Abstract
Hyperlipidemia is an enduring metabolic ailment that affects glucose and lipid processing.The research objective was to measure the total phenolic, flavonoid, and tannin contents in Olea europaea L. leaves and to to identify their antioxidant and antihyperlipidemic potential. The study included an in silico model of interaction for hydroxytyrosol, oleuropein, and xanthine dehydrogenase. The in vivo experiment involved rabbits that received olive leaves (150 mg/kg) and 10 mL of egg yolk as a high-fat diet. At the end of the experimental period, blood samples were tested for lipid profile, and tissue specimens were used for liver histology.
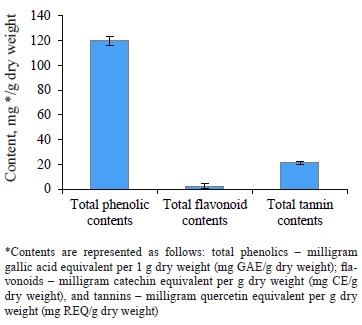
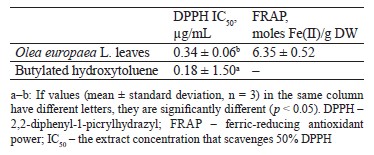
The total phenolic content was 119.84 ± 3.86 mg GAE/g, the total flavonoid content was 2.22 ± 0.07 mg CE/g, and the total tannin content was 21.25 ± 1.24 mg REQ/g dry weight. According to DPPH and FRAP analyses, the antioxidant capacities were 0.34 ± 0.06 μg/mL and 6.35 ± 0.52 μmol Fe(II)/g dry weight, respectively. In the experimental animals, O. europaea leaves reduced such parameters as total cholesterol, low-density lipoprotein, total triglycerides, total cholesterol vs. high-density lipoprotein, and low-density lipoprotein vs. high-density lipoprotein. The histopathological liver assay showed no signs of tissue damage while the samples obtained from the control group demonstrated steatosis deposits and cellular necrosis. Based on the energy and RMSD results, hydroxytyrosol proved an effective xanthine dehydrogenase inhibition.
These findings constitute a good scientific basis for the complementary future research on the potential of O. europaea leaves as ingredients of functional foods or medical drugs.
Keywords
Olea europaea L., hyperlipidemia, phenolic compounds, antioxidant activity, antihyperlipidemic activity, xanthine dehydrogenaseINTRODUCTION
Hyperlipidemia refers to increased presence of one or more lipid types in the bloodstream. This condition is evident through high cholesterol, sometimes accompanied by triglycerides [1–3]. Hyperlipidemia can be classified into two groups as either primary (familial) or secondary (acquired) hyperlipidemia. Primary hyperlipidemia originates from various genetic disorders while secondary hyperlipidemia typically arises as a result of inadequate diet, certain medications (amiodarone, glucocorticoids, etc.), hypothyroidism, uncontrolled diabetes, and unhealthy lifestyle [4]. The global incidence of hyperlipidemia has been progressively rising, which is commonly attributed to lifestyle and dietary factors [5].
As a multifaceted and enduring metabolic ailment, hyperlipidemia encompasses disruptions in glucose and lipid processing, along with broader systemic imbalances within the body. These imbalances include thickening of blood vessel walls, obesity, and heigh blood sugar [6]. Hyperlipidemia can directly trigger metabolic disorders, thus causing significant disruptions in metabolism and exerting adverse effects on intestinal well-being [7, 8]. Hyperlipidemia could be linked to an increased cumulative risk of myocardial infarction, ischemic stroke, and premature mortality [9]. High cholesterol is a significant factor that contributes to a third of global ischemic (coronary) heart disease cases. In total, high cholesterol is accountable for 2.6 million deaths, making up 4.5% of the total mortality [10, 11].
Managing hyperlipidemia includes such strategies as dietary regulation, physical activity, and medication. According to Gold et al., only a quarter of adults with significantly elevated low-density lipoprotein cholesterol (LDL-C) managed to attain the target reduction suggested by LDL-C guidelines [12]. The Spanish COHORT Familial Hypercholesterolemia Study provided individuals with familial hypercholesterolemia with lipid-lowering therapy; however, only a minority of 11.2% patients achieved the treatment objective of reducing low-density lipoprotein cholesterol below 100 mg/dL [13]. Several studies showed that hypercholesterolaemia correlates with the generation of reactive oxygen species that are known to cause oxidative damage to human cells. This process can be inhibited by antioxidant agents, e.g., polyphenols. They inhibit the propagation of free radicals either directly or indirectly, i.e., by reacting with enzymes involved in the production of reactive oxygen species [14].
The past few decades have seen a significant upsurge in alternative anti-hypercholesterolaemia remedies, particularly herbal medicines and their supplements [15]. Plants tend to yield compounds with fewer toxic side effects than synthetic medications, which makes them prospective raw materials for novel therapeutic agents [16]. Aumeeruddy et al. conducted a major review of popular herbal remedies utilized worldwide to manage high cholesterol [17]. The review covered a total of 174 surveys from 2001–2020 and revealed records of 390 plant species, encompassing 109 families and 294 genera. The studies were published by research teams from 37 countries and involved leaves (29%) and fruit (15%) as antihypercholesterolaemia remedies. Many of them featured Olea europaea L., commonly known as the olive tree. This species was reported across six countries: Algeria, Argentina, Greece, Palestine, Portugal, and Turkey. Olive (O. europaea) leaves contain a plethora of potentially bioactive compounds with antioxidant, antihypertensive, antiatherogenic, hypoglycemic, anti-cancer, and anti-inflammatory properties [18, 19].
Presumably, olive leaf extracts owe their biological properties to such potentially bioactive compounds as oleuropein and hydroxytyrosol [20, 21].
This article introduces the anti-hypercholesterolaemia potential of O. europaea leaves. We measured the total phenolic, flavonoid, and tannin contents to define their antioxidant and antihyperlipidemic potential. In literature sources, we did not find research on the antihyperlipidemic activity of O. europaea leaves using a high-fat diet and induced hypercholesterolaemia in rabbits as model animals. This research also featured an in silico interaction model of such phenolic compounds as hydroxytyrosol and oleuropein with xanthine dehydrogenase, which is a key enzyme in generating reactive oxygen species.
STUDY OBJECTS AND METHODS
Collecting the plant material. The olive leaves (Olea europaea L.) of a local Hamri variety were harvested in the Skikda region, North-East of Algeria, in early 2022. After a thorough cleaning, the leaves were dried at 40°C for 10 days to be ground to fine powder. The resulting powder was kept in a hermetically sealed bottle at room temperature in a dry and dark place until further use.
Preparing the extract. For extraction, we used a method described by Bhatia et al. [22]. After soaking 20 g of the olive leaf powder in 100 mL of 70% methanol at room temperature for 24 h, we filtered the mix and performed a triplicate maceration with solvent renewal in order to extract the maximum of the bioactive product. Then, we removed methanol in a rotary vacuum evaporator at 40°C and stored the resulting extract in airtight bottles at 4°C until use.
Total phenolics assay. To assess the total phenolic content, we applied the spectrophotometric method as described by Al-Farsi et al. [23]. In line with the procedure, we added 200 µL of the extract to 1.5 mL of the Folin-Ciocalteu reagent. After mixing and incubating the solutions in the dark for 5 min, we added 1.5 mL of sodium bicarbonate (60 g/L) to the reaction medium. Following 90 min of incubation at room temperature, we measured the absorbance with an ultraviolet-visible spectrophotometer at 725 nm against the blank without extract. The phenolic content was expressed as mg GAE/g dry weight, i.e., milligrams of gallic acid equivalent per 1 g dry weight.
Total flavonoids assay. Here, we followed the protocol described by Biglari et al. [24]. To assess the total amount of flavonoids in the extract, we added 4.0 mL of distilled water and 1 mL of the extract to 0.3 mL of 5.0% sodium nitrite (NaNO2) and 0.3 mL of 2.0% aluminum chloride (AlCl3) dissolved in methanol. After 5-min incubation at room temperature, we added 2 mL of 1.0% sodium hydroxide (NaOH) dissolved in methanol. The mix was then diluted to 10 mL with distilled water, and the absorbance was measured at 510 nm against the blank. The flavonoid content was expressed as mg CE/g dry weight, i.e., milligrams of catechin equivalent per 1 g dry weight.
Total tannins assay. To determine the total amount of tannins, we used the acidic vanillin method as described by Qaisar et al. [25]. We prepared the vanillin reagent by mixing the same volumes of 8% (v/v) HCl, 37% (v/v) methanol, and 4% vanillin in methanol (w/v). After storing the mix at 30°C, we added 200 μL of the extract to 1000 μL of the vanillin reagent. The mix was agitated and incubated in the dark at 30°C for 20 min. To measure the absorbance at 500 nm, we used a blank that consisted of a mix of methanol (37%) and HCl (8%) in equal volumes. The results were expressed as mg QE/g dry weight, i.e., milligrams of quercetin equivalent per 1 g dry weight.
Antioxidant activity. DPPH assay. As recommended by Blois, we measured the antioxidant potential of the olive leaf extract to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical [26]. We mixed 60 µL of different concentrations of the extract with 1500 mL of the DPPH solution (6×10–5 M). After 30 min of incubation at room temperature, we monitored the absorbance at 517 nm. The percentage inhibition (I, %) of the DPPH radical was calculated according to the Equation below:
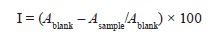
where Ablank is the absorbance of the control; Asample is the absorbance of the test extract with the DPPH solution.
The same procedure was repeated for butylated hydroxytoluene solutions as positive control. The antioxidant activity of the extract was expressed as IC50, which represents the concentration (µg/mL) of the extract required to scavenge 50% of the DPPH free radical.
FRAP assay. The ferric-reducing antioxidant power (FRAP) of the methanolic extract of O. europaea leaves was measured according the method described by Benzie & Strain [27]. In line with the protocol, we added 20 µL of the extract to 1.5 mL of the FRAP reagent. The obtained blue solutions remained at room temperature at 37°C for 20 min for the absorbance to be measured at 593 nm. We used ferrous sulfate heptahydrate (FeSO4∙7H2O) concentrations (100–2000 mmol/L) to calibrate the standard curve. The ferric-reducing antioxidant power was expressed as mmol Fe(II) per 1 g dry weight.
Experimental animals. Ethic. All the experiments on animals were confirmed and approved by the Department of Natural and Life Sciences, University of Skikda, Algeria. The experiments were conducted in line with the Guide for the Care and Use of Laboratory Animals.
Animals. The research involved healthy male rabbits (Oryctolagus cuniculus L.). The animals were eight weeks old and weighed 1.6–2.5 kg. They were obtained from a local supplier (Hama Bouziane, Constantine, Algeria). All the animals were kept under standard environmental conditions: 12 h light/12 h dark cycle, 20 ± 2°C. They had free access to tap water and food. The animals were quarantined for ≥ 10 days before the experiment.
The antihyperlipidemic activity of O. europaea leaves was measured based on the method proposed by Djerrou [28]. We divided a total of 25 rabbits randomly into five experimental groups with five animals in each (n = 5):
– group 1 represented normal control and received a standard diet;
– group 2 were subjected to oral feeding (gavage) with O. europaea leaves (150 mg/kg);
– group 3 received standard diet and 10 mL of egg yolk, i.e., a high-fat diet;
– group 4 received atorvastatin (2.5 mg/kg) followed by 10 mL of egg yolk after 30 min; and
– group 5 animals were fed with olive leaf powder (150 mg/kg) followed by 10 mL of egg yolk after 30 min.
Assay of plasma lipid and hepatic parameters. At the end of the experimental period (45 days), the rabbits were sacrificed to collect blood samples. Within 1 h, the samples were sent to a diagnostic laboratory for a lipid profile test using a BS-240 Mindray autoanalyzer. The test involved the following parameters: total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total triglycerides, total cholesterol vs. high-density lipoprotein, and low-density lipoprotein vs. high-density lipoprotein.
Histopathological study. The livers were washed with ice normal saline and fixed in a 10% formalin solution for histological assessment. The sections were assessed on hematoxylin and eosin to be examined with optic microscopy (Carl Seiss Microlmaging GmbH, Germany).
In silico study of the interaction of xanthine dehydrogenase and phenolic compounds. We used a method described by Patel & Kukol to study the interaction between xanthine dehydrogenase and phenolic molecules [29]. Xanthine dehydrogenase is a key enzyme in generating reactive oxygen species while hydroxytyrosol and oleuropein are the major phenolic compounds in O. europaea leaves.
Preparing protein. The structure of xanthine dehydrogenase was downloaded from the Protein Data Bank Archive (www.pdb.org) with PDB ID: 1N5X at 2.80 Ångström resolutions. Then, we used the AutodockTools software to add Gasteiger-type atomic charges and hydrogen atoms. The structures were saved in the PDBQT format.
Preparing ligand. This part of the research involved two phenolic compounds, i.e., hydroxytyrosol and oleuropein. We downloaded the ligands from the Pub-Chem site (https://pubchem.ncbi.nlm.nih.gov) in the SDF format to be reformatted into PDB using the PyMOL software. To increase the energy evaluation of the system, the receptor was immersed in a three-dimensional grid, which largely encompassed the active site of the protein and allowed the ligand to rotate freely in this site. The center of the grid box was determined by the coordinates X, Y, and Z with the dimensions of 3 Ångström. The spacing of the grid was fixed at 1 Ångström. The grid box was then centered on the ligand, its dimensions being proportional to the size of all the ligands in the study.
Calculating root mean square deviation. The reliability of a docking program is evaluated in terms its ability to reproduce protein-ligand complexes. Using the docking program, we determined the root mean square deviation (RMSD) between the conformation and the orientation of the ligand. The place it occupied in the experimental complex had to be as small as possible. The allowed ratio was a maximal difference of 2 Ångström. We used Discovery Studio 4.0 Client to calculate the root mean square deviation while AutoDock Vina served to determine the root mean square deviation and perform the visual analysis.
Statistical analysis. The results were given as mean ± standard deviation. The statistical analysis involved SPSS Statistics 16.0 (Chicago, USA). The analysis of variance (ANOVA) made it possible to identify the differences in the mean values. We applied the Tukey’s test to register statistically significant difference. The significance level was p < 0.05.
RESULTS AND DISCUSSION
Bioactive content. Figure 1 shows that the methanolic extract of Olea europaea L. leaves was rich in phenolic compounds. The total phenolic, flavonoid, and tannin contents were 119.84 ± 3.65 mg GAE/g dry weight, 2.22 ± 1.86 mg CE/g dry weight, and 21.25 ± 1.24 mg REQ/g dry weight, respectively.
Our results were in agreement with those obtained by Ines et al., who reported the phenolic contents of olive leaf extract (China) as 151.74 mg GAE/g [30]. Bixia et al., who worked with Tunisian O. europaea leaves, reported a higher total phenolic content of 905.96 mg GAE/g [31]. Results of Turkish and Saudi Arabian researchers for the total phenols were lower than ours, namely 37.8 and 45.48 mg GAE/g, respectively [32, 33]. Sirajudheen et al., who also worked with O. europaea leaves from Saudi Arabia, reported the total flavonoid content between 3.11 ± 0.67 and 6.44 ± 0.12 mg CE/g [33]. Our findings more or less coincided with these results. However, Nashwa & Abdel-Aziz reported the total flavonoid content as 21.45 mg EQ/g for the methanolic extract of olive leaves from Egypt [34]. These results exceeded those obtained in our study. To our best knowledge, no data regarding the total tannin contents in O. europaea leaf extracts have been published so far.
Chromatographic separation of aqueous extracts from Egyptian genotypes of the O. europaea plant revealed the presence of gallic acid, hydroxytyrosol, catechol, p-hydroxy benzoic acid, caffeine, vanillic acid, caffeic acid, syringic acid, oleuropein, vanillin, p-coumaric acid, ferulic acid, rutin, ellagic acid, benzoic acid, o-coumaric acid, salicylic acid, and cinnamic acid [35, 36]. Several studies identified oleuropein as a primary compound in O. europaea. These phytochemical components are known for their potent biological activities [37, 38]. Sirajudheen et al. mentioned 6-C-glucopyranosyl-8-cxylopyranosylchrysoeriol, quercetin 3-galactoside-7-rhamnoside, isovitexin, 6-hydroxyluteolin 5-rhamnoside, melanoxetin, calomelanol D-1, monotropein, tephrodin, robinetin 3-rutinoside, isovitexin 7-O-rhamnoside, isovitexin, and kaempferol 3-(2’’-(Z)-p-coumaroylglucoside) as the dominant flavonoids in the methanolic extract of O. europaea leaves [33]. The phytochemical composition of O. europaea leaf extracts depends on the geographic origin, climate, variety, growing conditions, maturity, season, soil, cultural specifics, processing methods, and solvent [39].Antioxidant activity. Table 1 summarizes the values of the antioxidant activities of O. europaea leaves based on DPPH and FRAP assays. The butylated hydroxytoluene, which was used as standard, had a stronger capacity to scavenge 2,2-diphenyl-1-picrylhydrazyl (0.18 ± 1.5 µg/mL) than the extract of O. europaea leaves (0.34 ± 0.06 µg/mL). As for the ferric-reducing antioxidant power, the olive extract demonstrated a rather high antioxidant potential of 6.35 ± 0.52 moles Fe(II)/g dry weight, as well as an ability to reduce the ferric iron (Fe+3) to ferrous iron (Fe+2).
Mansour et al.studied three O. europaea cultivars from Egypt, namely, picual, tofahi, and shemlali [36]. Their IC50 ranged from 48.14 ± 0.15 to 56.00 ± 0.13 μg/mL. As for the Turkish variety, its IC50 was 3.80 mg/mL [40].
Our findings agree with those reported by Cheurfa et al., whose team studied the antioxidant activity of the ethanolic and aqueous extracts of O. europaea leaves cultivated in Chlef, Algeria [41]. Their FRAP assay rendered the values of 07.53 ± 0.06 and 4.01 ± 0.01 moles Fe(II)/g dry weight, respectively. Presumably, O. europaea leaves owe their antioxidant properties to their high oleuropein [42]. This natural phytochemical protects human cells against free radicals that react with cellular molecules of fats, proteins, and DNA, thus producing oxidative damage [43]. The antioxidant potencies of oleuropein may be due to its capacities to chelate copper and iron. Ions of these metals generate reactive oxygen species, which are known to trigger cancer, hypertension, cardiovascular diseases, and inflammatory disturbances [37].
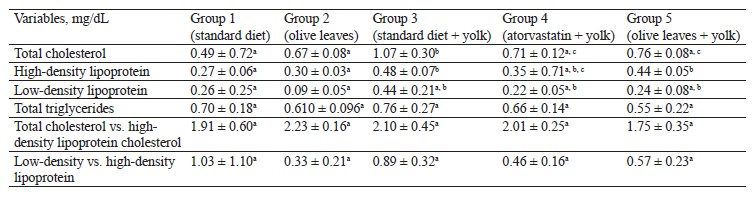
Antihyperlipidemic activity of Olea europaea L. leaves. The concentration of total cholesterol significantly increased (p < 0.05) in the group of rabbits fed with egg yolk (1.07 ± 0.306 g/dL) compared to the untreated control rabbits (0.49 ± 0.72 g/dL). The concentration of total cholesterol also increased compared to the group of rabbits treated with olive leaf powder (0.67 ± 0.08 g/dL). However, both groups fed with egg yolk and treated with either atorvastatin (0.71 ± 0.12 g/dL) or olive leaf powder (0.76 ± 0.08 g/dL) showed a significant decrease (p < 0.05) compared to Group 3, which received a standard diet and 10 mL of egg yolk. We detected a significant increase (p < 0.05) in high-density lipoprotein in the rabbits that received a high-fat diet (0.482 ± 0.070 g/dL) compared to the control (0.27 ± 0.06 g/dL) and Group 2 (0.30 ± 0.03 g/dL), which received olive leaves. Furthermore, we observed a significant decrease in Group 4 (0.35 ± 0.71 g/dL), treated with atorvastatin and egg yolk, compared to Group 3, which received olive leaves and egg yolk. However, the group of rabbits fed with olive leaves showed some decrease (0.44 ± 0.05 g/dL), although it was not significant, compared to Group 3.The concentration of low-density lipoprotein-cholesterol dropped (p < 0.05) in Group 3 (0.44 ± 0.21 g/dL) compared to Group 2 (0.09 ± 0.05 g/dL) and increased insignificantly compared to Group 1 (0.26 ± 0.25 g/dL), which received a standard diet. In contrast, Groups 4 and 5, which both received egg yolk and atorvastatin or olive leaf powder, showed an insignificant decrease (p > 0.05) compared to Group 3, which received egg yolk and a standard diet, with respective levels of 0.22 ± 0.05 and 0.24 ± 0.08 g/dL.
The concentration of total triglycerides increased insignificantly in Group 3 (0.76 ± 0.27 g/dL) compared to Group 2 (0.61 ± 0.09 g/dL) and Group 1 (0.70 ± 0.18 g/dL). However, the triglyceride level decreased insignificantly in Group 4 (0.66 ± 0.14 g/dL) and the group fed with olive leaf powder (0.55 ± 0.22 g/dL) compared to Group 3, which received egg yolk and a standard diet.
The results showed no significant increase (p > 0.05) in the ratio of total triglycerides and high-density lipoprotein in Group 3 (2.10 ± 0.45 g/dL), compared to control (1.91 ± 0.60 g/dL). However, we observed no significant decrease in the group of rabbits fed with olive leaves (1.75 ± 0.35 g/dL) and atorvastatin (2.01 ± 0.25 g/dL), compared to Group 3. We also detected no significant increase in the low-density lipoprotein vs. high-density lipoprotein ratio in Group 3 (0.89 ± 0.32 g/dL), compared to Group 2 (0.33 ± 0.21 g/dL). No significant decrease (p > 0.05) was registered in Group 3 compared to Group 1 (1.03 ± 1.10 g/dL). Similarly, we found no significant decrease (p > 0.05) in the low-density lipoprotein vs. high-density lipoprotein ratio in Group 4 (0.46 ± 0.16 g/dL) and Group 5 (0.57 ± 0.23 g/dL), which received atorvastatin or olive leaves with egg yolk, compared to Group 3, which received a standard diet and 10 mL of egg yolk.
Epidemiological data correlate a high consumption of plant extracts with a lower risk of several cardiovascular and degenerative diseases, hence the increasing scientific interest in their therapeutic properties as sources of health-promoting phytochemical molecules. Several experiments on people, mice, and rats reported that the extract of O. europaea leaves improved the plasma lipids profile (Table 2). However, to our best knowledge, no publications have so far reported the antihyperlipidemic activity of O. europaea leaves using a high-fat diet and induced hypercholesterolaemia in rabbits as model animals.
Our findings were in line with several previous studies. For instance, Cheurfa et al. studied administered aqueous extract of O. europaea leaf cultivated in Chlef, Algeria, on serum total cholesterol, triglycerides, high-density lipoproteins, low-density lipoproteins, and very low-density lipoproteins in hypercholesterolemic mice [41]. The mice treated with the extracts showed lower levels of total cholesterol, low-density lipoproteins, and triglycerides. In addition, they reported rutin and luteolin to be naturally present in the leaves of O. europaea. These substances were anchored against HMG-CoA reductase, the enzyme that limits cholesterol metabolism.
Atorvastatin is a synthetic lipid-lowering agent; it is a competitive inhibitor of HMG-CoA-reductase. It reduces total cholesterol, triglyceride levels, and low-density lipoprotein cholesterol [44].
Jang et al. reported that such phenolic compounds as gallic acid and linoleic acid, as well as their mixes, improved the serum lipid profile in hypolipidemic C57BL/6 mice by decreasing serum triglyceride and low-density lipoprotein cholesterol [45].
Hadrich et al. studied the effect of orally-administered oleuropein (50 mg/kg) on adiponectin secretion [46]. Oleuropein exerts hypocholesterolemic effect by inhibiting peroxisome proliferator-activated receptor γ, sterol regulatory element-binding protein-1c, and fatty-acid synthase expression. The team reported a protective effect of oleuropein and hydroxytyrosol derived from olive leaves on high-fat diet-induced lipid metabolism disorders. These phenolic compounds exerted their hypolipidemic and hepatoprotective effects by improving antioxidative defense system and blocking the expression of proteins involved in inflammation and liver damage [30].
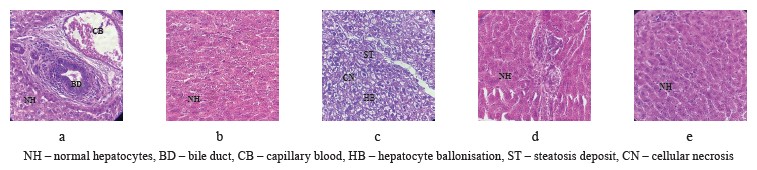
Hepatoprotective activity of Olea europaea L. leaves. Figure 2 illustrates the results of the histopathological tests. The morphological features of the liver in the positive control group showed a normal architecture of hepatocytes (Fig. 2a). The histology of the rabbits that received a standard diet together with olive leaves also demonstrated a normal architecture of hepatocytes (Fig. 2b). The microscopic observation of the liver obtained from the rabbits that received a hyper-lipid diet (control group) revealed some changes in the tissue architecture, namely, steatosis deposits, bloating, and cellular necrosis (Fig. 2c). However, the histopathological architecture of the liver sections obtained from the rabbits treated with the olive leaves (test group, Fig. 2d) and atorvastatin (reference group, Fig. 2e) demonstrated no histological changes in comparison with Group 3, which received a standard diet with egg yolk.
Increasing incidences of some chronic diseases, including hyperlipidemia, have raised awareness regarding the importance of diet. Numerous investigations determined that animals fed with high-fat and high-cholesterol feeds developed nonalcoholic fatty liver diseases. This pathology is characterized by steatosis, necroinflammation, ballooning, and fibrosis. Gaube et al. reported that the oral administration of ethanolic olive leaf extract induced no changes in histopathology, biochemical profile, and hematological parameters after single or repeated doses in a rat model [47]. According to Taamalli et al., the attenuation of hepatic tissues correlated quite well with the biochemical contents [48]. They explained the hepatoprotective potentials of O. europaea leaves by the high phenolic contents that were able to reduce the inflammatory and oxidant disorders in hepatic cells.
The hepatoprotective activity of O. europaea leaves has been elucidated against hepatic damages induced by cadmium, carbon tetrachloride (CCl4), and diazinon. Jemai et al. evaluated the hepatoprotective potential of oleuropein at 16 mg/kg boby weight against cadmium-induced hepatotoxicity in mice [49]. Orally-administered oleuropein restored significantly such biomarkers of liver injury as alanine transaminase, aspartate transaminase, lactate dehydrogenase, and phosphatase alkaline, compared to the animals that received cadmium alone. The histological and immunohistochemical tests showed a significant suppression of the inflammation scores, as well as the oxidative damage induced by cadmium in hepatic tissues. Rats pretreated with olive leaf extracts showed less oxidative damage in ischemic and nonischemic parts of liver, as well as a significant improvement in physiological and histopathological disorders. The predominant mechanism of hepatoprotective action is due to the high antioxidant potential and the capacity to scavenge free radicals [50]. Vidičević et al. studied the protective mechanisms of dry olive leaf extract in hepatotoxic rats treated with carbon tetrachloride (CCl4) [51]. The simultaneous treatment with CCl4 and the extract of O. europaea leaves significantly reduced the expression of protein kinase activated by adenosine monophosphate (AMPK) and inhibited the expression of autophagy-related protein LC3II compared with the control group. Omagari et al. confirmed the protective effect of oleuropein in reducing numerous hepatic genes involved in the production of reactive oxygen species and the regulation of cholesterol or lipid metabolism [52]. These effects are due to the properties of antioxidants that act as reducing agents by donating hydrogen and quenching singlet oxygen or by acting as chelators and trapping free radicals.
Interaction in silico between xanthine dehydrogenase and phytochemicals. Binding free energy. In this test, oleuropein formed the most stable complex with xanthine dehydrogenase with binding free energy (∆G) of –7.5 kcal/mol, followed by hydroxytyrosol with an energy of –6.4 kcal/mol.
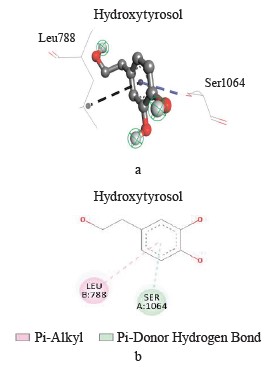
Interaction between hydroxytyrosol and xanthine dehydrogenase. A three-dimensional visualization of the interactions between the active site of xanthine dehydrogenase and hydroxytyrosol showed that the amino acids involved in the interaction were represented by leucine 788 and serine 1064 (Fig. 3).
According to the Discovery Studio model, hydroxytyrosol penetrated well into the active site of xanthine dehydrogenase by forming different pi-cation, pi-alkyl, hydrogen, pi-anion, and hydrogen carbon interactions (Table 3).
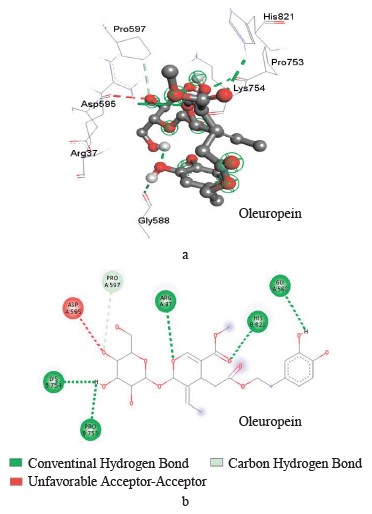
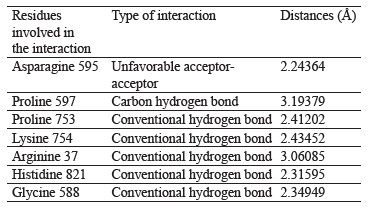
Interaction between oleuropein and xanthine dehydrogenase. A three-dimensional visualization of the interactions between the active site of xanthine dehydrogenase and oleuropein showed that the amino acids involved in the interaction were represented by glycine 38, glycine 588, glycine 35, lysine 95, and proline 753 (Fig. 4).
According to the Discovery Studio model, oleuropein penetrated well into the active site of xanthine dehydrogenase by forming hydrogen and carbon hydrogen bonds (Table 4).
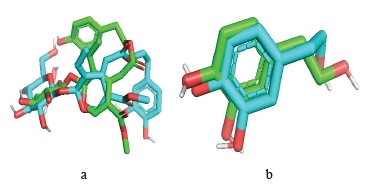
Reliability testing of the molecular docking program. Root mean square deviation. Interaction mode prediction consists of determining the correct positioning of the ligand in relation to its receptor. The ability of a program to perform this work is usually judged by means of the root mean square deviation (RMSD) of the model designed by the Vis-à-vis software regarding the structure of the reference (co-crystallized) ligand. The permissible limit is 2A°, beyond which the prediction is considered inadequate [53]. The enzyme complex used in this study is xanthine dehydrogenase (pdb: 1n5x). This procedure is followed by extracting the co-crystallized ligand from the given target and repositioning it by docking in the active site. The root mean square deviation of the best exposure of the reference ligand (after docking) is calculated with its crystallographic binding mode (before docking). The RMSD value of hydroxytyrosol of the software-designed model does not exceed 2 Å (Table 5).
Visual analysis is an essential step in judging the results described by the numerical value of the root mean square deviation. Visualization of the complexes selected, namely, xanthine dehydrogenase (pdb: 1n5x) and ligands made in the PyMOL molecular visualization system showed that the ligands predicted by AutoDock-Vina and the reference ligand were well positioned. Indeed, these results confirmed the root mean square deviation values.
The design of new plant-based computer-aided products (in silico study) is a relatively recent method for high-throughput screening of an extensive chemical database. It produces results for major compounds in less time and at lower costs. The in silico virtual screening method facilitates the development of innovative medicines. Based on the root mean square deviation results, the selected ligands, i.e., hydroxytyrosol and oleuropein, proved quite effective in inhibiting xanthine dehydrogenase (Fig. 5).
Xanthine oxidase results from the oxidation and/or proteolytic conversion of xanthine dehydrogenase. Xanthine oxidase is expressed in vascular cells and can circulate in plasma and bind to the extracellular matrix of endothelial cells. This enzyme catalyzes the metabolism of NADH, i.e., nicotinamide adenine dinucleotide (NAD) + hydrogen (H), molecular oxygen, hypoxanthine, and xanthine to produce O and HO. It is an important source of reactive oxygen species [54]. Most evidence about the involvement of xanthine oxidase in endothelial dysfunction and in the development of vascular diseases stems from the studies where the use of inhibitors of this enzyme (oxypurinol, allopurinol, febuxostat, topiroxostat) indicated improved endothelial function and vascular reactivity [29].
Recent studies showed that the inhibition of this enzyme protects against diabetic kidney disease and endometrial hyperplasia through the amelioration of oxidative stress by improving uterine-reduced glutathione and superoxide dismutase, as well as inhibiting the expressions of phosphatidylinositol-3-kinase (PI3K), Akt, and VEGF [54, 55]. Phenolic acids and flavonoids are antioxidants that can inhibit xanthine oxidase activity. The method was validated by attaching the inhibitory ligand to xanthine oxidase. Tran Minh et al. reported the root mean square deviation values ranging from 1.019 to 2.35 [56]. Cinnamon phenolic acids showed a remarkable activity of xanthine dehydrogenase inhibition [57]. Similarly, Serrano et al. inhibited xanthine oxidase with cinnamic acid [58]. Mehmood et al. studied the xanthine oxidase inhibition mechanism of eight structurally diverse phenolic compounds commonly present in fruit plant (quercetin, quercetin-3-rhamnoside, 4,5-O-dicaffeoylquinic acid, 3,5-O-dicaffeoylquinic acid, 3,4-O-di-caffeoylquinic acid, 4-O-caffeoylquinic acid, 3-O-caffeoylquinic acid, and caffeic acid) [59]. They used proton nuclear magnetic resonance (1H NMR), atomic force microscopy, and various computational techniques. The study suggested that these phytochemicals had a potent inhibition and interaction with this enzyme. The inhibition of xanthine oxidase by phenolic compounds can be used to prevent several pathologies.
CONCLUSION
In the present study, Olea europaea L. leaves were rich in phenolic compounds, which possess potent antioxidant and antihyperlipidemic activities. O. europaea leaves improved the lipid profile in test animals by reducing such parameters as total cholesterol, low-density lipoprotein cholesterol, total triglycerides, total cholesterol vs. high-density lipoprotein cholesterol, and low vs. high density lipoprotein cholesterol. According to the histopathological tests, the liver of rabbits that received a hyper-lipid diet revealed changes in tissue architecture represented by steatosis deposits, bloating, and cellular necrosis. The histopathological architecture of liver sections obtained from rabbits treated with olive leaf powder demonstrated no damage in comparison with the group which received a high-fat diet only. Based on the energy and root mean square deviation, hydroxytyrosol was effective in inhibiting xanthine dehydrogenase. Thus, O. europaea leaves proved to contain a wide array of phytochemicals that could be used in the therapeutic context as an effective antihyperlipidemic agent and an inhibitor of xanthine dehydrogenase responsible for the production of free radicals. This potential can be used to prevent a number of diseases.
Contribution
A. Amraoui and Z. Djerrou conceived and designed the analysis. A. Amraoui and S. Ali Haimoud collected the data. A. Amraoui, Z. Djerrou, K. Zerouki, and S. Elmokli worked with the data and analysis tools. A. Amraoui and Z. Djerrou performed the analysis. A. Amraoui and S. Ali Haimoud wrote the manuscript.
CONFLICTS OF INTEREST
The authors declared no conflict of interests related to the publication of this article.
REFERENCES
- Makshood M, Post WS, Kanaya AM. Lipids in South Asians: Epidemiology and management. Current Cardiovascular Risk Reports. 2019;13. https://doi.org/10.1007/s12170-019-0618-9
- Karr S. Epidemiology and management of hyperlipidemia. The American Journal of Managed Care. 2017;23(9):S139–S148.
- Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Primary Care: Clinics in Office Practice. 2013;40(1):195–211. https://doi.org/10.1016/j.pop.2012.11.003
- Ballantyne CM, Grundy SM, Oberman A, Kreisberg RA, Havel RJ, Frost PH, et al. Hyperlipidemia: Diagnostic and therapeutic perspectives. The Journal of Clinical Endocrinology and Metabolism. 2000;85(6):2089–2092. https://doi.org/10.1210/jcem.85.6.6642-1
- Moszak M, Szulinska M, Bogdanski P. You are what you eat – The relationship between diet, microbiota, and metabolic disorders – A review. Nutrients. 2020;12(4). https://doi.org/10.3390/nu12041096
- Wang K, Liao M, Zhou N, Bao L, Ma K, Zheng Z, et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Reports. 2019;26(1):222–235. https://doi.org/10.1016/j.celrep.2018.12.028
- Wen J-J, Li M-Z, Chen C-H, Hong T, Yang J-R, Huang X-J, et al. Tea polyphenol and epigallocatechin gallate ameliorate hyperlipidemia via regulating liver metabolism and remodeling gut microbiota. Food Chemistry. 2023;404. https://doi.org/10.1016/j.foodchem.2022.134591
- Poornima IG, Indaram M, Ross JD, Agarwala A, Wild RA. Hyperlipidemia and risk for preclampsia. Journal of Clinical Lipidology. 2022;16(3):253–260. https://doi.org/10.1016/j.jacl.2022.02.005
- Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Current Drug Targets. 2009;10(4):328–335. https://doi.org/10.2174/138945009787846434
- Global Health Observatory [Internet]. [cited 15 Sep 2023]. Available from: https://www.who.int/data/gho
- Uchendu IK, Ikebunwa OA, Okpagu CB. Cardiorenal protective effects of extracts of bitter leaf (Vernonia amygdalina L.) in animal model of metabolic syndrome. Foods and Raw Materials. 2024;12(2):264–272. https://doi.org/10.21603/2308-4057-2024-2-607
- Gold ME, Nanna MG, Doerfler SM, Schibler T, Wojdyla D, Peterson ED, et al. Prevalence, treatment, and control of severe hyperlipidemia. American Journal of Preventive Cardiology. 2020;3. https://doi.org/10.1016/j.ajpc.2020.100079
- Perez de Isla L, Alonso R, Watts GF, Mata N, Saltijeral-Cerezo A, Muñiz O, et al. Attainment of LDL-cholesterol treatment goals in patients with familial hypercholesterolemia: 5-year SAFEHEART Registry follow-up. Journal of the American College of Cardiology. 2016;67(11):1278–1285. https://doi.org/10.1016/j.jacc.2016.01.008
- Yoshioka Y, Ohishi T, Nakamura Y, Fukutomi R, Miyoshi N. Anti-cancer effects of dietary polyphenols via ROS-mediated pathway with their modulation of microRNAs. Molecules. 2022;27(12). https://doi.org/10.3390/molecules27123816
- Sharma P, Hajam YA, Kumar R, Rai S. Complementary and alternative medicine for the treatment of diabetes and associated complications: A review on therapeutic role of polyphenols. Phytomedicine Plus. 2022;2(1). https://doi.org/10.1016/j.phyplu.2021.10018
- Harnafi H, Serghini-Caid H, El Houda Bouanani N, Aziz M, Amrani S. Hypolipemic activity of polyphenol-rich extracts from Ocimum basilicum in Triton WR-1339-induced hyperlipidemic mice. Food Chemistry. 2008;108(1):205–212. https://doi.org/10.1016/j.foodchem.2007.10.062
- Aumeeruddy MZ, Mahomoodally MF. Global use of folk medicinal plants against hypercholesterolemia: A review of ethnobotanical field studies. Journal of Herbal Medicine. 2022;32. https://doi.org/10.1016/j.hermed.2022.100536
- El SN, Karakaya S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutrition Reviews. 2009;67(11):632–638. https://doi.org/10.1111/j.1753-4887.2009.00248.x
- Boss A, Bishop KS, Marlow G, Barnett MPG, Ferguson LR. Evidence to support the anti-cancer effect of olive leaf extract and future directions. Nutrients. 2016;8(8). https://doi.org/10.3390/nu8080513
- Kabbash EM, Abdel-Shakour ZT, El-Ahmady SH, Wink M, Ayoub IM. Comparative metabolic profiling of olive leaf extracts from twelve different cultivars collected in both fruiting and flowering seasons. Scientific Reports. 2023;13. https://doi.org/10.1038/s41598-022-27119-5
- Hassen I, Casabianca H, Hosni K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation – A mini review. Journal of Functional Foods. 2015;18:926–940. https://doi.org/10.1016/j.jff.2014.09.001
- Bhatia A, Singh B, Arora R, Arora S. In vitro evaluation of the α-glucosidase inhibitory potential of methanolic extracts of traditionally used antidiabetic plants. BMC Complementary Medicine and Therapies. 2019;19. https://doi.org/10.1186/s12906-019-2482-z
- Al-Farsi M, Alasalvar C, Morris A, Baron M, Shahidi F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun dried date (Phoenix dactylifera L.) varieties grown in Oman. Journal of Agricultural and Food Chemistry. 2005;53(19):7592−7599. https://doi.org/10.1021/jf050579q
- Biglari F, Alkarkhi AFM, Easa AM. Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits from Iran. Food Chemistry. 2008;107(4):1636–1641. https://doi.org/10.1016/j.foodchem.2007.10.033
- Qaisar MN, Chaudhary BA, Sajid MU, Hussain N. Evaluation of α-glucosidase inhibitory activity of dichloromethane and methanol extracts of Croton bonplandianum Baill. Tropical Journal of Pharmaceutical Research. 2014;13(11):1833–1836. https://doi.org/10.4314/tjpr.v13i11.9
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. https://doi.org/10.1038/1811199a0
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry. 1996;239(1):70–76. https://doi.org/10.1006/abio.1996.0292
- Djerrou Z. Anti-hypercholesterolemic effect of Pistacia lentiscus fatty oil in egg yolk-fed rabbits: A comparative study with simvastatin. Chinese Journal of Natural Medicines. 2014;12(8):561–566. https://doi.org/10.1016/S1875-5364(14)60086-8
- Patel H, Kukol A. Integrating molecular modelling methods to advance influenza A virus drug discovery. Drug Discovery Today. 2021;26(2):503–510. https://doi.org/10.1016/j.drudis.2020.11.014
- Fki I, Sayadi S, Mahmoudi A, Daoued I, Marrekchi R, Ghorbel H. Comparative study on beneficial effects of hydroxytyrosol- and oleuropein-rich olive leaf extracts on high-fat diet-induced lipid metabolism disturbance and liver injury in rats. BioMed Research International. 2020;2020. https://doi.org/10.1155/2020/1315202
- Wang B, Qu J, Feng S, Chen T, Yuan M, Huang Y, et al. Seasonal variations in the chemical composition of Liangshan olive leaves and their antioxidant and anticancer activities. Foods. 2019;12(8). https://doi.org/10.3390/foods8120657
- Acar-Tek N, Ağagündüz D. Olive leaf (Olea europaea L. folium): Potential effects on glycemia and lipidemia. Annals of Nutrition and Metabolism. 2020;76(1):10–15. https://doi.org/10.1159/000505508
- Anwar S, Saleem H, Khurshid U, Ansari SY, Alghamdi S, Al-Khulaidi AWA, et al. Comparative phytochemical composition, oleuropein quantification, antioxidant and cytotoxic properties of Olea europaea L. leaves. Natural Product Research. 2023;37(6):1023–1029. https://doi.org/10.1080/14786419.2022.2097230
- Morsy NFS, Abdel-Aziz ME. Efficiency of olive (Olea europaea L.) leaf extract as antioxidant and anticancer agents. Journal of Agroalimentary Processes and Technologies. 2014;20(1):46–53.
- Vogel P, Machado IK, Garavaglia J, Zani VT, de Souza D, Bosco SMD. Polyphenols benefits of olive leaf (Olea europaea L) to human health. Nutricion Hospitalaria. 2015;31(3):1427–1433. https://doi.org/10.3305/nh.2015.31.3.8400
- Mansour HMM, Zeitoun AA, Abd-Rabou HS, El Enshary HA, Dailin DJ, Zeitoun MAA, et al. Antioxidant and anti-diabetic properties of olive (Olea europaea) leaf extracts: In vitro and in vivo evaluation. Antioxidants. 2023;12(6). https://doi.org/10.3390/antiox12061275
- Orak HH, Karamać M, Amarowicz R, Orak A, Penkacik K. Genotype-related differences in the phenolic compound profile and antioxidant activity of extracts from olive (Olea europaea L.) leaves. Molecules. 2019;24(6). https://doi.org/10.3390/molecules24061130
- Zhang C, Xin X, Zhang J, Zhu S, Niu E, Zhou Z, et al. Comparative evaluation of the phytochemical profiles and antioxidant potentials of olive leaves from 32 cultivars grown in China. Molecules. 2022;27(4). https://doi.org/10.3390/molecules27041292
- Alesci A, Miller A, Tardugno R, Pergolizzi S. Chemical analysis, biological and therapeutic activities of Olea europaea L. extracts. Natural Product Research. 2021;36(11):2932–2945. https://doi.org/10.1080/14786419.2021.1922404
- Batçıoğlu K, Küçükbay F, Alagöz MA, Günal S, Yilmaztekin Y. Antioxidant and antithrombotic properties of fruit, leaf, and seed extracts of the Halhalı olive (Olea europaea L.) native to the Hatay region in Turkey. Foods and Raw Materials. 2023;11(1):84–93. https://doi.org/10.21603/2308-4057-2023-1-557
- Cheurfa M, Abdallah HH, Allem R, Noui A, Picot-Allain CMN, Mahomoodally F. Hypocholesterolaemic and antioxidant properties of Olea europaea L. leaves from Chlef province, Algeria using in vitro, in vivo and in silico approaches. Food and Chemical Toxicology. 2019;123:98–105. https://doi.org/10.1016/j.fct.2018.10.002
- Fernández-Poyatos MP, Ruiz-Medina A, Llorent-Martínez EJ. Phytochemical profile, mineral content, and antioxidant activity of Olea europaea L. cv. Cornezuelo table olives. Influence of in vitro simulated gastrointestinal digestion. Food Chemistry. 2019;297. https://doi.org/10.1016/j.foodchem.2019.05.207
- Lins PG, Pugine SMP, Scatolini AM, de Melo MP. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon. 2018;4(9). https://doi.org/10.1016/j.heliyon.2018.e00805
- Malhotra HS, Goa KL. Atorvastatin: an updated review of its pharmacological properties and use in dyslipidaemia. Drugs. 2001;61(12):1835–1881. https://doi.org/10.2165/00003495-200161120-00012
- Jang A, Srinivasan P, Lee NY, Song HP, Lee JW, Lee M, Jo C. Comparison of hypolipidemic activity of synthetic gallic acid-linoleic acid ester with mixture of gallic acid and linoleic acid, gallic acid, and linoleic acid on high-fat diet induced obesity in C57BL/6 Cr Slc mice. Chemico-Biological Interactions. 2008;174(2):109–117. https://doi.org/10.1016/j.cbi.2008.05.018
- Hadrich F, Mahmoudi A, Bouallagui Z, Feki I, Isoda H, Feve B, et al. Evaluation of hypocholesterolemic effect of oleuropein in cholesterol-fed rats. Chemico-Biological Interactions. 2016;252:54–60. https://doi.org/10.1016/j.cbi.2016.03.026
- Guex CG, Reginato FZ, Figueredo KC, da Silva ARH, Pires FB, Jesus RS, et al. Safety assessment of ethanolic extract of Olea europaea L. leaves after acute and subacute administration to Wistar rats. Regulatory Toxicology and Pharmacology. 2018;95:395–399. https://doi.org/10.1016/j.yrtph.2018.04.013
- Taamalli A, Feriani A, Lozano-Sanchez J, Ghazouani L, El Mufti A, Allagui MS, et al. Potential hepatoprotective activity of super critical carbon dioxide olive leaf extracts against CCl4-induced liver damage. Foods. 2020;9(6). https://doi.org/10.3390/foods9060804
- Jemai H, Mahmoudi A, Feryeni A, Fki I, Bouallagui Z, Choura S, et al. Hepatoprotective effect of oleuropein-rich extract from olive leaves against cadmium-induced toxicity in mice. BioMed Research International. 2020;2020. https://doi.org/10.1155/2020/4398924
- Uyanoğlu M. Prevention of tissue injury with Olea europaea L. leaf extract after partial liver ischemia/reperfusion. Biology Bulletin. 2021;48(5):536–545. https://doi.org/10.1134/S1062359021050150
- Vidičević S, Tošić J, Stanojević Ž, Isaković A, Mitić D, Ristić D, et al. Standardized Olea europaea L. leaf extract exhibits protective activity in carbon tetrachloride-induced acute liver injury in rats: The insight into potential mechanisms. Archives of Physiology and Biochemistry. 2020;126(5):399–407. https://doi.org/10.1080/13813455.2018.1550095
- Omagari K, Kato S, Tsuneyama K, Hatta H, Sato M, Hamasaki M, et al. Olive leaf extract prevents spontaneous occurrence of non-alcoholic steatohepatitis in SHR/NDmcr-cp rats. Pathology. 2010;42(1):66–72. https://doi.org/10.3109/00313020903434389
- Ahmed M, Shakeel M, Raza MA, Kumar U, Ansar M, Shah GA, et al. Models calibration and evaluation. In: Ahmed M, editors. Systems modeling. Singapore: Springer; 2020. pp. 151–178. https://doi.org/10.1007/978-981-15-4728-7_5
- Yang K-J, Choi WJ, Chang Y-K, Park CW, Kim SY, Hong YA. Inhibition of xanthine oxidase protects against diabetic kidney disease through the amelioration of oxidative stress via VEGF/VEGFR axis and NOX-FoxO3a-eNOS signaling pathway. International Journal of Molecular Sciences. 2023;24(4). https://doi.org/10.3390/ijms24043807
- Mohamed MZ, Abed El Baky MF, Hassan OA, Mohammed HH, Abdel-Aziz AM. PTEN/PI3K/VEGF signaling pathway involved in the protective effect of xanthine oxidase inhibitor febuxostat against endometrial hyperplasia in rats. Human and Experimental Toxicology. 2020;39(9):1224–1234. https://doi.org/10.1177/0960327120914977
- Ngoc TM, Khoi NM, Ha DT, Nhiem NX, Tai BH, Don DV, et al. Xanthine oxidase inhibitory activity of constituents of Cinnamomum cassia twigs. Bioorganic and Medicinal Chemistry Letters. 2012;22(14):4625–4628. https://doi.org/10.1016/j.bmcl.2012.05.051
- Hendriani R, Nursamsiar, Tjitraresmi A. In vitro and in silico evaluation of xanthine oxidase inhibitory activity of quercetin contained in Sonchus arvensis leaf extract. Asian Journal of Pharmaceutical and Clinical Research. 2017;10(14):50–53. https://doi.org/10.22159/ajpcr.2017.v10s2.19486
- Serrano JL, Figueiredo J, Almeida P, Silvestre, S. From xanthine oxidase inhibition to in vivo hypouricemic effect: An integrated overview of in vitro and in vivo studies with focus on natural molecules and analogues. Evidence-Based Complementary and Alternative Medicine. 2020;2020. https://doi.org/10.1155/2020/9531725
- Mehmood A, Li J, Rehman AU, Kobun R, Llah IU, Khan I, et al. Xanthine oxidase inhibitory study of eight structurally diverse phenolic compounds. Frontiers in Nutrition. 2022;9. https://doi.org/10.3389/fnut.2022.966557