Abstract
One of the problems in sea farming is infections that cause mass mortality of crustaceans. To fight infections and improve sanitary conditions, farmers are actively using probiotic preparations. We aimed to study the effect of a new probiotic based on Bacillus toyonensis B-13249 and Bacillus pumilus B-13250 strains on the incubation of Artemia franciscana cysts. Another purpose was to test a possibility of using a convolutional neural network for fast automatic counting of cysts, nauplii, and embryos.A pilot batch of the probiotic was prepared at the Prombiotech Engineering Center, Altai State University, from two strains of spore bacteria from the Center’s collection: B. toyonensis B-13249 and B. pumilus B-13250.
The recommended amount of the probiotic was experimentally determined as 0.1 per 2 g of cysts. This concentration increased the number of hatched cysts by 1.4 and 10% in the batches from Lake Bolshoye Yarovoye (Z29.04) and from Lake Kuchuk (C9). It also increased the biomass yield to 7.40 ± 0.69 and 6.80 ± 0.43 g in these two batches, respectively, compared to the control samples where the yields were 5.30 ± 0.60 and 4.60 ± 0.50 g, respectively. The robot counter reduced the sample processing time 15 times and saved the data for further use.
The probiotic based on B. toyonensis B-13249 and B. pumilus B-13250 had a positive effect on the hatching rate and biomass yield of A. franciscana. The new method for rapid counting of Artemia, which was based on the convolutional neural network and developed as an application of the Artemeter-1 robot, reduced the processing time and lowered labor costs.
Keywords
Bacillus pumilus, Bacillus toyonensis, Artemia franciscana, aquaculture, probiotics, convolutional neural network, bacteria countingINTRODUCTION
Aquaculture, one of the most promising branches of agriculture, is becoming an important source of nutrition for many people. Aquatic animals are widely bred and grown in coastal countries, such as India, Thailand, Mexico, and others [1–3]. Shrimp production exceeds millions of tons per year but fish farming is still the most common kind of aquaculture [4, 5].
Aquatic animals are grown in artificial ecosystems which have their peculiarities. For example, the larvae of many commercially cultivated species of marine fish, mollusks, and shrimps need live food at the early stages of their development (from a few days to 3–5 weeks).
Grouper, bream, and other popular species show excellent growth when feeding on live plankton as a starter feed. Yet, this is only critical to shrimps, since there are no alternative artificial feeds. Shrimps actively feed (and, therefore, grow) on independently-moving aquatic organisms, ignoring motionless or free-floating food particles. This is true not only of predatory species, such as the tiger shrimp (Penaeus monodon Fabricius, 1798), but also of popular omnivorous species, such as the whitelegged shrimp (Penaeus vannamei Boone, 1931) or the Rosenberg shrimp (Macrobrachium rosenbergii De Man, 1879) [6].
Considering the nutrition needs of the crustaceans, manufacturers began to use planktonic halophilic crustaceans of the genus Artemia as an optimal starter feed. These planktonic crustaceans are native to the saline lakes of Western Siberia (Russia and Kazakhstan), China, Tibet, and Iran. They can also be found in the Mediterranean region, New Zealand, Canada, and the USA [7, 8]. Brine shrimps are convenient to use since they can propagate under adverse conditions with cysts covered with a thick chitin membrane. Simple technical manipulations can preserve their cysts for a long time (up to 14 years) to be stored or transported. Biochemical processes can be easily activated in the cysts (salt water, light, aeration, etc.) to obtain, within 24 h, microscopic (0.4–0.45 mm) nauplii, an ideal food for the cultivated shrimp larvae.
Niu et al. explored alternatives to using brine shrimp as a starter feed for the Litopenaeus vannamei shrimp most commonly farmed in China and in the West [9]. During cultivation, its larvae get infected with diseases, including those carried by live organisms they feed on (rotifers and brine shrimp). In other words, these live organisms are a source of both food and disease (parasites) in the aquaculture. Therefore, the lack of efficient commercial methods is still the main obstacle to a sustainable, healthy farming of this species of shrimp. To overcome it, the scientists created an alternative fortified artificial food without live organisms. They found that the shrimp had a survival rate of 81–87% under constant cholesterol level monitoring. According to Malkova et al., a probiotic preparation for Artemia crustaceans could facilitate the commercial production of the shrimp [10]. As a result, they could still be used as a live starter feed.
Large-scaled sea farming (invertebrates and fish) is accompanied by various infections that may cause mass mortality. Among the most common pathogenic microorganisms in the aquatic environment are those of the genera Vibrio, Salmonella, Escherichia, and others [11]. Antibiotics have been used for many years to prevent large losses, which has made a lot of microflora resistant to them [12]. Thus, we need to seek safer ways to fight microbes so that bacterial resistance to antibiotics does not lead to an ecological catastrophe [13–15].
The above-mentioned problems in aquaculture can be overcome with modern probiotic preparations [16–18]. Ahmadifard et al. fortified brine shrimp with a probiotic based on Bacillus subtilis. They found that it had a positive effect on the growth, reproduction, and microflora of ornamental fish Poecilia latipinna, as well as its resistance to Aeromonas hydrophila. At the same time, there were no significant differences in the ontogeny of the fortified and non-fortified Artemia groups [19].
A research team from Korea found that strains B. subtilis KA1 and B. subtilis KA3 improved food absorption by the shrimp Palaemon paucidens and contributed to their survival from a white spot syndrome virus [20]. A group of Chinese scientists proved that the probiotic bacteria [21]. Bacillus coagulans ATCC 7050 improved the growth, intestinal morphology, immune response, and resistance to Vibrio parahaemolyticus in the shrimp L. vannamei. Fernandes et al. found that the bacteria B. subtilis, Bacillus amyloliquefaciens, Bacillus licheniformis, and Pseudomonas sp. were safe as biological inoculants. In addition, they increased enzymatic activity in the intestines of L. vannamei shrimp, contributing to higher weight gain [23]. According to Thai researchers, the Bacillus aryabhattai TBRC8450 was not only antagonistically active against Vibrio harveyi and V. parahaemolyticus, but also improved antioxidant activity in animal plasma [4].
Wang et al. from Taiwan experimentally confirmed that a multicomponent probiotic based on Lactobacillus pentosus BD6, Lactobacillus fermentum LW2, B. subtilis E20, and Saccharomyces cerevisiae P13 improved shrimp health and performance more effectively than monocomponent preparations with the same strains [23]. A team of Spanish scientists fortified Artemia metanauplii with a Lactobacillus rhamnosus-based probiotic and found its positive effect against potentially pathogenic microorganisms of the Vibrionaceae family [11].
A group of Indian scientists fortified brine shrimp, to be fed as live food to freshwater shrimp M. rosenbergii, with a probiotic supplement based on Lactobacillus sporogenes at different concentrations. The experiments showed that the fortification resulted in M. rosenbergii’s better survival, rapid growth, and higher contents of protein, amino acids, carbohydrates, and lipids. The L. sporogenes concentration of 6×108 CFU/g was found to be optimal for feed fortification [24].
In a similar study by scientists from India, brine shrimp nauplii fortified with a probiotic based on Saccharomyces boulardii showed an improved resistance to Vibrio bacteria they had been artificially infected with [25].
Another group of Indian scientists fortified Artemia parthenogenetica nauplii with L. rhamnosus and B. coagulans [26]. They studied the probiotic supplement’s load on the intestines of brine shrimp nauplii and its retention time in their intestines. According to the results, the nauplii fortified with L. rhamnosus and B. coagulans had a full intestine after 39 and 39.5 min, respectively. The load on the intestines and the retention time varied in the experimental groups.
As can be seen in the above studies, probiotic preparations are mainly made from well-studied microorganisms of the Lactobacillus and Bacillus genera. For the sustainable development of aquaculture, however, we need to expand the pool of probiotic microorganisms and make more preparations based on microbial consortia, rather than monocultures.
Until now, brine shrimp farmers have done the counting visually, using a binocular and/or a microscope, as well as with the naked eye. Time-consuming and tiring, these methods do not always produce accurate results. Moreover, additional devices have to be used to save the data.
To reduce statistical errors, experiments need to be conducted in at least eight repetitions. In order to shorten the counting time, improve accuracy, and save data, we used a brine shrimp counter robot, for the first time in Russia, based on a convolutional neural network that used the YOLO (You Only Look Once) algorithm [27].
The main difference between the YOLO algorithm and other neural network algorithms lies in its instantaneous detection of objects in real time. In the YOLO algorithm, a full-size image completely passes through the convolutional neural network only once, while other algorithms involve many repetitions.
When we started our study, YOLOv4 (2020) was one of the most productive neural networks for object detection. We tested several neural networks from the MS COCO (2017) set of 127 287 images and found YOLOv4 to be the most efficient. Its algorithm works twice as fast as EfficientDet, one of the most accurate models. Compared to the earlier version (YOLOv3), the Average Precision metric and performance (FPS) of YOLOv4 have been improved by 10 and 12%, respectively [28]. Therefore, YOLOv4 was used as a platform for creating an Artemeter-1 robot for counting organisms.
We aimed to study the effectiveness of a new probiotic preparation based on a consortium of rhizosphere strains Bacillus toyonensis B-13249 and Bacillus pumilus B-13250 for the incubation of Artemia franciscana cysts. We also explored a possibility of using a counter robot based on a convolutional neural network for fast automatic counting of cysts, nauplii, and embryos after decapsulation.
STUDY OBJECTS AND METHODS
Microorganisms in probiotics. A probiotic was based on two strains of spore bacteria from the collection of the Prombiotech Engineering Center. Both strains are patented and deposited in the Russian National Collection of Industrial Microorganisms. They were isolated in 2017 (Novye Zori village, Altai Krai, Russia). The Bacillus toyonensis В-13249 strain was isolated from the rhizosphere of the genus Helianthus, while the Bacillus pumilus В-13250 strain was isolated from the rhizosphere of the Cichorium genus.
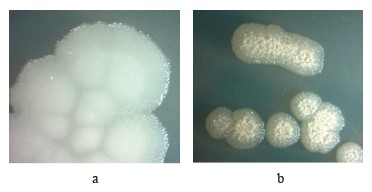
These strains form oval spores located terminally or subterminally and withstand heating at 80°C for 30 min. The morphology of their colonies is shown in Fig. 1.
Culture media for probiotic production. A pilot batch of probiotics was prepared using the following culture media:
1. Solid L(Luria)-medium for counting bacteria;
2. Liquid L-medium for cultivating the inoculum in flasks;
3. Endo medium for checking the samples for coliform bacteria;
4. A protective (cryoprotective) medium for protecting cells and spores during the freezing period; and
5. A fermentation (molasses-corn) medium used as the main nutrient medium in the fermentation apparatus.
A pilot batch of probiotics. A pilot batch of probiotics to be tested on Artemia crustaceans was produced at the Prombiotech Engineering Center (Barnaul, Russia), using the technology developed by this institution earlier [29].
The inoculum (seed) was cultivated in shake flasks in an Innova 44 shaker-incubator (New Brunswick). The cultivation methods were scaled on the laboratory batch fermentation units with a capacity of 15 and 250 L. After 24 h of cultivation, the accumulated biomass was concentrated in a GTGQ-1251 tubular centrifuge at 15 600 rpm. Then, the bacterial concentrate was mechanically removed from the centrifuge rotor, mixed with a cryoprotective medium (1:1), frozen, and subsequently freeze-dried in an SP Scientific 25L Genesis SQ Super ES-55 semi-industrial freeze-dryer. The resulting concentrate was mixed with a filler (maltodextrin) until the final titer of the probiotic preparation (at least 1×1010 CFU/g) [29].
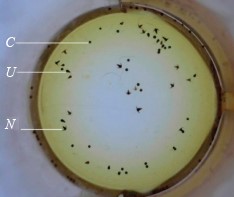
Incubation and analysis of Artemia cysts. We used cysts of the branchial crustacean Artemia franciscana from two different lakes: batch Z29.04 from Lake Bolshoye Yarovoye and batch C9 from Lake Kuchuk. The cysts were incubated in cones (Fig. 2) for 48 h with constant air bubbling under standard conditions: incubation solution – 1 L, salinity – 30%, temperature – 25–30°C, pH – 8.0–8.5, redox potential – 148–220 mV, illumination – 1500–2000 lux, cysts – 2.0 g, and activator – 0.2 cm3 of 3% H2O2 solution.
We produced one control and three test samples for each batch of cysts in 24 repetitions. At the start of nauplii incubation, probiotics were introduced directly into the cones at the following amounts: 0 (control) and 0.05, 0.1, and 0.2 g. After 48 h of incubation, we counted the number of crustaceans or nauplii (N), unhatched cysts (C), and half-hatched embryos or umbrellas (U) in each cone. According to the standard method, the crustaceans were counted in two stages [30].
At the first stage, a few drops (about 0.1 cm3) of concentrated Lugol’s solution was added to a liquid sample from the incubator (0.05–0.1 cm3) in order to immobilize the crustaceans and make formed elements more contrasting in color. Some 20–30 min later, nauplii (N) and embryos (U) were counted, then proceeding to the second stage of cyst count.
At the second stage, a sodium hypochlorite (NaOCl) solution was added to the stained samples to dissolve empty shells from hatched cysts, leaving only the interior of unhatched cysts (C). The amount of hypochlorite (and the exposure time) depended on the number of cysts in the sample, averaging 0.2–0.3 cm3 of 8% sodium hypochlorite for each sample.
As a result, we obtained the counts of nauplii (N), embryos (E), and cysts (C) in 0.05–0.1 cm3 of incubation suspension. Analyzing the data, we calculated the relative quality indicators of cysts (HR– and HR+).
The hatching rate (HR) is of great importance when using brine shrimp in farming. This indicator shows the percentage of nauplii hatched from the total number of cysts incubated. Thus, the higher the hatching rate, the greater the amount of live feed in the water. There are two HRs for each sample: HR– indicates the percentage of fully-hatched nauplii (N) and HR+ shows the percentage of fully-hatched (N) and half-hatched (U) nauplii. The HRs were calculated using the following formulas:
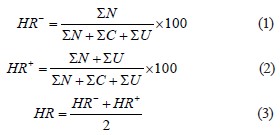
where HR– is the hatching rate without embryos, %; HR+ is the hatching rate with embryos, %; HR is the average between HR– and; ΣN is the sum of nauplii; ΣC is the sum of cysts; ΣU is the sum of embryos (umbrellas), half-hatched nauplii.
To determine the biomass yield, we turned off aeration at the end of incubation, while leaving the lighting on. After 10 min , the biomass (live nauplii) was separated from waste (shells and unhatched cysts on the bottom and walls of the cone) by sedimentation and simultaneous concentration of mobile nauplii in the illuminated middle part of the cone. Then, the liquid with nauplii was drained through a cone-shaped sieve (100 μm pores) with a 4–6 mm silicone tube from the middle part of the cone, leaving the waste on its walls and bottom. The sieved biomass was washed in fresh water and squeezed out at least five times. Then, it was left to drain excess moisture for 2 min and weighed on a laboratory balance with an accuracy of 0.01 g.
Biomass yield. Biomass yield is an indicator of incubation effectiveness that shows the amount of Artemia biomass incubated. This indicator is used in farming because of its simplicity: dry cysts are weighed at the beginning of incubation and compared with the weight of nauplii at the end of incubation. Although its accuracy is much lower than that of the classical method, we used it to double-check the results, trying to minimize possible errors [30]. The main problem in measuring biomass is that water runs down differently from the samples. Before weighing, the samples are kept in a sieve (100–150 µm) for some time to minimize the effect of water mass on the Artemia biomass.
In our study, we used the same kind of 50 cm3 gas net, which was twisted 720 degrees twice (to squeeze out most of the water), and then left for 5 min to drain excess residual liquid. Only after this double manipulation (the same for all samples), the resulting Artemia biomass was weighed on a laboratory balance. Then, we calculated the biomass coefficient corresponding to a multi-fold difference between the final wet weight and the weight of dry cysts introduced at the beginning of incubation.
Neural network operation. Nowadays, many farmers of Artemia crustaceans do the counting by using binoculars, microscopes, or magnifiers. To save data, they have to use additional devices, such as cameras or smartphones, and manually enter the data into logbooks or spreadsheets. All these manipulations take a lot of time and effort.
The Artemeter-1 robot counter based on a convolutional neural network that uses the YOLO algorithm is designed to significantly reduce the counting time, as well as improve the quality and accuracy of measurements. It ensures a one-time graphic fixation of Artemia and storage of all data, as well as saves researchers having to use other instruments. Therefore, we employed this robot counter in our study and we know of no other studies in Russia that have used it before.
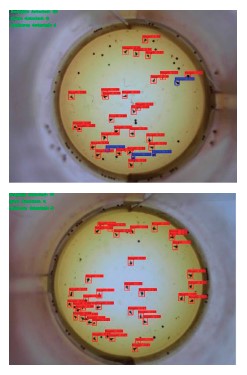
The neural network was trained using a dataset of 1200 manually labeled microphotographs of Artemia samples (Fig. 3). Each sample amounted to 0.1 cm3 of incubator water with a random number of unhatched cysts, embryos, and hatched nauplii.
All the objects that were to be counted by the neural network were marked on each sample. The total number of labeled images (dataset) was divided into two unequal parts: 1) a training sample (about 800 images) that the network learns from, and 2) a test sample (400 images) that the network uses to check itself [31]. Figure 4 shows some examples of the images in the corresponding resolution.
The images were enlarged in size. The number of pixels per unit area was sufficient for the neural network to operate, since the real size of the objects in the detector’s visibility was commensurate with that of the samples used to train it.
The neural network was trained in the GoogleColab cloud service connected to Nvidia K80s, T4s, P4s, and P100s GPUs. As a result, so-called “neural network weights” were obtained, i.e. parameter settings that the neural network used for most accurate detection of objects in the image. Further, the weights were downloaded from the cloud service to be used offline.
The mechanical part of the Artemeter-1 robot for nauplii counting was also designed and manufactured in Russia (Fig. 5).
The network detects three types of objects (nauplii, cysts, and umbrellas), whose ratio indicates the quality of the product. It also marks these objects in the original JPEG file and saves the data in both EXCEL tables and processed images. The robot is designed for both producers and large consumers of Artemia cysts [27].
RESULTS AND DISCUSSION
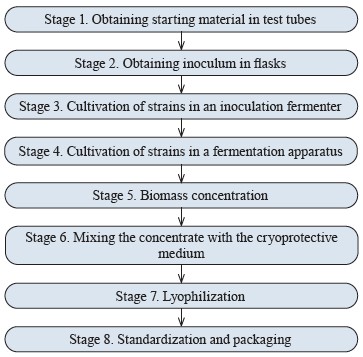
Probiotic production. We developed a technology for producing a new probiotic for aquaculture (Fig. 6).
Each stage of the process produced an intermediate result, namely:
Stage 1. Starting mother culture for further scaling in stepwise increasing inoculation containers.
Stage 2. Primary inoculum for sowing a small (1 L) fermentation apparatus, which was about 10% of the loaded medium (inoculation apparatus – 15 L, estimated nutrient medium – 10 L).
Stage 3. Inoculum for deep cultivation in commercial media on a semi-industrial bioreactor, with a volume of about 5% of the main fermenter (bioreactor – 250 L, estimated fermentation medium – about 120 L).
Stage 4. Each strain fermented in 120 L of nutrient medium separately, under the same cultivation conditions.
Stage 5. About 3 kg of concentrated biomass from two fermentations (one per each strain) with a residual moisture of 15–20% after flow centrifugation.
Stage 6. Тhe concentrated biomass mixed with 3 L of cryoprotective medium (1:1), poured into trays, and frozen.
Stage 7. The freeze-dried concentrate of bacteria with a total weight of about 2.5 kg and at least 1×1011
CFU/g for each strain, additionally ground in a blixer to homogeneous powder.
Stage 8. The concentrate standardized to a titer of 1×1010 CFU/g, packaged in plastic bags, sealed, and placed in three-layer kraft bags, which were sewn up to prevent the probiotic from getting wet.
As a result, we obtained a pilot batch of the probiotic with a titer of at least 1×1010 CFU/g to be used in fullscale tests.
The probiotic’s effect on the incubation of Artemia franciscana cysts. Shrimp need live feed in the early stages of development. Therefore, shrimp prelarvae are fed on live, freshly hatched Artemia nauplii. However, the incubation solution for A. franciscana is a favorable environment for any microorganisms, including pathogenic microflora, which affects the quality of shrimp. The number of pathogenic microorganisms in Artemia increases exponentially while the nauplii are hatching and becoming enriched with nutrients. For this reason, producers add probiotic and prebiotic preparations to reduce the development of pathogens in shrimp prelarvae [32].
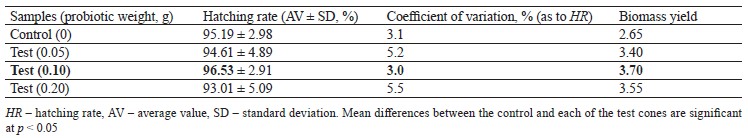
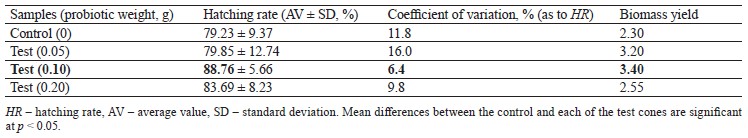
Artemia nauplii begin to hatch from cysts in large numbers after 20 h of incubation and start feeding actively 6–8 h after birth. Thus, during 48 h of incubation, Artemia cysts not only inseminate the surface of nauplii, but also populate their gastrointestinal tract with probiotic bacilli. Our study showed a positive effect of the probiotic we had developed on both batches of Artemia cysts, which manifested in increased biomass yield compared to the control. In addition, the consortium of Bacillus toyonensis B-13249 and Bacillus pumilus B-13250 appeared to favorably change the habitat conditions for Artemia, stimulating the hatching process. This could also be associated with the antagonistic properties of these bacilli against pathogens. The test results for cyst batches Z29.04 and batch C9 are shown in Tables 1 and 2, respectively. For both batches, the highest biomass yield was provided by 0.1 g of the probiotic per 2 g of dry cysts.
The probiotic added to batch Z29.04 increased the hatching rate by 1.4%, compared to the control (Table 1). The coefficient of variation from 3 to 5% indicated a slight spread of values and the validity of the results.
The probiotic added to batch C9 increased the hatching rate by about 10%, compared to the control (Table 2). The coefficient of variation for this batch was significantly higher (6.4–11.8%) than the one for batch Z29.04, but it was within an allowable range and therefore indicative of reliable results. The smallest variation was noted in the test group with 0.1 g of the probiotic.
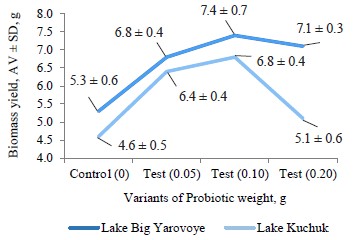
The biomass yield shows the mass of A. francisca- na incubated. Figure 7 presents the correlation between the amount of the probiotic and the biomass yield from two independent batches taken from two different lakes.
As can be seen in Fig. 7, the maximum biomass yield was obtained with a probiotic weight of 0.1/2 g of cysts in both experimental batches, which corresponded to the data in Tables 1 and 2.
A. franciscana cysts from batches C9 and Z29.04 had different hatching rates and biomass yield in the control cones. However, these indicators decreased in both batches with the addition of 0.2 g of the probiotic, compared to the best result in the cone with 0.1 g of the probiotic. The effect of the probiotic was more pronounced in batch C9 with the initially low hatching rate and biomass yield. Test batch Z29.04 (Lake Bolshoye Yaro- voye) showed a significantly higher hatching rate, which was normal for this batch and was not associated with the probiotic. Batch C9 (Lake Kuchuk) had minimum differences in the indicators when we added 0.1 g of the probiotic. This might indirectly indicate the stabilizing effect of the probiotic on hatching. The differences in the hatching rates between the two batches of Artemia cysts were due to the fact that they were obtained from different salt lakes, i.e., different ecosystems where they evolved.The biomass yield indicates the number of times that the mass of Artemia increased after incubation. Tables 1 and 2present the correlation between the amount of the probiotic and the biomass yield in the studied batches.
Thus, the new probiotic preparation based on B. toyo- nensis B-13249 and B. pumilus B-13250 had a positive effect on the hatching rate and biomass yield of A. fran- ciscana. According to our results, the optimal concentration of the probiotic was 0.1 per 2 g of cysts. This concentration increased the hatching rates by 1.4 and 10% in batches Z29.04 and C9, respectively. The biomass yields in the control cones were 5.30 ± 0.60 and 4.60 ± 0.50 g in batches Z29.04 and C9, respectively. The addition of 0.1 g of the probiotic increased the biomass yield to 7.40 ± 0.69 and 6.80 ± 0.43 g, respectively.
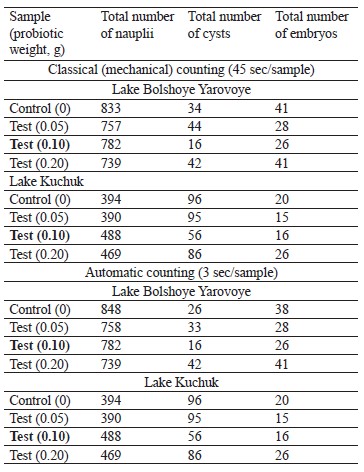
Counting reliability. To ensure the reliability of automatic counting results, we used the classical (mechanical) method for the entire batch of 192 images (Table 3).
We found no significant differences between the automatic and mechanical methods of counting. However, the automatic counting proved to be faster and more accurate. Slight differences were observed in the control and the sample with 0.05 g of the probiotic in the batch from Lake Bolshoye Yarovoye. In cone 1 (control), the mechanical and automatic methods revealed 833 and 848 nauplii, 34 and 26 cysts, and 41 and 38 embryos, respectively. The sample with the lowest concentration of the probiotic (0.05 g), also showed slightly different counts. These differences could be explained by a large number of objects to be observed in each sample, as well as the impossibility of marking the observed objects during mechanical counting. The different counts of morphologically different objects (nauplii, cysts, and embryos) could also be explained by higher accuracy of the robot counter, which compared the objects under observation with those it was trained on. The robot can instantly count all the objects and mark them on each sample, and if it fails to identify the objects correctly, it can be retrained. However, the differences between the counts were too insignificant to affect the final results. Both methods showed similar counts in the other samples. The entire set of images was saved for further research.
Figure 8 shows an example of saved images from the test set. The robot can determine and count both individual morphological forms (for more specific identification) and their different types (nauplii, cysts, and embryos).
The advantages of the Artemia robot counter based on the convolutional neural network outweigh its limitations. These advantages include a simultaneous use of multiple devices, a reduced data processing time, a possibility of saving data for later use, and less operator presence.
The absence of significant differences between the mechanical and automatic counting methods confirms high accuracy of the robot counter. Its large-scale use will optimize the counting of Artemia nauplii, cysts, and embryos in further research.
Thus, our study was the first in Russia that successfully tested the new robotic system for counting nauplii based on machine learning. It significantly reduced the processing time (15 times per 1 sample) and labor costs, as well as saved the data for further use.
CONCLUSION
We found that the new probiotic preparation based on Bacillus toyonensis B-13249 and Bacillus pumilus B-13250 had a positive effect on the biomass yield of Artemia franciscana. In particular, it increased the hatching rate in batches Z29.04 and C9 by 1.4 and 10%, respectively. While the biomass yields in the control cones were 5.30 ± 0.60 and 4.60 ± 0.50 g for batches Z29.04 and C9, respectively, the probiotic increased this indicator to 7.40 ± 0.69 and 6.80 ± 0.43 g, respectively.
As a result of our tests, we recommend 0.1 g of the probiotic per 2 g of cysts.
Just as important was the development and testing of the Artemeter-1 robot based on the convolutional neural network for rapid and accurate counting of Artemia during decapsulation. Our study was the first in Russia that utilized this new robotic system based on machine learning. It reduced the sample processing time 15 times, lowered labor costs, and saved the data in electronic form for further use.
Our results may be useful to farmers working in aquaculture, as well as researchers interested in this field.
Contribution
The authors were equally involved in writing the manuscript and are equally responsible for any potential plagiarism.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Salunke M, Kalyankar A, Khedkar CD, Shingare M, Khedkar GD. A review on shrimp aquaculture in India: Historical perspective, constraints, status and future implications for impacts on aquatic ecosystem and biodiversity. Reviews in Fisheries Science and Aquaculture. 2020;28(3):283–302. https://doi.org/10.1080/23308249.2020.1723058
- Sampantamit T, Ho L, Lachat C, Sutummawong N, Sorgeloos P, Goethals P. aquaculture production and its environmental sustainability in Thailand: Challenges and potential solutions. Sustainability. 2020;12(5). https://doi.org/10.3390/su12052010
- Cortes A, Casillas-Hernandez R, Cambeses-Franco C, Borquez-Lopez R, Magallon-Barajas F, Quadros-Seiffert W, et al. Eco-efficiency assessment of shrimp aquaculture production in Mexico. Aquaculture. 2021;544. https://doi.org/10.1016/j.aquaculture.2021.737145
- Tepaamorndech S, Chantarasakha K, Kingcha Y, Chaiyapechara S, Phromson M, Sriariyanun M, et al. Effects of Bacillus aryabhattai TBRC8450 on vibriosis resistance and immune enhancement in Pacific white shrimp, Litopenaeus vannamei. Fish and Shellfish Immunology. 2019;86:4–13. https://doi.org/10.1016/j.fsi.2018.11.010
- Naylor RL, Hardy RW, Buschmann AH, Bush SR, Cao L, Klinger DH, et al. A 20-year retrospective review of global aquaculture. Nature. 2021;591:551–563. https://doi.org/10.1038/s41586-021-03308-6
- Joshua WJ, Kamarudin MS, Ikhsan N, Yusoff FM, Zulperi Z. Development of enriched Artemia and Moina in larviculture of fish and crustaceans: A review. Latin American Journal of Aquatic Research. 2022;50(2):144–157.
- Kovacheva NP, Litvinenko LI, Saenko EM, Zhigin AV, Kryahova NV, Semik AM. Current state and prospects of aquaculture artemia in Russia. Trudy VNIRO. 2019;178:150–171. (In Russ.). https://doi.org/10.36038/2307-3497-2019-178-150-171
- Sellami I, Naceur HB, Kacem A. Study of cysts biometry and hatching percentage of the brine shrimp Artemia salina (Linnaeus, 1758) from the Sebkha of Sidi El Hani (Tunisia) according to successive generations. Aquaculture Studies. 2020;21(1):41–46. https://doi.org/10.4194/2618-6381-v21_1_05
- Niu J, Chen P-F, Tian L-X, Liu Y-J, Lin H-Z, Yang H-J, et al. Excess dietary cholesterol may have an adverse effect on growth performance of early post-larval Litopenaeus vannamei. Journal of Animal Science and Biotechnology. 2012;3(1). https://doi.org/10.1186/2049-1891-3-19
- Malkova A, Evdokimov I, Shirmanov M, Irkitova A, Dementyev D. New bacilli-based probiotic for aquaculture: Efficacy study on Macrobrachium rosenbergii. BIO Web of Conferences. 2022;42. https://doi.org/10.1051/bioconf/20224201011
- Ofelio C, Planas M, Pintado J. Administration of the probiotic Lactobacillus rhamnosus IMC 501 as a strategy for the control of Vibrio bacteria in the brine shrimp Artemia. Letters in Applied Microbiology. 2021;73(3):336–342. https://doi.org/10.1111/lam.13518
- Schar D, Zhao C, Wang Yu, Larsson DGJ, Gilbert M, Boeckel van TP. Twenty-year trends in antimicrobial resistance from aquaculture and fisheries in Asia. Nature Communications. 2021;12(1). https://doi.org/10.1038/s41467-021-25655-8
- Wall S. Prevention of antibiotic resistance – an epidemiological scoping review to identify research categories and knowledge gaps. Global Health Action. 2019;12. https://doi.org/10.1080/16549716.2020.1756191
- Uddin TM, Chakraborty AJ, Khusro A, Zidan BMRM, Mitra S, Emran TB, et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. Journal of Infection and Public Health. 2021;14(12):1750–1766. https://doi.org/10.1016/j.jiph.2021.10.020
- Larsson DGJ, Flach C-F. Antibiotic resistance in the environment. Nature Reviews Microbiology. 2022;20:257–269. https://doi.org/10.1038/s41579-021-00649-x
- Goh JXH, Tan LT-H, Law JW-F, Khaw K-Y, Zengin G, Chan K-G, et al. Probiotics: Comprehensive exploration of the growth promotion mechanisms in shrimps. Progress in Microbes and Molecular Biology. 2023;6(1). https://doi.org/a10.36877/pmmb.0000324
- da Costa Sousa N, do Couto MVS, Paixão PEG, dos Santos Medeiros E, de Souza JCN, Barros FAL, et al. Enriched Artemia nauplii with commercial probiotic in the larviculture of angelfish Pterophyllum scalare Lichtenstein (1823). Journal of Fisheries Science. 2020;2(1):17–21. https://doi.org/10.30564/jfsr.v2i1.1569
- Volkova GS, Serba EM. New Multistrain Bacterial Consortium for Feed Probiotics. Food Processing: Techniques and Technology. 2021;51(2):260–269. (In Russ.). https://doi.org/10.21603/2074-9414-2021-2-260-269.
- Ahmadifard N, Aminlooi VR, Tukmechi A, Agh N. Evaluation of the impacts of long-term enriched Artemia with Bacillus subtilis on growth performance, reproduction, intestinal microflora, and resistance to Aeromonas hydrophila of ornamental fish Poecilia latipinna. Probiotics Antimicrobial Proteins. 2019;11:957–965. https://doi.org/10.1007/s12602-018-9453-4
- Sekar A, Kima M, Jeonb H, Kima K. Screening and selection of bacteria inhibiting white spot syndrome virus infection to Litopenaeus vannamei. Biochemistry and Biophysics Reports. 2019;19. https://doi.org/10.1016/j.bbrep.2019.100663
- Amoah K, Huang Q-C, Tan B-P, Zhang S, Chi S-Y, Yang Q-H, et al. Dietary supplementation of probiotic Bacillus coagulans ATCC 7050, improves the growth performance, intestinal morphology, microflora, immune response, and disease confrontation of Pacific white shrimp, Litopenaeus vannamei. Fish and Shellfish Immunology. 2019;87:796–808. https://doi.org/10.1016/j.fsi.2019.02.029
- Fernandes V, Sabu EA, Shivaramu MS, Gonsalves MJBD, Sreepada RA. Dynamics and succession of plankton communities with changing nutrient levels in tropical culture ponds of whiteleg shrimp. Aquaculture Environment Interactions. 2019;11:639–655. https://doi.org/10.3354/aei00341
- Wang Y-C, Hu S-Y, Chiu C-S, Liu C-H. Multiple-strain probiotics appear to be more effective in improving the growth performance and health status of white shrimp, Litopenaeus vannamei, than single probiotic strains. Fish and Shellfish Immunology. 2019;84:1050–1058. https://doi.org/10.1016/j.fsi.2018.11.017
- Seenivasan C, Bhavan PS, Radhakrishnan S, Shanthi R. Enrichment of Artemia nauplii with Lactobacillus sporogenes for enhancing the survival, growth and levels of biochemical constituents in the post-larvae of the freshwater prawn Macrobrachium rosenbergii. Turkish Journal of Fisheries and Aquatic Sciences. 2012;12:23–31.
- Patra SK, Mohamed KS. Enrichment of Artemia nauplii with the probiotic yeast Saccharomyces boulardii and its resistance against a pathogenic Vibrio. Aquaculture International. 2003;11:505–514. https://doi.org/10.1023/b:aqui.0000004193.40039.54
- Isamma A, Divya, KR, Ramasubramanian V, Arunjith TS, Sureshkumar S. Standartization of the bioencapsulation of probiotics and oil emulsion in Artemia parthenogenetica. International Journal of Research in Fisheries and Aquaculture. 2014;4(3):122–125.
- Dementʹev DV, Komyshev GB, Semyonov PA. The convolutional neural network for determining the quality of Artemia. Patent RU 2021663036. 2021. https://www.elibrary.ru/ERHHLL
- Bochkovskiy A, Wang C-Y, Liao H-YM. YOLOv4: Optimal speed and accuracy of object detection. https://doi.org/10.48550/arXiv.2004.10934
- Malkova AV, Evdokimov IYu, Shirmanov MV, Irkitova AN, Dudnik DE. Development of a probiotic for animals and aquaculture based on Bacillus toyonensis B-13249 and Bacillus pumilus B-13250 strains. Proceedings of Universities. Applied Chemistry and Biotechnology. 2021;11(3):393–402. (In Russ.). https://doi.org/10.21285/2227-2925-2021-11-3-393-402
- Baert P, Bosteels T, Sorgeloos P. Pond production. In: Lavens P, Sorgeloos P, editors. Manual on the production and use of live food for aquaculture. Rome: FAO; 1996. pp. 196–251.
- Jabir B, Rabhi L, Falih N. RNN- and CNN-based weed detection for crop improvement: An overview. Foods and Raw Materials. 2021;9(2):387–396. https://doi.org/10.21603/2308-4057-2021-2-387-396
- Hamsah, Widanarni, Alimuddin, Yuhana M, Zairin M. The nutritional value of Artemia sp. enriched with the probiotic Pseudoalteromonas piscicida and the prebiotic mannan-oligosaccharide. AACL Bioflux. 2017;10(1):8–17.