Abstract
Yogurt is known as a suitable carrier of probiotics. Its supplementation with Iranian grape syrup used as a prebiotic can enhance its sensory and physicochemical properties, as well as improve the viability and growth of probiotics. Therefore, we aimed to investigate the effect of Iranian grape syrup on stirred probiotic yogurt’s rheological, physicochemical, and microbial properties.Probiotic yogurt samples were fortified with 3, 6, and 9% of Iranian grape syrup and evaluated in terms of pH, acidity, syneresis, viscosity, total phenolic and anthocyanin contents, as well as probiotic bacterial counts during 21 days of storage in a refrigerator at 4°C.
The results revealed that increasing concentrations of grape syrup inversely affected the yogurt’s pH, so the lowest and highest pH levels were recorded in the samples with the highest syrup concentration and the control (without syrup), respectively. No general trend was observed in acidity despite significant differences in acidity among the syrup-supplemented yogurts and the control (p ≤ 0.05). Syneresis demonstrated an inverse correlation, while viscosity exhibited a direct relationship, with a grape syrup concentration. Monitoring microbial changes in the samples throughout storage revealed a better growth in microbial colonies in the yogurts with higher grape syrup concentrations.
According to consumer preferences and physicochemical qualities, the optimal concentration of Iranian grape syrup was found to be 9%. Supplementing yogurt with grape syrup enhances its probiotic viability and metabolic activity. Considering its positive impact on both consumer preferences and product properties, Iranian grape syrup can be utilized as a prebiotic in future research to develop functional and symbiotic yogurts.
Keywords
Fermentation, grape syrup, probiotics, prebiotics, yoghurt, bacterial countINTRODUCTION
Yogurt is a widely consumed dairy product with high nutritional value. It is produced through the fermentation of milk by various bacteria, mainly lactic acid bacteria and bifidobacteria [1, 2]. The consumption of yogurts containing probiotic bacteria has significantly increased in recent years. In general, yogurt and other dairy products are referred to as suitable carriers of probiotic microorganisms. Recently, attention has been drawn to various compounds that can positively affect yogurt’s bacterial, rheological, and physicochemical properties and increase its nutritional value.
Grape syrup is a highly viscose honey-like product that is widely used in Iran. It is usually produced by heating the juice from grapes that are not suitable for fresh consumption. In some cases, unfermented grape syrup, which has a °Brix value of about 68, is used as a natural sweetener instead of sugar. According to the classical method of grape syrup production, white soil is added to the grape juice, and the mixture is boiled, cooled, and filtered. The filtered liquid is further boiled and concentrated to obtain syrup. Iranian grape syrup contains iron, phosphorus, calcium, and potassium. It is also rich in organic acids, vitamins A, C, B1, and B2, as well as some antioxidants, including flavonoids [3]. In some communities, Iranian grape syrup is used as a dessert – alone or in combination with other foods such as yogurt. Adding prebiotic compounds, such as syrup or fruit extracts, can lead to better consumer acceptance of dairy products, especially yogurt, as well as increases the viability of probiotic bacteria. The Iranian Askari grape is a common type of grape in Iran that is cultivated for fresh consumption and preparing Iranian grape syrup. Previous studies have revealed that adding grape extract to probiotic yogurt can increase the product’s nutritional value and improve its rheological, microbial, and sensory properties [4, 5].
When adding different compounds to probiotic yogurt, attention should be paid to the subsequent changes in the product’s physical, chemical, and sensory properties as an essential factor in consumer acceptance [4, 6]. In this study, we aimed to evaluate the effect of Iranian grape syrup used as a prebiotic at different concentrations (3, 6, and 9%) on the rheological, physicochemical, and microbial properties of stirred probiotic yogurt throughout storage.
STUDY OBJECTS AND METHODS
Probiotic cultures. The probiotic cultures Lactobacillus rhamnosus, Bifidobacterium animalis subsp. lactis BB-12 and Lactobacillus acidophilus La-5 in the freezedried form were obtained from Chr. Hansen (Denmark).
Preparing Iranian grape syrup. To prepare 4 kg of Iranian grape syrup, Asgari grapes purchased from the local market in Qazvin (Qazvin province, Iran) were washed and cleaned with distilled water. Then, we prepared grape juice (3 kg), transferred it to cooking utensils, added 100 g of white yogurt. The cooking utensils were heated to the boil and then left to reach room temperature. After filtering the juice through filter paper and measuring its pH, we heated it further until grape syrup was extracted at a high concentration [3].
Preparing yogurt. Fresh milk needed to prepare yogurt was obtained from the dairy workshop at the Department of Food Science and Technology (University of Tehran, Karaj, Iran). It had a pH of 6.4 and contained 3% of fat, 0.906% of ash, and 13% of total acids. The initial culture containing Streptococcus thermophiles and Lactobacillus delbrueckii spp. bulgaricus in the freeze-dried form (YO-FAST-88) was purchased from Chr. Hansen (Denmark). The milk was heated at 80°C for 15 min and then cooled to 42 ± 1°C to add the starter. After the temperature decreased, Iranian grape syrup was added to the milk in a ratio of 3:9 (v/v). Then, the samples were incubated at 42 ± 1°C and transferred to plastic containers. The incubation process continued until the pH of the samples reached 4.6. Both the control and experimental (with Iranian grape syrup) samples were stored at 4°C until used in the experiment.
Measuring the total titratable acidity and pH. The value of total titratable acidity was calculated on the °Dornic scale by adding 9 g of distilled water to a 9-g yogurt sample and titrating it with 0.1 N NaOH to the endpoint with the phenolphthalein detector [7]. The pH was measured by the direct electrode immersion method and the pH meter (WTW inoLab® 720, Weilheim, Germany).
Measuring the total phenolic content. To measure the total phenolic content of the samples, we employed a method described by Latifi et al. with some modifications [8]. First, we mixed 100 μL of the test solution and 100 μL of Folin-Ciocalteu’s phenol reagent (1 N solution) and allowed the mixture to react at room temperature for 3 min. Then, we added 300 μL of 1 N Na2CO3 to each tube and incubated them for 90 min at room temperature. Finally, 1 mL of distilled water was added to the tubes to complete the reaction. The absorbance was determined at 725 nm using a spectrophotometer. The results were expressed as mg of gallic acid equivalents per 10 g sample.
Microbiological examination of yogurt. To determine bacterial count in the yogurt, 10 g of a sample was homogenized in 90 mL of peptone water (0.1% peptone) in a Stomacher 400 homogenizer (Type BA7021, Seward Medical, UK) for a minimum of 2 min. The samples were diluted in peptone water and inoculated in other containers of 0.1 mL. The plate count method was employed for S. thermophiles and L. delbrueckii subsp. bulgaricus on M17 agar and MRS agar, respectively (Merck, Iran). The samples were incubated for two days at 37°C (aerobic) and three days at 43°C (anaerobic) with anaerocult sachets (Merck). The L. acidophilus LA-5 and Bifidobacterium BB-12 were counted on MRS-Sorbitol agar (1% sorbitol) and MRS-NNLP agar (100 mg neomycin sulfate, 15 mg nalidixic acid, and 3 g LiCl), respectively. The results of counting plates containing 20–200 colonies were expressed in colony-forming units per gram of sample (CFU g-1) [9].
Measuring the total anthocyanin content. The pH differential method described by Jaafar et al. was employed to determine the total anthocyanin content with a UV-601spectrophotometer (Shimadzu, Japan) [10]. The wavelengths of 520 and 700 nm were used for the samples in 0.025 M potassium chloride buffer (pH 1.0) and 0.4 M sodium acetate buffer (pH 4.5) to measure the anthocyanin content (A – (A λ520–A λ700) pH 1.0 – (A λ520–A λ700) pH 4.5). Accordingly, the anthocyanin content of each sample was obtained in mg of malvidin-3-O-glucoside equivalent per kg of the sample.
Measuring the syneresis. The Syphon method was applied to calculate the spontaneous whey separation of yogurt. For this, three tubes containing 5 g of yogurt each were weighed before and after syneresis [11]. The amount of spontaneous whey separation, %, was calculated using the following equation:
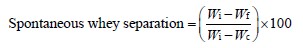
where Wi is the initial weight of the tube containing yogurt, g; Wf is the final weight of the tube containing yogurt after syneresis, g; Wc is the weight of the empty tube, g
Viscosity. A cylindrical programmable viscometer (Rheomat RM 100, France) was employed to calculate the viscosity. The shear velocity was 70.8 rpm. Accordingly, the viscosity of each sample was obtained in cP/Poises.
Sensory evaluation. A 7-point scale was used to specify the sweetness and color of the samples, with 1 and 7 being the lowest and highest scores, respectively (ISO 4121, 2003). In the evaluation of sweetness, monochromatic illumination was applied to hide the color differences. The samples (6°C) were evaluated by a test panel of 15 panelists aged 22–35. According to ISO 8589 (2007), the evaluations were performed in a standardized test room. According to ISO 8587 (2006), yogurt acceptance was evaluated by a ranking test from 1 (lower tendency) to 3 (highest tendency) by 60 students aged 22–26.
Statistical analysis. The samples were randomly selected in all the experiments with three repetitions. The means were classified based on a significance level of 0.05 using a one-way ANOVA test with SPSS software (Version 19). The graphs were drawn using Excel 2010 software.
RESULTS AND DISCUSSION
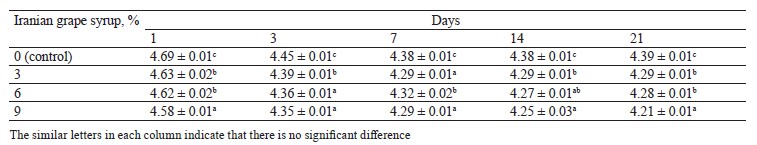
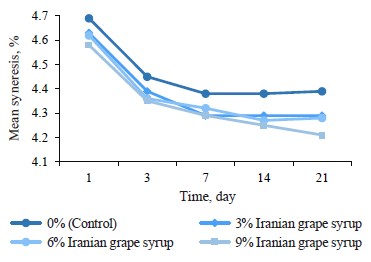
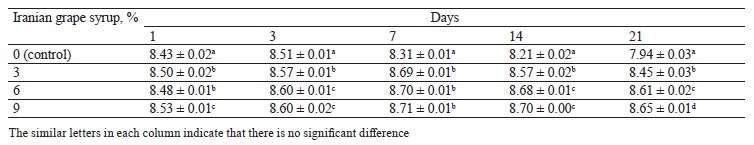
pH changes. The evaluation of pH changes in the samples with Iranian grape syrup and the control showed significant differences at each of the evaluation points (p ≤ 0.05) (Table 1). The mean pH of all the samples significantly decreased from day 1 to day 3, followed by a gentler decline until day 7. Afterwards, the sample with 3% Iranian grape syrup, along with the control, showed constant pH values until day 14, while the samples with 6 and 9% Iranian grape syrup continued to decrease (Fig. 1). The lowest pH (4.21 ± 0.01) was recorded in the sample with 9% Iranian grape syrup throughout storage, while the highest pH (4.69 ± 0.01) was registered in the control sample on day 1 (Table 1). The other samples ended the storage period on day 21 with an increase or no change in pH values. The trend of pH changes showed that the concentration of Iranian grape syrup and the storage time were in inverse relation to pH (Fig. 1).
Our results did not agree with some previous studies. In particular, Sarwar et al. found that the introduction of inulin alongside Saccharomyces boulardii demonstrated no notable impact on the alteration of pH or acidity in synbiotic yogurt [13]. This observation suggests that neither the prebiotic (inulin) nor yeast (S. boulardii) exerted a discernible influence on the acid production capacity of the starter lactic acid bacteria in yogurt.
However, numerous studies showed similar trends to the ones we observed in our study. For example, Ghasem- pour et al. reported a decrease in pH during the storage of functional Manuka honey yogurts containing probiotics [14]. The authors attributed the decreasing trend to post-acidification, resulting from the continuous production of organic acids by the starter culture bacteria throughout the product’s shelf life.
Shahein et al., who examined probiotic-fermented camel milk fortified with date syrup, reported a significant decrease in pH values and a concurrent increase in acidity across all treatments during the 15-day storage period [15]. The authors attributed these results to the influence of camel milk on microorganism growth impacting pH values over time. Another study, which formulated probiotic yogurt with basil seed gum and red beet extract, found a more conspicuous trend of pH reduction in the samples characterized by an elevated quantity of basil seed gum at 0.4%, particularly when supplemented with 0.1% red beet extract [14]. In a study fortifying probiotic yogurt with almond milk, higher concentrations of almond milk significantly attenuated pH reduction, which was attributable to the absence of its buffering capacity [16]. Similarly, the incorporation of passion fruit juice into set yogurt during the initial fermentation phase was found to induce a decline in pH, effectively inhibiting the proliferation of diverse bacterial strains [14].
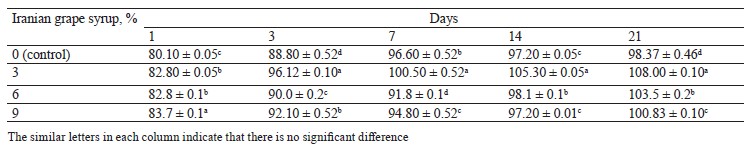
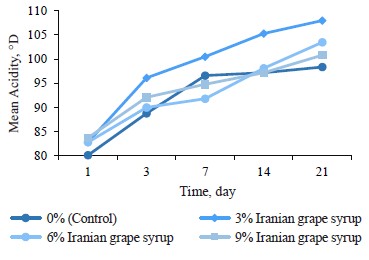
Acidity changes. The changes in titratable acidity of the samples during storage indicated significant differences between the samples with Iranian grape syrup and the control at each of the evaluation points (p ≤ 0.05) (Table 2). The highest increase in average acidity was observed from day 1 to day 3, followed by different slopes for each sample until the end of the storage period (Fig. 2). The sample containing 3% Iranian grape syrup consistently had the highest acidity throughout storage, reaching 108.0 ± 0.1°D on day 21. The control sample showed the lowest acidity at the end of storage (98.37 ± 0.46°D) and on the first day of storage (80.10 ± 0.05°D). A study consistent with our research showed a rise in titratable acidity of camel milk fermented with date syrup compared to the control during storage. This rise was attributed to the conversion of lactose to lactic acid by the yogurt starter culture, coupled with a stimulating effect of date syrup’s composition (simple sugars, glucose and fructose, and nutrients) on bacterial activity [15]. Nevertheless, this elevation in acidity was not tangible due to the same reason in a study by Mohan et al. on functional manuka honey. The authors reported that inherent sweetness and supplementary flavor constituents in the stingless bee honey proficiently obscured the heightened acidity generated throughout fermentation [12].
A number of previous studies were consistent with our results. In the study of synbiotic yogurt containing inulin and S. boulardii, all the samples exhibited a slight decrease in pH and an increase in titratable acidity during storage [13]. In the study that fortified probiotic yogurt with almond milk, an elevation in milk concentration induced a surge in acidity [16]. In another study, the addition of apple peel-derived phenolic extract marginally increased the acidity of probiotic yogurt compared to the control [18].
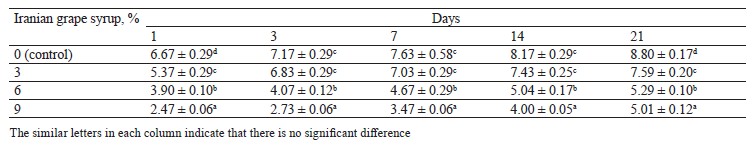
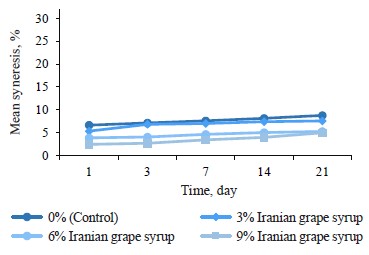
Syneresis changes. The syneresis changes among the samples during storage indicated significant differences between the samples with Iranian grape syrup and the control at each of the evaluation points (p ≤ 0.05) (Table 3). Furthermore, we found an inverse relationship between the concentration of Iranian grape syrup in the sample (3, 6, and 9%) and its syneresis percentage during the storage period (Fig. 3). According to these results, the samples with 9% Iranian grape syrup consistently recorded lower syneresis percentages than the other samples throughout storage, with the lowest value on day 1 (2.47 ± 0.06%). In contrast, the control samples consistently showed higher percentages of syneresis than the Iranian grape syrup yogurts, with the highest value on day 21 (8.80 ± 0.17%). In the study of probiotic yogurt fortified with manuka honey, the cohesiveness values exhibited no significant differences among the sweetened samples over the storage period and remained lower than those of the unsweetened control sample [12]. Notably, the unsweetened samples displayed a pronounced visual syneresis, a phenomenon associated with elevated cohesiveness readings in yogurts.
Karaca et al. concluded that larger amounts of apricot fiber in yogurt increased its water-holding capacity, whereas prolonging the storage period caused a decrease in this capacity [19]. Some studies have also reported similar findings. For example, introducing higher concentrations of inulin into yogurt to create a synbiotic product reduced its syneresis due to its enhanced waterholding capacity [13]. Similarly, lower syneresis resulted from incorporating polymerized whey protein with xanthan gum and pectin as gelation agents into a symbiotic almond yogurt alternative [20]. In another study, adding apple peel-derived phenolic extract to probiotic yogurt decreased the product’s syneresis while enhancing its firmness and viscosity. The study also underscored the potential for syneresis control through the augmentation of total solid contents in the yogurt formulation [18].
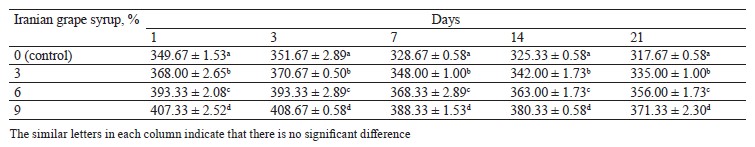
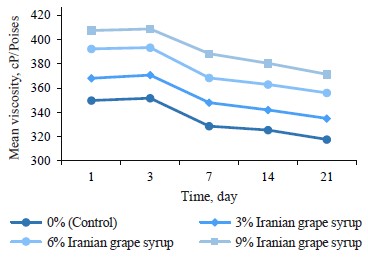
Viscosity changes. Evaluating viscosity changes throughout the storage period revealed that the samples containing Iranian grape syrup and the control sample had significant differences at each of the evaluation points (p ≤ 0.05) (Table 4). We also found a direct relationship between the concentration of Iranian grape syrup in the samples (3, 6, and 9%) and their average viscosity at all evaluation points (days 1, 3, 7, 14, and 21). During the entire storage period, the yogurt with 9% grape syrup and the yogurt without grape syrup (control) showed the highest and lowest viscosity values, respectively. On the whole, after a slight increase in the samples’ viscosity on day 3, the viscosity trend for all the samples was downward until the end of the storage period (Fig. 4). Accordingly, the highest viscosity (408.67 ± 0.58 cP/Poises) was recorded in the sample with 9% Iranian grape syrup on day 3, while the lowest viscosity (317.67 ± 0.58 cP/Poises) was observed in the control sample on day 21.
The results of some studies entirely or partly align with our findings. In particular, incorporating inulin into yogurt augmented its viscosity by effectively binding and orienting water molecules that did not integrate into the protein network. This consequently impeded wheying off and fostering robust aggregation of casein micelles [13]. Conversely, adding invert syrup to yogurt resulted in markedly higher viscosity compared to manuka honey samples at equivalent concentrations, with a discernible reduction observed over the storage period [12]. Furthermore, introducing date syrup to camel milk yielded synbiotic products with significantly higher viscosity, possibly due to the presence of oligosaccharides in date syrup [15]. In the study that fortified yogurt with basil seed gum and red beet extract, the interplay between basil seed gum and storage time significantly influenced yogurt viscosity, with concentration-dependent effects (higher gum levels corresponded to higher yogurt viscosity) [14]. Similarly, a symbiotic almond yogurt-like product formulated with polymerized whey protein as a gelation agent alongside pectin and xanthan gum exhibited non-significant variations in viscosity throughout storage [20]. In another study, addition of apple peelderived phenolic extract to probiotic yogurt demonstrated a direct relationship between yogurt viscosity and extract concentration, with higher concentrations leading to notably elevated viscosity [18].
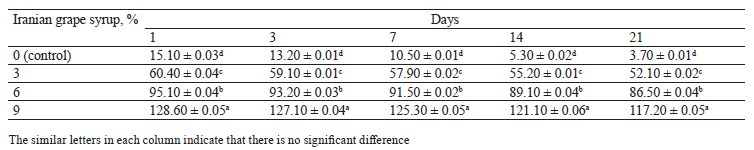
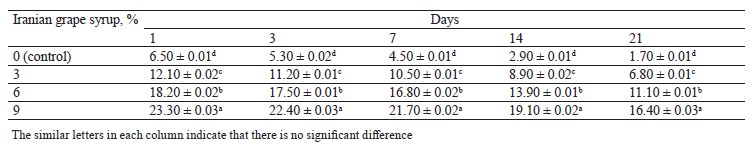
Total polyphenol and anthocyanin contents. The total polyphenol contents of the probiotic yogurt samples supplemented with Iranian grape syrup are presented in Table 5. As can be seen, the total polyphenol content was influenced by the concentration of Iranian grape syrup (Table 5). The yogurt fortified with 9% Iranian grape syrup had a higher polyphenol content (128.6 mg gallic acid equivalents/mL) than the other samples on day 1 (p < 0.01). Generally, phenolic compound levels in the yogurt samples decreased during 21 days of storage.
Table 6 presents total anthocyanin contents of the yogurts containing different concentrations of Iranian grape syrup and the control sample. As can be seen, the highest anthocyanin content (23.3 mg malvidin-3-O-glucoside equivalents/mL) was recorded in the sample with 9% Iranian grape syrup on day 1, while the lowest anthocyanin content (1.7 mg malvidin-3-O-glucoside equivalents/mL) was observed in the control sample on day 21.
Numerous studies have indicated that phenolic compounds can enhance probiotics’ ability to stick to surfaces and endure conditions resembling the digestive tract. Phenolic compounds also seem to positively influence the composition of gut microbiota, benefiting markers and risks related to chronic illnesses. Additionally, metabolites produced by gut microbes (including probiotics) from phenolic compounds offer health advantages surpassing the original compounds [21]. Our findings demonstrated that increased amounts of Iranian grape syrup in the yogurt samples led to higher total anthocyanin contents. We also found that increasing the storage time over 14 days reduced the anthocyanin contents. Thus, storage time had a significant effect on the polyphenol and anthocyanin contents of the yogurt samples.
Silva et al. reported that goat yogurt formulated with fiber- and phenolic-rich ingredients from the integral valorization of Isabel grape had a positive effect on gut microbiota and human health [22]. Our findings were consistent with the results obtained by Fuhrman et al., who determined total phenolic and anthocyanin contents in red and white grape varieties during storage [23]. According to Jackman et al., phenolic and anthocyanin compounds are readily degradable and form colorless or brown-colored compounds [24]. The stability of anthocyanin is influenced by pH, microbial activity, storage temperature, enzymes, and metallic ions in the environment [24]. Okogeri and Tasioula-Margari also reported that phenolic compounds degraded during storage in virgin olive oils [25]. Similarly, we found significant decreases in polyphenol and anthocyanin contents in the yogurt samples.
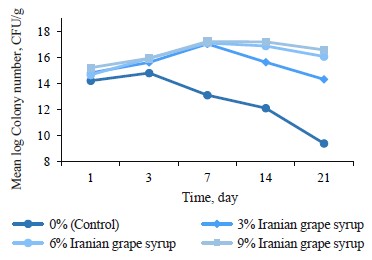
Microbial changes. Evaluating microbial changes in the yogurts showed significant differences in the samples containing Iranian grape syrup and the control sample at each of the evaluation points (p ≤ 0.05) (Table 7). As can be seen, the average number of bacterial colonies increased from day 1 to day 3. This trend continued up to day 7 for all the samples except the control, with the highest number of colonies (8.71 ± 0.01 CFU/g) recorded in the sample containing 9% Iranian grape syrup. After day 7, the number of bacterial colonies in the 3, 6, and 9% samples had a decreasing trend until the end of the storage period. The control sample had the lowest number of colonies (7.94 ± 0.03 CFU/g) on day 21. Throu ghout the entire storage period, the control yogurt showed consistently lower numbers of colonies, while the 9% Iranian grape syrup yogurt showed consistently higher numbers of bacterial colonies than the other samples at all the evaluation points (Fig. 5).
A number of studies align with some of our findings. Sarwar et al. reported that the survivability of S. boulardii and lactic acid bacteria in synbiotic yogurt notably decreased over a four-week storage period, while inulin was a crucial factor that preserved the viability of the yeast in the yogurt matrix [13].
In the study of manuka honey-added yogurt, the total counts of viable probiotics exhibited a decline over a three-week refrigerated storage period, with an exception noted in the yogurt containing invert syrup. Nevertheless, the viable counts of probiotics remained consistently above the recommended minimum dosage of 6 log CFU/mL throughout storage [12]. Another study, where date syrup was added to camel milk, observed a significant increase in total bacterial and bifidobacterial counts, with the degree of augmentation correlating with the quantity of date syrup added. This augmentation was potentially attributed to certain components in date syrup, particularly oligosaccharides, which can act as prebiotics and stimulate bacterial growth [15]. Additionally, a study focusing on almond milk added to probiotic yogurt revealed that while the content of fermentable sugars in almond milk supported probiotic bacterial growth, it was insufficient for metabolic activity. Increasing the amount of almond milk substantially elevated the viability of probiotics, suggesting its potential as a substrate for diverse functional product development [16]. Finally, incorporating apple peel polyphenol extract as a prebiotic ingredient into yogurt not only resulted in viable counts above the minimum requirement for probiotics but also demonstrated the highest viable counts throughout fermentation and a subsequent 21-day refrigerated storage period, indicating an enhancement in probiotic survival [18].
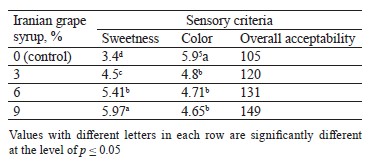
Sensory changes. Evaluating sweetness values in the yogurts with Iranian grape syrup and the control revealed significant difference (p ≤ 0.05). We found a direct relationship between the concentration of Iranian grape syrup in the sample and its sweetness, with the highest sweetness (5.97) recorded in the 9% Iranian grape syrup yogurt. We also observed significant differences in the color of the Iranian grape syrup samples and the control (p ≤ 0.05). Based on the scores given by the panelists, there was no significant difference between the samples containing 3, 6, and 9% Iranian grape syrup. In the ranking test for sample acceptance, the total scores for the control sample and the samples containing 3, 6, and 9% Iranian grape syrup were equal to 105, 120, 131, and 149, respectively. According to Table 8, the values did not differ significantly (p ≥ 0.05), and the acceptance of the samples by the panelists was identical.
Our results were consistent with some previous studies. In particular, introducing date syrup into camel milk resulted in a marked enhancement of sensory scores (flavor, consistency, appearance, and overall evaluation) in the resulting synbiotic products [15]. Furthermore, the yogurts fortified with apple peel extract showed notable distinctions in taste and color compared to the control yogurt throughout the storage period [18]. The addition of inulin to yogurt was found to enhance mouthfeel and taste, while the incorporation of the probiotic yeast S. boulardii resulted in decreased scores for yogurt texture. This reduction in texture scores was attributed to the production of alcohol and carbon dioxide, contributing to an overall improvement in flavor and taste [13]. In contrast, the sensory evaluation of manuka honey-fortified synbiotic yogurts containing Lactobacillus reuteri DPC16 showed no statistically significant differences in consumer ratings for color, appearance, mouthfeel, and smoothness. However, overall acceptance appeared to be dependent on consumer preferences for sweetness and sourness levels in the yogurts [12]. Additionally, higher levels of red beet extract and basil seed gum in the yogurts led to pronounced antioxidant activity but a decline in the sensory scores during storage [14].
CONCLUSION
Our study demonstrated that high concentrations of Iranian grape syrup could increase the number of probiotic bacterial colonies in yogurt. According to our findings, higher concentrations of Iranian grape syrup increased the viscosity and decreased the syneresis of the yogurt, so the amount of grape syrup has a significant impact on achieving the desired rheological properties. The activity of bacteria affected by different concentrations of Iranian grape syrup can lead to changes in the physicochemical properties of yogurt. Our study indicated that the acidity and sweetness of the probiotic yogurt containing Iranian grape syrup and fermented with a mixture of starter cultures are directly related to the concentration of Iranian grape syrup, whereas the product’s syneresis and pH are in inverse relation to Iranian grape syrup concentration. Particularly, higher concentrations of Iranian grape syrup showed a decrease in syneresis and pH but an increase in acidity and sweetness. We found that the yogurt containing 9% of grape syrup received the highest consumer’s ratings and had the best physicochemical properties. Our study also showed that supplementing yogurt with Iranian grape syrup can improve the viability and metabolic activity of probiotic bacteria. Thus, Iranian grape syrup can be used as a prebiotic in further research to develop functional and symbiotic yogurts.
Contribution
F. Abdolmaleki wrote the manuscript. R. Rezaei Mokarram conceived and designed the analysis. M. Daneshniya and M.H. Maleki collected and analyzed the data.CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- Hadjimbei E, Botsaris G, Chrysostomou S. Beneficial effects of yoghurts and probiotic fermented milks and their functional food potential. Foods. 2022;11(17):2691. https://doi.org/10.3390/foods11172691
- Baskar N, Varadharajan S, Rameshbabu M, Ayyasamy S, Velusamy S. Development of plantbased yogurt. Foods and Raw Materials. 2022;10(2):274–282. https://doi.org/10.21603/2308-4057-2022-2-537
- Naderi Beni B, Naderi Beni A. An experimental study of the effects of the mud boil-up process on the physico-chemical properties of grape syrup. Iranian Journal of Chemistry and Chemical Engineering. 2019;38(1):155–161. https://doi.org/10.30492/IJCCE.2019.29494
- Felix da Silva D, Junior NNT, Gomes RG, dos Santos Pozza MS, Britten M, Matumoto-Pintro PT. Physical, microbiological and rheological properties of probiotic yogurt supplemented with grape extract. Journal of Food Science and Technology. 2017;54:1608–1615. https://doi.org/10.1007/s13197-017-2592-x
- Silva FA, de Oliveira MEG, de Figueirêdo RMF, Sampaio KB, de Souza EL, de Oliveira CEV, et al. The effect of Isabel grape addition on the physicochemical, microbiological and sensory characteristics of probiotic goat milk yogurt. Food and Function. 2017;8:2121–2132. https://doi.org/10.1039/C6FO01795A
- Jovanović M, Petrović M, Miočinović J, Zlatanović S, Laličić Petronijević J, Mitić-Ćulafić D, et al. Bioactivity and sensory properties of probiotic yogurt fortified with apple pomace flour. Foods. 2020;9(6):763. https://doi.org/10.3390/foods9060763
- de Brito AA, Campos F, dos Reis Nascimento A, Damiani C, da Silva FA, de Almeida Teixeira GH, et al. Non-destructive determination of color, titratable acidity, and dry matter in intact tomatoes using a portable Vis-NIR spectrometer. Journal of Food Composition and Analysis. 2022;107:104288. https://doi.org/10.1016/j.jfca.2021.104288
- Latifi Y, Chaharlang M, Daneshniya M, Khaki Arani S, Barzanooni M, Boghori P. Antioxidant and antimicrobial properties of methanolic extract of green walnut skin. Journal of Food Science And Technology. 2021;17(108):127–136. https://doi.org/10.52547/fsct.17.108.127
- Torabi M, Abdolmaleki F. Investigation of physicochemical and antioxidant properties of goat milk enriched with barley malt extract fermented with some lactic acid bacteria. Research and Innovation in Food Science and Technology. 2023;12(1):77–93. https://doi.org/10.22101/JRIFST.2022.340359.1353
- Jaafar NF, Ramli ME, Mohd Salleh R. Optimum extraction condition of Clitorea ternatea flower on antioxidant activities, total phenolic, total flavonoid and total anthocyanin contents. Tropical Life Sciences Research. 2020;31(2):1–17. https://doi.org/10.21315/tlsr2020.31.2.1
- Cui L, Chang SKC, Nannapaneni R. Comparative studies on the effect of probiotic additions on the physicochemical and microbiological properties of yoghurt made from soymilk and cow's milk during refrigeration storage (R2). Food Control. 2021;119:107474. https://doi.org/10.1016/j.foodcont.2020.107474
- Mohan A, Hadi J, Gutierrez-Maddox N, Li Y, Leung IKH, Gao Y, et al. Sensory, microbiological and physicochemical characterisation of functional manuka honey yogurts containing probiotic Lactobacillus reuteri DPC16. Foods. 2020;9(1):106. https://doi.org/10.3390/foods9010106
- Sarwar A, Aziz T, Al-Dalali S, Zhao X, Zhang J, Din J, et al. Physicochemical and microbiological properties of synbiotic yogurt made with probiotic yeast Saccharomyces boulardii in combination with inulin. Foods. 2019;8(10):468. https://doi.org/10.3390/foods8100468
- Ghasempour Z, Javanmard N, Langroodi AM, Alizadeh-Sani M, Ehsani A, Kia EM. Development of probiotic yogurt containing red beet extract and basil seed gum; techno-functional, microbial and sensorial characterization. Biocatalysis and Agricultural Biotechnology. 2020;29:101785. https://doi.org/10.1016/j.bcab.2020.101785
- Shahein MR, Atwaa E-SH, Elkot WF, Hijazy HHA, Kassab RB, Alblihed MA, et al. The impact of date syrup on the physicochemical, microbiological, and sensory properties, and antioxidant activity of bio-fermented camel milk. Fermentation. 2022;8(5):192. https://doi.org/10.3390/fermentation8050192
- El-Sayed SM, El-Sayed HS, Elgamily HM, Youssef AM. Preparation and evaluation of yogurt fortified with probiotics jelly candy enriched with grape seeds extract nanoemulsion. Journal of Food Processing and Preservation. 2022;46(7):e16713. https://doi.org/10.1111/jfpp.16713
- Ning X, Luo Z, Chen Z, Zhou C, Xie C, Du W, et al. Fortification of set yogurt with passion fruit juice: Effects on fermentation kinetics, physicochemical properties, and functionality. Journal of Dairy Science. 2021;104(4):4084–4093. https://doi.org/10.3168/jds.2020-19261
- Ahmad I, Khalique A, Shahid MQ, Ahid Rashid A, Faiz F, Ikram MA, et al. Studying the influence of apple peel polyphenol extract fortification on the characteristics of probiotic yoghurt. Plants. 2020;9(1):77. https://doi.org/10.3390/plants9010077
- Karaca OB, Güzeler N, Tangüler H, Yaşar K, Akın MB. Effects of apricot fibre on the physicochemical characteristics, the sensory properties and bacterial viability of nonfat probiotic yoghurts. Foods. 2019;8(1):33. https://doi.org/10.3390/foods8010033
- Shi H, Kraft J, Guo M. Physicochemical and microstructural properties and probiotic survivability of symbiotic almond yogurt alternative using polymerized whey protein as a gelation agent. Journal of Food Science. 2020;85(10):3450–3458. https://doi.org/10.1111/1750-3841.15431
- de Souza EL, de Albuquerque TMR, dos Santos AS, Massa NML, de Brito Alves JL. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities – A review. Critical Reviews in Food Science and Nutrition. 2019;59(10):1645–1659. https://doi.org/10.1080/10408398.2018.1425285
- Silva FA, Queiroga RCRE, de Souza EL, Voss GB, Pintado MME, da Silva Vasconcelos MA. Ingredients from integral valorization of Isabel grape to formulate goat yogurt with stimulatory effects on probiotics and beneficial impacts on human colonic microbiota in vitro. Food Science and Human Wellness. 2023;12(4):1331–1342. https://doi.org/10.1016/j.fshw.2022.10.034
- Fuhrman B, Volkova N, Suraski A, Aviram M. White wine with red wine-like properties: Increased extraction of grape skin polyphenols improves the antioxidant capacity of the derived white wine. Journal of Agricultural and Food Chemistry. 2001;49(7):3164–3168. https://doi.org/10.1021/jf001378j
- Jackman RL, Yada RY, Tung MA, Speers TRA. Anthocyanins as food colorants – A review. Journal of Food Biochemistry. 1987;11(3):201–247. https://doi.org/10.1111/j.1745-4514.1987.tb00123.x
- Okogeri O, Tasioula-Margari M. Changes occurring in phenolic compounds and α-tocopherol of virgin olive oil during storage. Journal of Agricultural and Food Chemistry. 2002;50(5):1077–1080. https://doi.org/10.1021/jf010895e