and an Amylograph device (Brabender OGH, Duisburg, Germany). ICC method 107/1 was used to evaluate the α-amylase activity of the wheat flour through the Falling number values (FN, s). Such parameters as gelatinization temperature (Tg, °C), peak viscosity (PVmax, BU), and temperature at peak viscosity (Tmax, °C) were determined according to ICC method 126/1.
Dough rheological properties during fermentation were measured with a Chopin Rheofermentometer (Chopin Rheo, type F3, Villeneuve- La- Garenne Cedex, France). The parameteres were: maximum height of gaseous production (H’m, mm), total CO2 volume production (VT), volume of the gas retained in the dough at the end of the test (VR), and retention coefficient (CR, %).
Fundamental dough rheological properties were analysed using a HAAKE MARS 40 rheometer. The dough samples had the optimum dough consistency according to the water absortion values previosly established by the Farinograph device. Each sample was placed between the rheometer plates. The excess margins of the samples was removed and vaseline oil was used to prevent drying of the dough samples. The gap was setted to 2 mm, and a plate system with a diameter of 40 mm was used. Before analysis, the dough samples were left between plates for 10 min in order to allow its relaxation and to eliminate the stress rsulting from the mixing process. Frequency sweep tests from 0.00 to 20 Hz were performed at 25°C for all the dough samples. For the temperature sweep test, the samples were heated from 20 to 100°C at a heating rate of 4°C per min at a fixed frequency of 1 Hz and a strain of 0.001. During the frequency sweep tests and during heating storage modulus (G’) and loss modulus (G”) were analysed.
Statistical analysis of the triplicate results obtained was done using the XLSTAT statistical package (free trial version 2016, Addinsoft, Inc., Brooklyn, NY, USA), at a significance level of p < 0.05.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
Table 1 demonstrates the empirical rheological properties of dough samples with or without iron ions during mixing which were analysed by the Farinograph device.
As one can see in Table 1, water absortion values did not signifcantly change in the samples with the iron ions. A slightly decrease of these values were noticed in the samples with large amounts of iron ions. This might be due to the fact that salt ions are able to modify hydrogen and hydrophobic interactions with the wheat flour components and lead to protein-water interactions instead of protein ones [26].
Increased amounts of iron ions addition decreased the dough development time significantly (p < 0.001) for both types of salts. An explanation of that was probably gluten proteins interactions modified by iron salts. They would possibly present more positive electric charge which might favor a less interaction in a shorter mixing time. Also, dough stability decreased more significqantly at high levels of iron ions addition in the case of gluconate salt than in the case of lactate one. This behaviour may be atribuited to the anion salt type.
According to Codină et al. [27], the same level of iron ions addition contains lactate anion in a less amount that the gluconate anion. This will lead to a more compacted dough in the case of gluconate salt than in the case of lactate one. It is well known fact that the cation salt has a less effect on wheat flour components of dough system than the anion salt. As Miller and Hoseney reported [28], anion from a salt added in wheat flour might decrease electrostatic repulsion between gluten proteins, allowing them to connect and thus forming more stable dough. An increase in the dough stability with the increase in the level of iron salts has also been reported by Akhtar et al. and Rebellato et al. [29, 30].
The degree of softening values at 10 min decreased to a larger extent in the case of ferrous lactate than in the case of ferrous gluconate, which indicated a more weakening effect when lactate salt was incorporated in the wheat flour dough.
The dough viscometric rheological properties on Falling Number and Amylograph values are shown in Fig. 1. The value decreased with the increase in level of iron salts, with no significant differences between the dough samples with different types of iron ions incorporated (Fig. 1a). These decreased values indicated an increase in the wheat flour slurry viscosity, which could be correlated to decreased α-amylase activity in the wheat flours samples [31]. Falling number values increased up to 413 s and the mean values of the samples were slightly higher than 330 s. This indicated that the flour with iron ions additions showed a low α-amylase activity, which agreed well with the results obtained in [32].
All the parameters in the experimental samples analysed by Amylograph presented higher or similar values compared to the control sample (Fig. 1b and c). However, no significant difference were noticed between the samples with different type of iron salt addition. These results were somewhat predictable due to the fact that the Amylograph device was also a viscometric method which could be used to predict the α-amylase activity of wheat flour [27, 33] which is highly connected with these parameters. A lower α-amylase activity in wheat flour led to a lower starch hydrolysis and therefore to a lower amount of simple sugars and dextrins [34], which in turn caused an increase in all Amylograph parameters values [35].
Dough rheological properties during fermentation was analysed by a Chopin Rheofermentometer (Table 2). The maximum height of gaseous production were recorded by a Rheofermentometer pressure sensor, and the total CO2 volume production were determined by means of a pneumatic circuit which measured an increase in the pressure of the fermentation gases. The iron salts addition increased the total CO2 volume production from the dough system, which was probably due to the fact that iron ions stimulated the growth of yeast cells and therefore the total amount of the CO2 volume production.
However, the volume of the gas retained in the dough at the end of the test (VR) and the retention coefficient (CR) decreased with the increased level of iron ions addition. This increase was greater in for the samples with ferrous lactate salt than for those with ferrous gluconate. The cause of that might be weakening effect that iron salts exerted on the wheat flour dough which was not capable to retain the gas formed. Similar results were alo obtained by Codină et al. [26]. The maximum height of gaseous production (H’m) varied with the increase in level of iron ions addition. This was probably due to the fact that iron ions additions in increased amounts initiated an increased gas production in the dough, but the wheat flour dough was not capabale to retain it.
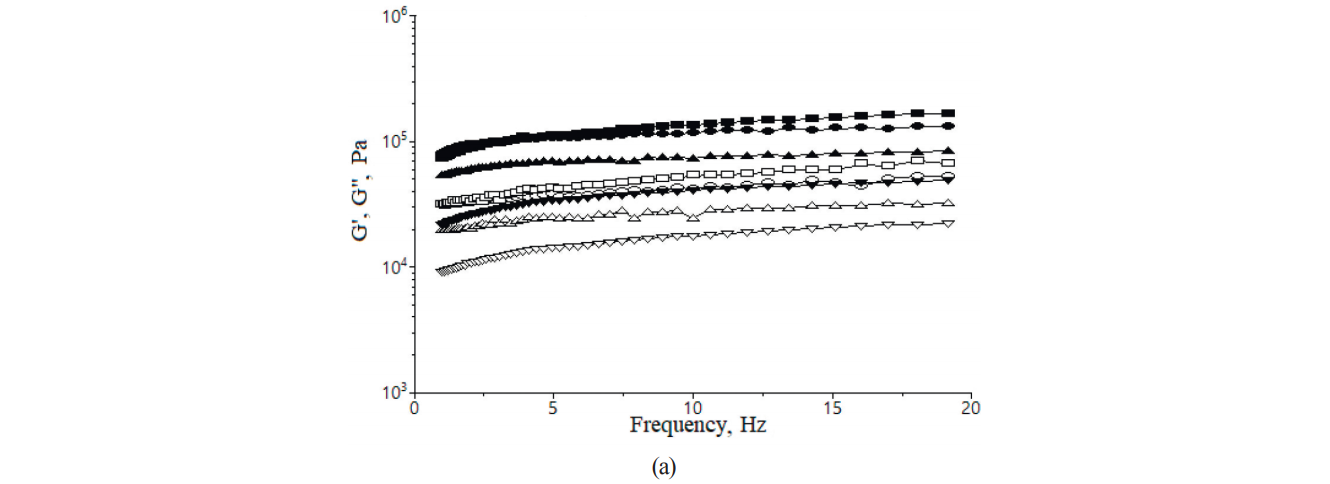
Fig. 2 shows effects of the iron ions additions from the two types of salts on the storage/elastic module G’ and the loss/viscous module values G”. All the experimental dough samples, as expected, presented G’ > G” at all frequency ranges, which indicated a solid elastic-like behavior of wheat flour dough according to [36]. The G’ and G” values increased slightly with the increase in frequency from 1 to 20 Hz. The dough samples with 3 mg of iron ions addition showed a decrease in the G’ and G”, which implied that the samples demonstrated visco-elasticity characteristics to less extent than the control sample. However, high levels of iron ions increased the G’ and G” values compared to the control sample. An explanation of this might be dehydration effect that iron salts could exert on gluten network that might lead to a more compacted dough with higher visco-elasticity properties.
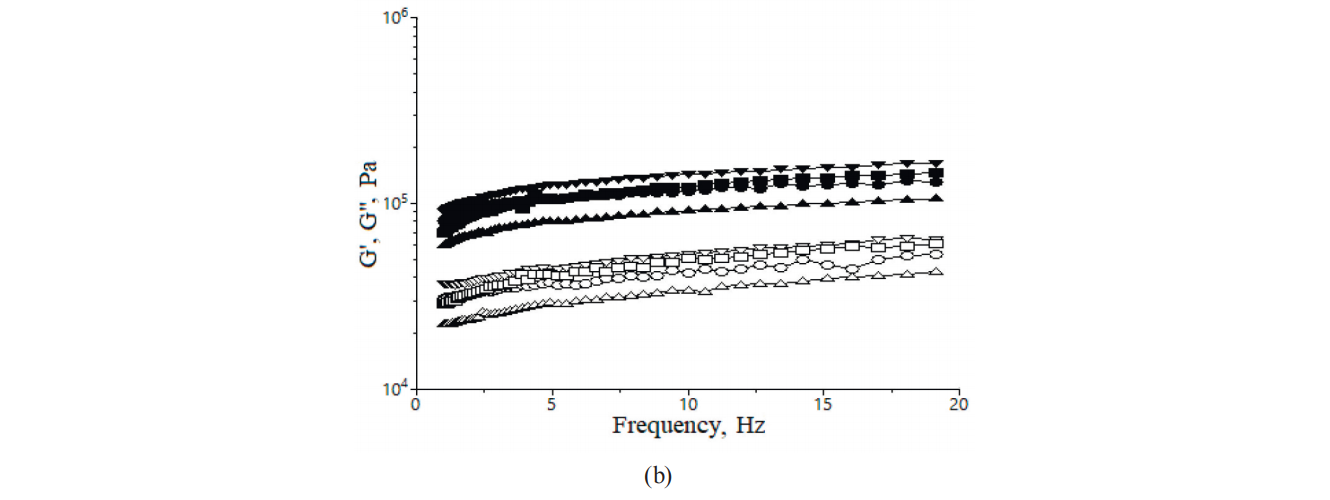
The influence of the iron ions addition on dynamic moduli during heating is shown in Fig. 3. G’ and G” values were lower in the samples with the maximum amount of iron ions (5 mg/100 g). It seemed that both ferrous lactate and ferrous glucanate displayed a significant effect during dough heating. At the begining of heating the moduli decreased for all the samples due to protein denaturation which seemed to increase with the increase in the amount of iron ion addition. Thus, proteins lost their capacity to retain water, starch granules began to absorb the water and to gelatinise as temperature increased. This fact is obvious, since an increase in dough elasticity and viscosity is manifasted in the increase of the G’ and G” after the temperature exceeds 50°C.
The principal components analysis (PCA) of the wheat flour dough rheological characteristics determined by the Farinograph and Rheofermentometer is shown in Fig. 4. The two plots represent 99.72% of the total variance. The plot of PC1 vs. PC2 loadings shows a close association between the dough sample with 3 mg of iron ions from the lactate salt addition and the volume of the gas retained in the dough at the end of the test (VR). The dough samples with 3 mg/100 g addition from gluconate salt is closed positioned to the retention coefficient (CR). This facts shows that the samples with iron ions addition in the aount of 3 mg/100 g presents a prositive effect on the dough rheological properties during the fermentation process.

The second PC axis show a close association between the samples with 4 mg of the iron ions per 100 g of the wheat flour, which indicate that both types of salts at this amount have a similar effect on dough rheological properties. However, both ferrous lactate and ferrous gluconate in an increased amounts show a different effect, from a statistical point of view, on the dough rheological properties, since they are differently positioned in the PCA plot.
According to the dought rheological properties results obtained with the help of Farinograph and Rheofermentometer devices, good correlation may be observed between CR and DS, CR and DT, ST and VR, as well as between VT and VR Rheofermentometer values.
ВЫВОДЫ
The effect of iron ions from lactate and gluconate salts in amounts of 3, 4, and 5 mg/100 g on wheat flour dough empirical and fundamental rheological properties was analyzed. It seems that the 3 mg/100 g iron ions addition did not affect adversely the dough rheological properties since dough stability, dough development time, and total CO2 volume production increased. In addition, such dough rheological properties as the degree of softening at 10 min, Amylopgrah paramter values, volume of the gas retained in the dough at the end of the test, retention coefficient, and dynamic rheological properties did not decrease significantly. The 4 mg/100 g the iron ions addition weakened the dough rheological properties, namely decreased dough stability, the degree of softening at 10 min, and the retention coefficient value. Despite the increase of the total CO2 volume production, the wheat flour dough was not capable of retaining a high amount of CO2 released.
However, the 5 mg/100 g iron ions addition impaired the dough rheological properties in the case of lactate salt more significantly than in the case of gluconate salt. According to the data obtained, ferrous gluconate in the amount of up to 4 mg/100g was optimal to use in bread making wheat flour to ensure good rheological properties of dough.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare no conflict of interest.
БЛАГОДАРНОСТИ
This work was supported by a grant of the Romanian National Authority for Scientific Research and Innovation. CNCS/CCCDI – UEFISCDI. project number PN-III-P2-2.1-BG-2016-0079. within PNCDI III.
СПИСОК ЛИТЕРАТУРЫ
- Noort M.W.J., Mattila O., Katina K., and van der Kamp J.W. HealthBread: Wholegrain and high fibre breads with optimized textural quality. Journal of Cereal Science, 2017, vol. 78, pp. 57–65. DOI: https://doi.org/10.1016/j. jcs.2017.03.009.
- Hoppe M., Hulthen L., and Hallberg L. The relative bioavailability in humans of elemental iron powders for use in food fortification. European Journal of Nutrition, 2006, vol. 45, no. 1, pp. 37–44. DOI: https://doi.org/10.1007/ s00394-005-0560-0.
- Bryszewska M.A., Tomás-Cobos L., Gallego E., et al. In vitro bioaccessibility and bioavailability of iron from breads fortified with microencapsulated iron. LWT – Food Science and Technology, 2019, vol. 99, pp. 431–437. DOI: https:// doi.org/10.1016/j.lwt.2018.09.071.
- Amiri R., Bahraminejad S., and Cheghamirza K. Estimating genetic variation and genetic parameters for grain iron, zinc and protein concentrations in bread wheat genotypes grown in Iran. Journal of Cereal Science, 2018, vol. 80, pp. 16–23. DOI: https://doi.org/10.1016/j.jcs.2018.01.009.
- Alzaheb R. and Al-Amer O. The Prevalence of Iron Deficiency Anemia and its Associated Risk Factors Among a Sample of Female University Students in Tabuk, Saudi Arabia. Clinical Medicine Insights: Women’s Health, 2017, vol. 10, pp. 1–8. DOI: https://doi.org/10.1177/1179562X1774508/117.
- Bryszewska M., Laghi L., Zannoni A., et al. Bioavailability of Microencapsulated Iron from Fortified Bread AssessedUsing Piglet Model. Nutrients, 2017, vol. 9, no. 3, pp. 272. DOI: https://doi.org/10.3390/nu9030272.
- Martinsson A., Andersson C., Andell P., et al. Anemia in the general population: Prevalence, clinical correlates and prognostic impact. European Journal of Epidemiology, 2014, vol. 29, no. 7, pp. 489–498. DOI: https://doi.org/10.1007/ s10654-014-9929-9.
- Rodriguez-Ramiro I., Brearley C.A., Bruggraber S.F.A., et al. Assessment of iron bioavailability from different bread making processes using an in vitro intestinal cell model. Food Chemistry, 2017, vol. 228, pp. 91–98. DOI: https://doi. org/10.1016/j.foodchem.2017.01.130.
- Sadeghi H. and Eatye Salehi E. Effects of iron supply on the rheological properties and sensory characteristics of bread dough enriched with micronutrients. Journal of Fundamental and Applied Sciences, 2016, vol. 8, no. 3S, pp. 203–229. DOI: https://doi.org/10.4314/jfas.v8i3s.177.
- Blanco-Rojo R. and Vaquero P. Iron bioavailability from food fortification to precision nutrition. A review. Inno- vative Food Science and Emerging Technologies, 2019, vol. 51, pp. 126–138. DOI: https://doi.org/10.1016/j. ifset.2018.04.015.
- Hurrell R.F. Fortification: overcoming technical and practical barriers. The Journal of Nutrition, 2002, vol. 132, no. 4,pp. 806S–812S. DOI: https://doi.org/10.1093/jn/132.4.806S.
- Gharibzahedi S.M.T. and Jafari S.M. The importance of minerals in human nutrition: Bioavailability, food fortifica- tion, processing effects and nanoencapsulation. Trends in Food Science & Technology, 2017, vol. 62, pp. 119–132. DOI: https://doi.org/10.1016/j.tifs.2017.02.017.
- Lynch S.R. Why Nutritional Iron Deficiency Persists as a Worldwide Problem. The Journal of Nutrition, 2011,vol. 141, no. 4, pp. 763S–768S. DOI: https://doi.org/10.3945/jn.110.130609.
- Rebellato A.P., Castro Lima J., Silva J.G.S., Steel C.J., and Lima Pallone J.A. Mineral bioaccessibility in French breads fortified with different forms iron and its effects on rheological and technological parameters. Journal of Cereal Science, 2017, vol. 74, pp. 56–63. DOI: https://doi.org/10.1016/j.jcs.2017.01.020.
- Rebellato A.P., Bussi J., Silva J.G.S., et al. Effect of different iron compounds on rheological and technological pa- rameters as well as bioaccessibility of minerals in whole wheat bread. Food Research International, 2017, vol. 94, pp. 65–71. DOI: https://doi.org/10.1016/j.foodres.2017.01.016.
- Richins A.T., Burton K.E., Pahulu H.F., Jefferies L., and Dunn M.L. Effect of iron source on color and appearance of micronutrient-fortified corn flour tortillas. Cereal Chemistry, 2008, vol. 85, no. 4, pp. 561–565. DOI: https://doi. org/10.1094/CCHEM-85-4-0561.
- Walter T., Pizarro F., Abrams S.A., and Boy E. Bioavailability of elemental iron powder in white wheat bread. Euro- pean Journal of Clinical Nutrition, 2004, vol. 58, pp. 555–558. DOI: https://doi.org/10.1038/sj.ejcn.1601844.
- Hurrell R. Use of ferrous fumarate to fortify foods for infants and young children. Nutrition Reviews, 2010, vol. 68, no. 9, pp. 522–530. DOI: https://doi.org/10.1111/j.1753-4887.2010.00312.x.
- Nielsen A.V., Tetens I., and Meyer A.S. Potential of phytase-mediated iron release from cereal-based foods: A quanti- tative view. Nutrients, 2013, vol. 5, no. 8, pp. 3074–3098. DOI: https://doi.org/10.3390/nu5083074.
- Prentice A.M., Mendoza Y.A., Pereira D., et al. Dietary strategies for improving iron status: Balancing safety andefficacy. Nutrition Reviews, 2017, vol. 75, no. 1, pp. 49–60. DOI: https://doi.org/10.1093/nutrit/nuw055.
- Hurrell L., Bothwell C., Glahn H., et al. Enhancing the absorption of fortification iron: A SUSTAIN task force re- port. International Journal for Vitamin and Nutrition Research, 2004, vol. 74, no. 6, pp. 387–401. DOI: https://doi. org/10.1024/0300-9831.74.6.387.
- Li Y.O., Yadava D., Lo K.L., Diosady L. and Wesley A. Feasibility and optimization study of using cold-forming extrusion process for agglomerating and microencapsulating ferrous fumarate for salt double fortification with iodine and iron. Journal of Microencapsulation, 2011, vol. 28, no. 7, pp. 639–649. DOI: https://doi.org/10.3109/02652048. 2011.604434.
- dos Santos Vieira D.A., Steluti J., Verly-Jr E., Marchioni D.M., and Fisberg R.M. Brazilians’ experiences with iron fortification: evidence of effectiveness for reducing inadequate iron intakes with fortified flour policy. Public Health Nutrition, 2016, vol. 20, no. 2, pp. 363–370. DOI: https://doi.org/10.1017/S1368980016001981.
- Codina G.G., Zaharia D., Stroe S.G. Effect of different iron type on bread quality from white wheat flour. In Vitro Cellular & Developmental Biology-Plant, 2018, vol. 54, pp. S33–S34.
- Allen L., de Benoist B., Dary O., and Hurrel R. Guidelines on food fortification with micronutrients. Geneva, Switzer- land: World Health Organization Publ., 2006, 341 p.
- Codină G.G., Dabija A., Stroe S.G. and Ropciuc S. Optimization of iron–oligofructose formulation on wheat flour dough rheological properties. Journal of Food Processing and Preservation, 2018. DOI: https://doi.org/10.1111/ jfpp.13857.
- Codină G.G., Zaharia D., Stroe S.G., and Ropciuc S. Influence of calcium ions addition from gluconate and lactate salts on refined wheat flour dough rheological properties. CyTA – Journal of Food, 2018, vol. 16, no. 1, pp. 884–891. DOI: https://doi.org/10.1080/19476337.2018.1498129.
- Miller R.A. and Hoseney R.C. Role of salt in baking. Cereal Foods World, 2008, vol. 53, pp. 4–6. DOI: https://doi. org/10.1094/CFW-53-1-0004.
- Akhtar S., Anjum F., Rehman S., and Sheikh M.A. Effect of mineral fortification on rheological properties of whole wheat flour. Journal of Texture Studies, 2009, vol. 40, no. 1, pp. 51–65. DOI: https://doi.org/10.1111/j.1745- 4603.2008.00169.x
- Rebellato A.P., Bussi J., Silva J.G.S., et al. Effect of different iron compounds on rheological and technological pa- rameters as well as bioaccessibility of minerals in whole wheat bread. Food Research International, 2017, vol. 94, pp. 65–71. DOI: https://doi.org/10.1016/j.foodres.2017.01.016.
- Codină G.G., Mironeasa S., and Mironeasa C. Variability and relationship among Mixolab and Falling Number evalu- ation based on influence of fungal α-amylase addition. Journal of the Science of Food and Agriculture, 2012, vol. 92, no. 10, pp. 2162–2170. DOI: https://doi.org/10.1002/jsfa.5603.
- Ji T., Penning B., and Baik B.K. Pre-harvest sprouting resistance of soft winter wheat varieties and associated grain characteristics. Journal of Cereal Science, 2018, vol. 83, pp. 110–115. DOI: https://doi.org/10.1016/j.jcs.2018.08.006.
- Donelson J.R., Gaines C.S., Donelson T.S., and Finney P.L. Detection of wheat preharvest sproutingusing a prege- latinized starch substrate and centrifugation. Cereal Chemistry, 2001, vol. 78, no. 3, pp. 282–285. DOI: https://doi. org/10.1094/CCHEM.2001.78.3.282.
- Gray J.A. and Bemiller, J.N. Bread stailing: molecular basis and control. Comprehensive Reviews in Food Science and Food Safety, 2003, vol. 2, no. 1, pp. 1–21. DOI: https://doi.org/10.1111/j.1541-4337.2003.tb00011.x.
- Codină G.G., Zaharia D., Mironeasa S., and Ropciuc, S. Evaluation of wheat flour dough rheological properties by magnesium lactate salt addition. Bulletin UASVM. Food Science and Technology, 2018, vol. 75, no. 1, pp. 21–26. DOI: https://doi.org/10.15835/buasvmcn-fst:0019.
- Codină G.G., Arghire C., Rusu M., Oroian M.A., and Sănduleac E.T. Influence of two varieties of flaxseed flour ad- dition on wheat flour dough rheological properties. Annals of the University Dunarea de Jos of Galati, Fascicle VI: Food Technology, 2017, vol. 41, no. 2, pp. 115–126.