Abstract
Peptides of plant and animal origin have good anti-diabetic prospects. The research objective was to use bovine colostrum peptides to reduce hyperglycemia in diabetic rats.Bovine colostrum peptides were obtained by trypsin hydrolysis of colostrum proteins with preliminary extraction of triglycerides. The study involved four groups of Wistar rats with seven animals per group. Group 1 served as control; group 2 received 300 mg/kg of trypsin hydrolysate of bovine colostrum as part of their daily diet for 30 days. Groups 3 and 4 had diabetes mellitus caused by intraperitoneal injections of 110 mg/kg of nicotinamide and 65 mg/kg of streptozotocin. Group 4 also received 300 mg/kg trypsin hydrolysate of bovine colostrum intragastrically five times a week for 30 days.
Three peptides were isolated from the trypsin hydrolysate of bovine colostrum and tested for the sequence of amino acids and molecular weight. Their identification involved the Protein NCBI database, followed by 2D and 3D modeling, which revealed their chemical profile, pharmacological properties, and antioxidant activity. The diabetic rats treated with colostrum peptides had lower glucose, glycated hemoglobin, malondialdehyde, and catalase activity but a higher content of glutathione in the blood. Their leukocytes and erythrocytes also demonstrated less deviation from the standard. The antioxidant effect of colostrum protein hydrolysate depended on a peptide with the amino acid sequence of SQKKKNCPNGTRIRVPGPGP and a mass of 8.4 kDa.
Colostrum peptides reduced hyperglycemia and oxidative stress in diabetic rats. The research revealed good prospects for isolating individual colostrum peptides to be tested for antidiabetic properties.
Keywords
Peptides, bovine colostrum, diabetes mellitus, glucose, glycated hemoglobin, antioxidant activityINTRODUCTION
Diabetes mellitus, or type II diabetes, is the most common type of diabetes [1–3]. It causes such chronic complications as cardiovascular disease and neurological disorders, which have become a real pandemic of the XXI century. Medical science is constantly looking for new methods to curb the pathogenic factors that determine this socially significant disease and its chronic complications.
The list of targeted antidiabetic compounds includes dipeptidyl peptidase 4 (DPP4), intestinal maltase glumylase, hepatic receptor homologue 1 (NR5A2), pancreatic alpha-amylase, peroxisome proliferator-activated receptor alpha (PPARA), protein tyrosine phosphatase, and retinol-binding protein-4 (RBP4) [4–6].
Protein hydrolysates of plant and animal origin contain a number of biologically active regulatory peptides with antidiabetic properties [7–13]. Antidiabetic peptides inhibit DPP4 activity, which prolongs the insulin-secreting action of incretins. Antioxidant peptides inactivate reactive oxygen species, scavenge free radicals, chelate prooxidant transition metals, and increase the activity of intracellular antioxidant enzymes [10]. Angiotensinconverting enzyme inhibitors have a hypotensive effect and reduce the risk of atherosclerosis, which is one of the main chronic complications of diabetes mellitus. Peptides that inhibit DPP-IV and angiotensin-converting enzyme have been found in protein hydrolysates obtained from chlorella, spirulina, amaranth, tuna milk, and lactic acid products [7–9, 12, 14, 15].
The peptide obtained from Chlorella pyrenoidosa contained ten amino acids: Leu-Leu-Val-Val-Try-Pro-Trp-Thr-Gln-Arg. It was reported to inhibit the activity of pancreatic lipase and prevent the accumulation of fatty acids and triglycerides in cells [16]. Peptide hydrolysates synthesized from quinoa (Chenopodium quinoa L.) and chickpea (Cicer arietinum L.) proteins were able to inhibit the activity of α-amylase and α-glucosidase, thus reducing glucose uptake and hyperglycemia [17, 18].
Bovine colostrum is the first cow’s milk which is obtained after calving. It is a promising source of peptides. Colostrum contains more protein, peptides, lipids, hormones, minerals, and vitamins than whole milk [19, 20].
Colostrum contains antioxidants (vitamins C, A, E, β-carotene, selenium), as well as vitamins B1, B2, PP, B5, B6, B12, D, orotic acid, and enzymes with peroxidase activity [21]. The protein component of colostrum is represented mainly by such whey proteins as albumins and globulins. Casein appears only on lactation days 3–4, and its amount gradually increases but never prevails.
The whey fraction of colostrum contains factors that provide passive immunity: immunoglobulins, lactoferrin, interferon, lysozyme, and defensins are partially synthesized by udder cells and partially formed by milk lymphocytes. Numerous studies have confirmed the antiviral, antifungal, and antibacterial properties of colostrum [21].
Colostrum contains such growth factors and protein hormones as epidermal growth factor (EGF), IGF-1, IGF-2, TGFα, TGFβ, fibroblast growth factor, gonadotropin-releasing hormone, somatotropin, and growth hormone releasing factor (GHRF) [21, 22]. Growth factors promote regeneration, regulate blood glucose levels, and stimulate lipolysis. Glucagon-like peptide 1 (GLP-1) regulates glucose homeostasis by stimulating insulin secretion. A long-term colostrum diet increased the level of GLP-1 in the blood plasma of calves [23]. Colostrum casein hydrolysate appears under the action of lactic acid bacterial proteinases. Hydrolysis yields peptides with hypotensive, antimicrobial, antifungal, and immunoprotective effects. Bovine colostrum whey hydrolysates were reported to contain angiotensin-converting enzyme inhibitors, as well as proteins with antioxidant, antimicrobial, immunomodulatory, and opioid effects [24].
Agarkova et al. hydrolyzed casein by digestive enzymes to obtain 23 peptides with DPP-IV inhibitory activity, which exhibited no side effects typical of DPP-IV inhibitors [25]. A greater degree of hydrolysis reduces the allergenic properties of proteins. Colostrum hydrolysate contains peptides with a lower molecular weight compared to milk hydrolysate. Golovach et al. detected antioxidant activity in colostrum peptides, which increased together with the degree of hydrolysis [26].
Colostrum is safe as it cannot be overdosed and has demonstrated no side effects of clinical significance so far. Colostrum and its protein hydrolysates have a potential therapeutic and preventive use due to their antibacterial and immunoregulatory peptides, as well as growth factors. Colostrum-based functional products may accompany synthetic drugs in the prevention and treatment of diabetes mellitus. These encouraging results require further studies to confirm these effects, as well as to define the optimal amount and treatment time.
The present research objective was to reveal the possibility of reducing hyperglycemia in diabetic rats under the action of bovine colostrum peptides.
STUDY OBJECTS AND METHODS
Colostrum peptides were obtained on the first day after calving by enzymatic hydrolysis of colostrum with trypsin. We removed the fat fraction by centrifugation at 3900 rpm for 10 min in an SM-12-06 centrifuge (TAGLER, Russia). After that, we introduced the enzyme trypsin (Samson-Med, Russia) at 0.15% colostrum weight in a phosphate buffer solution at pH 7.4. The solution consisted of disodium hydrogen phosphate dodecahydrate (Rosspolymer, Russia). The hydrolysis lasted 12 h at a temperature of 36°C until it was raised to 75°C to inactivate the enzyme.
The molecular weight distribution of the peptides was carried out by mass spectrometry and identified by MALDI-TOF and MS/MS mass spectrometry using an Ultraflex MALDI time-of-flight mass spectrometer (Bruker, Germany). The mass spectra analysis involved the Mascot program, Peptide Fingerprint option (Matrix Science, USA), and the Protein NCBI database.
The score was calculated by the following formula:
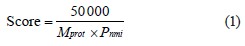
where Mprot is the molecular weight for each matched protein; Pnmi is the product calculated from the Mowse matrix of weights M for each match with the experimental data and peptide masses calculated based on the Protein NCBI genomic database. The spatial structure of the isolated peptides was modeled using the Schrodinger Maestro molecular modeling program (USA)
The antioxidant activity of the peptides was determined by their ability to scavenge free radicals DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonate), as well as by their reducing power when interacting with the Fe(III)-2,4,6-tripyridyl-s-triazine complex bythe ferricreducing antioxidant power method (FRAP). The experiment followed the procedure described in [27] with some modifications.
To determine the antioxidant activity by the DPPH method, we mixed 20 μL of protein hydrolysate or standard solution with 300 μL of a fresh 0.1 mm solution of 2,2-diphenyl-1-picrylhydrazyl. The mix was incubated in the dark at room temperature for 30 min. The optical density decreased at 515 nm, compared to the control.
To determine antioxidant activity by the ABTS method, we used a ABTS radical solution. The ABTS radical was generated by mixing aliquots of 7.0 mM ABTS solution and 2.45 mM potassium persulfate solution. The solution was kept in the dark at room temperature for 16 h. Then, we added 20 µL of the hydrolysate or standard to 300 μL of the solution of the ABTS·+ radical cation. The absorbance was measured at 734 nm after the mix was incubated for 15 min at 37°C in the dark.
To determine the restoring power of the peptides, we prepared a FRAP reagent by mixing 10 parts of 0.3 M acetate buffer (pH 3.6), one part of a 10 mM solution of 2,4,6-tripyridyl-s-triazine in 40 mM HCl, and one part aqueous 20 mM solution of iron chloride FeCl3×6H2O. The reaction was initiated by mixing 300 µL of the FRAP reagent and 20 µL of the test peptide sample or standard solution. The reaction lasted 10 min at 37°C in the dark. The increase in optical density occurred at 593 nm, compared to the control.
We used a trolox solution (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) of known concentration as a standard solution in all the methods. The results of the analyzes were expressed in mmol Trolox equivalents/l.
All the spectrophotometric measurements were performed using a CLARIOstar microplate reader (BMG Labtech, Germany).
The experiment involved twelve-week-old male Wistar rats that weighed 345 ± 11 g. The rats were purchased from the Institute of Immunology and Physiology of the Ural Branch of the Russian Academy of Sciences. They lived five animals per cage under standard laboratory conditions at 20 ± 2°C and a 12-h light-dark cycle, with free access to water and food. They were fed on 2020X Teklad rodent food without soy protein (Envigo, Huntingdon, UK). All manipulations followed the EU Council Directive 2010/63/EU and were approved by the ethics committee of the Institute of Physics, Ural Branch of the Russian Academy of Sciences.
The rats were divided into four groups with seven animals in each. Group 1 included control animals. Group 2 received 300 mg/kg of trypsin hydrolysate of bovine colostrum every day for 30 days. Rats in groups 3 and 4 acquired diabetes mellitus. After 16 h of fasting, they were administered 65 mg/kg of streptozotocin in citrate buffer pH 4.5 intraperitonially, following a preliminary administration of an aqueous solution of 110 mg/kg of nicotinamide [28]. Group 4 received 300 mg/kg of trysin hydrolysate of bovine colostrum for 30 days. A DE006A 18G×50 mm probe (Great Britain) was used for intragastric administration of colostrum peptides. All the animals were weighed every week.
All the animals were withdrawn from the experiment by intramuscular administration of 40 mg/kg of sodium pentobarbital. Blood samples for biochemical studies were collected from the tail vein with anticoagulant heparin. The content of glycated hemoglobin (HbA1c) was determined in whole blood by affinity gel chromatography using a GLYCOHEMOTEST kit (ELTA, Russian Federation). Blood plasma was separated from the new elements by centrifugation at 1000 g for 10 min. The content of glucose in blood plasma was determined by the glucose oxidase method using a Glucose-Novo reagent kit (Vector-Best, Russian Federation). The blood plasma was tested for free radical oxidation products that react with thiobarbituric acid, including malondialdehyde malondialdehyde (MDA) [29]. The amount of reduced glutathione (GSH) and other thiols in the blood plasma was defined using Ellman’s reagent (5,5’-dithiobis(2-nitrobenzoic) acid) [30].
Erythrocytes were mixed with distilled water in a ratio of 1:18, cooled at 4°C for 1 h, and centrifuged again at 1000 g for 10 min. After that, the hemoglobin content was determined to recalculate the indicators of the free radical oxidation – antioxidant protection system (FRO-AOD) per gram of hemoglobin. The procedure involved a Vital reagent kit (Vital Development Corporation, Russian Federation). In the erythrocyte hemolysate, the catalase activity (Enzyme Commission number EC 1.11.1.6) was determined by the decrease in the amount of hydrogen peroxide, which formed a dyed complex with ammonium molybdate [31].
The optical density was measured with a Beckman DU-800 spectrophotometer (USA).
The hematological parameters were obtained using an automated hematological analyzer Celly 70 (Biocode Hycel), designed to study experimental animal blood.
The statistical analysis relied on the OriginPro 9.0 software (OriginlabCorporation, USA). The data were presented as mean ± standard error. The statistical significance of differences in the data obtained was assessed using the nonparametric Mann-Whitney test (U). The difference in the mean values within a group had a 5% significance level (p < 0.05).
RESULTS AND DISCUSSION
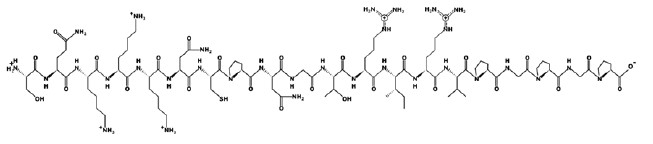
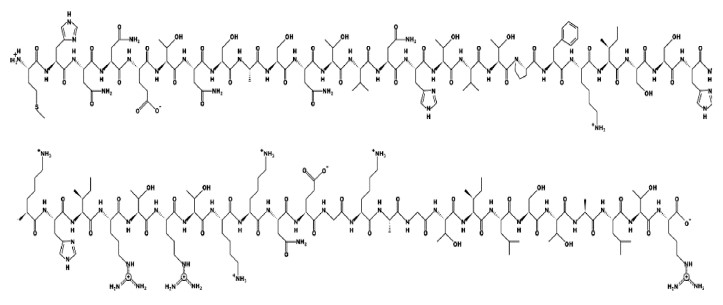
Table 1 describes three biologically active peptides isolated from hydrolyzed bovine colostrum proteins. Peptide 1 with a mass of 8.4 kDa contained 20 amino acids and demonstrated a significant antioxidant activity in comparison with other samples, as revealed by the FRAP method.
The antioxidant activity against DPPH and ABTS radicals demonstrated by peptide 1 indicated that the mix of peptides and amino acids was able to suppress DPPH and ABTS. Probably, the result was due to the pairing of one unpaired electron present in these radicals. We found ambiguous data regarding the FRAP-measured relationship between the average molecular weight of the peptide and its iron-reducing capacity. Sonklin et al. found that peptides with a low molecular weight (< 3 kDa) demonstrated a lower FRAP value while peptides with a molecular weight of 3–5 and 5–10 kDa had a higher FRAP level [32]. However, Meza-Espinoza et al. reported that soybean hydrolysate with an average molecular weight of peptides ≤ 1 kDa had a greater FRAP-measured antioxidant activity than those with higher molecular weight fractions [33]. Thus, both our results and review proved that molecular weight may not be the most important factor affecting the ability of peptides to reduce ferric ions.
Scientific publications report peptides with hydrophobic amino acids to have a higher antioxidant activity:alanine (Ala), lysine (Lys), proline (Pro), leucine (Leu), histidine (His), tyrosine (Tyr), and methionine (Met) [33]. Methionine (Met), cysteine (Cys), tyrosine (Tyr), tryptophan (Trp), histidine (His), and lysine (Lys) increased the iron-reducing ability of peptides while the aromatic amino acids enhanced the antiradical activity of peptides [34–36].
A comparative analysis of ABTS, DPPH, and FRAP antioxidant tests revealed that the antioxidant activity of peptides depended on the amino acid composition of peptide sequences. The low molecular weight peptides exhibited higher antioxidant activity against the DPPH radical compared to those with a high molecular weight. The samples with a lower average molecular weight consisted of shorter and more active peptides that acted as electron donors. They reacted with free radicals and turned them into more stable substances, thus stopping free radical chain oxidation reactions.
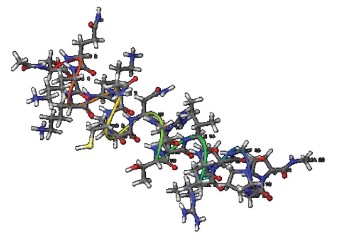
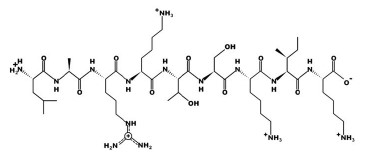
Figures 1–6 show simulated 2D and 3D structures of the peptides.
The spatial models of peptides isolated from fermented bovine colostrum hydrolysate established that the amino acid sequences formed secondary structures. These structures were mostly represented by an alpha helix since they contained little aromatic amino acid residues. This fact explained the lack of antiradical activity in peptides 2 and 3. According to Wang et al., aromatic amino acids enhance the antioxidant activity of peptides [36].
All the peptides in this study had the isoelectric point in a highly-alkaline medium. Peptide 1 had the isoelectric point at pH 11.62 while peptide 2 had it at pH 11.79. The points depended not on the number of amino acids but on the predominance of amine or carboxyl groups in the peptide.
The peptide structure modeling revealed the range of peptide hydrophilicity from +16.95 Kcal/mol in peptide 2 to +48.34 Kcal/mol in peptide 3. Peptide 1 had a hydrophilicity level of +25.51 Kcal/mol.
The 3D model showed that peptide 3 with its charge of +6 had a higher chemical activity than the rest: peptide 1 had a charge of +4, and peptide 2 had a charge of +5.
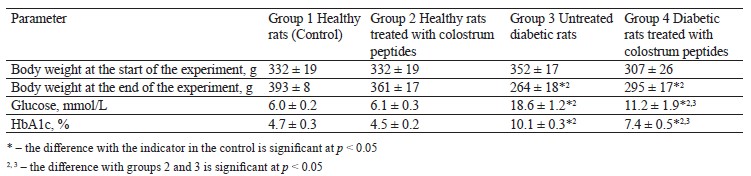
The intragastric administration of colostrum peptides caused no significant changes in body weight, glucose, and glycated hemoglobin in healthy rats, compared to the control (Table 2).
Unlike the healthy and control rats, the experimental diabetic rats demonstrated a lower body weight, higher glucose levels, and more glycated hemoglobin by day 30 (Table 2). The administration of colostrum peptides to diabetic rats did not prevent weight loss, but the hyperglycemia was less severe, unlike in group 3 (untreated diabetic rats), which included untreated diabetic rats (Table 2).
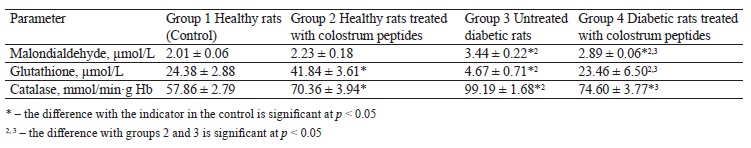
The administration of colostrum peptides to healthy animals did not increase the amount of malondialdehyde in the blood plasma (Table 3). However, group 2, which included healthy rats treated with colostrum peptides, had a higher content of reduced glutathione and catalase activity compared to the control. The intragastric administration procedures definitely caused stress, which inevitably disturbed homeostasis in the free radical oxidation-antioxidant protection system. The fact that the malondialdehyde did not increase in group 2 indicated a slight increase in the free radical oxidation and a compensation for this process.
Diabetic rats started accumulating malondialdehyde, and the level of reduced glutathione dropped by 5.2 times. They demonstrated a greater catalase activity than the healthy rats (Table 3). Despite the catalase activation, the diabetic rats lacked the non-enzymatic link of antioxidant protection but accumulated secondary products of free radical oxidation, which indicated the development of oxidative stress.
The diabetic rats treated with colostrum peptides (Group 4) had a lower content of malondialdehyde and catalase activity while their level of reduced glutathione stabilized, compared to the untreated diabetic rats (Table 3). While glutathione was the only indicator that stabilized in Group 4, this change can still be assessed as a decrease in the severity of oxidative stress under the action of colostrum. Glutathione contains γ-glutamic acid, cysteine, and glycine. In the diabetic rats treated with colostrum peptides, it could increase because its synthesis increased under the action of colostrum peptide amino acids.
The diabetic rats treated with colostrum hydrolysate demonstrated a less severe oxidative stress because the hydrolysate contained a peptide with antioxidant properties, namely peptide 1.
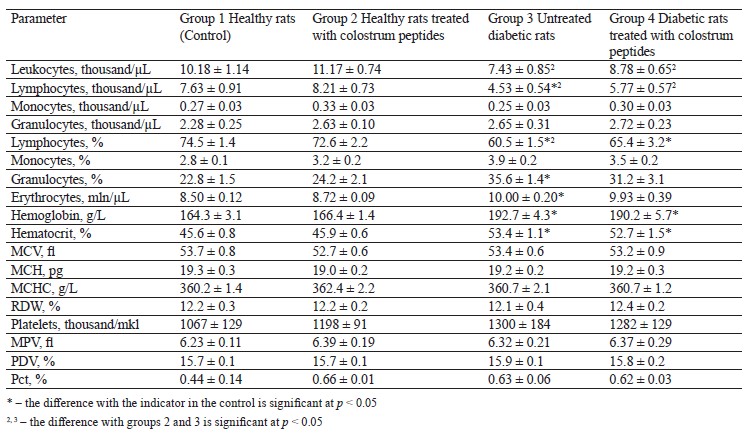
The hematological analysis revealed no abnormalities in leukocyte, erythrocyte, or platelet parameters in healthy rats treated with colostrum peptides (Table 4). The untreated diabetic rats demonstrated a lower absolute and relative number of leukocytes due to the fraction of lymphocytes with a greater proportion of granulocytes.
Leukopenia and lymphopenia in diabetic rats might have been caused by the destructive processes in the organs of the immune system [37]. The untreated diabetic rats also had a greater number of erythrocytes, and the hemoglobin content also increased by 1.2 times, affected by a larger number of erythrocytes. Therefore, the hematocrit increased by 1.2 times relative to the healthy rats. Other hematological parameters remained within the norm (Table 4). The high content of erythrocytes and hemoglobin in the diabetic rats may be a consequence of hypoxia caused by diabetes mellitus. In the diabetic rats treated with colostrum peptides, the values of leukocytes and erythrocytes approached those in the healthy rats, but the relative number of granulocytes and the number of erythrocytes were the only parameters that stabilized (Table 4).
CONCLUSION
1.The intragastric administration of 300 mg/kg of bovine colostrum peptides to the healthy rats caused minor and compensated changes in the antioxidant system but did not affect the level of glycemia and hematological parameters.
2.The rats with diabetes mellitus developed hyperglycemia, oxidative stress, leukopenia, and a slight increase in the number of red blood cells and hemoglobin.
3. The diabetic rats treated with 300 mg/kg of colostrum peptides had a lower hyperglycemia and oxidative stress, as well as better hematological parameters.
4. The peptide with the amino acid sequence SQKKKNCPNGTRIRVPGPGP and a mass of 8.4 kDa added the antioxidant effect to colostrum hydrolysate.
Thus, the isolation of individual colostrum peptides is relevant for studying their antidiabetic properties in the future.
Contribution
The authors were equally involved in the writing of the manuscript and bear equal responsibility.
CONFLICTS OF INTEREST
The authors declared no conflict of interests regarding the publication of this article.
REFERENCES
- International Diabetes Federation: Diabetes Atlas 8th edition. 2017.
- Ademosun AO. Glycemic properties of soursop-based ice cream enriched with moringa leaf powder. Foods and Raw Materials. 2021;9(2):207–214. https://doi.org/10.21603/2308-4057-2021-2-207-214
- Zaytseva LV, Ruban NV, Tsyganova TB, Mazukabzova EV. Fortified confectionery creams on vegetable oils with a modified carbohydrate profile. Food Processing: Techniques and Technology. 2022;52(3):500–510. (In Russ.). https://doi.org/10.21603/2074-9414-2022-3-2377
- Artasensi A, Pedretti A, Vistoli G, Fumagalli L. Type 2 diabetes mellitus: A review of multi-target drugs. Molecules. 2020;25(8). https://doi.org/10.3390/molecules25081987
- Holst JJ. From the incretin concept and the discovery of GLP-1 to today's diabetes therapy. Frontiers in Endocrinology. 2019;10. https://doi.org/10.3389/fendo.2019.00260
- Pereira ASP, Banegas-Luna AJ, Peña-García J, Pérez-Sánchez H, Apostolides Z. Evaluation of the anti-diabetic activity of some common herbs and spices: Providing new insights with inverse virtual screening. Molecules. 2019;24(22). https://doi.org/10.3390/molecules24224030
- Lin Y-H, Chen G-W, Yeh CH, Song H, Tsai J-S. Purification and identification of angiotensin I-converting enzyme inhibitory peptides and the antihypertensive effect of Chlorella sorokiniana protein hydrolysates. Nutrients. 2018;10(10). https://doi.org/10.3390/nu10101397
- Andreeva A, Budenkova E, Babich O, Sukhikh S, Ulrikh E, Ivanova S, et al. Production, purification, and study of the amino acid composition of microalgae proteins. Molecules. 2021;26(9). https://doi.org/10.3390/molecules26092767
- Li Y, Aiello G, Bollati C, Bartolomei M, Arnoldi A, Lammi C. Phycobiliproteins from Arthrospira platensis (spirulina): A new source of peptides with dipeptidyl peptidase-IV inhibitory activity. Nutrients. 2020;12(3). https://doi.org/10.3390/nu12030794
- Novoselova MV, Prosekov AYu. Technological options for the production of lactoferrin. Foods and Raw Materials. 2016;4(1):90–101. https://doi.org/10.21179/2308-4057-2016-1-90-101
- Manzanares P, Gandía M, Garrigues S, Marcos JF. Improving health-promoting effects of food-derived bioactive peptides through rational design and oral delivery strategies. Nutrients. 2019;11(10). https://doi.org/10.3390/nu11102545
- Suo S-K, Zheng S-L, Chi C-F, Luo H-Y, Wang B. Novel angiotensin-converting enzyme inhibitory peptides from tuna byproducts-milts: Preparation, characterization, molecular docking study, and antioxidant function on H2O2-damaged human umbilical vein endothelial cells. Frontiers in Nutrition. 2022;9. https://doi.org/10.3389/fnut.2022.957778
- Ryazantseva KA, Agarkova EYu, Fedotova OB. Continuous hydrolysis of milk proteins in membrane reactors of various configurations. Foods and Raw Materials. 2021;9(2):271–281. https://doi.org/10.21603/2308-4057-2021-2-271-281.
- Ayala-Niño A, Rodríguez-Serrano GM, González-Olivares LG, Contreras-López E, Regal-López P, Cepeda-Saez A. Sequence identification of bioactive peptides from amaranth seed proteins (Amaranthus hypochondriacus spp.). Molecules. 2019;24(17). https://doi.org/10.3390/molecules24173033
- Rubak YuT, Nuraida L, Iswantini D, Prangdimurti E. Angiotensin-I-converting enzyme inhibitory peptides in milk fermented by indigenous lactic acid bacteria. Veterinary World. 2020;13(2):345–353. https://doi.org/10.14202/vetworld.2020.345-353
- Zhang R, Chen J, Mao X, Qi P, Zhang X. Separation and lipid inhibition effects of a novel decapeptide from Chlorella pyenoidose. Molecules. 2019;24(19). https://doi.org/10.3390/molecules24193527
- Valenzuela Zamudio F, Segura Campos MR. Amaranth, quinoa and chia bioactive peptides: a comprehensive review on three ancient grains and their potential role in management and prevention of Type 2 diabetes. Critical Reviews in Food Science and Nutrition. 2020;62(10):2707–2721. https://doi.org/10.1080/10408398.2020.1857683
- Quintero-Soto MF, Chávez-Ontiveros J, Garzón-Tiznado JA, Salazar-Salas NY, Pineda-Hidalgo KV, Delgado-Vargas F, et al. Characterization of peptides with antioxidant activity and antidiabetic potential obtained from chickpea (Cicer arietinum L.) protein hydrolyzates. Journal of Food Science. 2021;86(7):2962–2977. https://doi.org/10.1111/1750-3841.15778
- Uyama T, Kelton DF, Winder CB, Dunn J, Goetz HM, LeBlanc SJ, et al. Colostrum management practices that improve the transfer of passive immunity in neonatal dairy calves: A scoping review. PLoS ONE. 2022;17(6). https://doi.org/10.1371/journal.pone.0269824
- Tikhonov SL, Tikhonova NV, Tursunov KhKh, Danilova IG, Lazarev VA. Peptides of trypsin hydrolyzate in bovine colostrum. Food Processing: Techniques and Technology. 2023;53(1):150–158. (In Russ.). https://doi.org/10.21603/2074-9414-2023-1-2422
- Playford RJ, Weiser MJ. Bovine colostrum: Its constituents and uses. Nutrients. 2021;13(1). https://doi.org/10.3390/nu13010265
- Brenmoehl J, Ohde D, Wirthgen E, Hoeflich A. Cytokines in milk and the role of TGF-beta. Best Practice and Research Clinical Endocrinology and Metabolism. 2018;32(1):47–56. https://doi.org/10.1016/j.beem.2018.01.006
- Inabu Y, Pyo J, Pletts S, Guan LL, Steele MA, Sugino T. Effect of extended colostrum feeding on plasma glucagon-like peptide-1 concentration in newborn calves. Journal of Dairy Science. 2019;102(5):4619–4627. https://doi.org/10.3168/jds.2018-15616
- Ashok NR, Aparna HS. Empirical and bioinformatic characterization of buffalo (Bubalus bubalis) colostrum whey peptides & their angiotensin I-converting enzyme inhibition. Food Chemistry. 2017;228:582–594. https://doi.org/10.1016/j.foodchem.2017.02.032
- Agarkova EYu, Ryazantseva KA, Kruchinin AG. Anti-diabetic activity of whey proteins. Food Processing: Techniques and Technology. 2020;50(2):306–318. (In Russ.). https://doi.org/10.21603/2074-9414-2020-2-306-318
- Golovach TN, Kurchenko VP, Tarun EI. Protein-peptide composition and radical reducing properties of fermented bovine colostrum. Food Industry: Science and Technologies. 2016;33(3):57–63. (In Russ.). https://elibrary.ru/XAMKJF
- Feduraev P, Skrypnik L, Nebreeva S, Dzhobadze G, Vatagina A, Kalinina E, et al. Variability of phenolic compound accumulation and antioxidant activity in wild plants of some Rumex species (Polygonaceae). Antioxidants. 2022;11(2). https://doi.org/10.3390/antiox11020311
- Spasov AA, Vorohkova MP, Snegur GL, Cheplyaeva NI, Chepurnova MV. Experimental model of a type 2 diabetes. Journal Biomed. 2011;(3):12–18. (In Russ.). https://elibrary.ru/OJBTOH
- Stalʹnaya ID, Garishvili TG. Determining malondialdehyde with thiobarbituric acid. In: Orekhovicha VN, editor. Modern methods in biochemistry. Moscow: Meditsina; 1977. pp. 66–68. (In Russ.).
- Verevkina IV, Tochilkin AI, Popova NA. Colorimetric determination of SH-groups and –SS-bonds in proteins with 5,5'-dithiobis(2-nitrobenzoic) acid. In: Orekhovicha VN, editor. Modern methods in biochemistry. Moscow: Meditsina; 1977. pp. 223–231. (In Russ.).
- Korolyuk MA, Ivanova LN, Mayorova IG, Tokarev VE. Determining catalase activity. Laboratory Sphere. 1988;(4):44–47. (In Russ.).
- Sonklin C, Laohakunjit N, Kerdchoechuen O. Assessment of antioxidant properties of membrane ultrafiltration peptides from mungbean meal protein hydrolysates. PeerJ. 2018;6. https://doi.org/10.7717/peerj.5337
- Meza-Espinoza L, Sáyago-Ayerdi SG, García-Magaña ML, Tovar-Pérez EG, Yahia EM, Vallejo-Cordoba B, et al. Antioxidant capacity of egg, milk and soy protein hydrolysates and biopeptides produced by Bromelia pinguin and Bromelia karatas-derived proteases. Emirates Journal of Food and Agriculture. 2018;30(2):122–130. https://doi.org/10.9755/ejfa.2018.v30.i2.1604
- Samaranayaka AGP, Li-Chan ECY. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. Journal of Functional Foods. 2011;3(4):229–254. https://doi.org/10.1016/j.jff.2011.05.006
- Carrasco-Castilla J, Hernández-Álvarez AJ, Jiménez-Martínez C, Jacinto-Hernández C, Alaiz M, Girón-Calle J, et al. Antioxidant and metal chelating activities of Phaseolus vulgaris L. var. Jamapa protein isolates, phaseolin and lectin hydrolysates. Food Chemistry. 2012;131(4):1157–1164. https://doi.org/10.1016/j.foodchem.2011.09.084
- Wang Y-Y, Wang C-Y, Wang S-T, Li Y-Q, Mo H-Z, He J-X. Physicochemical properties and antioxidant activities of tree peony (Paeonia suffruticosa Andr.) seed protein hydrolysates obtained with different proteases. Food Chemistry. 2021;345. https://doi.org/10.1016/j.foodchem.2020.128765
- Queiroz LAD, Assis JB, Guimarães JPT, Sousa ESA, Milhomem AC, Sunahara KKS, et al. Endangered lymphocytes: The effects of alloxan and streptozotocin on immune cells in type 1 induced diabetes. Mediators Inflammation. 2021;2021. https://doi.org/10.1155/2021/9940009