Abstract
Diseases associated with metabolic disorders seem to affect more and more people worldwide. Biologically active supplements may prevent or relieve metabolic disorders. Quercetin is known for its potential to inhibit metabolic syndrome. This paper introduces an in vivo experiment on rodents. It featured hypoglycemic, hypocholesterolemic, and hepatotoxic properties of quercetin.Quercetin was obtained from the hairy root extract of Hedysarum neglectum Ledeb. Two doses (50 and 100 mg/kg) were used to evaluate its hypoglycemic potential. Rats with induced diabetes were tested for body weight, glucose, and cholesterol while mice with induced hypercholesterolemia were checked for blood cholesterol changes. Potential biochemical and pathological changes in the liver were also studied on rats.
Quercetin treatment caused neither significant health problems nor death in the model animals. It had no effect on body weight, even in the animals with induced diabetes. In addition, quercetin did not increase glucose and cholesterol in the blood and triggered no pathological changes in the liver.
Quercetin isolated from H. neglectum hairy root extract demonstrated no hepatotoxicity. Unfortunately, it showed no beneficial effect on cholesterol and glucose levels and had no efficacy against metabolic syndrome. Further research is needed to assess the effect of quercetin on other metabolic markers, e.g., genes associated with the metabolism of lipids, carbohydrates, etc.
Keywords
Quercetin, in vivo, Hedysarum neglectum, metabolic syndrome, hepatotoxicity, hypercholesterolemic activity, hypoglycemic activityINTRODUCTION
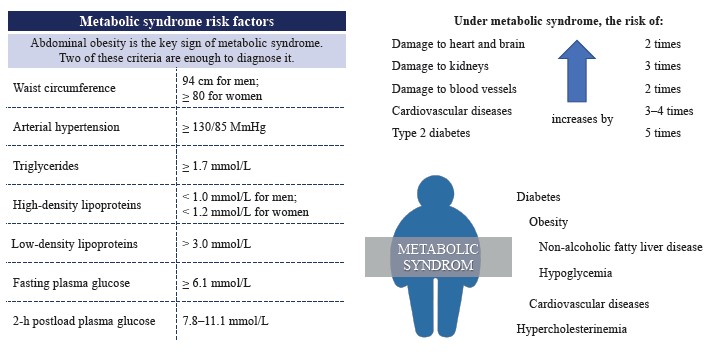
Metabolic syndrome is a cluster of metabolic conditions that lead to dyslipidemia, hyperglycemia, insulin resistance, oxidative stress, inflammations, and, ultimately, to non-alcoholic fatty liver disease, obesity, diabetes mellitus, cardiovascular diseases, etc. [1]. Figure 1 illustrates the risk factors most closely tied to metabolic syndrome as evaluated by the All-Russian Association of Cardiologists [2, 3].
The diseases in Fig. 1 pose a threat to healthcare worldwide: for instance, cardiovascular diseases are the leading cause of death globally [4]. The number of people newly diagnosed with metabolic disorders has increased in the recent years (Fig. 2) [5–7].
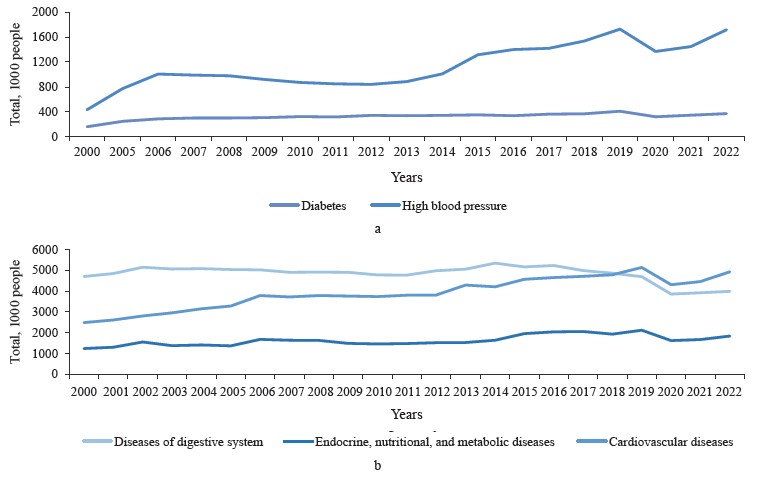
On the one hand, metabolic conditions, e.g., diabetes and hypertension, accelerate age-related pathologies as they facilitate inflammatory and oxidative processes [9–11]. On the other hand, metabolic syndrome itself is age-related. According to Dominguez & Barbagallo, it is global population aging that stands behind the high incidence of metabolic syndrome because senior citizens often experience cardiovascular diseases, diabetes, and metabolic conditions all together (Fig. 3) [12].
Preventive measures against metabolic disorders involve healthy lifestyle combined with functional foods and biologically active additives that contain metabolites of plant origin with antioxidant and anti-inflammatory properties [13, 14]. Such additives reduce cholesterol and glucose in the blood. For example, Sotiropoulou et al. and Serba et al. reported some effective biologically active substances of plant origin that improve human metabolism [15, 16].
Shabbir et al. highly praised quercetin, curcumin, and resveratrol as substances that inhibit inflammatory reactions, reduce insulin resistance, and counteract pathogenic and opportunistic strains that harm gastrointestinal microbiota [17]. All these beneficial properties make them potential preventers of metabolic diseases. Xu et al. studied berberine (Berberis aristata, Berberis vulgaris, Coptis chinensis), an alkaloid found in many medicinal plants of India and China [18]. Berberine proved able to stimulate insulin secretion, facilitate insulin resistance, inhibit lipogenesis, and prevent adipose tissue fibrosis. In addition, it reduced liver steatosis and improved gastrointestinal microbiota.
Quercetin is a wide-spread plant flavonoid with antioxidant, anti-inflammatory, hypoglycemic, hypolipidemic, and hepatoprotective properties that have good potential against metabolic syndrome [17, 19, 20].
Belovol et al. gave quercetin to patients with arterial hypertension [4]. The additive improved the effectiveness of antihypertensive therapy, e.g., reduced cholesterol and triglycerides. Other studies in vivo reported quercetin as able to remove free radicals. It also regulated enzymatic defenses by affecting such enzymes as superoxide dismutase, catalase, glutathione peroxidase, etc. Quercetin had a positive impact on such properties of red blood cells as deformability, nitric oxide production, and osmotic resistance. It regulated metabolic dysfunction of glucose and lipids while exhibiting hepatoprotective properties, thus preventing metabolic syndrome [15, 21, 22]. Quercetin owes this activity to the effect that it exerts on the expression of some genes associated with inflammatory reactions, e.g., SIRT1, NF-κB p65, iNOS, etc., as well as on lipid and carbohydrate metabolism, e.g., PON1, PPARG, ALDH1B1, APOA4, etc. [15].
As their systematic intake causes few or no side effects, herbal biologically active substances are popular as remedies against metabolic disorders. Such substances as stanines or biguanides often cause gastrointestinal problems, liver dysfunction, weight gain, and other effects that restrict healthy life activities [18].
In our previous study, we established that the hairy root extract of Hedysarum neglectum Ledeb., also known as sweet vetch, exhibits cardioprotective potential. We used soil nematode Caenorhabditis elegans to test the extract, and the model organisms demonstrated better survival under oxidative stress and accumulated less lipid fractions [23]. We linked this activity to quercetin metabolite and its ability to prevent metabolic syndrome. However, as part of dietary supplements and functional foods, the extracts’ efficacy depended on the extraction conditions, the initial composition of the raw materials, and the eventual variability of the quantitative and qualitative composition. Therefore, we extracted individual components from the extracts to be used in dietary supplements and functional foods. Thus, quercetin we isolated from the hairy root extract of H. neglectum increased the survival rate of nematodes under oxidative stress and reduced the accumulation of lipid inclusions. In addition, quercetin also increased the expression of the antioxidant defense gene SOD-3 [24].
In this in vivo study, we evaluated the bioactivity of quercetin isolated from the H. neglectum hairy root extract, i.e., its ability to reduce cholesterol and glucose, protect the liver, and prevent metabolic syndrome.
We tested its effect on hypoglycemic properties, hy- pocholesterolemic activity, and hepatotoxicity.
STUDY OBJECTS AND METHODS
The research featured 95% quercetin obtained as a secondary metabolite of Hedysarum neglectum Ledeb. hairy roots. We described the cultivation, extraction, and purification processes in our previous publication [24].
All studies in vivo were conducted on the premises of Ifar Company, Tomsk. For the study, we used rodents that are similar to humans in physiological, cellular, and other functions. They were chosen for their small size, high reproduction rate, low maintenance, and short average life expectancy [4, 25].
For the hypoglycemic tests, we used 50 male rats (Rattus sp., 12 weeks old, 219–272 g) with induced diabetes mellitus. The model organisms were recognized as healthy and free from pathogenic microflora. The hypocholesterolemic experiments involved 29 male mice (Mus musculus, 19 weeks old, 40.8–49.6 g) with induced hypercholesterolemia. The animals were healthy and unaffected by pathogenic microflora. The hepatotoxicity tests involved 15 male rats (Rattus sp., 28 weeks old, 623– 755 g). The model organisms were recognized as healthy and free from pathogenic microflora.
Male podens are preferable for laboratory experiments because they have no estrous cycle that could affect the susceptibility to etiological factors in females (Guidelines for preclinical studies of drugs. Part 1).
A bioethical committee proved that the animal test conditions complied with the Policy for Working with Laboratory Animals in Ifar Company and state standards of Humane Care and Use of Laboratory Animals (Guidelines for preclinical studies of drugs. Part 1, State Standard 33215-2014, State Standard 33216-2014, State Standard R ISO 10993-2-2009, SP 2.2.1.3218-14).
The hypoglycemic and hypocholesterolemic experiments followed similar procedures:
– we took blood samples from the tail vein, obtained serum, and measured the concentration of glucose and total cholesterol;
– we took blood samples from the inferior vena cava, obtained serum, and measured the concentration of total cholesterol.
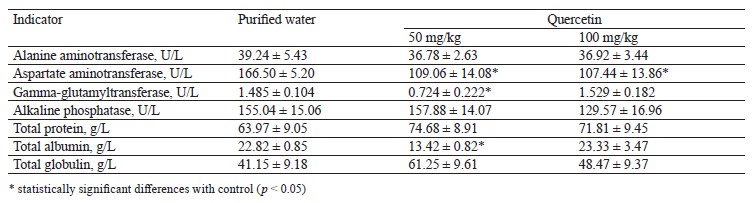
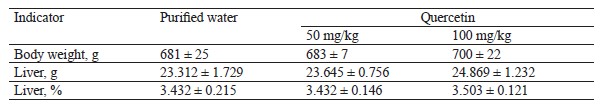
In the hepatotoxicity experiment, we took blood samples from the jugular vein, obtained serum, and measured alanine aminotransferase, aspartate aminotransferase, gamma-glutamyltransferase, and alkaline phosphatase, as well as total protein, albumin, and globulin. The latter was determined as the difference between the concentrations of total protein and albumin. The experiment involved an incomplete necropsy to study liver tissues. After the euthanasia, the liver was weighed, and its weight was compared to the body weight as percentage. The obtained liver tissues underwent a histological analysis.
In the hypoglycemic experiment, the male rats were anaesthetized by CO2 inhalation with further cervical dislocation. As part of the hypocholesterolemic experiment, the mice were anesthetized with CO2 for blood collection and euthanized. The male rats that participated in the hepatotoxicity tests were euthanized by exsanguination.
The blood biochemistry measurements involved a Minitecno LIND 126 biochemical analyzer (I.S.E. Srl, Italy) with commercial kits purchased from Vector-Best, Novosibirsk. The histological analysis of the liver microslides involved an Axio Lab A1 binocular light microscope with an Axiocam 105 digital camera (Carl Zeiss, Germany).
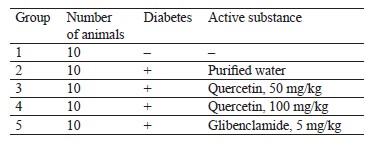
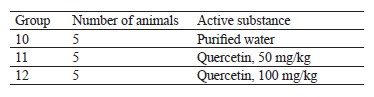
The hypoglycemic tests of 50 and 100 mg/kg quercetin were performed on male rats with diabetes mellitus induced by alloxan. The tests followed the protocol described in Table 1. Purified water served as negative control substance and medium for the test substances. Glibenclamide (Ozon, Russia), a glucose-lowering substance, was used as positive control.
This experiment involved 50 rats, which were divided into five groups of ten animals in each (Guidelines for preclinical studies of drugs. Part 1). The animals in groups 2–5 remained without food for 16 h, upon which we induced diabetes mellitus with a single intraperitoneal injection of alloxan solution at a dose of 150 mg/kg per 1 mL in line with experimentally selected conditions [26]. To confirm the development of diabetes mellitus, we measured the body weight and the concentration of glucose in the blood 48 h before and after the alloxan injection. Only animals with ≥ 11 mmol/L blood glucose stayed in the experiment. The test substances were administered two weeks later to let the animals stabilize. They received the test substance once a day during the last week of the experiment. We measured the fasting body weight and the concentration of glucose and total cholesterol in the blood once a week for 21 days. The hypoglycemic agent glibenclamide served as positive control at an effective dose of 5 mg/kg, which was administered intragastrically as often as the test substance [27].
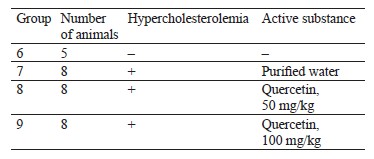
The hypocholesterolemic tests involved 50 and 100 mg/kg of quercetin. The male mice received a lipoprotein lipase inhibitor to develop hypercholesterolemia. Table 2 shows the research protocol. Purified water served as a negative control substance and a medium for the substances under analysis. Alloxan (Diaem LLC, Russia) and Poloxamer P 407 (Koliphor) (BASF, USA) were used as pathology inducers.
The intact group included five animals while the experimental groups included eight animals each (Guidelines for preclinical studies of drugs. Part 1). To simulate hypercholesterolemia, the animals in groups 7–9 were intraperitoneally injected three times a week (Monday, Wednesday, and Friday) for two weeks with aqueous solution of a lipoprotein lipase inhibitor at 400 mg/kg per 1 mL. Poloxamer P 407 (Koliphor) disrupted the clearance of lipoproteins. The intact animals in group 6 received no substances. The test substances and the negative control substance were injected into stomachs daily for two weeks in a volume of 1 mL. After the last administration, the animals were anesthetized for blood collection and euthanized. After that, we determined the concentration of total cholesterol in the blood serum.
To study hepatotoxicity, quercetin was administered intragastrically to male rats at 50 and 100 mg/kg daily for 14 days. Purified water served as negative control and medium for the test substances. Table 3 illustrates the research protocol.
The animals were monitored daily; the body weight and stool consistency tests took place once a week. The animals were euthanized 24 h after the last administration and fasting. The histological analysis made it possible to assess the potential toxic damage to the liver. The weight coefficient was determined as the percentage of liver weight to the body weight. The blood serum was tested in a semi-automatic biochemical analyzer for markers of damage to hepatocytes and biliary tract, i.e., alanine aminotransferase, aspartate aminotransferase, gamma-glutamyltransferase, alkaline phosphatase, and total protein and albumin content. The globulin concentration was represented as the difference between the concentrations of total protein and albumin.
The literature review made it possible to determine the experimental doses as 50 and 100 mg/kg [19, 28].
Each group consisted of ≤ 10 animals, so we applied the nonparametric Mann-Whitney test to compare the indicators from different groups [29]. In the experiment on hypoglycemic activity, we used the Grubbs criterion as in [31] to define outliers (State Standard R ISO 162694-2017). Differences were considered statistically significant at p < 0.05. The data obeyed the normal distribution law, so we used the Shapiro-Wilk test to assess the hypoglycemic and hypocholesterolemic activity. The final results were presented as the mean of trait X and the mean error SE.
RESULTS AND DISCUSSION
In this research, quercetin caused neither severe health problems nor death in the model animals. During the entire experiment, the animals demonstrated no health problems associated with any potential toxic effects of quercetin. No changes in stool consistency were recorded. Thus, intragastric 50 and 100 mg/kg quercetin caused no major health problems after 14 days of treatment.
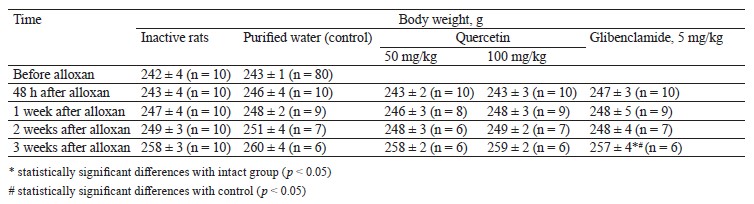
Hypoglycemic activity test results. We tested the rats’ body weight after oral administration of quercetin and glibenclamide (Table 4).
The body weight changes in the male rats with induced diabetes after 50 and 100 mg/kg of quercetin were almost the same as in the intact and control groups (p > 0.05). The diabetic rats treated with 5 mg/kg of glibenclamide for 7 days showed body weight results similar to the intact and control groups (p > 0.05). Thus, a single intraperitoneal injection of 150 mg/kg alloxan and intragastric administration of 50 and 100 mg/kg quercetin did not affect the body weight in the experimental animals.
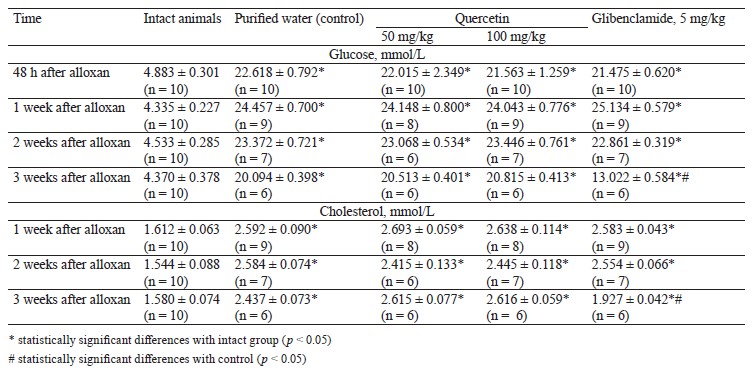
After oral administration of quercetin and glibenclamide, we also assessed the blood biochemistry of the rats (Table 5).
Before alloxan was administered, the concentration of glucose in the blood serum of the intact animals (5.264 ± 0.374 mmol/L, n = 10) and the grouping pool (5.254 ± 0.163 mmol/L, n = 80) conformed to standards. These indicators were similar (p > 0.05).
The rats developed diabetes 48 h after the intra-peritoneal injection of alloxan solution at a dose of 150 mg/kg (p < 0.05). These animals had a higher concentration of glucose and cholesterol in the blood serum than the intact animals. The effect persisted in experimental animals throughout the experiment (p < 0.05).
The rats received quercetin into the stomach for 7 days at doses of 50 and 100 mg/kg, which did not reduce the concentration of glucose and cholesterol in the blood serum (p > 0.05). Glibenclamide administered at a dose of 5 mg/kg reduced the concentrations of glucose and cholesterol in the blood serum, but their levels still exceeded those in the intact group (p < 0.05).
Therefore, 50 and 100 mg/kg of quercetin produced no hypoglycemic activity under these experimental conditions.
Hypocholesterolemic activity test results. Table 6 shows the biochemistry results of the blood of mice that received quercetin orally in doses of 50 and 100 mg/kg.
Poloxamer P 407 (Koliphor) is a lipoprotein lipase inhibitor that disrupts the clearance of lipoproteins. The mice received it intraperitoneally as a 400 mg/kg aqueous solution for 14 days three times a week (Monday, Wednesday, and Friday). As a result, they developed hypercholesterolemia. The experimental mice were intragastrically injected with 50 and 100 mg/kg of quercetin for 14 days, but the concentration of cholesterol in the blood serum did not go down.
Thus, 50 and 100 mg/kg of quercetin had no hypocholesterolemic effect under the experimental conditions.
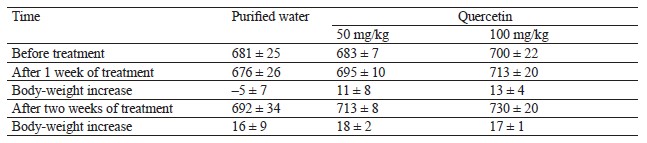
Hepatotoxicity activity test results. Table 7 illustrates the body weight of animals during the intragastric treatment with quercetin.
During the entire treatment, the animals that received quercetin demonstrated the same body-weight increase as the control animals (p > 0.05).
In this research, 14 days of intragastric 50–100 mg/kg quercetin did not affect the body weight of the test animals and, therefore, had no impact on their general health condition.
Table 8 shows the blood biochemistry indicators at the end of the intragastric treatment.
The animals that received 50 mg/kg of quercetin demonstrated a significantly lower gamma-glutamyltransferase activity, but this indicator remained the same in the animals that received 100 mg/kg. Both quercetin doses reduced the aspartate aminotransferase activity.
High alanine aminotransferase and aspartate aminotransferase activities are a characteristic biochemical sign of hepatotoxicity. Therefore, the low liver enzyme activity could not be interpreted as hepatotoxicity, and we observed no critical liver damage in this experiment. The decrease in gamma-glutamyltransferase alanine aminotransferase, and aspartate aminotransferase proved that quercetin was not hepatotoxic.
To sum it up, 50 and 100 mg/kg of intragastric quercetin had no effect on blood biochemistry that would signal of hepatotoxicity after 14 days of treatment.
The necropsy revealed no pathological changes. Therefore, 14 days of intragastric treatment with 50– 100 mg/kg of quercetin caused no pathological changes in the liver.
Table 9 demonstrates the absolute and relative liver weight at the end of the intragastric treatment with quercetin.
We detected no statistically significant differences in body and liver weight between the intact group and the test group. Therefore, 14 days of treatment with 50– 100 mg/kg of quercetin caused no pathological damage to liver tissues.
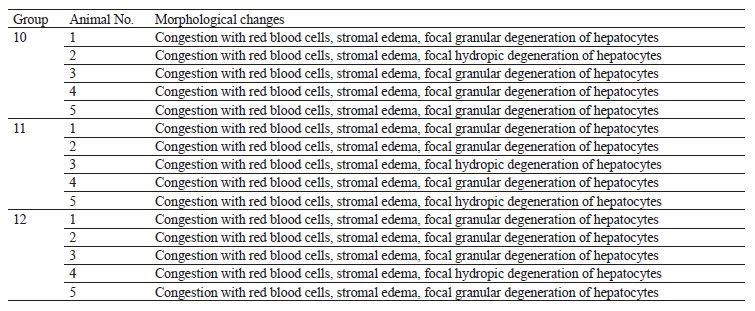
Table 10 demonstrates the histological results.
All experimental animals revealed congestion accompanied by red blood cell stasis, stromal edema, and focal granular or hydropic degeneration of hepatocytes. These phenomena occurred in all groups, including the control, and were caused by the acute circulatory disorders during euthanasia. Thus, 14 days of intragastric treatment with 50 and 100 mg/kg of quercetin triggered no morphological changes in liver tissues.
Our results contradicted those published by Hosseini et al., who reported the positive effect of quercetin on glucose, triglycerides, cholesterol, and weight [3]. Vessal et al. gave 10 and 15 mg/kg of quercetin to rats with streptozotocin-induced diabetes [30]. After 10 days, glucose, triglycerides, and cholesterol went down whereas some liver enzymes became more active. Kılıçarslan & Dönmez also experimented on rats with streptozotocin-induced diabetes [31]. They administered 15 mg/kg of quercetin for 28 days and observed low activity of liver enzymes, lipid peroxidation, and increased expression of SOD and GSH, i.e., genes responsible for antioxidant cell defense. Zhang et al. experimented on mice with genetic obesity and diabetes [32]. The
animals received quercetin in doses of 50–200 mg/kg for 35 days and demonstrated a decrease in blood glucose, triglycerides, and total cholesterol. Presumably, this effect was associated with increased insulin secretion. Bhaskar et al. injected female rabbits with 25 mg/kg of quercetin: after 90 days of hypercholesterolemic diet, their lipid profile significantly improved [33]. Rivera et al. assessed the effect of 2 and 10 mg/kg of quercetin on Zucker rats [34]. The treatment relieved symptoms of dyslipidemia, hypertension, and hyperinsulinemia; 10 mg/kg of quercetin promoted weight loss. Zhao et al. studied Wistar rats on a high-fat diet [35]. The animals received resveratrol at a dose of 120 mg/kg/day and quercetin at a dose of 240 mg/kg/day. The combination of quercetin and resveratrol reduced insulin resistance and inflammation in adipose tissue.
Our data differed from those reported by other authors because we used different model objects, quercetin doses, and markers of metabolic syndrome.
In general, our results do not refute other studies. After administering 50 and 100 mg/kg of quercetin, we observed no increase in body weight, blood glucose, or cholesterol in male rats with alloxan-induced diabetes, nor did we detect anything like that in male mice with hypercholesterolemia induced by a lipoprotein lipase inhibitor.
CONCLUSION
Our in vivo experiments on male rats and mice featured the hypoglycemic, hypocholesterolemic, and hepatotoxic properties of quercetin isolated from the hairy root extract of Hedysarum neglectum Ledeb. The results could be summarized as follows:
1. Treatment with quercetin in doses of 50 and 100 mg/kg caused neither serious health problems nor death, which means that quercetin produced no toxic effect on the test subjects.
2. The same treatment caused no changes in the body weight of the model animals with induced diabetes;
3. It did not affect the levels of glucose and cholesterol in the blood of the model animals with induced diabetes. Therefore, quercetin exhibited no hypoglycemic activity: it did not reduce the high blood glucose levels that caused diabetes. However, quercetin did not increase serum glucose and cholesterol either.
4. Administration of 50 and 100 mg/kg of quercetin had no impact on the cholesterol in the blood of the model animals with induced hypercholesterolemia. Therefore, quercetin demonstrated no hypocholesterolemic activity as it did not reduce the concentration of cholesterol in the blood serum. Still, the treatment did not increase the serum cholesterol levels in the hypercholesterolemic animals.
5. The treatment inhibited the activity of aspartate aminotransferase; at 50 mg/kg, quercetin led to a statistically significant decrease in the activity of gamma-glutamyltransferase.
6. Administration of 50 and 100 mg/kg of quercetin neither affected blood biochemistry nor caused any pathological changes in liver tissue.
This in vivo research on rodents showed that quercetin can be safely used in dietary supplements because it has no toxic effect on liver cells. However, quercetin demonstrated no preventive activity against metabolic syndrome, i.e., it did not reduce cholesterol or blood glucose. Further research is needed to assess the effect of quercetin on other markers of hypoglycemia and hypocholesterolemia, such as antioxidant defense genes or those involved in carbohydrate and lipid metabolism, e.g., PON1, PPARG, ALDH1B1, APOA4, etc.
Contribution
All authors have contributed equally to this project.
CONFLICTS OF INTEREST
The authors declared no potential conflict of interests regarding the publication of this article.
FUNDING
The research was part of State assignement No. FZSR-2024-0008: Development of biologically active additives consisting of metabolites of plant objects in vitro to protect the population from premature aging.REFERENCES
- Uchendu IK, Ikebunwa OA, Okpagu CB. Cardiorenal protective effects of extracts of bitter leaf (Vernonia amygdalina L.) in animal model of metabolic syndrome. Foods and Raw Materials. 2024;12(2):264–272. https://doi.org/10.21603/2308-4057-2024-2-607
- Li H-Y, Zhou D-D, Gan R-Y, Huang S-Y, Zhao C-N, Shang A, et al. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: A narrative review. Nutrients. 2021;13(9):3211. https://doi.org/10.3390/nu13093211
- Hosseini A, Razavi BM, Banach M, Hosseinzadeh H. Quercetin and metabolic syndrome: A review. Phytotherapy Research. 2021;35(10):5352–5364. https://doi.org/10.1002/ptr.7144
- Vesnina A, Prosekov A, Atuchin V, Minina V, Ponasenko A. Tackling atherosclerosis via selected nutrition. International Journal of Molecular Sciences. 2022;23(15):8233. https://doi.org/10.3390/ijms23158233
- Bilovol AN, Kniazkova II, Tverytinov AB, Korniichuk VI, Nesen AA, Zorenko NB. Therapeutic efficiency of quercetinum forin patiens with arterial hypertension and metabolic syndrome. West Kazakhstan Medical Journal. 2021;63(2):56–62. (In Russ.). https://doi.org/10.24412/2707-6180-2021-63-56-62; https://elibrary.ru/RESSVN
- Reznik EV, Nikitin IG. Hypertension management in metabolic syndrome. Archive of Internal Medicine. 2019;9(5):327–347. (In Russ.). https://doi.org/10.20514/2226-6704-2019-9-5-327-347; https://elibrary.ru/MYBTFH
- Bogdanova OG, Mylʹnikova IV. Metabolic syndrome: Situation in the world, clinical-diagnostic criteria and risk factors (review of literature). Hygiene and Sanitation. 2020;99(10):1165–1169. (In Russ.). https://doi.org/10.47470/0016-9900-2020-99-10-1165-1169; https://elibrary.ru/OJEAGH
- Healthcare [Internet]. [cited 2024 Jan 15]. Available from: https://rosstat.gov.ru/folder/13721
- Zhang K, Ma Y, Luo Y, Song Y, Xiong G, Ma Y, et al. Metabolic diseases and healthy aging: identifying environmental and behavioral risk factors and promoting public health. Frontiers in Public Health. 2023;11. https://doi.org/10.3389/fpubh.2023.1253506
- Juárez-Fernández M, Porras D, García-Mediavilla MV, Román-Sagüillo S, González-Gallego J, Nistal E, et al. Aging, gut microbiota and metabolic diseases: Management through physical exercise and nutritional interventions. Nutrients. 2020;13(1):16. https://doi.org/10.3390/nu13010016
- Baek SJ, Hammock BD, Hwang I-K, Li QX, Moustaid-Moussa N, Park Y, et al. Natural products in the prevention of metabolic diseases: Lessons learned from the 20th KAST frontier scientists workshop. Nutrients. 2021;13(6):1881. https://doi.org/10.3390/nu13061881
- Dominguez LJ, Barbagallo M. The biology of the metabolic syndrome and aging. Current Opinion in Clinical Nutrition and Metabolic Care. 2016;19(1):5–11. https://doi.org/10.1097/MCO.0000000000000243
- Babich O, Sukhikh S, Prosekov A, Asyakina L, Ivanova S. Medicinal plants to strengthen immunity during a pandemic. Pharmaceuticals. 2020;13(10):313. https://doi.org/10.3390/ph13100313
- Milentyeva IS, Vesnina AD, Fedorova AM, Ostapova EV, Larichev TA. Chlorogenic acid and biohanin a from Trifolium pratense L. callus culture extract: Functional activity in vivo. Food Processing: Techniques and Technology. 2023;53(4):754–765. (In Russ.). https://doi.org/10.21603/2074-9414-2023-4-2475
- Sotiropoulou M, Katsaros I, Vailas M, Lidoriki I, Papatheodoridis GV, Kostomitsopoulos NG, et al. Nonalcoholic fatty liver disease: The role of quercetin and its therapeutic implications. The Saudi Journal of Gastroenterology. 2021;27(6):319–330. https://doi.org/10.4103/sjg.sjg_249_21
- Serba EM, Yuraskina TV, Rimareva LV, Tadzibova PYu, Sokolova EN, Volkova GS. Microbial biomass as a bioresource of functional food ingredients: A review. Food Processing: Techniques and Technology. 2023;53(3):426–444. (In Russ.). https://doi.org/10.21603/2074-9414-2023-3-2446
- Shabbir U, Rubab M, Daliri EB-M, Chelliah R, Javed A, Oh D-H. Curcumin, quercetin, catechins and metabolic diseases: The role of gut microbiota. Nutrients. 2021;13(1):206. https://doi.org/10.3390/nu13010206
- Xu X, Yi H, Wu J, Kuang T, Zhang J, Li Q, et al. Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence. Biomedicine and Pharmacotherapy. 2021;133:110984. https://doi.org/10.1016/j.biopha.2020.110984
- Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, et al. Quercetin, inflammation and immunity. Nutrients. 2016;8(3):167. https://doi.org/10.3390/nu8030167
- Faskhutdinova ER, Sukhikh AS, Le VM, Minina VI, Khelef MEA, Loseva AI. Effects of bioactive substances isolated from Siberian medicinal plants on the lifespan of Caenorhabditis elegans. Foods and Raw Materials. 2022;10(2):340–352. https://doi.org/10.21603/2308-4057-2022-2-544
- Jasenovec T, Radosinska D, Kollarova M, Balis P, Ferenczyova K, Kalocayova B, et al. Beneficial effect of quercetin on erythrocyte properties in type 2 diabetic rats. Molecules. 2021;26(16):4868. https://doi.org/10.3390/molecules26164868
- Gao X-R, Chen Z, Fang K, Xu J-X, Ge J-F. Protective effect of quercetin against the metabolic dysfunction of glucose and lipids and its associated learning and memory impairments in NAFLD rats. Lipids in Health and Disease. 2021;20:164. https://doi.org/10.1186/s12944-021-01590-x
- Vesnina AD, Milentyeva IS, Dmitrieva AI, Prosekov AYu, Neverova OA. Prospects for the application of Hedysarum neglectum Ledeb as a cardioprotector. Agro-Industrial Complex of Russia. 2023;30(5):677–682. (In Russ.). https://doi.org/10.55934/10.55934/2587-8824-2023-30-5-677-682; https://elibrary.ru/OXZVNP
- Vesnina A, Milentyeva I, Minina V, Kozlova O, Asyakina L. Evaluation of the in vivo anti-atherosclerotic activity of quercetin isolated from the hairy roots of Hedysarum neglectum Ledeb. Life. 2023;13(8):1706. https://doi.org/10.3390/life13081706
- Prosekov AYu, Vesnina AD, Kozlova OV. The methodology of food design. Part 2. Digital nutritiology in personal food. Theory and Practice of Meat Processing. 2021;6(4):328–334. https://doi.org/10.21323/2414-438X-2021-6-4-328-334; https://elibrary.ru/KUFMRE
- Ighodaro OM, Adeosun AM, Akinloye OA. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina. 2017;53(6):365–374. https://doi.org/10.1016/j.medici.2018.02.001
- Rabbani SI, Devi K, Khanam S. Protective role of glibenclamide against nicotinamide-streptozotocin induced nuclear damage in diabetic Wistar rats. Journal of Pharmacology and Pharmacotherapeutics. 2010;1(1):18–23. https://doi.org/10.4103/0976-500X.64531
- Muthian G, Bright JJ. Quercetin, a flavonoid phytoestrogen, ameliorates experimental allergic encephalomyelitis by blocking IL-12 signaling through JAK-STAT pathway in T lymphocyte. Journal of Clinical Immunology. 2004;24:542–552. https://doi.org/10.1023/B:JOCI.0000040925.55682.a5
- Glushanko VS. Basic medical statistics. Vitebsk: VGMU; 2012. 155 p. (In Russ.).
- Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology. 2003;135(3):357–364. https://doi.org/10.1016/s1532-0456(03)00140-6
- Kılıçarslan G, Dönmez N. The effects of quercetin on antioxidant system and some blood parameters in experimental diabetic rats. Bulletin of Environment, Pharmacology and Life Sciences. 2016;5(6):28–32.
- Zhang R, Yao Y, Wang Y, Ren G. Antidiabetic activity of isoquercetin in diabetic KK -Ay mice. Nutrition and Metabolism. 2011;8:85. https://doi.org/10.1186/1743-7075-8-85
- Bhaskar S, Kumar KS, Krishnan K, Antony H. Quercetin alleviates hypercholesterolemic diet induced inflammation during progression and regression of atherosclerosis in rabbits. Nutrition. 2013;29(1):219–229. https://doi.org/10.1016/j.nut.2012.01.019
- Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity. 2008;16(9):2081–2087. https://doi.org/10.1038/oby.2008.315
- Zhao L, Cen F, Tian F, Li M-J, Zhang Q, Shen H-Y, et al. Combination treatment with quercetin and resveratrol attenuates high fat diet-induced obesity and associated inflammation in rats via the AMPKα1/SIRT1 signaling pathway. Experimental and Therapeutic Medicine. 2017;14(6):5942–5948. https://doi.org/10.3892/etm.2017.5331