Abstract
Biodegradable polymers, specifically polylactide, are an important part of food packaging and medical devices. Microbiological synthesis uses cheap renewable raw materials and industrial waste to produce a high yield of lactic acid, the monomer of polylactide. This method needs new effective lactic acid producing strains, e.g., thermophilic bacteria.The research involved thermophilic bacterial strains isolated from soil and compost samples. Their ability to produce organic acids and extracellular enzymes was tested using the method of high-performance liquid chromatography (HPLC) and microbiological tests respectively. The real-time polymerase chain reaction method (PCR) detected L-lactate dehydrogenase structural genes of L-lactate dehydrogenase of Bacillaceae. Strain T7.1 was fermented using glucose and yeast extract as carbon and nitrogen sources, respectively. The optical purity of lactic acid was evaluated using quantitative gas chromatography on a chiral column to separate lactate isomers. The molecular genetic analysis of the 16S rRNA gene sequence was applied to identify strain T7.1.
The chromatographic analysis proved that 10 out of 13 isolated thermophilic strains were effective lactic acid producers. They demonstrated proteolytic, amylolytic, or cellulase activities. During the fermentation, strain T7.1 produced 81 g/L of lactic acid with a peak productivity at 1.58 g/(L·h). The optical purity of the product exceeded 99.9% L-lactate. The genetic analysis identified strain T7.1 as Weizmannia coagulans (Bacillus coagulans).
The research revealed a promising thermophilic producer of optically pure L-lactic acid. Further research is needed to optimize the cultivation conditions, design an effective and cheap nutrient medium, and develop engineering and technological solutions to increase the yield.
Keywords
Thermophilic bacteria, Bacillus, Weizmannia coagulans, lactic acid, L-lactate, polylactideINTRODUCTION
Lactic acid is widely used in food production, pharmacy, and cosmetics. The global production of lactic acid amounted to 1.39 million tons in 2021 and will have reached 2.65 million tons by 2029 [1]. The demand for local acid keeps increasing, following the increase in production of foods, beverages, and biodegradable polymers [2]. Lactic acid serves as a preservative and acidity regulator in pickled and fermented foods, cheeses, yoghurts, fermented dairy products, bakery, confectionery, etc. [3]. In addition, environmentally-aware consumers, manufacturers, and scientists encourage the development of sustainable products and processes. This trend affects packaging methods, since environmental pollution by synthetic polymers is approaching a critical level [4, 5].
Lactic acid is a precursor for the synthesis of green solvents, lactide and polylactide. It should be noted that lactic acid can at least partially solve the tremendous problem of plastic pollution because it is a source of polylactide, which is a biodegradable polymer of high transparency and excellent mechanical strength. Polylactide has good barrier properties to food smell, as well as chemical resistance to fats and oils [6, 7]. It is a prospective material for biodegradable films and plasticware [2].
Lactic acid has two stereoisomers. As a result, it can produce poly-L-lactide, poly-D-lactide, and poly-L,D-lactides with different isomer content. The mechanical properties of particular polymer depend on the ratio of L- and D-isomers. For instance, polylactide with 90% of poly-L-lactide has a semi-crystalline structure, while polylactide with a higher content of D-lactate is an amorphous material with poor mechanical properties [8].
Chemical and microbiological syntheses are two commercial methods of lactic acid production. However, chemical synthesis forms racemic L,D-lactic acid, which makes it difficult to produce polylactide with required properties. Biotechnological synthesis yields optically pure isomers of lactic acid from cheap and renewable raw materials, e.g., industrial waste [9]. A proper pretreatment, such as grinding, chemical or enzymatic hydrolysis, etc., can increase the yield. For example, Alexandri et al. used a hydrolysate of bread production waste as a substrate to obtain optically pure L-lactic acid and cultivate Bacillus coagulans [10]. They also substituted yeast extract with a more accessible alfalfa juice. The productivity for batch cultivation was 2.59 g/(L·h) with the final lactic acid titer of 62.2 g/L. The combination of these two substrates (50:50) in a continuous process with cell recycling raised the efficiency up to 11.28 g/(L·h).
Some lignocellulosic substrates are fit for simultaneous saccharification and fermentation. The pretreatment occurs, wholly or partly, in a fermenter, where the lactic acid producer is cultivated. Hu et al. used the method of simultaneous saccharification and fermentation, as well as corn straw as substrate, to cultivate Lactobacillus pentosus FL0421 (37°C, pH 6.0) [11]. They also added cellulases and yeast extract to the medium with the pretreated substrate. The final lactic acid titer and the product efficiency were 92.30 g/L and 1.92 g/(L·h), respectively.
The yield and optical purity of microbiologically-synthesized lactic acid depends on gene expression, enzymatic activity, stereospecificity of lactate dehydrogenases (LDH), lactate racemases, and some enzymes of amino acid metabolism. For instance, enzymes L-LDH and D-LDH catalyze the conversion of pyruvic acid to L-lactic acid and D-lactic acid, respectively. They can be present, together or separately, in different types of microorganisms [12].
Lactate racemases provide the mutual conversion of lactate isomers. They can be found in some lactic acid bacteria, but not necessarily in Bacillaceae, which also can produce lactic acid [13]. Bacillaceae strains are known as effective producers of optically pure lactic acid. Unlike the more popular Lactobacillaceae, Bacillaceae are thermotolerant and/or thermophilic. Thermophilic lactic acid producers have some advantages over mesophilic microorganisms. They demonstrate accelerated metabolism and increase the solubility of substrates in the fermentation medium during thermal processing, thus boosting the yield. In addition, they reduce the risk of contamination during fermentation [14]. Thermophilic microorganisms have the same optimal growth temperatures as commercial enzyme preparations, which increases the efficiency of saccharification of complex raw materials in a bioreactor [15]. Thermotolerant Bacillaceae, e.g., B. coagulans (Weizmannia coagulans), are prospective lactic acid producers as they have a low need for growth factors and can utilize a wide range of substrates [16]. Some Bacillaceae are resistant to inhibitory substances contained in some hydrolysates, e.g., to furfural and its derivatives [17]. In addition, Bacillaceae have their own hydrolytic enzymes, i.e., proteases, amylases, and cellulases. As a result, saccharification requires less enzymes during simultaneous saccharification and fermentation [9].
Fed-batch culture, as well as semi-continuous and continuous fermentation, can increase the lactate yield during biosynthesis. Continuous cultivation methods, including those based on a membrane bioreactor, facilitate substrate conversion in the fermentation medium into the target product and reduce inhibitory effects [18]. The efficiency of bioreactors depends on how well they are able to maintain the culture in a certain physiological state at a given medium supply rate [19]. Longterm cultivation during continuous fermentation requires process stability, a low contamination risk, and a highly-active and stable producer. As a result, the food science keeps looking for new lactic acid producers with high optical purity and their assessment methods. The research objective was to isolate and identify thermophilic bacterial strains capable of homofermentative conversion of carbohydrates into lactic acid, as well as to evaluate the optical purity of the product.STUDY OBJECTS AND METHODS
Microorganisms and culture conditions. The research featured strains of thermophilic bacteria isolated from various samples of natural origin on nutrient media with different glucose and lactate amounts as a carbon source and elective element. To obtain enrichment cultures, we added 1 g of the sample to nutrient media and cultivated at 50°C for 24 h. After that, we seeded the cultures into a liquid medium and its tenfold dilutions on agar nutrient medium. The medium composition, g/L, was as follows: glucose – 20, tryptone – 20, yeast extract – 5, K2HPO4·3H2O – 2, MgSO4·7H2O – 0.2, MnSO4·5H2O – 0.05, and CaCO3 – 10. For further research, we selected those colonies that demonstrated zones of calcium carbonate dissolution caused by organic acids. The profiling of low molecular weight metabolites was carried out using a similar but tryptone-free liquid medium after 24 h of cultivation in test tubes under anaerobic or microaerophilic conditions. The nature of colony growth on agar media revealed its ability to synthesize extracellular proteases, amylases, and cellulases. The medium contained skimmed milk powder, starch, and carboxymethyl cellulose, respectively [20].To produce lactic acid, the strains were cultivated in a five-liter Minifors fermenter (INFORS, Switzerland). The inoculum was grown at 50°C under microaerophilic conditions on a nutrient medium for 24 h. The medium included, g/L: glucose – 20, yeast extract – 5, K2HPO4·3H2O – 2, MgSO4·7H2O – 0.2, and MnSO4·5H2O – 0.05. All the fermentation medium components were sterilized separately to avoid the formation of inhibiting compounds. The operating volume of the medium in the fermenter was 3 L, and the concentrations of the components, g/L, were as follows: glucose – 100, yeast extract – 5, K2HPO4·3H2O – 2, MgSO4·7H2O – 0.2, and MnSO4·5H2O – 0.05. The inoculum was added as 10% of the medium volume. The fermentation occurred under anaerobic conditions while the medium was stirred at 250 rpm. During cultivation, temperature and pH were recorded and controlled using the Iris V5 software. The fermentation medium was automatically titrated to neutralize lactic acid with 25% ammonia solution until pH reached 7.0. The bacterial biomass was measured by the optical density of tenfold dilutions at λ = 590 nm.
Determining the content of organic acids and glucose. To analyze the metabolites in the culture broth of thermophilic strains and glucose consumption, we used the method of high-performance liquid chromatography (HPLC). The procedure involved an Agilent 1220 Infinity chromatograph (Agilent, USA) with an Agilent Hi-Plex H column (250×4.6 mm). The concentration was determined by the refractometric signal after standard calibration. As pretreatment, the culture broth was centrifuged at 12 000 rpm for 15 min and filtered through 0.45-µm cellulose acetate membranes (MF-Millipore, USA). The analysis was performed at 50°C and a mobile phase flow rate (0.002 M H2SO4) of 0.3 mL/min.
Evaluating the optical purity of lactate. To determine the individual optical isomers of lactic acid in the fermentation products, the culture broth was purified from biomass by ultrafiltration on an AR-100 hollow fiber device with a cut-off limit of 100 kDa. Nonutilized components of the nutrient medium and interfering metabolic products were adsorbed: the cell-free broth was filtered through a layer of activated carbon. After that, the lactate was converted into salt, and from salt into acid. This process involved ion-exchange H-form resin Amberlyst 70. The entire lactic acid was converted into the trimethylsilyl derivative using N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA) as a silylating agent. We took 0.03 g of the sample, thoroughly mixed it with 1 mL of ethyl acetate, and added extra BSTFA. The mix was incubated at 60°C for 30 min. We used the same conditions to convert all forms of lactic acid to derivatives of ditrimethylsilyl ethers (diTMS esters) suitable for gas chromatography.
The optical isomers of the obtained lactic acid derivatives were determined by quantitative gas chromatography using a column for the separation of chiral isomers Cyclosil-B, 30 m×0.25 mm×0.25 μm (Agilent, USA). The procedure also involved a Kristall 5000.1 gas chromatograph (Khromatek, Russia) with a flame-ionization detector and Khromatek Analyst 2.6 software. Nitrogen served as the mobile phase at a pressure of 75 kPa. The initial temperature of the column (35°C) was maintained for 20 min, then the temperature was raised to 100°C at a rate of 1°C/min and then to 250°C at a rate of 10°C/min. The optical purity (OP) of L-lactic acid was calculated as follows:
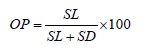
where SL is the peak area of the diTMS-ester of L-lactic acid; and SD is the peak area of the diTMS-ester of D-lactic acid.
Screening L-LDH genes. The structural genes of L-lactate dehydrogenase were detected using the realtime polymerase chain reaction method (PCR). Degenerate primers for genetic screening were selected based on the sequences typical of Bacillaceae, in particular Bacillus coagulans and Bacillus subtilis: 5’-CGGSCTGCCGAAAGAAC-3’ (forward primer) and 5’-GCCGTGYTCGCCGATAAT-3’ (reverse primer). The genomic DNA isolation involved a 24-h culture and a genomic DNA isolation kit (Dia-M, Russia). The PCR analysis was performed in a CFX96 Touch Deep Well Real-Time PCR Detection System (Bio-Rad Laboratories, USA).
The reaction mix had a volume of 25 µL and included 5 µL of 5x qPCRmix-HS SYBR reagent (Evrogen, Russia). In total the reaction mix contained Taq DNA Polymerase, dNTPs, buffer, MgCl2 and SYBR Green I, 1 μL of each primer with final concentration of 200 nM, 1 μL of the culture lysate, and bidistilled water.
The PCR had the following temperature conditions: preliminary denaturation at 95°C for 3 min followed by 35 cycles of denaturation at 95°C for 15 s, annealing at 55°C for 15 s, and elongation at 72°C for within 25 s.
The PCR data analysis involved melting curves constructed when the samples were heated at 65–95°C at 0.5°C intervals.
Strain identification. The bacteria were identified by their morphological and biochemical features, as well as by molecular genetic analyses of the 16S rRNA gene nucleotide sequence. The genomic DNA isolation involved a 24-h culture and a kit for isolating genomic DNA from bacterial cells.
The classical PCR was performed in a CFX96 Touch Deep Well Real-Time PCR Detection System with a Hot Start Taq-DNA polymerase PCR kit (Dia-M, Russia). The universal primers 8F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 1492R (5’-TACGGYTACCTTGTTACGACTT-3’) were used at a final concentration of 240 nM to amplify the 16S rRNA gene region.
The process proceeded under the following conditions: pre-denaturation at 95°C for 5 min followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 54°C for 30 s, elongation at 72°C for 90 s, and the final elongation at 72°C for 10 min.
The sequencing of the obtained PCR fragments of the 16S rRNA genes followed the Sanger method with preliminary purification (Evrogen, Russia). The sequencing involved the same universal primers as those used for amplification. The amplicons were sequenced in forward and reverse directions. The resulting nucleotide sequences were compared to the 16S rRNA gene sequences from the NCBI GenBank database using the BLAST search tool. The phylogenetic analysis relied on the neighbor-joining method in the MEGA software [19].
RESULTS AND DISCUSSION
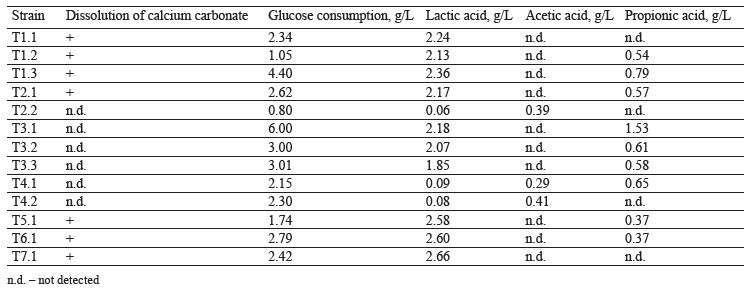
Isolating the thermophilic strains and their metabolite profiling. We isolated and profiled 13 thermophilic bacterial strains using the enrichment culture method. The samples were obtained from soils of Moscow urban forests, summer gardens, and natural ecosystems of the Moscow Region. Seven strains developed calcium carbonate dissolution zones on agar media with glucose as a carbon source, which proved that they released organic acids. The isolated acid-forming strains were characterized by rod-shaped morphology. They were classified as aerotolerant anaerobes and facultative anaerobes. The chromatography of the thermophilic culture broth grown under microaerophilic conditions showed that the bacteria released lactic, acetic, and propionic acids (Table 1).
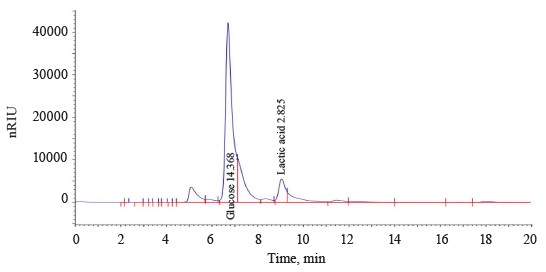
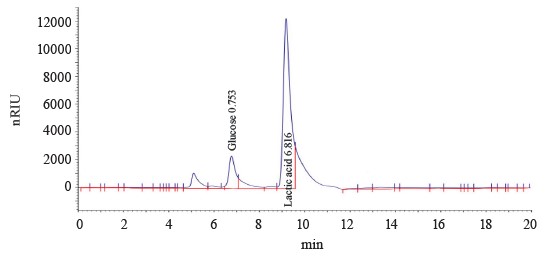
Figure 1 shows a typical chromatogram of T6.1 culture broth grown on a glucose medium under microaerophilic conditions. The bacteria proved able to perform homofermentative lactic acid fermentation.
Strains T1.1, T5.1, T6.1, and T7.1 converted glucose into lactic acid in large quantities with minimal byproduct organic acids. The lactic acid yield and fertation selectivity depended both on the strain and culture conditions.
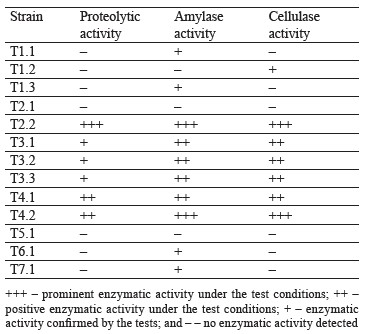
Further research involved detection of extracellular enzymatic activities on agar media with respective substrates. Table 2 introduces the screening results for extracellular hydrolases in cultures.
The experiments demonstrated that six out of thirteen strains possessed proteolytic activity, ten strains showed amylase activity, and seven strains had cellulase activity. These results could be used to develop a new nutrient medium for synthesis of lactic acid, e.g., for acidforming strains T3.1. T6.1, and T7.1. Starch-containing vegetable raw materials could serve as a potential source of carbon.
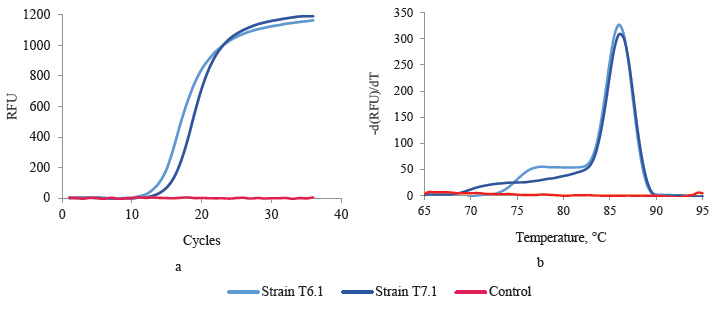
Genetic screening for LDH genes. Promising lactic acid producers T6.1 and T7.1 underwent genetic screening. According to their morphological and physiological characteristics, they were previously assigned to Bacillaceae. The PCR analysis detected the bacillary L-ldh gene in both strains. It is responsible for the synthesis of L-lactate (Fig. 2a). The melting curves showed that the fragments were homogeneous and contained no PCR by-products (Fig. 2b).
Thermophilic strains T6.1 and T7.1 were close to some representatives of the Bacillus genus and were found potentially capable of synthesizing the L-enantio- mer of lactic acid. Genome of some Bacillus representatives, e.g., in particular Bacillus coagulans, contains both structural genes. However, L-lactate-producing B. coagulans have a much more expressed L-ldh than D-ldh [20].
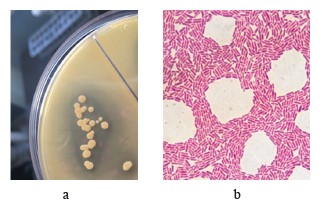
Profiling the promising thermophilic acid-forming strains. Strain T7.1 was as a spore-forming facultative anaerobe, resistant to slightly acidic pH. On agar, it developed white colonies with a diameter of 2–4 mm with a smooth surface. Strain T7.1 hydrolyzed starch but not casein. On the medium with calcium carbonate, it formed clean zones, which indicated the release of acids (Fig. 3a). The cell morphology was rod-shaped and typical for Bacillaceae (Fig. 3b).
For strain T7.1 identification, the 16S rRNA gene was amplified with standard bacterial primers, purified, and sequenced by the Sanger method.
The bioinformatic analysis of the nucleotide sequence included sequence alignment of reference bacterial 16S rRNA and a phylogenetic analysis.
The phylogenetic analysis relied on the 16S rRNA sequences deposited in the GenBank NCBI. The strains were selected with ≥ 92% similarity of the gene. Strain T7.1 shared 99.86% with Weizmannia coagulans NBRC 12583, which was the maximal similarity achieved for this gene (Fig. 4).
Thus, strain T7.1 was identified as W. coagulans. The result correlated with the morphological, physiologica and biochemical characteristics of the test culture.
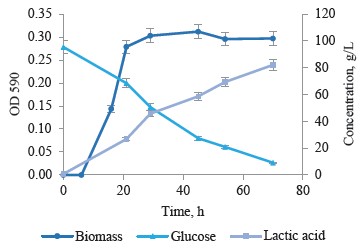
Simple batch cultivation of strain T7.1 on the medium with high initial glucose content. Strain T7.1 was chosen for further studies of lactic acid synthesis by thermophilic bacteria. It underwent batch fermentation for 70 h. Figure 5 illustrates the main results of the fermentation: culture growth, consumption, and lactic acid synthesis.
Thermophilic strain T7.1 utilized 86 out of 95 g/L glucose to form 81 g/L of lactic acid with a peak productivity at 1.58 g/(L·h). The substrate conversion exceeded 90% while the yield of lactate was 94%. The chromatography showed no by-product organic acids at the end of cultivation (Fig. 6).
The fermentation data indicated that the sugars in the medium were metabolized by homofermentative lactic acid fermentation. Both in lactic acid and glucose conversion, the maximal productivity was observed at a residual glucose concentration of ≈ 50 g/L and lactate accumulation ≤ 45 g/L in the medium. The process occurred when the culture entered the stationary phase. The subsequent productivity decreased because the final product inhibited it. Further research is required to explain the effect of the medium composition and cultivation physicochemical parameters on the lactic acid synthesis.
Fed-batch cultivation and continuous fermentation, e.g., membrane bioreactors, could remove the inhibitory effect of metabolites and maintain cells in the required physiological state [23]. This engineering and technological approach is known to boost the efficiency of mesophilic cultures in lactic acid production. Logically, isolated thermophilic cultures should also demonstrate high process performance, especially because strain T7.1 produced exclusively lactic acid under the conditions of batch fermentation. In addition, T7.1 was thermophilic and had much lower requirements for the growth factor content in the medium, compared to Lactobacilli.
Lactic acid optical purity analysis. Lactic acid intended for the food industry or polylactide synthesis requires excellent optical purity. As a result, the next step was to determine the content of L- and D-isomers formed during fermentation.
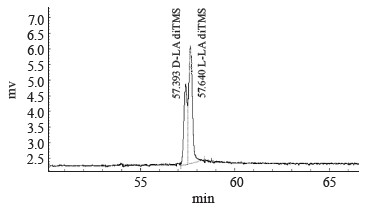
We used the method of gas chromatography of volatile acid derivatives using an optically active column to identify and analyze the optical isomers of lactic acid in the fermentation products. We tested the method on standard samples of L-lactic acid and a racemic mix of D,L-lactic acid obtained by mesolactide hydrolysis. Table 3 and Fig. 7 illustrate the separation of optical isomers of lactic acid. The chromatogram shows separate yields of D-lactic acid (peak 1 at 57.393 min) and L- lactic acid (peak 2 at 57.640 min).
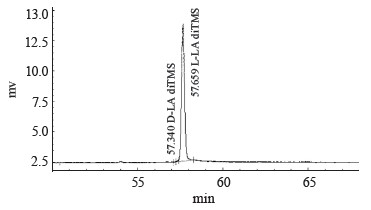
Table 4 and Fig. 8 represent the chromatographic data for the sample of the culture broth obtained during the fermentation of the thermophilic strain T7.1. The D-lactate peak was hardly visible.
The optical purity of lactate produced by strain T7.1 during fermentation at a high content of glucose in the medium correlated with other studies of B. coagulans. For example, Zhou et al. fermented B. coagulans WCP10-4 with glucose (source of carbon) and yeast extract (source of nitrogen) [24]. The optical purity of the obtained lactic acid was 99.8%, and the total concentration of by-product organic acids was below 1 g/L. Other publications [25, 26] explained the high optical purity of the obtained L-lactate by the absence of the lactate racemase gene in B. coagulans and related species. Further research on the development of a new technology for high optical purity lactic acid production will test various carbon sources, including plant hydrolysates, and analyze their effect on the optical purity.
CONCLUSION
The research resulted in a highly effective thermophilic acid-forming strain. Strain T7.1, which possesses the bacillary L-lactate dehydrogenase gene, proved able to ferment homofermentative lactic acid. The molecular genetic analysis identified this strain as Weizmannia coagulans (Bacillus coagulans). The process of batch fermentation provided L-lactate of high optical purity (≥ 99.9%) with a yield of 94% glucose. Therefore, strain T7.1 is a promising thermophilic producer of lactic acid. It could be part of a highly efficient process for obtaining optically pure L-lactic acid in a membrane bioreactor. Lactic acid is intended for the food and chemical industries, namely for polylactide synthesis, biodegradable materials and green packaging prodicing, etc.Contribution
M.V. Romanova, N.Yu. Khromova, and A.V. Beloded developed the research concept. N.Yu. Khromova and A.V. Beloded were responsible for data curation, wrote the review and proofread the manuscript. N.Yu. Khromova, Yu.M. Epishkina, A.V. Beloded, and A.Ye. Kuznetsov performed the formal analy sis. M.V. Romanova, A.N. Dolbunova, A.Ye. Kuznetsov, M.R. Kozlovskiy and S.A. Evdokimova conducted the laboratory research. M.V. Romanova worked with the software, provided infographics, wrote the original draft. N.Yu. Khromova provided research funds, supervised the project, responsible for the validation of the results. A.V. Beloded developed the methodology. All the authors discussed the results and contributed to the final manuscript. All the authors read and agreed to the published version of the manuscript.CONFLICTS OF INTEREST
The authors declare that there is no conflict of interests regarding the publication of this article.FUNDING
The research was supported by the foundation of Dmitry Mendeleev University of Chemical Technology of Russia (Mendeleev University) as part of strategic academic leadership Program Priority 2030 (project no. 2022-040).REFERENCES
- Lactic acid market key players, sales, demand, business strategy and forecast 2030 [Internet]. [cited 2023 Mar 6]. Available from: https://www.digitaljournal.com/pr/lactic-acid-market-key-players-sales-demand-business-strategy-and-forecast-2030#ixzz7uLkASYIk
- Lactic acid market – Global industry analysis, size, share, growth, trends, regional outlook, and forecast 2022–2030 [Internet]. [cited 2023 Mar 6]. Available from: https://www.precedenceresearch.com/lactic-acid-market
- Krasnova IS, Ganina VI, Semenov GV. Fruit and vegetable purees as cryoprotectants for vacuum freeze-dried fermented milk products. Foods and Raw Materials. 2023;11(2):300–308. https://doi.org/10.21603/2308-4057-2023-2-578
- Panseri S, Martino PA, Cagnardi P, Celano G, Tedesco D, Castrica M, et al. Feasibility of biodegradable based packaging used for red meat storage during shelf-life: A pilot study. Food Chemistry. 2018;249:22–29. https://doi.org/10.1016/j.foodchem.2017.12.067
- Kryuk RV, Kurbanova MG, Kolbina AYu, Plotnikov KB, Plotnikov IB, Petrov AN, et al. Color sensors in smart food packaging. Food Processing: Techniques and Technology. 2022;52(2):321–333. (In Russ.). https://doi.org/10.21603/2074-9414-2022-2-2366
- Ncube LK, Ude AU, Ogunmuyiwa EN, Zulkifli R, Beas IN. Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to polylactic acid based materials. Materials. 2020;13(21). https://doi.org/10.3390/ma13214994
- Singha S, Hedenqvist MS. A review on barrier properties of poly(lactic acid)/clay nanocomposites. Polymers. 2020;12(5). https://doi.org/10.3390/polym12051095
- Farah S, Anderson DG, Langer R. Physical and mechanical properties of PLA, and their functions in widespread applications – A comprehensive review. Advanced Drug Delivery Reviews. 2016;107:367–392. https://doi.org/10.1016/j.addr.2016.06.012
- Aulitto M, Fusco S, Bartolucci S, Franzén CJ, Contursi P. Bacillus coagulans MA-13: a promising thermophilic and cellulolytic strain for the production of lactic acid from lignocellulosic hydrolysate. Biotechnology for Biofuels and Bioproducts. 2017;10. https://doi.org/10.1186/s13068-017-0896-8
- Alexandri M, Blanco-Catalá J, Schneider R, Turon X, Venus J. High L(+)-lactic acid productivity in continuous fermentations using bakery waste and lucerne green juice as renewable substrates. Bioresource Technology. 2020;316. https://doi.org/10.1016/j.biortech.2020.123949
- Hu J, Lin Y, Zhang Z, Xiang T, Mei Y, Zhao S, et al. High-titer lactic acid production by Lactobacillus pentosus FL0421 from corn stover using fed-batch simultaneous saccharification and fermentation. Bioresource Technology. 2016;214:74–80. https://doi.org/10.1016/j.biortech.2016.04.034
- Kilcawley K, O'Sullivan M. Cheese flavour development and sensory characteristics. In: Papademas P, Bintsis T, editors. Global cheesemaking technology: Cheese quality and characteristics. Wiley; 2017. pp. 45–70. https://doi.org/10.1002/9781119046165.ch0c
- Poudel P, Tashiro Y, Sakai K. New application of Bacillus strains for optically pure L-lactic acid production: general overview and future prospects. Bioscience, Biotechnology, and Biochemistry. 2016;80(4):642–654. https://doi.org/10.1080/09168451.2015.1095069
- Urbieta MS, Donati ER, Chan K-G, Shahar S, Sin LL, Goh KM. Thermophiles in the genomic era: Biodiversity, science, and applications. Biotechnology Advances. 2015;33(6):633–647. https://doi.org/10.1016/j.biotechadv.2015.04.007
- Blumer-Schuette SE, Brown SD, Sander KB, Bayer EA, Kataeva I, Zurawski JV, et al. Thermophilic lignocellulose deconstruction. FEMS Microbiology Reviews. 2014;38(3):393–448. https://doi.org/10.1111/1574-6976.12044
- Wang Y, Cao W, Luo J, Wan Y. Exploring the potential of lactic acid production from lignocellulosic hydrolysates with various ratios of hexose versus pentose by Bacillus coagulans IPE22. Bioresource Technology. 2018;261:342–349. https://doi.org/10.1016/j.biortech.2018.03.135
- Bischoff KM, Liu S, Hughes SR, Rich JO. Fermentation of corn fiber hydrolysate to lactic acid by the moderate thermophile Bacillus coagulans. Biotechnology Letters. 2010;32:823–828. https://doi.org/10.1007/s10529-010-0222-z
- Fan R, Ebrahimi M, Czermak P. Anaerobic membrane bioreactor for continuous lactic acid fermentation. Membranes. 2017;7(2). https://doi.org/10.3390/membranes7020026
- Ma K, Hu G, Pan L, Wang Z, Zhou Y, Wang Y, et al. Highly efficient production of optically pure l-lactic acid from corn stover hydrolysate by thermophilic Bacillus coagulans. Bioresource Technology. 2016;219:114–122. https://doi.org/10.1016/j.biortech.2016.07.100
- Thebti W, Riahi Y, Gharsalli R, Belhadj O. Screening and characterization of thermo-active enzymes of biotechnological interest produced by thermophilic Bacillus isolated from hot springs in Tunisia. Acta Biochimica Polonica. 2016;63(3):581–587. https://doi.org/10.18388/abp.2016_1271
- Tamura K, Stecher G, Kumar S. MEGA11: Molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution. 2021;38(7):3022–3027. https://doi.org/10.1093/molbev/msab120
- Sun L, Zhang C, Lyu P, Wang Y, Wang L, Yu B. Contributory roles of two l-lactate dehydrogenases for l-lactic acid production in thermotolerant Bacillus coagulans. Scientific Report. 2016;6. https://doi.org/10.1038/srep37916
- Kuznetsov A, Beloded A, Derunets A, Grosheva V, Vakar L, Kozlovskiy R, et al. Biosynthesis of lactic acid in a membrane bioreactor for cleaner technology of polylactide production. Clean Technologies and Environmental Policy. 2017;19:869–882. https://doi.org/10.1007/s10098-016-1275-z
- Zhou X, Ye L, Wu JC. Efficient production of L-lactic acid by newly isolated thermophilic Bacillus coagulans WCP10-4 with high glucose tolerance. Applied Microbiology and Biotechnology. 2013;97:4309–4314. https://doi.org/10.1007/s00253-013-4710-7
- Wang L, Cai Y, Zhu L, Guo H, Yu B. Major role of NAD-dependent lactate dehydrogenases in the production of l-lactic acid with high optical purity by the thermophile Bacillus coagulans. Applied and Environmental Microbiology. 2014;80(23). https://doi.org/10.1128/AEM.01864-14
- Bosma EF, van de Weijer AHP, van der Vlist L, de Vos WM, van der Oost J, van Kranenburg R. Establishment of markerless gene deletion tools in thermophilic Bacillus smithii and construction of multiple mutant strains. Microbial Cell Factories. 2015;14. https://doi.org/10.1186/s12934-015-0286-5