Abstract
Every year, metabolic syndrome and cardiorenal diseases cause many deaths worldwide. African bitter leaf (Vernonia amygdalina L.) is known for its numerous therapeutic effects. Potentially, it can lower plasma lipid and glucose levels, which, in turn, may improve the condition of patients with the abovementioned diseases. This research featured the antihyperlipidemic and antihyperglycemic effects of methanol extract of V. amygdalina in an animal model of metabolic syndrome.Twenty albino rats were divided into four groups. Groups A to C were orally administered with ghee (3 mL/kg) + high-cholesterol diet (500 mg/kg) + high-sugar diet (10 mL/kg) to induce metabolic syndrome. Group A served as negative control and received no treatment with bitter leaf methanol extract. Groups B and C received 200 and 400 mg/kg of V. amygdalina methanol extract, respectively. Group D received no administration. The cardiorenal injuries and alterations in blood lipids and sugar levels wereassessed via various biochemical analyses.
The combination of ghee + high-cholesterol diet + high-sugar diet triggered a significant elevation of creatine kinase myocardial band, lactate dehydrogenase, aspartate aminotransferase, triglycerides, total cholesterol, low density lipoprotein-cholesterol, glucose, urea, creatinine, and potassium levels. The histopathological results agreed with the biochemical findings. However, the treatment with 200 and 400 mg/kg of V. amygdalina methanol extract was able to inhibit the adverse alterations causing a dosedependent significant antihyperlipidemic and antihyperglycemic effect (p < 0.05).
Bitter leaf (V. amygdalina) demonstrated cardiorenal protective effects and may be used to manage metabolic syndrome.
Keywords
Bitter leaf, Vernonia amygdalina, methanol extract, metabolic syndrome, animal model, hyperlipidemia, hyperglycemiaINTRODUCTION
Metabolic syndrome is an umbrella term for such disorders as hyperlipidemia, hyperglycemia, hypertension, and obesity, rather than a single condition [1]. As a result, it is among the biggest causes of morbidity and mortality worldwide [2]. In economically developing countries like Nigeria, inadequate early diagnosis and poor management often lead to a sequence of complications and death. Metabolic syndrome triggers a group of risk factors that, if not addressed properly, can lead to more serious metabolic problems, such as Type II diabetes and nonalcoholic fatty liver disease [3–5].
Sedentary lifestyle and consumption of low-fiber, high-fat food products are two primary contributors to this condition [2]. However, the pathogenic processes behind metabolic syndrome remain unclear and understudied. For instance, scientists cannot explain whether its numerous symptoms are evidence of separate diseases or of a single, shared pathogenic mechanism. The enormous geographic heterogeneity of metabolic syndrome highlights the extent to which social variables, including consuming too many calories and not exer cising enough, contribute to its etiology [6]. Most of the mechanisms involved in metabolic syndrome are primarily triggered by visceral fat, which means that a high calorie intake is a primary cause of disease [7]. Out of all the potential mechanisms, chronic inflammation, neurohormonal activation, and insulin resistance seem to be the key elements in the onset, development, and transformation of metabolic syndrome into cardiovascular diseases (Fig. 1) [8].
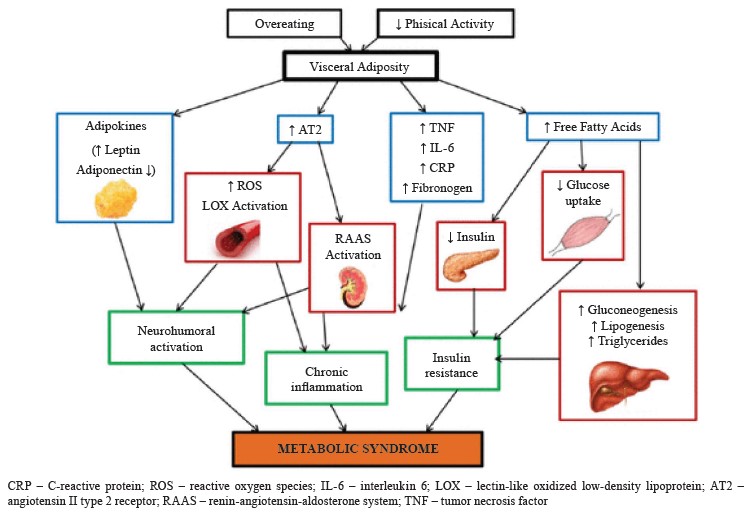
Numerous studies have established that metabolic syndrome can cause alterations in the cardiovascular system and renal structure [10]. Cardiovascular and kidney diseases are among the foremost causes of mortality in the world, despite the rapid progress in medical care [11]. Heart failure, hypertension, chronic kidney disease, high cholesterol, Type II diabetes, and cardiorenal metabolic disease are interconnected medical conditions [12, 13]. All these illnesses are associated with systemic inflammation, central obesity, and insulin resistance. Diets that are rich in fat and sugar cause degeneration in heart, kidney, and liver tissues [3, 14].
According to established scientific principles, a medical food is defined as a food prescribed by a physician and prepared to be eaten or delivered enterally. This food is designed specifically for dietary treatment of a particular medical condition or disease for which scientifically-proven dietary requirements are available [15].
Extracts of bitter leaf (Vernonia amygdalina L.) are known to have nutritional and therapeutic benefits that depend on its constituents [16–18]. Bitter leaf is a shrub or a small tree that can grow to be 23 feet tall when fully developed. Bitter leaf has flaking and gritty grey or brown bark. V. amygdalina is a native African plant that can be found throughout Sub-Saharan Africa. Bitter leaf has long been used as a medicinal herb against renal toxicity and oxidative stress [18]. In addition, it contains vitamins and trace elements that minimize oxidative stress and promote healing. Cu, Fe, and Zn values in V. amygdaline were reported as 0.0375, 0.5352, and 0.1916 mg/kg, respectively [19]. African bitter leaf is a popular food component in eastern Nigeria. Tradition has it that bitter leaf is good for hyperglycemic patients due to its bitter taste, or that it can reduce cholesterol and relieve fever, joint pain, intestinal colic, stomach ache, and malaria. The total effect of the bitter leaf can be determined experimentally by analyzing various biochemical parameters.
This research featured the lipid profile and glucose level in a rat model with hyperlipidemia and hyperglycemia after treatment with V. amygdalina extract. We defined metabolic syndrome as three abnormal outcomes from five components: increased triglycerides, increased blood pressure, decreased high-density lipoproteincholesterol, increased fasting plasma glucose, and increased waist circumference [20].
We believe that a locally available herbal product derived from bitter leaf may be an effective solution to the metabolic syndrome epidemic, which affects population of all age in a country where most people cannot afford drugs required for the treatment and control of obesity, hypertension, and other complications.
STUDY OBJECTS AND METHODS
Plant raw materials. Fresh Vernonia amygdalina L. leaves were purchased on the Ogbete market, Enugu, Nigeria. A consulting taxonomist verified their authenticity. A voucher specimen (no. PCG/UNN/030) was deposited at the Department of Pharmacognosy and Environmental Medicines, University of Nigeria, Nsukka.
Chemicals and reagents. Vegetable oil ghee, coconut oil, sucrose, and cholesterol powder were acquired from the Ogbete Market, Enugu, Nigeria. All reagents were obtained from Randox Laboratory Ltd., UK.
High-fat, high-sucrose diet for inducing metabolic syndrome. We mixed vegetable oil ghee and coconut oil in a 3:1 (v/v) ratio to create a high-fat diet which we administered orally to rats at a dose of 3 mL/kg per day for 28 days. The high-sugar diet of 30% sucrose solution was given orally to rats at a dose of 10 mL/kg per day for 28 days. The high-cholesterol diet involved 50 g of readily available cholesterol powder plus 9 g of sodium deoxycholate with bile salt to improve bioavailability. The components were mixed together and dissolved in coconut oil before being diluted to 200 mL with coconut oil to produce 250 mg/mL. The rats received it at the amount of 500 mg/kg per day for 28 days.
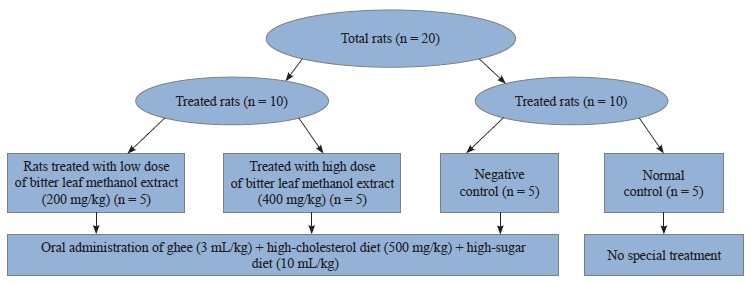
Laboratory animals. Twenty adult albino Wistar rats (110 ± 10 g) were procured from the College of Veterinary Medicine, University of Nigeria. The rats lived in a metallic cage with a regular temperature of 22 ± 3°C and a 12-h light-dark cycle. The animals were monitored for 14 days prior to the experiment date in order to allow them to acclimatize to the environment. The experimental design and management complied with the institutional guidelines for the care and use of laboratory vertebrates published by the American Physiological Society [21].
Preparing the plant extracts. A 400-g fresh plant of V. amygdalina was cleaned, cut into little pieces, and fully homogenized in a Waring blender. The final mix was immersed in 2 L of 80% methanol followed by mixing at room temperature for 24 h while being shaken intermittently. We used a low-pressure rotary evaporator to concentrate the filtrate to dryness at 40°C after filtration. After that, we extracted the residue once more in line with the same method. The entire amount of methanol was extracted from the collected filtrates using a rotary evaporator, and then the resulting crude extract was made and stored at 4°C until use.
Phytoconstituents analyses of bitter leaf. The sample of V. amygdalina was analyzed for flavonoids, glycosides, saponins, tannins, steroids, proteins, carbohydrates, and terpenoids at the Department of Pharmacognosy, University of Nigeria, Nsukka. We followed the methodology developed by Trease & Evans [22].
Acute toxicity. Tijjani et al. wrote that the intraperitoneal LD50 of ethanolic V. amygdalina leaf extract was 3721 mg/kg [23]. They reported no deaths after administering 5000 mg/kg extract orally, which means that the extract was deemed safe to use. In the current study, we applied methanol extract of bitter leaf as high and low doses of 400 and 200 mg/kg, respectively.
Experimental design. We divided 20 albino rats into four groups (A–D) of five rats in each. They received the following treatments for 28 days:
1. Group A (negative control) received ghee (3 mL/kg) + high-cholesterol diet (500 mg/kg) + sucrose solution (10 mL/kg), orally;
2. Group B ras were given ghee (3 mL/kg) + high-cholesterol diet (500 mg/kg) + sucrose solution (10 mL/kg),and low dose of V. amygdalina methanol extract (200 mg/kg), orally;
3. Group C rats were subjected to oral administration of ghee (3 mL/kg) + high-cholesterol diet (500 mg/kg) + sucrose solution (10 mL/kg), and high dose of V. amygdalina methanol extract (400 mg/kg); and
4. Group D (normal control) received no special treatment.
Sample collection. Blood samples for biochemical analysis were taken from the left ventricle of the heart under chloroform anesthesia. Heart and kidney tissues were excised for histopathological analyses.
Biochemical analyses. Serum lipid profile. We followed the cholesterol oxidase method described by Fredrickson et al. to measure total cholesterol [24]. The precipitation technique to determine high-density lipoprotein-cholesterol was described by Albers et al. [25]. The triglyceride test was in line with the glycerol phosphate oxidase method specified in [26]. The low-density lipoprotein-cholesterol was calculated using the Friedewald formula: Low-density lipoprotein-cholesterol = Total cholesterol ̶ (Very low-density lipoprotein-cholesterol + High-density lipoprotein-cholesterol) [27].
Plasma glucose. The blood glucose levels were determined using the glucose oxidase method as in [28].
Measuring the cardiac biomarkers. The level of creatine kinase myocardial band was determined in line with the kinetic colorimetric technique [29]. A Randox kit made it possible to measure the lactate dehydrogenase. We adopted Reitman & Frankel’s colorimetric approach to determine the aspartate transaminase [30].
Measuring the renal function biomarkers. Electro lyte, urea, and creatinine levels in the blood were measured to assess the renal function. A Perlong Medical PL1000A electrolyte analyzer served to study serum K+ and Na+. The urea level was estimated using diacetyl monoxime technique with protein precipitation, while the creatinine level was estimated by the Jaffe creatinine method as described in [31, 32].
Histopathological analysis. The paraffin wax embedding method was employed to prepare the removed heart and kidney tissues. The sections of 5 μm were subjected to the hematoxylin and eosin staining and examined under an OlympusTM light microscope [33].
Statistical analysis. The data analysis involved Graph Pad Prism 7.0 (San Diego, CA, USA). The results of the biochemical experiments were presented as mean ± SEM (standard error of mean). We used the one-way analysis of variance (ANOVA) to determine the degree of significance with probability levels below 0.05 (p < 0.05) as significant.
Ethical approval was issued by the Ethics Committee of the Department of Veterinary Medicine, University of Nigeria (Approval number: UNN/eTC/14/67485).
RESULTS AND DISCUSSION
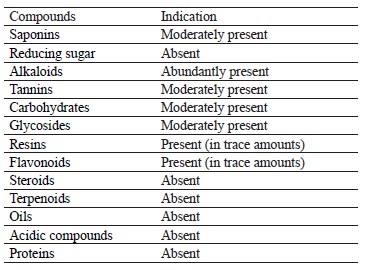
Phytochemical results. Table 1 illustrates the phytochemical examination of Vernonia amygdalina L. methanol extract, which appeared to be rich in alkaloids. Carbohydrates, glycosides, saponins, and tannins were present in moderate amounts. Flavonoids and resins were detected in trace amounts while proteins, acidic compounds, oils, terpenoids, and steroids were found absent.
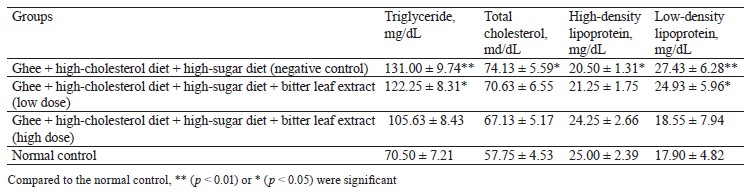
Biochemical results. This experiment featured the antihyperlipidemic and antihyperglycemic benefits of bitter leaf extract in albino Wistar rats with high-fat and high-glucose metabolic syndrome. The negative control rats that received ghee (3 mg/kg), high-cholesterol diet (500 mg/kg), and 30% sucrose solution (10 mg/kg) had the greatest levels of blood triglycerides, total cholesterol, and low-density lipoprotein (Table 2). This combination was able to induce significant dyslipidemia, thus giving the rats metabolic syndrome.
Low and high doses of bitter leaf methanol extract reduced triglyceride, total cholesterol, and low-density lipoprotein compared to the normal control group. Our results were comparable with those reported by Ogunrinola et al., who detected a significant (p < 0.05) hypolipidemia in rats treated with bitter leaf extract at all doses [34]. Ogbuabu et al. also posited that V. amygdalina leaves could substantially reduce triglyceride and total cholesterol, with no effect on high-density lipoprotein-cholesterol [35].
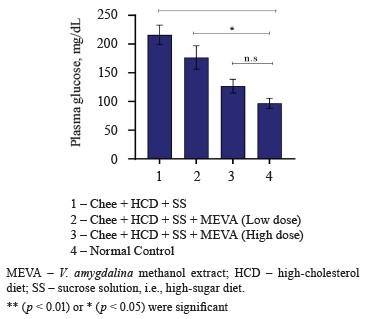
The negative control rats that received a combination of ghee + high-cholesterol diet + high-sugar diet showed the highest plasma glucose levels (Fig. 3). Therefore, the treatment with ghee, high-cholesterol, and sucrose solution induced hyperglycemia, i.e., a metabolic syndrome condition, in the rats.
In comparison with the normal control, the rats treated with low or high doses of bitter leaf extract demonstrated non-significant and significant declines in plasma glucose levels, respectively. This result also corroborates with other publications that reported the hypoglycemic properties of bitter leaf extract [36]. For instance, Olooto et al. studied the hypoglycemic potential of V. amygdalina in albino rats fed with a high-sucrose diet. In their study, the mean plasma glucose level was significantly elevated (p < 0.05) in the rats that followed a high-sucrose diet while those treated with bitter leaf demonstrated a small decrease [37]. Bawa & Ayobola proved the antidiabetic and anti-hyperlipidemic properties of V. amygdalina methanol extract [38]. Probably, that is why bitter leaf has historically been used to combat diabetes mellitus and its complications [39]. This beneficial effect may be attributed to such bioactive phytoconstituents as alkaloids, tannins, saponin, glycosides, and flavonoids [40].
Figure 3 clearly demonstrates that the bitter leaf extract was able to reduce blood glucose levels in a dosedependent manner.
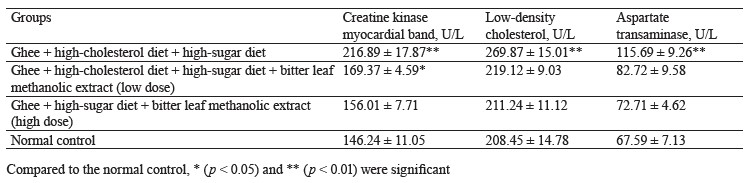
The negative control rats that received ghee + highcholesterol diet + sucrose solution showed a statistically significant (p < 0.05) elevated levels of creatine kinase myocardial band, low-density cholesterol, and aspartate transaminase when compared to the high-dose treatment group and the normal control, separately. Evidently, the combination of ghee, high-cholesterol diet, and sucrose solution could induce cardiac muscle damage in the rats. Both the low and high dose treatments caused a significant reduction of serum creatine kinase myocardial band, low-density cholesterol, and aspartate transaminase, compared to the negative control (Table 3).
Our finding is consistent with that made by Wijaya et al., who also reported that rats treated with 500 mg/kg of ethanolic extract of V. amygdalina had the lowest levels of creatine kinase myocardial band and low-density cholesterol [41]. Syahputra et al. similarly observed that rats treated with V. amygdalina had lower creatine kinase myocardial band, low-density cholesterol, and aspartate transaminase [42]. The cardioprotective effect of bitter leaf ethanol extract could be attributed to luteolin. This flavonoid inhibits apoptosis, improves systolic and diastolic function of the heart, and potentiates nitric oxide synthesis, thereby improving the overall function of the heart [43].
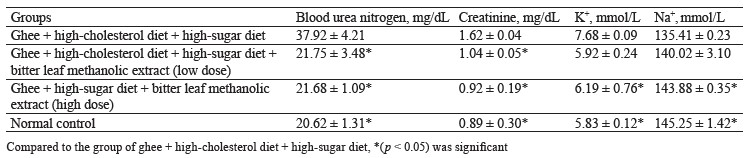
The negative control rats that received a combination of ghee + high-cholesterol diet + sucrose solution for 28 days showed a significant (p < 0.05) increase in blood urea nitrogen, creatinine, and potassium (K+), compared to the high-dose treatment group and the normal control, separately. Obviously, the combination of ghee, highcholesterol diet, and sucrose solution was able to induce renopathy secondary to metabolic syndrome in the rats. When compared to the negative control, the treatment with both low and high doses of bitter leaf extract lowered the levels of blood urea nitrogen, creatinine, and potassium (K+) (Table 4).
Our renal assessment was similar to that published by Barnes et al., who fed renal dysfunctional rats with V. amygdalina extract for 3 weeks to achieve normal restoration of creatinine and urea level [44]. Onwubiko et al. also treated rats with 400 mg/kg of V. amygdalina extract and reported lower urea and creatinine levels [45].
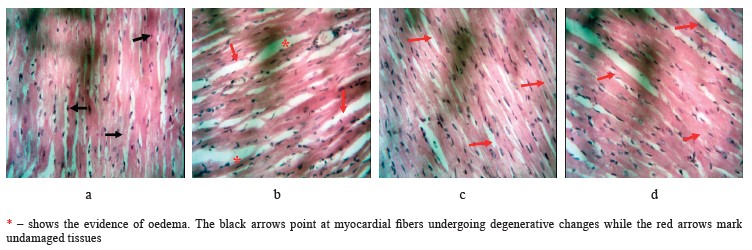
Histopathological results. In this research, metabolic syndrome damaged cardiac muscles, which could potentially lead to a variety of cardiovascular diseases. Figure 4 shows the photomicrograph of heart sections in the experimental groups following the treatments. The rats that received no special treatment showed unaffected myocardial fibers (Group D). The myocardial fibers of the negative control rats that received oral administration of ghee + high-cholesterol diet + sucrose solution showed some degenerative changes, e.g., poor striations (Group A). However, the rats subjected to the low dose of V. amygdalina treatment demonstrated normal myocardial fibers (Group B) with some evidence of edema seen as increased spaces between the fibers. Furthermore, the rats that received the high dose of V. amygdalina extract had normal-looking myocardial fibers (Group C).
The histopathological findings were in tandem with the biochemical results. This result was similar to the research conducted by Syahputra et al., where V. amygdalina extract also reduced cardiac degeneration [42].
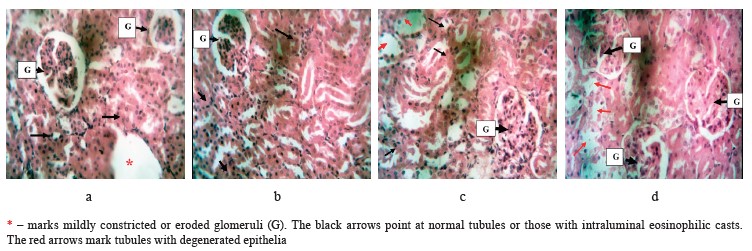
In Fig. 5, the glomeruli appeared normal while some tubules seemed to have slightly degenerated epithelia in the untreated rats (Group D). The kidney section of the negative control rats that received ghee + highcholesterol diet + sucrose solution showed some constricted or eroded glomeruli while the tubules appeared normal (Group A). However, the rats that were treated with the low-dose of bitter leaf extract demonstrated standard glomeruli while occasional tubules looked mildly eroded, some with intraluminal eosinophilic casts (Group B). The high-dose group had normal glomeruli while the tubules appeared eroded with occasional intraluminal eosinophilic casts (Group C).
This histopathological finding showed a dose-dependent effect, with the high dose having a better renal protective function than the low one. This result was in tandem with the study published by Barnes et al., where V. amygdalina extract improved the renal tissues of rats damaged by heavy metals [44]. The positive effect could be attributed to vitamins, antioxidants, and minerals in the plant.
CONCLUSION
The methanol extract of Vernonia amygdalina L. reduced hyperlipidemia and hyperglycemia in rats with metabolic syndrome caused by increased glucose and fat intake. In the test group, the bitter leaf extract ameliorated the negative effects. Its anti-cardiotoxic properties provided important protection against cardiovascular diseases. In sufficient doses, V. amygdalina demonstrated a renoprotective effect.
Contribution
The authors are equally liable for any plagiarism because they all contributed equally to writing the contentCONFLICTS OF INTEREST
There are no disclosed conflicts of interests regarding the publication of this article.
REFERENCES
- Katsimardou A, Imprialos K, Stavropoulos K, Sachinidis A, Doumas M, Athyros V. Hypertension in metabolic syndrome: Novel insights. Current Hypertension Reviews. 2020;16(1):12–18. https://doi.org/10.2174/1573402115666190415161813
- Saklayen MG. The global epidemic of the metabolic syndrome. Current Hypertension Reports. 2018;20. https://doi.org/10.1007/s11906-018-0812-z
- Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? Journal of Hepatology. 2018;68(2):335–352. https://doi.org/10.1016/j.jhep.2017.09.021
- Debnath B, Manna K. Formulating anti-diabetic nutraceutical tablets based on edible plants from Tripura, India. Foods and Raw Materials. 2022;10(2):227–234. https://doi.org/10.21603/2308-4057-2022-2-532
- Zaytseva LV, Ruban NV, Tsyganova TB, Mazukabzova EV. Fortified confectionery creams on vegetable oils with a modified carbohydrate profile. Food Processing: Techniques and Technology. 2022;52(3):500–510. (In Russ.). https://doi.org/10.21603/2074-9414-2022-3-2377
- Silvestri E, Giacco A. Diet, exercise, and the metabolic syndrome: enrollment of the mitochondrial machinery. Nutrients. 2022;14(21). https://doi.org/10.3390/nu14214519
- Purwowiyoto SL, Prawara AS. Metabolic syndrome and heart failure: mechanism and management. Medicine and Pharmacy Reports. 2021;94(1):15–21. https://doi.org/10.15386/mpr-1884
- Muzurović E, Mikhailidis DP, Mantzoros C. Non-alcoholic fatty liver disease, insulin resistance, metabolic syndrome and their association with vascular risk. Metabolism. 2021;119. https://doi.org/10.1016/j.metabol.2021.154770
- Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Therapeutic Advances in Cardiovascular Disease. 2017;11(8):215–225. https://doi.org/10.1177/1753944717711379
- Tune JD, Goodwill AG, Sassoon DJ, Mather KJ. Cardiovascular consequences of metabolic syndrome. Translational Research. 2017;183:57–70. https://doi.org/10.1016/j.trsl.2017.01.001
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: Challenges, progress, and possibilities. Clinical Journal of the American Society of Nephrology. 2017;12(12):2032–2045. https://doi.org/10.2215/CJN.11491116
- Mattina A, Argano C, Brunori G, Lupo U, Raspanti M, Lo Monaco M, et al. Clinical complexity and diabetes: a multidimensional approach for the management of cardiorenal metabolic syndrome. Nutrition, Metabolism and Cardiovascular Diseases. 2022;32(12):2730–2738. https://doi.org/10.1016/j.numecd.2022.09.008
- Silveira Rossi JL, Barbalho SM, Reverete de Araujo R, Bechara MD, Sloan KP, Sloan LA. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes/Metabolism Research and Reviews. 2022;38(3). https://doi.org/10.1002/dmrr.3502
- Kadowaki T, Maegawa H, Watada H, Yabe D, Node K, Murohara T, et al. Interconnection between cardiovascular, renal and metabolic disorders: A narrative review with a focus on Japan. Diabetes, Obesity and Metabolism. 2022;24(12):2283–2296. https://doi.org/10.1111/dom.14829
- Holmes JL, Biella A, Morck T, Rostorfer J, Schneeman B. Medical foods: Science, regulation, and practical aspects. Summary of a workshop. Current Developments in Nutrition. 2021;5. https://doi.org/10.1093/cdn/nzaa172
- Ugbogu EA, Emmanuel O, Dike ED, Agi GO, Ugbogu OC, Ibe C, et al. The phytochemistry, ethnobotanical, and pharmacological potentials of the medicinal plant – Vernonia amygdalina L. (bitter Leaf). Clinical Complementary Medicine and Pharmacology. 2021;1(1). https://doi.org/10.1016/j.ccmp.2021.100006
- Uchendu IK. Effect of aqueous extract of bitter leaf (Vernonia amygdalina) against acetaminophen-induced liver damage in rats. Bioequivalence and Bioavailability International Journal. 2018;2(1). https://doi.org/10.23880/BEBA-16000122
- Achuba FI. Role of bitter leaf (Vernonia amygdalina) extract in prevention of renal toxicity induced by crude petroleum contaminated diets in rats. International Journal of Veterinary Science and Medicine. 2018;6(2):172–177. https://doi.org/10.1016/j.ijvsm.2018.07.002
- Aderinboye OE, Solanke AS. Inductively coupled plasma-atomic emission spectrophotometer (ICP-AES) determination of trace elements present in Telfairia occidentalis and Vernonia amygdalina obtained from orita market, Ilaro town, Yewa South local government, Ogun State. 1st National WITED Conference; 2019; Ilaro. Ilaro; 2019.
- Nilsson PM, Tuomilehto J, Rydén L. The metabolic syndrome – What is it and how should it be managed? European Journal of Preventive Cardiology. 2019;26(2):33–46. https://doi.org/10.1177/2047487319886404
- Guiding principles for research involving animals and human beings. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2002;283:R281–R283. https://doi.org/10.1152/ajpregu.00279.2002
- Trease GE, Evans SM. Pharmacognosy. 15th Edition. London: Bailliere Tindall; 2002; 600 p.
- Tijjani MA, Mohammed GT, Alkali YT, Adamu TB, Abdurahaman FI. Phytochemical analysis, analgesic and antipyretic properties of ethanolic leaf extract of Vernonia amygdalina Del. Journal of Herbmed Pharmacology. 2017;6(3):95–99.
- Fredrickson DS, Levy RI, Lees RS. Fat transport in lipoproteins – An integrated approach to mechanisms and disorders. The New England Journal of Medicine. 1967;276(3):148–156. https://doi.org/10.1056/NEJM196701192760305
- Albers JJ, Warnick GR, Chenng MC. Quantitation of high density lipoproteins. Lipids. 1978;13(12):926–932. https://doi.org/10.1007/BF02533852
- Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clinical Chemistry. 1982;28(10):2077–2080. https://doi.org/10.1093/clinchem/28.10.2077
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry. 1972;18(6):499–502. https://doi.org/10.1093/clinchem/18.6.499
- Trinder P. Enzymatic colorimetric method of cholesterol determination. Annals of Clinical Biochemistry. 1969;6.
- Gerhardt W, Ljungdahl L, Börjesson J, Hofvendahl S, Hedenas B. Creatine kinase B-subunit activity in human serum. I. Development of an immunoinhibition method for routine determination of S-creatine kinase B-sununit activity. Clinica Chimica Acta. 1977;78(1):29–41. https://doi.org/10.1016/0009-8981(77)90335-7
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. American Journal of Clinical Pathology. 1957;28(1):56–63. https://doi.org/10.1093/ajcp/28.1.56
- Natelson S, Scott ML, Beffa C. A rapid method for the estimation of urea in biologic fluids. American Journal of Clinical Pathology. 1951;21(3):275–281. https://doi.org/10.1093/ajcp/21.3_ts.275
- Fabiny DL, Ertingshausen G. Automated reaction-rate method for determination of serum creatinine with the CentrifiChem. Clinical Chemistry. 1971;17(8):696–700. https://doi.org/10.1093/clinchem/17.8.696
- Koivukoski S, Khan U, Ruusuvuori P, Latonen L. Unstained tissue imaging and virtual hematoxylin and eosin staining of histologic whole slide images. Laboratory Investigation. 2023;103(5). https://doi.org/10.1016/j.labinv.2023.100070
- Ogunrinola OO, Fajana OO, Adu OB, Otutuloro AM, Moses TA, Lediju K, et al. The effects of Vernonia amygdalina leaves on lipid profile in cadmium-induced rat. MOJ Toxicol. 2019;5(2):83–88. https://doi.org/10.15406/moji.2019.05.00159
- Ogbuagu EO, Airaodion AI, Ogbuagu U, Airaodion EO. Effect of methanolic extract of Vernonia amygdalina leaves on glycemic and lipidaemic indexes of Wistar rats. Asian Journal of Research in Medical and Pharmaceutical Sciences. 2019;7(3):1–14. https://doi.org/10.9734/ajrimps/2019/v7i330122
- Oluwayemi AT, Nwachuku EO, Holy B. Effects of the interation of metformin and Vernonia amygdalina (Bitter leaf) on steptozotocin-induced diabetic rats. Asian Journal of Biochemistry, Genetics and Molecular Biology. 2018;1(2):1–8. https://doi.org/10.9734/ajbgmb/2018/v1i227494
- Olooto WE, Ogunkoya OO, Alabi AO, Oyinloye EO. Hypolipidaemic potentials of Vernonia amygdalina (Bitter leaf) in male albino rats fed high-sucrose diet. Annals of Health Research. 2017;3(1):10–17.
- Bawa KB, Iyanda AA. Investigation of hypoglycemic and hypolipidemic effects of methanolic extracts of Bitter leaf (Vernonia Amygdalina Delile) in male rats. Asian Journal of Advanced Research and Reports. 2020;10(4):30–37. https://doi.org/10.9734/ajarr/2020/v10i430250
- Ebong PE, Atangwho IJ, Eyong EU, Egbung GE. The antidiabetic efficacy of combined extracts from two continental plants: Azadirachta indica (A. Juss) and Vernonia amygdalina (African bitter leaf). American Journal of Biochemistry and Biotechnology. 2008;4(3):239–244.
- Uchendu IK, Okoroiwu HU. Evaluation of blood oxidant/antioxidant changes and testicular toxicity after subacute exposure to cadmium in albino rats: Therapeutic effect of Nigella sativa seed extracts. Combinatorial Chemistry and High Throughput Screening. 2021;24(1):79–87. https://doi.org/10.2174/1386207323666200526134923
- Wijaya L, Ginting CN, Chiuman L, Lister INE. Cardio protective effect of ethanolic extract Vernonia amygdalina Delile on rats induced doxorubicin. 2020 3rd International Conference on Mechanical, Electronics, Computer, and Industrial Technology (MECnIT); 2020; Medan. Medan; 2020. p. 5–8. https://doi.org/10.1109/MECnIT48290.2020.9166668
- Syahputra RA, Harahap U, Dalimunthe A, Pandapotan M, Satria D. Protective effect of Vernonia amygdalina Delile against doxorubicin-induced cardiotoxicity. Heliyon. 2021;7(7). https://doi.org/10.1016/j.heliyon. 2021.e07434
- Luo Y, Shang P, Li D. Luteolin: A flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Frontiers in Pharmacology. 2017;8. https://doi.org/10.3389/fphar.2017.00692
- Barnes P, Yeboah JK, Gbedema W, Saahene RO, Amoani B. Ameliorative effect of Vernonia amygdalina plant extract on heavy metal-induced liver and kidney dysfunction in rats. Advances in Pharmacological and Pharmaceutical Sciences. 2020;2020. https://doi.org/10.1155/2020/2976905
- Onwubiko GN, Nwankwo NE, Egbuonu ACC, Soribe BA, Okeke ES, Eze CJ. Amelioration of high levels of serum kidney function biomarkers by Vernonia amygdalina in monosodium glutamate induced rats. Indian Journal of Natural Products and Resources. 2022;13(2):197–205. https://doi.org/10.56042/ijnpr.v13i2.36235