Аннотация
The present research features a natural polymer that can be used for immobilisation of bifidobacteria as well as a method of immobilisation. We described a modified method of microencapsulation of probiotics using sodi- um alginate. The experiment studied the effect of encapsulation on probiotic stability and involved an in vitro model of human digestive tract. The test sample of microencapsulated Bifidobacterium bifidum 791 showed a decrease in the activity from 3.0×107 to 2.2×105 CFU/ml in a mouse model with pH 1.2. By contrast, the control sample, unprotected by biodegradable polymer microcapsules, demonstrated a higher death rate of bifidobacteria: from 2.6×108 CFU/ml to 5.0×103 CFU/ml. The control sample demonstrated the same downward trend in in vitro gastrointestinal models with pH values of 4.5, 6.8, 7.2, and 5.8. Because the total plate count fell down to 4.0l g CFU/ml in acidity gradients, the titres of the initial microencapsulated biomass had to be increased up to > 109 CFU/ml. According to the results of scanning electron microscopy, the new type of microcapsules obtained by using a resistant starch had a closed sur- face. Prebiotics increased the resistance of bacteria to low pH and bile salts. Bifidobacteria encapsulated with natural biodegradable polymers proved to be well-tolerated and harmless for mice. The experiment revealed that biodegrad- able polymer microcapsules did not cause any chronic or acute toxicity when administered orally at 2×107 CFU per 1 gram of animal mass. The microcapsules demonstrated neither dermonecrotic properties nor any irritant effect on the ocular mucosa and, thus, can be used for food enforcement.ВВЕДЕНИЕ
The functional food market keeps holding the leading positions around the world as consumers tend to choose products that taste better and provide additional health benefits. Most consumers would like to prevent some diseases and cure the ones they already have. Therefore, they buy products with bioactive supplements that are able to support their health physiologically. It has been scientifically proven that non-microbial and microbial functional products have a therapeutic effect and can be used in preventive medicine. However, these biologically active ingredients are prone to rapid degradation during food processing, storage, and gastrointestinal transit. One of the best ways to prevent the degradation of these non-microbial and microbial bioactive components is to encapsulate them.
Recently, the popularity of functional foodstuffs on the global food market has increased significantly. The turnover of the global functional food market will reach several hundred billion dollars in the nearest future. In addition to the positive effect they exert on human health, functional foods correspond to and satisfy all basic nutritional needs. Functional food products with probiotics and prebiotics have gained significant market share worldwide, especially in Europe, Asia (Japan), Australia, and, more recently, in the United States.
Despite all their fundamental differences, probiotic and prebiotic approaches to functional foods are equally beneficial for gastrointestinal tract (GIT). As a result, a symbiotic approach, i.e. a combination of probiotic and prebiotic approaches, is becoming more and more popular. Therefore, a number of symbiotic products are currently being developed for functional food markets.
Low survival rate of potential probiotics during storage and intestinal passage may limit the positive qualities of food products. Microencapsulation helps reduce the adverse effects on the viability of probiotics in GIT, as well as during food or nutraceutical processing, storage, and consumption. Microencapsulation separates and protects probiotic cells from the environment before their release.
There are various methods of gel microencapsulation that involve polymers: extrusion method, emulsification method, spray drying technology, etc. The main advantage of microencapsulation is in the controlled release of bacteria.
Microencapsulation is the process of enclosing substances in microcapsules, i.e. a material or a mixture of materials covered, or encapsulated, by another material or system. The coated material is called active, or base, material. It can be solid, liquid, or gaseous. The coating material is called shell, wall material, carrier, or encapsulating agent. Microparticles have a multicomponent structure with a diameter of 1–1,000 micrometers [1]. As a rule, microspheres are described as a matrix system where the active ingredient is dispersed/dissolved in a carrier matrix. Microcapsules have, at least, one discrete domain of the active agent, sometimes more (reservoir system) [2]. As a result, every microcapsule consists of a layer of an encapsulating agent that isolates and protects the active substance from any negative impact. Microcapsules can have a regular (spherical, tubular, or oval) or irregular shape [3].
An analysis of scientific resources resulted in the following list of substances used for microencapsulation of probiotics in food industry: sodium alginate, pectin, chitosan, carrageenan, gelatin, xanthan-gelatin mixture, and cellulose acetyl phthalate. All these substances help mitigate the process of immobilisation, thus, preserving the biological properties of substances and cell integrity. The most common encapsulating material is sodium alginate: it is simple, biocompatible, non-toxic, and cost effective. Alginate is a polysaccharide extracted from algae. It consists of β-d-mannuronic and α-l-guluronic acids. Different amounts and sequential distribution of β-d-mannuronic and α-l-guluronic acids in the chain can affect the functional properties of alginate as an auxiliary material [17].
If a polymer base is chosen as a shell, it results in the formation of microcapsules of various sizes, as well as in a good packing degree, molecular weight, structure, and shape, which guarantees targeted delivery of viable probiotics into the GIT as a part of food matrix.
When microencapsulating probiotics, one should take into account the chemical nature of coating materials. The use of microencapsulation techniques increases the viability of probiotics, both within food products and during their passage through the GIT. However, coating materials behave differently, and, therefore, their ability to protect living microorganisms and deliver biologically active substances also varies. In addition, the effectiveness of material depends not only on its encapsulating properties and strength, but also on its low cost, availability, and biocompatibility [18].
Microcapsules are currently applied in food [4], textile, pharmaceutical [5, 6], cosmetic, and agrochemical [7] industries. This method allows the producers to improve and/or modify the characteristics and properties of the active material, as well as its protection, stabilisation and slow release.
Microencapsulation can modify the colour, shape, volume, pressure sensitivity, heat sensitivity, and photosensitivity of the encapsulated substance [8]. In addition, microencapsulation:
– protects the base material from ultraviolet rays, moisture, and oxygen;
– increases the shelf life of the volatile compound;
– reduces the rate of evaporation or transfer of active material from the core to the medium;
– prevents chemical reaction; reduces the problems of fine powders’agglomeration;
– improves the processing properties of sticky materials;
– controls the release of substances; and
– reduces toxicity.
Thus, a research in the following spheres seems very promising: immobilizing methods of bifidobacterial cells and their use in the development of enforced dairy products from goat or sheep milk. Microencapsulation of bifidobacteria is important since it allows one to preserve the useful properties of bifidobacteria in foodstuffs. In addition, it helps to protect the viable cells from gastric juice, bile, and other external conditions.
The research objective was to provide a scientific basis for choosing a natural polymer as a method of immobilisation of bifidobacteria; to evaluate their physical and chemical characteristics; to study the process of microencapsulation of probiotics with prebiotics; to study the morphological features of microparticles, formed by natural biodegradable polymer (sodium alginate), using optical and electron microscopy.
ОБЪЕКТЫ И МЕТОДЫ ИССЛЕДОВАНИЯ
The research featured extrusion technique in alginate gel microencapsulation of bifidobacteria. Thus, there were two study subjects: microcapsules with bifidobacteria and resistant starch (Hi-maize) as a part of the capsular structure and microcapsules without starch. Dehydrated and hydrated microcapsules were assessed for average diameter, size, and general morphology characteristics.
To obtain a ≥ 109 liquid bifidobacteria concentrate, we used a serial passage. For the first passage, we prepared a culture medium. A sublimated Bifidobacterium bifidum 791 was inoculated into the culture medium and incubated at 37–38°C to obtain microbacterial mass with the bifidobacteria content of 108 CFU/ml. During the second passage, 10% of the cultured Bifidobacterium bifidum 791 was inoculated into the second culture medium at 37–38°C. During the third passage, 10% of the cultured Bifidobacterium bifidum 791 was inoculated into the third culture medium at 37–38°C and incubated for 6 hours. Next, it was centrifuged at 4°C for 20 min at 5,000 rpm. The resulting bacterial concentrate contained at least 1010 CFU/ml and was used to obtain microcapsules.
The microcapsules were obtained using extrusion technique. Two kinds of solution were prepared: the first solution contained 1% of sodium alginate and 1% of prebiotic Hi-maize, the second – 1% of sodium alginate. After the polymers dispersed completely, Bifidobacterium bifidum 791 was added to the solutions. The multicomponent composition was sprayed from 30 cm into 0.1 M of CaCl2 using an airbrush (model EW 110) with a 0.3 mm nozzle that was attached to an air compressor (model Jas – 1203). The resulting particles were stirred for 30 min in a CaCl2 solution to ensure complete gelation. After that they were removed from the solution.
To define the specific activity of the encapsulated probiotics, we used Procedural Guidelines 1.2.2566-09.
The acidic gastric environment was modeled by adding two components into the sterile physiological solution. The first component was 0.5 mg/ml of acidin-pepsin manufactured by RUP Belmedpreparaty (Minsk, Belarus), registration number LS-001355. Each tablet contained 50 mg of proteolytic pepsin enzyme and 200 mg of acidin. The second component was a 0.1 mol solution of HCl (pH ≤ 2.0), which corresponds to the average gastric juice acidity [State Pharmacopoeia CCC ed. XI]. The small intestine environment was modeled by adding 2.5 mg/ml of panzinorm forte 20,000 produced byOOO KRKA-RUS (Istra, Moscow region, Russia), registration number P No. 014602/01. The pH level was adjusted by sterile 0.1 mol solutions of NaOH and HCl.
As a rule, survival studies of probiotic microorganisms are performed on a model in vitro, simulating the process of digestion in the human body. During the first stage, microorganisms are incubated at 37 ± 1°C first in an acidic model environment with acidin-pepsin (pH 2.0) and then in an alkaline model with panzinorm forte 20,000 (pH 6.8–5.8). The incubation time equals the period it takes mixed food to pass through the GIT. After that, the number of surviving microorganisms is assessed according to colony forming units of bifidobacteria (CFU/ml) in tenfold limiting dilutions, as recommended in Procedural Guidelines 4.2.999-00.
To study the protective properties of microcapsules, we used solutions with the following pH gradients: pH 2.0 – gastric environment model (exposure time = 30–120 min); pH 4.5 – duodenum environment model (15–60 min); pH 6.8 – jejunum environment (60–120 min); pH 7.2 – ileum environment (60–120 min); and pH 5.8 – large intestine environment (18 hours). The temperature was 37 ± 1°C. The sample vials were intermittently stirred with circular motions. At each time point, we tested the probiotic survivability using the titration method of tenfold serial dilutions from 10–9 to 10–1 CFU/ml in two parallel rows of test tubes. The cell cultures were thermostated at 37 ± 1°C for 72 hours.
If no significant differences were registered in the number of CFU/ml of control and test samples, it was concluded that the concentration of the substance had no effect on the in vitro GIT model. If the CFU/ml in the test samples decreased significantly (up to one logarithmic order) in comparison with the control sample, the effect was declared inhibitory.
The general morphology of the microcapsules was determined with the help of a scanning electron microscope (SEM) (MIRA3 TESCAN). The microcapsules were placed on the substrate of the mechanical microscope stage using a double-sided gold-flashed tape. The accelerating direction of the microscope was 5 kV. The diameters of the microcapsules were determined using the ImageJ software (NIH, USA). The average diameter was calculated by measuring 100 microcapsules.
Fifty white mice of the SHK line of both sexes were selected to assess the safety level of the microcapsules. The animals were provided by the Research Centre for Biomedical Technologies of the Russian Federal Medical-Biological Agency (certificate number 05815, 11.05.2017– 19.10.2017; veterinary certificate 250 № 0860445, 11.05.2017). The mice had a 21-day quarantine.
The dermonecrotic properties (irritant effect on mucous tissues) were studied on rabbits of Soviet Chinchilla breed. The animals were provided by the Federal State Unitary Enterprise, Experimental production farm (Manihino branch) (veterinary certificate 250 №0819660, 11.22.2016). The rabbits were quarantined for 30 days.
Three series of experiments were conducted to estimate the levels of safety, acute toxicity, chronic toxicity, and dermonecrotic properties.
Sample groups were formed according to body weight and age factor. The animals were kept in a vivarium according to Sanitary Rules 2.2.1.3218-14.
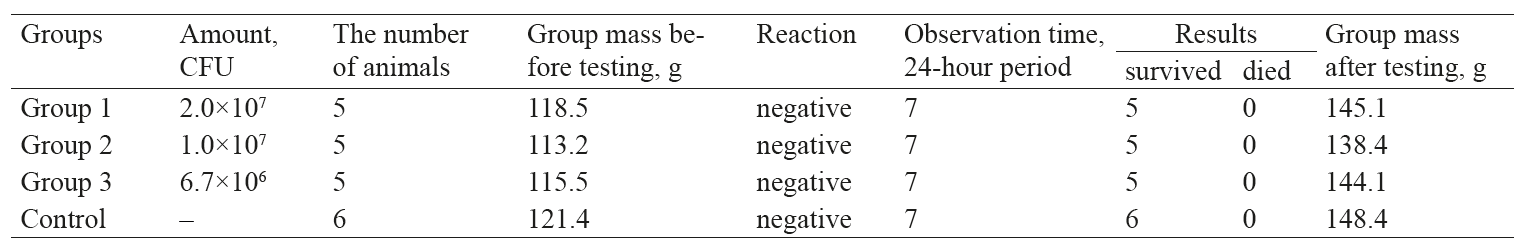
The analysis of safety and acute toxicity of microcapsules was conducted according to Procedural Guidelines 4.2.2602-10. As a result, three experimental groups of five animals were formed. According to the key selective factors, each laboratory rodent had to be clinically healthy, well-fed, active and mobile, with a good pelage, normally coloured mucous membranes, and formed stool. The average animal weight was 20 ± 5 g.
A paste of microcapsules with concentration of bifidobacteria 1×108 CFU of microbial cells per 1 g was diluted with saline C080812 (expiration date September 2017). The paste was prepared in three doses. The ratio of the paste and the saline in the first dose was 1:5 while the amount of microbial cells was 2×107 CFU/ml. In the second dose, the ratio was 1:10, and the amount of cells equalled 1×107 CFU/ml. As for the third dose, the ratio was 1:15, and the amount of microbial cells was 6.7×106 CFU/ml. The solutions were administered orally using a 1 ml feeding tube. Four hours before the manipulations, the animals had stopped taking food and water. Feeding was resumed two hours after the procedure. As a control measure, physiological saline C080812 (expiration date: September 2017) was orally administered to the control group. The control group of five mice was used simultaneously in parallel experiments in safety, acute toxicity, and chronic toxicity. All the animals belonged to the same lot.
The dermonecrotic properties of the microcapsules were tested on rabbits of Soviet Chinchilla breed.
In vivo dermal irritation tests were performed on the anterior segment of the eyes of three rabbits. The animals weighed 1,500–2,500 g and were kept in a standard vivarium at 22 ± 2°C with a 12-hour synchronised change of the light period (Sanitary Rules 2.2.1.3218-14). The animals were grouped according to body weight and age factor. During the whole research period, the animals received briquetted feed produced by OOO Laboratorkorm.
Animal testing was performed according to State Standard ISO 10993-10-2011.
The bifidobacteria microcapsules were tested for the chronic toxicity according to Procedural Guidelines 4.2.2602-10. Three groups of six animals were formed. All animals received specialized briquetted feed produced by OOO Laboratorkorm and water.
Group no. 4 was experimental. These animals received an experimental preparation, i.e. microcapsules composed of natural biodegradable polymers containing bifidobacteria.
Group no. 5 was control group. Animals received an alternative preparation, i.e. Bifidobakterin produced by ZAO Ecopolis (Kirov, Russia), series 792 (release date: March 2017; expiration date: April 2019).
Physiological saline C 080812 (expiration date: September 2017) was orally administered to the animals of the control group. To analyse the chronic toxicity, the preparations were administered orally once a day for 14 days to six animals in the experimental groups. The amount was equivalent to that proposed for humans. The dose was calculated according to the number of bifidobacteria in Bifidobacterin as stated in the product label. The dose of bifidobacteria in the microcapsules was identical to that in Bifidobacterin. For a 14 g mouse the concentration was 2.14×105 CFU. The animal behaviour and state of health were registered during the 14 days of administration and 7 days after the trial. Death rate of animals, state of hair, activity, colour of mucous membranes, body weight, and bowel movements were daily recorded.
РЕЗУЛЬТАТЫ И ИХ ОБСУЖДЕНИЕ
There are a lot of modern methods of microencapsulation, and this number continues to increase as companies keep patenting more and more innovative microencapsulation technologies [10, 11]. The methods make it possible to encapsulate active material. However, the final choice of microencapsulation method depends on the type of encapsulated material, the release characteristics of the encapsulated compound, application, and regulatory requirements, which can affect the final characteristics and properties of the microparticles. The whole spectre of microencapsulation methods can be divided into three main categories: chemical processes, which included interfacial and in situ polymerization methods; physicochemical processes, which involved coacervation (phase separation) and evaporation/emulsified solvent extraction; and physicomechanical processes,which involved air suspension method, spray drying, spraying, spray cooling, and fluidized bed coating.
Table 1 shows various methods of microencapsulation. The data presented in the table characterize the effectiveness of each encapsulation process.
Although complex and simple coacervation are the most effective methods, they are more costly. Spray drying and extrusion are second best according to the efficiency rating. Spray cooling and molecular incorporation are the least effective encapsulation techniques.
Table 2 demonstrates some of the most important and common methods of microencapsulation, the size of particles obtained by various methods of microencapsulation, as well as the advantages and disadvantages of every method.
According to Table 2, there are several advantageous techniques for immobilisation of bifidobacteria. However, extrusion proves to be the most acceptable variant, given the limitations described.
Polysaccharides are the most widely used materials for various encapsulation techniques. They are followed by proteins and lipids.
The following types of carriers were selected to obtain bifidobacteria microcapsules, based on the cost parameter, as well as on safety and technology indexes.
Alginate gels are quite suitable for encapsulation of eukaryotic and prokaryotic cells. Microencapsulation using alginate gel was evaluated as a possible method for improving the viability of probiotics during the low pH exposure and storage.
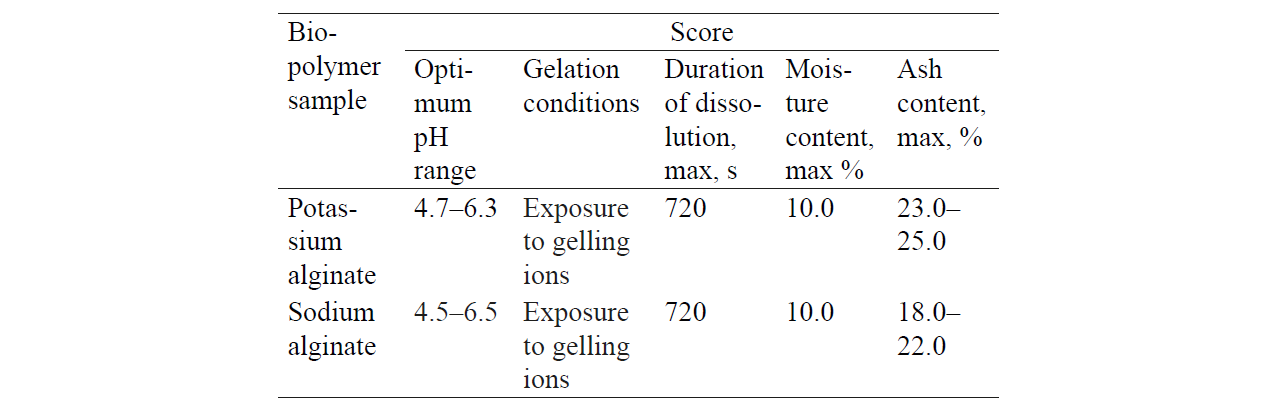
Table 3 presents some physicochemical parameters of natural non-toxic biodegradable biopolymers.
Thus, the analysis of the physicochemical properties presented in Table 3 allows one to conclude that all these samples of biological polymers can be used for the immobilisation of bifidobacteria.
As for the molecular aspect, the alginate creates a particularly strong molecular structure in the presence of Ca2+. As a result, one can obtain cold-prepared, thermoreversible, and freeze-thaw resistant microcapsules.
Probiotics are living microorganisms that help consumers to improve their health. However, such kinds of microorganisms lose their viability and stability rather easily due to various physical and physiological conditions and factors.
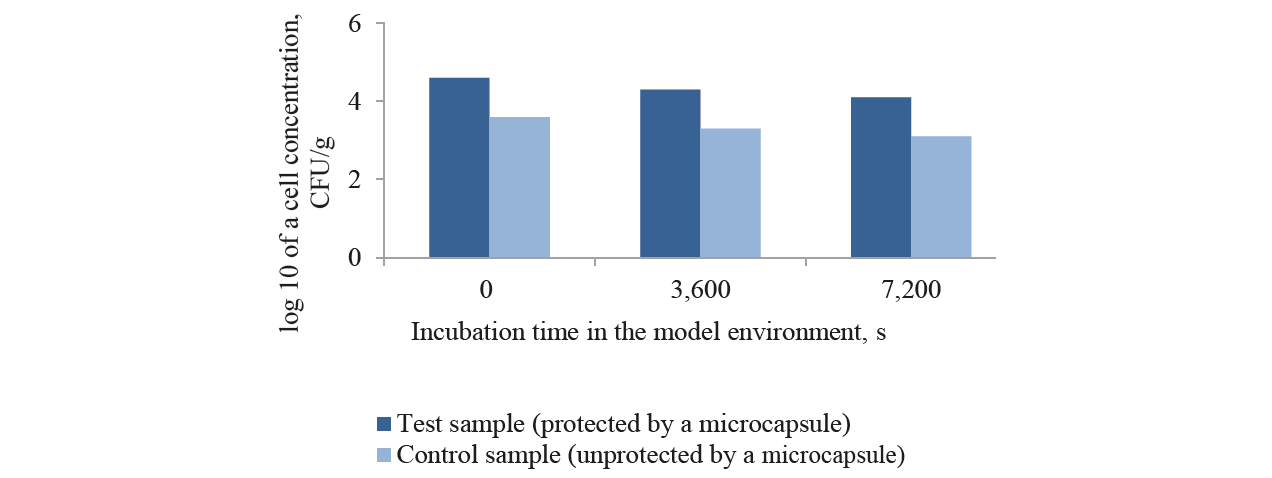
The selected immobilisation method has a great effect on the viability of associated probiotic bacteria. It proved to be an effective method of probiotic viability improvement. Figs. 1–4 show some results of probiotic survivability in vitro in a GIT model.
During the test on bifodobacteria survival rate in a simulated gastric environment at pH 1.2 (Fig.3), the microencapsulated test sample demonstrated a reduction from 3.0×107 to 2.2×105 CFU/g. The unprotected control sample showed a higher death rate – from 2.6×108 CFU/ml to 5.0×103 CFU/g. The same downward trends in the viability of unprotected bifidobacteria were registered in in vitro GIT models with pH 4.5; 6.8; 7.2; and 5.8 (Figs. 1–4). This confirms the protective effect of the biodegradable natural polymer on bifidobacteria during their passage throughthe in vitro gastric model. However, because the total plate count fell down to 4.0 lg CFU/g in acidity gradients (in vitro GIT model), the titers of the initial microencapsulated biomass had to be increased up to > 109 CFU/g. Probiotics must have a 106–107 level of living microorganisms per 1 gram of product when administered orally to maintain viability when passing through the GIT.
Alginate immobilisation of bifidobacteria protects them from aggressive external factors. To increase the stability of bifidobacteria, resistant starch (Hi-maize) was introduced into the composition of the biodegradable microcapsules. If prebiotics are introduced into the walls of probiotic microcapsules, it provides an improved protection for active microorganisms.
Some researchers also reported of a higher bacterial survival rate in alginate microcapsules containing prebiotics in a GIT model (fructo-oligosaccharides, galacto-oligosaccharides/inulin, fructo-oligosaccharides, monosaccharides, respectively) compared with alginate microcapsules without prebiotics [19, 20].
Some studies suggest that alginate-based microcapsules may provide a limited protection for probiotics due to its specific properties. For example, microcapsules obtained by extrusion using alginate as the main carrier and biopolymer are not stable in an acidic medium. Moreover, the microspheres obtained on the basis of alginate are characterized by a porous structure and provide an easy diffusion of acid into and out of the microspheres. These disadvantages can be effectively eliminated: alginate can be combined with other polymers or structurally modified using various additives [21].
Our method of obtaining microcapsules based on biodegradable non-toxic polymers of natural origin allows one to obtain microcapsules with a closed surface and specific sizes.
The optical microscopy of wet microparticles was performed using a microscope and a digital-still camera. The morphology of the lyophilized microparticles was evaluated using a scanning electron microscope. Microcapsules were mounted on aluminum plugs using a double-sided adhesive tape and then sprayed with gold.
The shape of the wet microparticles was close to spherical, and the core material was distributed throughout the matrix (Fig. 5). The optical micrographs show that alginate particles and microorganisms were found inside the microparticle. Thus, microencapsulation of Bifidobacterium bifidum 791 was effective for both treatments.
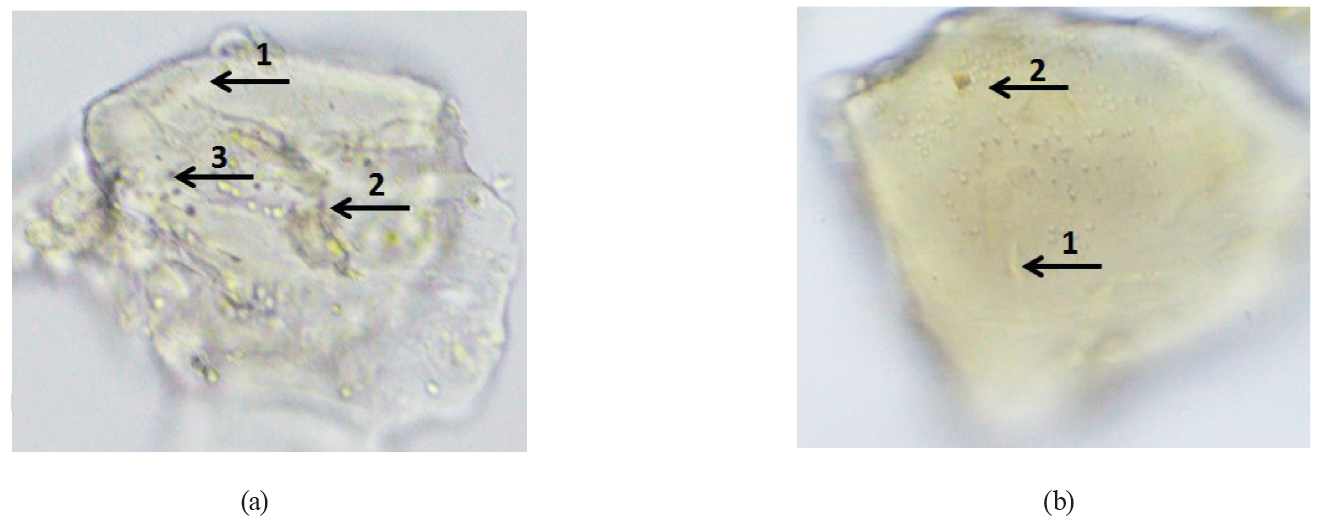
Scanning electron microscopy (Fig. 6) revealed that the morphology of freeze-dried microparticles had a high agglomeration of particles and a variety of particle size distribution for both treatments.
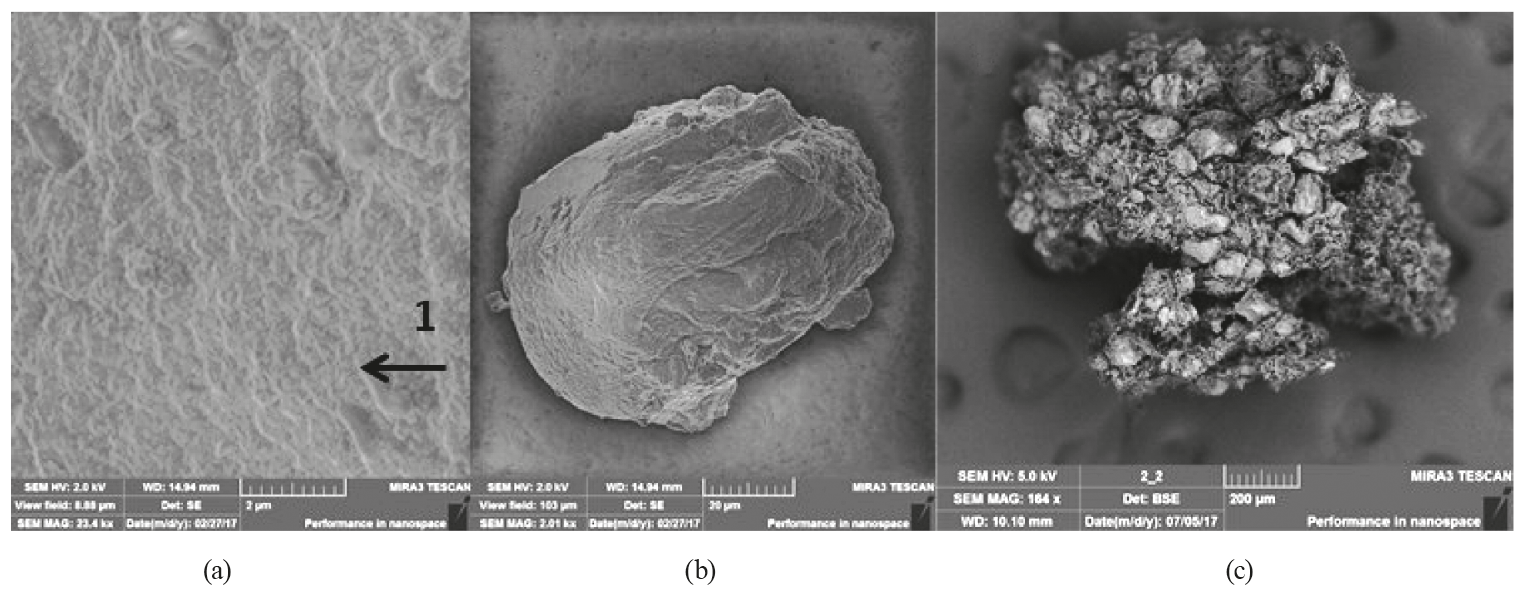
A sharp dehydration of lyophilized polysaccharide gels results in a porous matrix. In the process of lyophilization, all microcapsules were exposed to low temperatures. This led to the formation of ice crystals and the sublimation of ice under reduced pressure, resulting in a porous, dry product. The microparticles containing resistant Hi-Maize starch were more agglomerated if compared to the alginate microparticles.
The use of Hi-maize resistant starch in the process of microencapsulation did not significantly affect the diameters of wet microparticles.
As a result, the lyophilized microparticles had an average diameter of 150 and 97 micrometers for the matrix of microparticles alginate + Hi-maize and the alginate matrix, respectively. The structural changes caused by the process of freeze-drying increase the pore size, which results in a quick and full rehydration. Thus, freeze-dried microparticles swell quickly after being immersed in water and get larger than those wet microparticles that have not been lyophilized.
Nontoxicity and harmlessness of the new components, as well as the benefits to the human health, are the key factors in using additives in food dairy products.
For this purpose, acute toxicity, chronic toxicity, and dermonecrotic properties were tested in animals. The tests on the safety level and acute toxicity proved that microcapsules made of natural biodegradable polymers with bifidobacteria were harmless to white mice of the SHK line of both sexes.
The mice were observed for 7 days. The experiment lasted 14 days. All animals survived the tests.
All the animals looked healthy and active, had a good appetite and a nice white thick tight pelage. The abdominal zone was not enlarged. The urinary frequency and urine colour corresponded to the physiological norm. The colour of the mucous membranes and the bowel habits remained the same during the entire time of the experiment. The behaviour of the test animals did not differ from that of the control group. Table 4 demonstrates the results of weighing.
On day 14 after the administration of the preparations, the animals were euthanized with chloroform, and further morphological studies of the internal organs followed.
A macroscopic examination did not register any effect of the preparations on the state of the internal organs of mice; no differences were found between the control and experimental groups.
The location of the internal organs was proper. Free fluid in the pleural and abdominal cavities was not detected. The lumen of the trachea and bronchi was unobstructed; the mucous membrane was moist and slimy. The spleen was elongated, not enlarged, with a dense texture and smooth surface. The liver was not enlarged, had a proper shape, with a dense homogeneous smooth and slimy texture without inclusions.
When administered orally, the dose of LD50 was not determined since the administered doses caused no clinical signs of poisoning (dose limitation was due to the possibility of administering a concentrated preparation through a probe).
When determining the chronic toxicity of microcapsules, the biodegradable microcapsules did not produce any chronic toxicity effect on the white mice of the SHK-line of both sexes when administered orally.
When determining the dermonecrotic properties of microcapsules, the assessment of the local irritant effect on the ocular mucosa was carried out on rabbits: 1–2 drops (0.1 ml ≤ 100 mg) of suspended microcapsules were introduced into the conjunctival sac of the left eye in diluted form. The ratio of the paste and the saline was 1:5 while the amount of bacterial cells equalled 2×107 CFU/g. Five minutes after application, the eyes were rinsed with distilled water. The ocular mucosa was inspected 1 hour after the introduction of the preparation and on the next day. The right eye of the animal served as a control sample. Observation of animals continued for 14 days. 24 hours after the preparations were applied to the rabbits’ eyes, the following results were obtained: hyperemia – 0 points, swelling – 0 points, accumulation of serous secretions in the canthi – 0–1 points; cornea damages were not observed in any animal. The total score was 0–1. The results of irritation of the conjunctiva were assessed according to a 5-point scale, as recommended by Mikhailov [22].
On the second day after the exposure, the signs of eye irritation disappeared: the ocular mucosa recovered completely.
Thus, the preparation of biodegradable polymer microcapsules with bifidobacteria in diluted form 1:5 at a dose of 2×107 CFU/g had a slight irritant effect on the ocular mucosa. According to State Standard 1.12.007-76, it corresponds with the 4th hazard class.
ВЫВОДЫ
The research featured a comparative analysis of various methods for obtaining immobilised probiotic cultures and their analogues.
The results of the analysis determined the choice of the method and the carrier for the immobilisation of bifidobacteria.
The morphological characteristics of microparticles were studied by using optical and scanning electron microscopy. The shape of the wet microparticles was close to spherical, and the core material was distributed throughout the matrix. The optical micrographs showed that alginate particles and microorganisms were found inside the microparticle.
The study also featured the effect of resistant starch on the process of immobilisation of bifidobacteria. The resistant starch (Hi-maize) in combination with alginate had a synergistic effect on gelation, providing additional protection for the probiotic cells.
We analysed the structural changes of microparticles caused by the process of freeze-drying. The scanning electron microscopy proved that the morphology of the freeze-dried microparticles had a high extent of agglomeration of particles, as well as a variety of particle size distribution.
All the characteristics of the obtained microcapsules underwent a comparative assessment. In the process of lyophilization, the microcapsules were exposed to low temperatures, which led to the formation of ice crystals and ice sublimation under reduced pressure, resulting in a porous, dry product. The microparticles containing resistant Hi-Maize starch were more agglomerated compared to the alginate microparticles. The lyophilized microparticles had an average diameter of 150 and 97 micrometers, corresponding to alginate microparticles with resistant starch (Hi-maize) microparticles with alginate matrix. The structural changes caused by the process of freeze-drying increased the pore size, which resulted in a quick and full rehydration.
The study proved the biodegradable polymer microcapsules with bifidobacteria to be well-tolerated and harmless for laboratory rodents. The experiment revealed that the microcapsules did not cause any chronic or acute toxicity if administered orally at a dose of 2×107 CFU per 1 g of animal mass. The microcapsules demonstrated neither dermonecrotic properties nor irritant effect on the ocular mucosa and can be used for food enforcement.
The research revealed good prospects for studying the properties and structure of microcapsules with immobilised bifidobacteria and their use in the food industry. The functional characteristics of a biopolymer particle ultimately depend on its composition, physicochemical properties, and structural characteristics. Therefore, it is a priority to study the most important characteristics of biopolymer particles and their connection with the physicochemical and sensory properties of food products.
Immobilisation of bifidobacteria in microcapsules makes it possible to preserve the useful properties of bifidobacteria in foods. In addition, it helps protect the viable cells from the negative impact of gastric juice, bile, and other external conditions.
Based on the above, it seems promising to continue the studies of immobilizing methods of bifidobacterial cells in the structure of natural biodegradable polymers and their use in the development of fortified dairy products.
КОНФЛИКТ ИНТЕРЕСОВ
The authors declare that there are no conflicts of interest related to this article.
БЛАГОДАРНОСТИ
The authors would like to express their sincere gratitude to Olga G. Zhilenkova, Candidate of Biological Sciences, the acting head of the Laboratory of Bifidobacterial Biologyat G.N. Gabrichevsky Moscow Research Institute of Epidemiology and Microbiology (Rospotrebnadzor).
ФИНАНСИРОВАНИЕ
The research was carried out as part of the first stage (sub-stage № 1) of the grant program ‘Start’ financed by the Innovation Promotion Fund; contract №1402/GC1/22672 (20.07.2016) with OOO BIOMILKYUG; research topic ‘Development of the biotechnology of a fermented milk drink using immobilised probiotic cultures of microorganisms’.
СПИСОК ЛИТЕРАТУРЫ
- Lam K.H., Cheng S.Y., Lam P.L., et al. Microencapsulation: past, present and future. Minerva Biotecnologica, 2010, vol. 22, no. 1, pp. 23–28.
- Patravale V.B. and Mandawgade S.D. Novel cosmetic delivery systems: an application update. International Journal of Cosmetic Science, 2008, vol. 30, no. 1, pp. 19–33. DOI: https://doi.org/10.1111/j.1468-2494.2008.00416.x.
- Huang H.-J., Chen X.D., and Yuan W.-K. Microencapsulation based on emulsification for producing pharmaceutical products: a literature review. Developments in Chemical Engineering and Mineral Processing, 2006, vol. 14, no. 3–4, pp. 515–544.
- Onwulata C.I. Microencapsulation and functional bioactive food. Journal of Food Processing and Preservation, 2012, vol. 37, pp. 511–532. DOI: https://doi.org/10.1111/j.1745-4549.2012.00680.x.
- Scalia S., Coppi G., and Iannuccelli V. Microencapsulation of a cyclodextrin complex of the UV filter, butyl methoxy- dibenzoylmethane: In vivo skin penetration studies. Journal of Pharmaceutical and Biomedical Analysis, 2011, vol. 54, no. 2, pp. 345–350. DOI: https://doi.org/10.1016/j.jpba.2010.09.018.
- Lam P.-L., Lee K.K.-H., Wong R.S.-M., et al. Development of hydrocortisone succinic acid/and 5-fluorouracil/chi- tosan microcapsules for oral and topical drug deliveries. Bioorganic & Medicinal Chemistry Letters, 2012, vol. 22, no. 9, pp. 3213–3218. DOI: https://doi.org/10.1016/j.bmcl.2012.03.031.
- Alonso M.L., Laza J.M., Alonso R.M., et al. Pesticides microencapsulation. A safe and sustainable industrial process. Journal of Chemical Technology & Biotechnology, 2014, vol. 89, no. 7, pp. 1077–1085. DOI: https://doi.org/10.1002/ jctb.4204.
- Lamprecht A. and Bodmeier R. Microencapsulation. Weinheim: Wiley-VCH Publ., 2012, vol. 23, pp. 157–171.DOI: https://doi.org/10.1002/14356007.a16_575.pub2
- Singh M.N., Hemant K.S.Y., Ram M., and Shivakumar H.G. Microencapsulation: A promising technique for con- trolled drug delivery. Journal Research in pharmaceutical sciences, 2010, vol. 5, no. 2, pp. 65–77.
- Gamez-Garcia M. Fragrance delivery system for surface cleaners and conditioners. Patent, no. WO 2005/041918 A1, 2005.
- Weber H. and Raehse W. Cleaning agent contains fragrance microparticles. HAPPI, 2009, vol. 46, pp. 1–5.
- Xiao Z., Liu W., Zhu G., Zhou R., and Niu Y. A review of the preparation and application of flavour and essential oils microcapsules based on complex coacervation technology. Journal of the Science of Food and Agriculture, 2014, vol. 94, no. 8, pp. 1482–1494. DOI: https://doi.org/10.1002/jsfa.6491.
- Lam P.L. and Gambari R.J. Advanced progress of microencapsulation technologies: In vivo and in vitro models for studying oral and transdermal drug deliveries. Journal of Controlled Release, 2014, vol. 178, no. 1, pp. 25–45. DOI: https://doi.org/10.1016/j.jconrel.2013.12.028.
- Gharsallaoui A., Roudaut G., Chambin O., Voilley A., and Saurel R. Applications of spray-drying in microencapsula- tion of food ingredients: An overview. Food Research International, 2007, vol. 40, no. 9, pp. 1107–1121. DOI: https:// doi.org/10.1016/j.foodres.2007.07.004.
- Bansode S.S., Banarjee S.K., Gaikwad D.D., Jadhav S.L., and Thorat R.M. Microencapsulation: A review. Interna- tional Journal of Pharmaceutical Sciences Review and Research, 2010, vol. 1, no. 2, pp. 38–43.
- McClements D.J., Decker E.A., Park Y., and Weiss J. Structural Design Principles for Delivery of Bioactive Com- ponents in Nutraceuticals and Functional Foods. Critical reviews in food science and nutrition, 2009, vol. 49, no. 6, pp. 577–606. DOI: https://doi.org/10.1080/10408390902841529.
- Sadovoy V.V., Selimov M.A., Slichedrina T.V., and Nagdalian A.A. Usage of biological active supplements in tech- nology of prophilactic meat products. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 2016, vol. 7, no. 5, pp. 1861–1865.
- Barybina L.I., Voblikova T.V., Beloysova E.V., et al. Usage of Vegetable Stuff in Dry Sausage Production. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 2018, vol. 9, no. 4, pp. 1536–1540.
- Krasaekoopt W. and Watcharapoka S. Effect of addition of inulin and galactooligosaccharide on the survival of mi- croencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt and fruit juice. LWT – Food Science and Technology, 2014, vol. 57, no. 2, pp. 761–766. DOI: https://doi.org/10.1016/j. lwt.2014.01.037.
- Hassan A., Nawaz M., and Rasco B. Microencapsulation, survival and adherence studies of indigenous probi- otics. African Journal of Microbiology Research, 2014, vol. 8, no. 8, pp. 766–775. DOI: https://doi.org/10.5897/ AJMR2013.6182.
- Burgain J., Gaiani C., Linder M., and Scher J. Encapsulation of probiotic living cells: From laboratory scale to in- dustrial application. Journal of Food Engineering, 2011, vol. 104, no. 4, pp. 467–483. DOI: https://doi.org/10.1016/j. jfoodeng.2010.12.031.
- Mikhaylov P. Meditsinskaya kosmetika. Rukovodstvo [Medical cosmetics. Guide]. Moscow: Meditsina Publ., 1985. 208 p. (In Bul.).