Abstract
Metastable electrochemically-activated water solutions possess unique properties that make it possible to modify food emulsions. This comparative analysis featured the stability of model oil-in-water emulsions with anolyte or catholyte as a dispersion medium, as well as the physical and morphometric profile of the emulsion system.The research involved emulsions based on anolyte and catholyte. They consisted of refined sunflower oil, emulsifier (lecithin), and stabilizers, which were represented by sodium alginate, sodium carboxymethylcellulose, pectins, and agar. The study also covered such parameters as aggregative stability, viscosity, morphometry, oil particle size, and zeta potential.
Anolyte and catholyte affected the process of separation in the model emulsions. The samples stabilized with alginate and sodium carboxymethylcellulose proved to be the most stable emulsions while agar triggered gelation. The effect of substituting tap water with metastable electrolyzed water solutions depended on the oil proportion in the emulsion. Catholyte destabilized the samples with 20% of oil and liquified gel in the samples stabilized with agar. Anolyte was more aggressive in destabilizing emulsions with 30% of oil. The effective viscosity of these emulsions correlated with the stable phase fraction. The anolytebased samples had low effective viscosity. The opposite results for emulsions with different oil fractions may have been caused by interface changes, i.e., surface tension, adsorption, coalescence, etc. In the emulsions with 46% of oil and animal origin emulsifier, neither anolyte nor catholyte had any significant effect on the aggregative stability of the system.
The revealed patterns can be used to control the properties of emulsion products with oil phase ≤ 30%, e.g., low-fat mayonnaises, sauces, emulsion drinks, etc. Metastable electrolyzed water solutions may provide a reagent-free control of properties and patterns of finished or semi-finished foods and biological raw materials.
Keywords
Electrolyzed water, anolyte, catholyte, oil-in-water emulsion, aggregative stability, scanning electron microscopyINTRODUCTION
The structure and properties of food products depend on such technological factors as temperature, pressure, mixing rate, etc., as well as on the interaction between ingredients, including water. Logically, modified water can change the properties of semi-finished or finished product. The method of electrolyzed water solution is quite promising in this respect. Its metastable fractions accumulate in the anode or cathode chamber of the electrolyzer [1, 2]. The resulting electrolysis produces anolyte and catholyte. These aqueous solutions possess high oxidizing or reducing abilities, respectively.
Water nanoclusters based on catholyte or anolyte were reported to show a correlation between the number of bound water molecules and the energy of hydrogen bonds between them. Experimental studies proved that water surface tension depends on the energy of hydrogen bonds [3]. Mathematical modeling demonstrated a decrease in the number of clusters in catholyte and an increase in the number of water molecules per cluster in anolyte [4]. Compared to water, catholyte has greater average energy of hydrogen bonds. For anolyte, however, the average energy is lower. This phenomenon indicates an increase or decrease in energy density, respectively.
It depends on the physiological effects produced by electrolyzed water solutions. Anolyte has acidic pH and an anomalous positive redox potential while catholyte has alkaline pH and a unique negative redox potential [5, 6].
Electrolyzed water solutions owe their useful properties to hydrogen and oxygen bubbles. Bubbles are stable due to the uncompensated electric charges on the interface between liquid and gas [7, 8]. During electrolysis, bubbles appear, grow, and detach themselves from the electrode surface [7]. Their growth rate depends on the current density and water flow rate. For instance, high current density increases gas bubble diameter while low flow rate reduces it.
Electrolyzed water solutions were reported to have a lower surface tension on the interface between liquid and air than the initial water solution. In catholyte, the surface of hydrogen bubbles started to adsorb atomic hydrogen and Н3О2- ions when the surface tension on the liquid-air interface went down [9]. Anolyte accumulated hypochlorite ions and hypochlorous acid molecules on the surface of oxygen bubbles. During open storage, electrolyzed water solutions interacted with air, causing spontaneous restoration of the water solution. Catholyte demonstrated a correlation between the surface potential difference and the tension [8–10]. This effect was probably brought about by the neutralization of negatively charged hydroxyl ions on the solution surface. The fact that ions accumulate on the interface and reduce the surface tension could also explain the surface-active properties of catholyte and anolyte. As a result, electrolyzed water solution may obtain emulsifying properties.
Such dispersed systems as emulsions, suspensions, and foams are the main objects of directed modification in food production. Food producers control the water phase of dispersed systems by introducing food additives with certain functions and applying various physical and physicochemical methods, including the electrolyzed water solution technology [11, 12]. Ultrasound treatment was reported to facilitate the process in emulsions [12, 13]. Related studies usually feature such physicochemical properties as pH, redox potential, water activity, and color, as well as such technological parameters as yield, emulsion stability, and consistency. As a rule, the resulting emulsions prove stable and more abundant while the texture profile remain the same. For instance, ultrasound treatment combined with electrolyzed water was able to boost the extraction efficiency and solubility of krill proteins. Their emulsifying properties, foaming ability, and foam stability improved while the particle size decreased [14]. Other studies reported other factors that affected the conformation of molecules, their volume in the solution and, eventually, the rheological properties of the biomolecular solution. These factors include polyelectrolyte concentration, temperature, pressure, low molecular weight substances, pH, and electric field [15–20].
Emulsions with a surfactant, or emulsifier, are the most common dispersed systems in the food industry. By changing the physicochemical properties of the aqueous phase, food producers can adjust the emulsion, while giving it additional stability by applying protein or polysaccharide food thickeners. If a direct emulsion contains a high proportion of the hydrophilic phase and the emulsifier has an amphiphilic chemical structure, the system can become sensitive to such factors as pH, redox potential, electrical conductivity, ionic impurities, etc. [21]. As a result, dispersed systems based on electrolyzed water solutions have anomalous physicochemical parameters [22–25]. Their properties may differ from those of dispersed systems that were prepared using ordinary water.
Therefore, anolyte and catholyte require a deeper scientific insight into the way they affect emulsions. The current trends in fatty products dictate that food emulsions, e.g., mayonnaise and sauces, should have low energy value or that some animal ingredients should be replaced by their plant analogues [26–28]. This article introduces a comparative analysis of the aggregative stability of oil-in-water model emulsions with anolyte or catholyte as a hydrophilic dispersion medium.
STUDY OBJECTS AND METHODS
The experiment involved softened tap water (pH 7.2, redox potential +340 mV). We used a diaphragm modular electrochemical cell (Delfin Aqua, Russia) to obtain metastable fractions of electrolyzed water solution, an HI98120 device (Hanna, Germany) to measure pH, and a ST20R meter (Ohaus, China) to define redox potential. The anolyte had pH 4 and ORP +800 mV while the catholyte had pH 9 and ORP –400 mV.
Liquid fat-soluble soy lecithin (DENLEC, Louis Dreyfus Company, Brazil) served as emulsifier. To create the oil phase, we dissolved the lecithin in sunflower oil until 0.2% concentration (by weight). To obtain the aqueous phase, we dissolved plant polysaccharide in water/anolyte/catholyte until 0.5% concentration (by weight). This plant polysaccharide was a standard stabilizer for an oilin-water emulsion. For comparison, we used several polysaccharides, namely agar (GELAGAR IT+, B&V S.R.L., Italy), sodium carboxymethylcellulose (Acucell AF2985, Akzo Nobel Chemicals A.G., Netherlands), sodium alginate (Shandong Jeling Group Corporation Inspection Repart, China), as well as low-methoxylated apple pectin and apple fruit pectin (APA105, Yantai Andre Pectin Co. Ltd, China). We produced direct emulsions by pouring the oil phase into the water phase while stirring the mix with a mechanical stirrer at 1200 rpm for 5–10 min. All the samples were stored at room temperature.
To study the separation process, we left the emulsion to settle at room temperature for 6, 7, and 13 days. The stable component (Vst) was determined as a percentage from total emulsion volume. Emulsion stability depended on how well the polysaccharides were dissolved in water. The effective viscosity was measured using an RVDV II+Pro rotational viscometer (Brookfield, Spain). The morphological studies involved a light microscope (Altami, Russia) and a JSM-6390A scanning electron microscope (JEOL, Japan). The droplet size distribution (diameter, μm) was obtained by morphometric analysis of microphotographs of the emulsion monolayer using the ImagoJ software. We appealed to the dynamic light scattering method to study the hydrodynamic radius of droplets in a Zetasizer Nano ZS analyzer (Malvern, UK). The same device made it possible to define the zeta potential of emulsion droplets. The experimental data were processed using MS Excel and graphs.RESULTS AND DISCUSSION
Aggregative stability of 20% oil emulsion. We used polysaccharides because polar groups could respond to changes in the redox potential of the water solution. The hypothesis was that by replacing water with anolyte or catholyte with a modified redox potential value we would be able to affect the consistency, stability, and gelation of the resulting emulsion. However, unique properties of electrolyzed water solutions do not depend on the chemical composition of the water solution. As a result, they gradually return to the initial values of softened tap water.
The water emulsion samples (redox potential +340 mV, pH 7.2) contained alginate, sodium carboxymethylcellulose, or pectins. They were initially homogeneous, but the liquid part eventually became separated. The emulsion stabilized with agar formed a stable gel that did not separate even after 6 days of storage. Table 1 illustrates the comparative analysis of model emulsions stabilized by several different polysaccharides.
Sodium carboxymethylcellulose yielded the most stable model emulsion. Pectins, however, were responsible for the least stable emulsions, especially apple pectin, so we excluded them from further studies. Figure 1 demonstrates a comparative analysis of the experimental data.
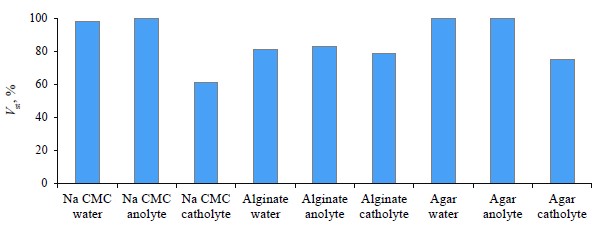
Anolyte and catholyte had different effects on the stability of the oil-in-water emulsion. However, the severity and direction of this effect depended on the polysaccharide.
The emulsion samples stabilized with sodium carboxymethylcellulose initially looked like homogeneous thick liquid. The samples with water or catholyte released the liquid component after 7 days of storage. Their aggregative stability dropped by 20% for the water sample and by 40% for the catholyte sample. The emulsion that contained anolyte maintained its initial state. These differences may be attributed to the chemical structure of sodium carboxymethylcellulose, which is an anionic polyelectrolyte with a functional acidic carboxymethyl group.
All the sodium alginate emulsions eventually lost their aggregation stability by approximately 20% (Fig. 1). The liquid volume was the same in all the samples.
The agar-stabilized emulsion exhibited two states. If the aqueous phase was water or anolyte, it was a stable gel. If the aqueous phase was represented by catholyte, the emulsion split into dense and liquid components. In the latter case, the aggregative stability decreased by 25% by the end of the experiment (Fig. 1). This effect took place because agar gel develops together with hydrogen bonds between agarose molecules, a process that depends entirely on electrostatic interaction, not chemical one [29]. Probably, the negative redox value of catholyte reduced the number of hydrogen bonds, which, in its turn, reduced the stability of agar gel. In terms of emulsion stability, the sample with sodium carboxymethylcellulose and anolyte demonstrated the best results, provided that the purpose was to maintain the liquid state of the emulsion.
Anolyte and catholyte failed to increase the stability of the sodium alginate emulsion.
Figure 2 demonstrates the state of the stable component in these two emulsions for 13 days, which gives an idea of how the composition stability may change during longer storage.
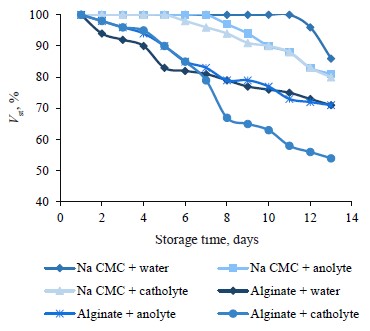
The curves in Fig. 2 clearly demonstrate that the stability trends for 7 days of storage persisted for 13 days. Catholyte triggered separation in both types of emulsions. This feature was most pronounced for the sample stabilized with sodium alginate. The samples with anolyte and softened water demonstrated the same changes. The emulsions stabilized with sodium carboxymethylcellulose had the best results.
In this experiment, we studied the effect of electrolyzed water solution on emulsions in a model oil-in-water system with 20% of oil and 0.2% of liquid lecithin as emulsifier. Catholyte, being the reduced fraction, accelerated separation, destroyed the emulsions stabilized by sodium carboxymethylcellulose or sodium alginate, and prevented gelation in the agar emulsion.
Aggregative stability of 30% oil emulsion. Low-calorie mayonnaises and sauces have the mass fraction of fat between 15 and 40%. We raised the fatty phase proportion to 30%. The hypothesis was that, by lowering the aqueous phase, we could affect the emulsion properties. This experiment involved only sodium carboxymethylcellulose because this polysaccharide had proved more active in stabilizing the oil-in-water system. The oil phase was prepared in line with the same method, and the mixing procedure also remained the same as described above, but the storage time was as long as 16 days (Fig. 3).
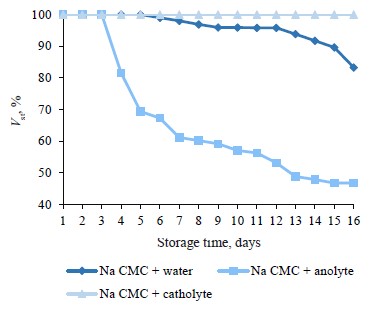
The catholyte-based sample had the best preservation effect on the emulsion with 30% of oil and sodium carboxymethylcellulose: no signs of separation were registered during the entire storage period. This effect was opposite to the one we observed when the oil phase was 20%. In that case, catholyte was more destructive. Anolyte also produced a different effect (Fig. 3), reducing the stable fraction to 30% of the total emulsion volume. The samples with water demonstrated the same kinetics at both oil concentrations.
By bringing up the oil phase proportion from 20 to 30%, we changed the conditions on the interfacial surface, i.e., surface tension, adsorption, coalescence, etc. Our results were consistent with the the effect of anolyte and catholyte on the structure and pattern of proteins and polysaccharides in water solutions reported in [30, 31].
A relatively high content of the dispersed medium might have affected the electrostatic interactions between the molecules of stabilizer biopolymers, their aggregation, and solvation. Probably, it also changed the properties of the hydrophilic molecules of the emulsifier on the interface between oil and water.
The first three days of storage saw no changes in the emulsion stability (Fig. 3) but the effective viscosity was significantly different. This indicator was 3706 mPa∙s for the catholyte sample, 3544 mPa·s for the water sample, and 3316 mPa·s for the anolyte sample, respectively. The stability of each sample depended quite strongly on its effective viscosity. Therefore, the higher the initial effective viscosity value, the greater the stable component volume during storage.
Under a light microscope, the samples with catholyte and anolyte demonstrated larger particles in the emulsion, compared to water-based emulsions. This trend was the strongest in the catholyte sample (Fig. 4).
This comparative experiment studied aggregative stability on model emulsions with 20 and 30% oil which were stabilized with plant polysaccharides. The aqueous phase consisted of softened water, anolyte, or catholyte.
Anolyte or catholyte affected the stability of the model system. The effect was multidirectional and depended on the concentration of oil.
Aggregative stability of mayonnaise. This part of the research featured emulsions that simulated low-energy mayonnaise or sauces and contained only plant ingredients. Such formulations follow the current trends in functional nutrition, e.g., developing new vegetarian fatty products. However, traditional mayonnaise is still in great demand.
Here, we studied the effect of replacing water with anolyte or catholyte on the aggregative stability of the emulsion system. Its composition was the same as in the target product, e.g., a popular Lyubitelsky mayonnaise (oil content of 46%) [32]. The samples were prepared according to the previously described procedure, which started by mixing together water-soluble ingredients.
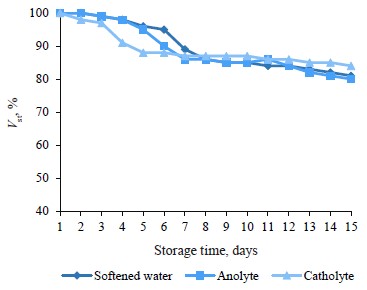
The obtained dependence (Fig. 5) made it possible to evaluate the stability of mayonnaise prepared with anolyte or catholyte during 15 days of storage.
The similarity between the aggregation stability curves (Fig. 5) indicates that the fractions of electrolyzed water solution had almost no effect on the multicomponent mayonnaise. The mayonnaise was a complex system with a high oil concentration (46%) and an emulsifier in the form of animal phospholipids. All the mayonnaise samples remained highly and equally stable throughout the entire storage period.
The zeta potential of mayonnaise particles confirmed this conclusion. This parameter characterizes the stability of dispersed systems. The method of electrophoretic light scattering showed the following values: –40 ± 3 mV for the mayonnaise with softened water, –42 ± 3 mV for the mayonnaise with catholyte, and –38 ± 2 mV for the mayonnaise with anolyte.
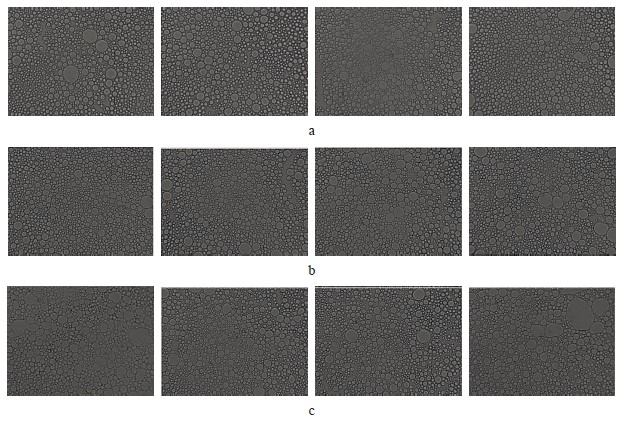
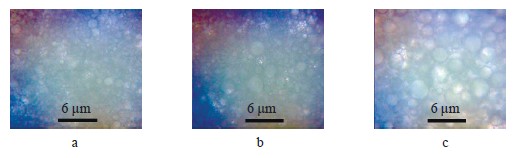
Figure 6 shows microphotographs of emulsion particles in the traditional mayonnaise.
Figure 7 illustrates the morphometric analysis of the microphotographs in Fig. 6. The mayonnaise monolayer revealed that the particles were distributed heterogeneously. However, we detected no significant difference between the samples. These data indirectly confirmed the results of the stability experiment. The particles could be divided into three size groups. The most representative group consisted of droplets with the diameter of 0.6–0.9 µm.
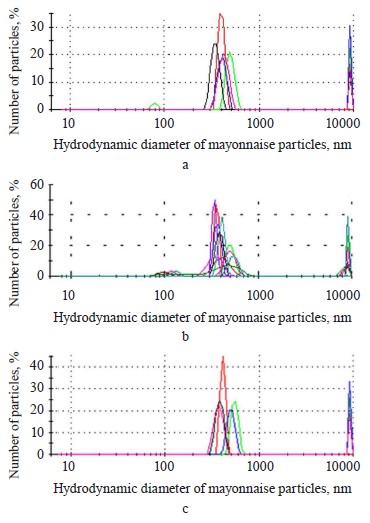
The dynamic light scattering method confirmed the morphometric results. Figure 8 shows that each sample contained several groups of particles. The largest group consisted of droplets with the hydrodynamic diameter of 300–700 nm. The fraction of micron particles was also quite large, which also confirmed the morphometric data (Fig. 7). In addition, the research revealed an insignificant number of nanometer-sized particles that the light microscope could not identify because the method was limited in spatial resolution.
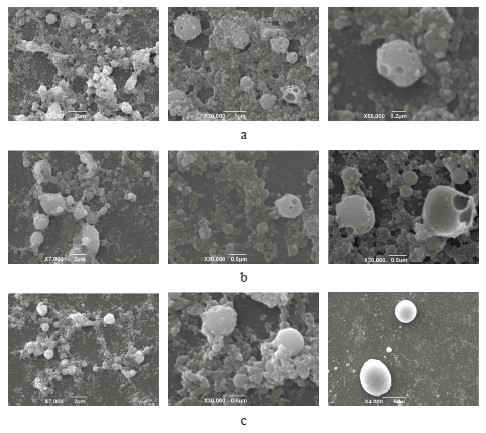
Figure 9 shows the results of the ultrastructural study. The microphotographs demonstrate the characteristic features of each mayonnaise sample.
The mayonnaise emulsion with water contained micron, submicron, and nano-sized particles. The high magnification revealed cavities that may point at fragmentation.
The ultrastructure of the emulsion particles with anolyte was similar to that of the previous sample but for larger particles of several microns in size. The catholyte sample also had similar visual characteristics but contained some large fat droplets which exceeded several microns.
These results were consistent with the micrographs and the dynamic light scattering method. Apparently, the pronounced effect of anolyte or catholyte on the aggregative stability of mayonnaise emulsion disappeared when the oil content was high and/or the mix involved a surfactant represented by phospholipids of animal origin. The effect of anolyte/catholyte on phospholipid emulsifier requires a more comprehensive study. Prospective research might include phosphatidylcholine or its derivatives, both fat-soluble and water-soluble. In addition, catholyte exhibited some antioxidant properties, which suggests that electrolyzed water solutions can protect emulsion-based products from oxidative processes during storage.
CONCLUSION
Electrolyzed water solution was able to serve as a hydrophilic phase in an oil-in-water model emulsified with fat-soluble lecithin and stabilized with polysaccharides of plant origin. It demonstrated a multidirectional effect depending on the oil phase proportion.
The catholyte fraction with a negative redox potential caused gradual separation in the emultions with a 20% share of sunflower oil stabilized by sodium carboxymethylcellulose or sodium alginate. A similar composition, but stabilized with agar, resulted in gelation, which did not occur when softened water was replaced with catholyte.
The samples with 30% of oil demonstrated an opposite effect: it was anolyte with high redox potential that destabilized the emulsion. After separation, the volume of the stable phase correlated with its initial effective viscosity. In other words, catholyte provided a more stable emulsion whereas anolyte lowered aggregation stability. Perhaps, the opposite results for the sample with 20 and 30% of oil could be explained by the changes in the interface conditions, i.e., surface tension, adsorption, coalescence, etc.
To sum it up, anolyte and catholyte fractions of electrolyzed water solution did not affect the stability of traditional high-fat mayonnaise emulsified by animal phospholipids.
Therefore, the practical application of anolyte and catholyte as means of reagent-free control of oil-in-water food emulsions is limited to low-fat products, e.g., mayonnaise sauces, emulsion drinks, and cocktails.
Contribution
A.G. Pogorelov developed the research concept and wrote the manuscript. L.G. Ipatova interpreted the obtained data, designed the experiment, and performed the experimental studies. A.I. Panait prepared the samples for optical microscopy and processed the obtained results. A.A. Stankevich was responsible for electron microscopy. V.N. Pogorelova provided the physicochemical measurements and obtained the electrolyzed water solutions. O.A. Suvorov processed the materials and proofread the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflict of interests regarding the publication of this manuscript.
FUNDING
The research was supported by Russian Science Foundation (RSF) , grant No. 20-16-00019 (https://rscf.ru/en/ project/20-16-00019).REFERENCES
- Henry M, Chambron J. Physico-chemical, biological and therapeutic characteristics of electrolyzed reduced alkaline water (ERAW). Water. 2013;5(4):2094–2115. https://doi.org/10.3390/w5042094
- Bakhir VM, Pogorelov AG. Universal electrochemical technology for environmental protection. International Journal of Pharmaceutical Research and Allied Sciences. 2018;7(1):41–57.
- Ignatov I, Gluhchev G, Karadzhov S, Yaneva I, Valcheva N, Dinkov G et al. Dynamic nano clusters of water on waters catholyte and anolyte: Electrolysis with nano membranes. Physical Science International Journal. 2020;24(1):46–54. https://doi.org/10.9734/psij/2020/v24i130173
- Ignatov I, Gluhchev G, Neshev N, Mehandjiev D. Structuring of water clusters depending on the energy of hydrogen bonds in electrochemically activated waters Anolyte and Catholyte. Bulgarian Chemical Communications. 2021;53(2):234–239.
- Chen B-K, Wang C-K. Electrolyzed water and its pharmacological activities: A mini-review. Molecules. 2022;27(4):1222. https://doi.org/10.3390/molecules27041222
- Rebezov M, Saeed K, Khaliq A, Rahman SJU, Sameed N, Semenova A, et al. Application of electrolyzed water in the food industry: A review. Applied Sciences. 2022;12(13):6639. https://doi.org/10.3390/app12136639
- Li Y, Yang G, Yu S, Kang Z, Mo J, Han B, et al. In-situ investigation and modeling of electrochemical reactions with simultaneous oxygen and hydrogen microbubble evolutions in water electrolysis. International Journal of Hydrogen Energy. 2019;44(52):28283–28293. https://doi.org/10.1016/j.ijhydene.2019.09.044
- Sipahi H, Reis R, Dinc O, Kavaz T, Dimoglo A, Aydın A. In vitro biocompatibility study approaches to evaluate the safety profile of electrolyzed water for skin and eye. Human and Experimental Toxicology. 2019;38(11):1314–1326. https://doi.org/10.1177/0960327119862333
- Yurkevich AB. Dependence of physicochemical parameters of anolyte and catholyte on the concentration of initial aqueous solutions of sodium chloride. Pharmacy Bulletin. 2002;4:66–72. (In Russ.).
- Iram A, Wang X, Demirci A. Electrolyzed oxidizing water and its applications as sanitation and cleaning agent. Food Engineering Reviews. 2021;13:411–427. https://doi.org/10.1007/s12393-021-09278-9
- Pinton MB, dos Santos BA, Lorenzo JM, Cichoski AJ, Boeira CP, Campagnol PCB. Green technologies as a strategy to reduce NaCl and phosphate in meat products: An overview. Current Opinion in Food Science. 2021;40:1–5. https://doi.org/10.1016/j.cofs.2020.03.011
- Sena Vaz Leães Y, Basso Pinton M, Terezinha de Aguiar Rosa C, Robalo SS, Wagner R, de Menezes CR, et al. Ultrasound and basic electrolyzed water: A green approach to reduce the technological defects caused by NaCl reduction in meat emulsions. Ultrasonics Sonochemistry. 2020;61:104830. https://doi.org/10.1016/j.ultsonch.2019.104830
- Sena Vaz Leães Y, Silva JS, Robalo SS, Basso Pinton M, dos Santos SP, Wagner R, et al. Combined effect of ultrasound and basic electrolyzed water on the microbiological and oxidative profile of low-sodium mortadellas. International Journal of Food Microbiology. 2021;353:109310. https://doi.org/10.1016/j.ijfoodmicro.2021.109310
- Li Y, Zeng Q-H, Liu G, Peng Z, Wang Y, Zhu Y, et al. Effects of ultrasound-assisted basic electrolyzed water (BEW) extraction on structural and functional properties of Antarctic krill (Euphausia superba) proteins. Ultrasonics Sonochemistry. 2021;71:105364. https://doi.org/10.1016/j.ultsonch.2020.105364
- Hu W, Li P, Guo D, Zhang B, Tao D, Li J, et al. Effect of solution pulsed plasma process on the degradation and physicochemical properties of pectin. Food Hydrocolloids. 2023;136:108236. https://doi.org/10.1016/j.foodhyd.2022.108236
- Zhang L, Lin W-F, Zhang Y, Tang C-H. New insights into the NaCl impact on emulsifying properties of globular proteins. Food Hydrocolloids. 2022;124:107342. https://doi.org/10.1016/j.foodhyd.2021.107342
- Han F, Shen Q, Zheng W, Zuo J, Zhu X, Li J, et al. The conformational changes of bovine serum albumin at the air/water interface: HDX-MS and interfacial rheology analysis. Foods. 2023;12(8):1601. https://doi.org/10.3390/foods12081601
- Banerjee A, De R, Das B. Hydrodynamic and conformational characterization of aqueous sodium alginate solutions with varying salinity. Carbohydrate Polymers. 2022;277:118855. https://doi.org/10.1016/j.carbpol.2021.118855
- Król Ż, Malik M, Marycz K, Jarmoluk A. Characteristic of gelatine, carrageenan and sodium alginate hydrosols treated by direct electric current. Polymers. 2016;8(8):275. https://doi.org/10.3390/polym8080275
- Yang Z, Yu S, Chen H, Guo X, Zhou J, Meng H. Effect of electrochemistry modification on the macromolecular, structural, and rheological characteristics of citrus peel pectin. Food Hydrocolloids. 2023;136:108246. https://doi.org/10.1016/j.foodhyd.2022.108246
- Antony P, Bhat SS, Tallur PN, Naik VM. Effect of activity coefficient of polyvalent ionic salt solution on demulsification of soy lecithin based oil-in-water emulsion. Asian Journal of Chemical Sciences. 2019;6(1):1–11. https://doi.org/10.9734/ajocs/2019/v6i118988
- Ito H, Kabayma S, Goto K. Effects of electrolyzed hydrogen water ingestion during endurance exercise in a heated environment on body fluid balance and exercise performance. Temperature. 2020;7(3):290–299. https://doi.org/10.1080/23328940.2020.1742056
- Nagamatsu Y, Nagamatsu H, Ikeda H, Shimizu H. Microbicidal effect and storage stability of neutral HOCl-containing aqueous gels with different thickening/gelling agents. Dental Materials Journal 2021;40(6):1309–1319. https://doi.org/10.4012/dmj.2020-454
- Bölek S, Tosya F, Dinç Ö. Effects of different types of electrolyzed waters on rheological characteristics of dough and quality properties of bread. Food Science and Technology International. 2023. https://doi.org/10.1177/10820132231170288
- Gorbacheva MV, Tarasov VE, Kalmanovich SA, Sapozhnikova AI. Electrochemical activation as a fat rendering technology. Foods and Raw Materials. 2021;9(1):32–42. https://doi.org/10.21603/2308-4057-2021-1-32-42
- Frolova YuV, Sobolev RV, Sarkisyan VA, Kochetkova AA. Effect of polysaccharide compounds on the stability of oil-in-water emulsions during storage. Food Processing: Techniques and Technology. 2022;52(1):32–45. (In Russ.). https://doi.org/10.21603/2074-9414-2022-1-32-45
- Bredikhin SA, Martekha AN, Andreev VN, Kaverina YuE, Korotkiy IA. Rheological properties of mayonnaise with non-traditional ingredients. Food Processing: Techniques and Technology. 2022;52(4):739–749. (In Russ.). https://doi.org/10.21603/2074-9414-2022-4-2402
- Abdullah, Liu L, Javed HU, Xiao J. Engineering emulsion gels as functional colloids emphasizing food applications: A review. Frontiers in Nutrition. 2022;9. https://doi.org/10.3389/fnut.2022.890188
- Pandya YH, Bakshi M, Sharma A. Agar-agar extraction, structural properties and applications: A review. The Pharma Innovation Journal 2022;11(6):1151–1157.
- Pogorelov AG, Ipatova LG, Pogorelova MA, Kuznetsov AL, Suvorov OA. Properties of serum albumin in electrolyzed water. Foods and Raw Materials. 2022;10(1):117–126. https://doi.org/10.21603/2308-4057-2022-1-117-126
- Pogorelov AG, Ipatova LG, Panait AI, Pogorelova MA, Gulin AA, Pogorelova VN. Spectrometry of plant polysaccharides in electrochemically activated water. Modern Trends in Biological Physics and Chemistry. 2021;6(3):511–515. (In Russ.).
- Kornena EP, Kalmanovich SA, Martovshchuk EV, Tereshchuk LV, Martovshchuk VI, Poznyakovskiy VM. Examining oils, fats, and their products. Quality and safety. Novosibirsk: Sibirskoye universitetskoye izdatel’stvo, 2017. 384 p.