Abstract
Chitosan reacts with amino acids and hydrolyzed whey proteins to produce biologically active complexes that can be used in functional foods. The research objective was to obtain chitosan biocomposites with peptides and amino acids with improved antioxidant and sensory properties.The research featured biocomposites of chitosan and succinylated chitosan with whey peptides and amino acids. The methods of pH metry and spectrophotometry were employed to study the interaction parameters between polysaccharides and peptides, while colorimetry and spectrophotometry served to describe the amino acids content. The antiradical effect was determined by the method of fluorescence recovery. Pure compounds and their complexes underwent a sensory evaluation for bitterness.
Chitosan and succinylated chitosan formed complexes with whey peptides and such proteinogenic amino acids as arginine, valine, leucine, methionine, and tryptophan. The equimolar binding of tryptophan, leucine, and valine occurred in an aqueous chitosan solution (in terms of glucosamine). Methionine appeared to be the least effective in chitosan interaction, while arginine failed to complex both with chitosan and succinylated chitosan. Chitosan and succinylated chitosan biocomposites with peptides and leucine, methionine, and valine proved to be less bitter that the original substances. The samples with arginine maintained the same sensory properties. Chitosan complexes with tryptophan and peptides increased their antioxidant activity by 1.7 and 2.0 times, respectively, while their succinylated chitosan complexes demonstrated a 1.5-fold increase.
Chitosan and succinylated chitosan biocomplexes with tryptophan and whey protein peptides had excellent antioxidant and sensory properties. However, chitosan proved more effective than succinylated chitosan, probably, because it was richer in protonated amino groups, which interacted with negatively charged amino acids groups.
Keywords
Chitosan, succinylated chitosan, whey peptides, proteinogenic amino acids, chitosan biocompositesINTRODUCTION
Milk proteins are a source of biologically active peptides that appear as a result of enzymic processes in the gastrointestinal tract. Milk proteins undergo enzymatic hydrolysis as part the commercial hypoallergenic foods [1, 2]. Proteolysis forms bioactive functional peptides [3]. Milk proteins lose their allergenicity because the antigenic determinants split in the structure of allergen proteins [4].
Hydrolyzed cow’s milk proteins are short chain peptides and amino acids. Histidine, proline, phenylalanine, tyrosine, and tryptophan give them a characteristic bitter taste, which limits the use of hypoallergenic hydrolysates in the food industry [5]. Hydrophobic chromatography improves the bitter taste of hydrolysates; other options include specific sorbents, isoelectric focus, and limited proteolysis [6]. Instrumental hydrolysate processing increases production costs and changes the amino acid composition because it removes essential amino acids. Moreover, instrumental processing requires specific enzymes and complex detoxication. Flavoring additives are an alternative solution that can camouflage the bitterness [7]. Still, food science is looking for new ways to reduce the bitterness of protein hydrolysates and amino acid mixes.
Cyclic oligosaccharides, or cyclodextrins, have a cone-like spatial structure with a hydrophobic inner cavity. As a result, they can form inclusion complexes with guest molecules, which are also called clathrates [8]. If bitter peptides and amino acids are encapsulated in cyclodextrins, their taste improves because the cone shields them from taste receptors [9, 10]. In addition, the method improves the biologically active potential of the encapsulated compounds [11, 12].
Aminopolysaccharide chitosan also can improve the sensory properties of bitter amino acids and peptides. Chitosan is a low-toxic substance, popular in the food industry as a filler, thickener, and stabilizer [13]. Food scientists obtain composites with more advantageous properties by complexing natural biopolymers and bioactive substances with chitosan and its derivatives.
Chitosan, a potential complexing agent, is able to interact with organic compounds due to its hydrophobic effect, as well as due to ionic and hydrogen bonds [14]. Although chitosan has good biocompatibility and adsorption, its solubility is rather limited [15]. As a result, chitosan is unpopular in the food industry and pharmacy. At pH < 6.5, its free amino groups are protonated, thus rendering it polycationic properties [16]. Soluble chitosan can be obtained by depolymerization and chemical modification [17]. For instance, Lee et al. introduced anionic succinate groups to produce succinylated chitosan [18]. This chitosan derivative was soluble at pH > 7.0 and < 4.5.
Molecular modeling demonstrated that the complexation occurs as a result of the electrostatic interaction between the amino acid carboxyl group and the chitosan amino group [19]. Chitosan and its derivatives interact with amino acids in three different ways [20–28]:
1. Chitosan produces a gel-like base. This hydrogel acts as films or spheres and immobilizes amino acids inside the base or adsorbs it on its surface. As part of the film, amino acids can also be chemically crosslinked with chitosan molecules [20–23];
2. Molecules of chitosan and amino acid are chemically crosslinked. The resulting biocomposites serve as transport for various molecules [24–26]; and
3. Chitosan and amino acids interact in a solution as compounds bind with NH2-groups of chitosan. Different chitosan modifications interact with target molecules, which are normally used in gene therapy, packaging, drug delivery, wastewater treatment, etc. [27, 28].
This research focused on the third type of interaction because of its technical simplicity and low cost. The main goal was to use the biocomposites of chitosan and amino acids as a food component. According to the hypothesis, these high-molecular-weight biocomposites interact with bitter-taste receptors to improve the sensory properties of whey protein hydrolysates. The biocomposite releases its peptides and amino acids in the gastrointestinal tract because of weak electrostatic forces.
Biocomposites of chitosan and its derivatives with peptides and amino acids are promising functional ingredients. Chitosan complexing affects the antioxidant and sensory properties of amino acids and peptides.
The research objective was to obtain biocomposites of chitosan with peptides and amino acids with high antioxidant activity and improved sensory properties.
STUDY OBJECTS AND METHODS
The study involved chitosan with a molecular weight of 100 kDa and a degree of deacetylation of 90%. The succinylated chitosan had a molecular weight of 200 kDa and a degree of substitution of 75.1% (Bioprogress, Russia). The peptide mix had a molecular weight of ≤ 10 kDa. It was part of the Peptigen IF 3080 WPH whey protein hydrolysate with protein mass fraction 80% (Arla Foods Ingredients Group, Denmark). The experiment also featured such amino acids as L-arginine, L-valine, L-leucine, L-methionine, and L-tryptophan (Sigma, USA).
Complexing chitosan with whey protein hydrolysate. We prepared 0.1% aqueous solutions of chitosan and succinylated chitosan and 15% aqueous solution of hydrolysate, which was a mix of whey protein peptides. After that, we added 250 µL of the 15% hydrolysate solution to 50 mL of 0.1% chitosan solution and stirred. The solution was tested for active acidity and optical density at a wavelength of 640 nm. Then, the 0.1% chitosan solution was titrated with the 15% hydrolysate solution in the protein concentration range of 0.08–1.34%. The optical density and active acidity of the samples were evaluated after each hydrolysate cycle. The experiment with the succinylated chitosan followed the same procedure. The active acidity was determined using a HANNA HI 83141 pH-meter (Hanna Instruments, Germany). The optical density was monitored using a Metertech UV/VIS SP 8001 device (Metertech, Taiwan).
Obtaining chitosan biocomposites with amino acids. Variant 1 included 0.1% aqueous solutions of chitosan/succinylated chitosan with 0.5% aqueous solutions of arginine, valine, leucine, methionine, and tryptophan. The chitosan solution (0.1%, 20 mL) was titrated with a 0.5% amino acid solution. The amino acids were added by 80 µL until their concentration in the mix reached 0.002–0.05%. After each titration stage, we monitored the optical density and active acidity. The solution of succinylated chitosan (0.1%) was titrated under similar conditions.
Variant 2 involved aqueous solutions of 0.5% chitosan/succinylated chitosan and 0.5% proteinogenic amino acid, namely arginine, valine, leucine, or methionine. The tryptophan experiment included aqueous solutions of 0.1% chitosan/succinylated chitosan and 0.05% the amino acid. The resulting solutions were stirred at 25°C for 1 h. After that, we tested the samples for optical density and active acidity.The chitosan and succinylated chitosan complexes with tryptophan were subjected to dialysis using a tubular cellulose membrane with a 14-kDa shutoff (Sigma, USA). The removal of unbound tryptophan took 4 h. After that, we measured amino acids in the dialysate and the optical density at a wavelength of 280 nm by using spectrophotometry. We also designed a calibration dependency curve for the optical density and tryptophan (0.0001–0.0016%). The curve made it possible to calculate the amount of tryptophan in the initial biocomposite solutions and dialysate samples.
The colorimetry test involved the Folin-Chocalteu reagent (Sigma, USA). We measured the optical density of the tryptophan complexes with chitosan/ succinylated chitosan (variant 2) and the tryptophan calibration samples at a wavelength of 620 nm. We introduced 1000 µL of 0.5 M sodium hydroxide solution into 200 µL of the test sample, stirred, and added 240 µL of the Folin Ciocalteu reagent. The resulting mix was kept in a dark place for 20 min. Subsequently, the dyed samples were tested for optical density. The effect of the tryptophan content (0.0001–0.010%) on the optical density of the calibration samples was expressed as a dependency curve, which made it possible to calculate the amino acid concentration in the biocomposite solutions and the corresponding dialysates.
We introduced 500 μL of the test complexes (variant 2) into test tubes with centrifuge filters with a cut-off at 10 kDa (Merck Millipore, USA) to determine the concentration of free and bound arginine, valine, leucine, or methionine. The filtrates were obtained by centrifugation at 14 000 rpm for 15 min. We used the Kjeldahl method (State Standard 23327-98) to determine the total amount of nitrogen in the filtrate samples.
The statistical processing involved the arithmetic mean value ± confidence interval (n = 3, α = 0.05).
Determining the bitterness of pure compounds and chitosan complexes. The sensory evaluation followed a 10-point scale of bitterness: 0 – no bitterness detected; 1–2 – very weak; 3–4 – weak; 5–6 – mild; 7–8 – strong; 9–10 – very strong.
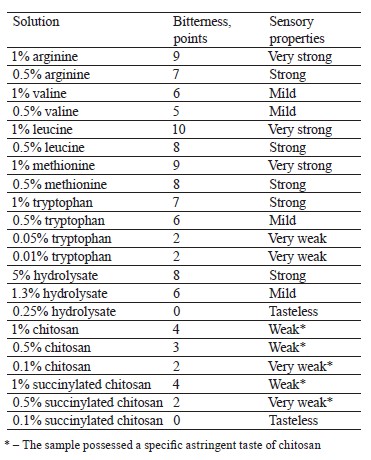
The severity of bitterness was determined using standard solutions of chitosan, amino acids, and peptides (Table 1). The mean value of bitterness was calculated after three repetitions.
Evaluating the antioxidant activity of chitosan biocomposites with tryptophan and hydrolysate. We used the Oxygen Radical Absorbance Capacity Assay (ORAC) to determine the antioxidant activity [29] following the procedure developed by Tarun et al., who measured the antioxidant activity based on the results of their interaction with hydroxyl radicals [30]. The radicals were generated using the Fenton system.
We determined the fluorescence fluorescein recovery (A, %) as the ratio of the signal intensity of the sample and the control fluorescein (100%). As a result, we obtained dependency curves for fluorescein fluorescence (A, %) and the solids. The curve made it possible to define the concentration of the sample necessary for a 50% suppression of fluorescein fluorescence (IC50). The experiments were conducted in triplicates to obtain the arithmetic mean ± confidence interval. We used the confidence interval method to calculate the significance of the differences between the samples.
RESULTS AND DISCUSSION
Interaction of chitosan and succinylated chitosan with whey protein hydrolysate. Chitosan and its succinylated derivative interacted with an extensive enzymatic hydrolysate of whey, which gave the resulting mix a bitter taste. The specific sensory properties of the hydrolysate resulted from the intensive breakdown of whey proteins, which released the bitter peptide and amino acid fraction.
Table 1 shows the bitterness of amino acids depending on their concentration in aqueous solutions.
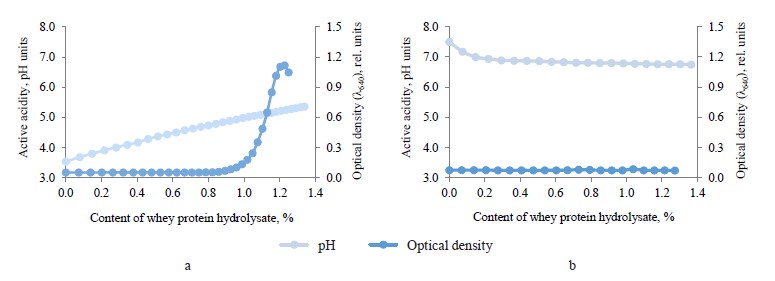
We added a 0.1% solution of chitosan with whey protein hydrolysate in the concentration range of 0.08– 1.34%, which increased the active acidity of the medium by 1.9 pH units (Fig. 1a).When chitosan mixed with whey protein hydrolysate, the resulting protonation raised the active acidity of the medium. The optical density of the system increased by 1.1 relative units while the content of the hydrolysate changed from 1.0 to 1.3%. These consequences occurred because macromolecules of chitosan with those of peptides formed aggregates, which had a neutral total charge. The spectrophotometry test registered an equivalence point at a peptide concentration of 1.3% as the chitosan – hydrolysate protein system titrated.
Figure 1b summarizes the binding of succinylated chitosan and whey protein hydrolysate. When we added 0.08–1.34% hydrolysate to the 0.1% succinylated chitosan solution, the pH of the medium dropped by 0.75 units but the optical density of the system remained the same. According to the pH analysis, 0.25% hydrolysate resulted in an equivalent binding of succinylated chitosan to the peptide fraction.
Therefore, when the succinylated chitosan and whey protein hydrolysate had a lower pH, protons entered the medium. No changes occurred in the optical properties of the succinylated chitosan solution and the peptide fraction. Apparently, the same surface charges prevented a large-scale aggregation of macromolecular composites of succinylated chitosan with peptides.
In general, 1.0 g of chitosan interacted with 13.0 g of hydrolysate, while 1.0 g of succinylated chitosan bound with 2.5 g of hydrolysate. Good ability of chitosan to bind peptides results from its polycationic properties. In succinylated chitosan, anionic succinate groups replace 75.1% of amino groups, which decreases the number of potential interaction sites.
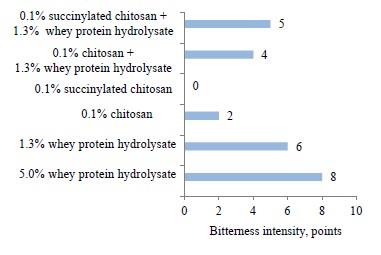
The next stage featured the sensory properties of protein hydrolysate, chitosan, its derivative, and their complexes (Fig. 2). We founded that it was hydrolysate that was responsible for the bitter taste. Chitosan and succinylated chitosan demonstrated a low bitterness and a specific astringency. The bitterness depended on the concentration of the ingredients. The bitterness of hydrolysates in biocomposites dropped by 1–2 points, compared with its pure form.
Complexing chitosan and succinylated chitosan with amino acids. This stage involved the physicochemical parameters of prototypes obtained by mixing 0.1% solutions of chitosan/succinylated chitosan with 0.002–0.05% arginine/valine/leucine/methionine/tryptophan. The experiment revealed no significant changes in the optical density and active acidity of the solutions.
Other studies reported that amino acids could bind with polycationic chitosan as a result of electrostatic interaction [19, 31]. Unlike such macromolecular structures as proteins and peptide fractions, amino acids with their low molecular weight neither formed coagulating complexes with chitosan/succinylated chitosan solutions nor affected the pH of the medium.
To determine the optimal conditions for the interaction of tryptophan with chitosan and succinylated chitosan, we obtained solutions of 0.05% amino acids and 0.1% polysaccharide. The molar ratio of NH2-groups and tryptophan was 2:1 based on the content of amino groups in the composition of glucosamine (chitosan monomer). When we used succinylated chitosan, the molar ratio of tryptophan:glucosamine equaled 1:0.25, and that of tryptophan:succinylglucosamine was 1:1.3.
We subjected the experimental samples of chitosan/ succinylated chitosan with tryptophan to dialysis to separate the unbound amino acid. Spectrophotometry and colorimetry made it possible to determine the proportion of tryptophan in the biocomposites. We determined the proportion of bound and free tryptophan in the complexes based on the amount of initial and dialyzed tryptophan.
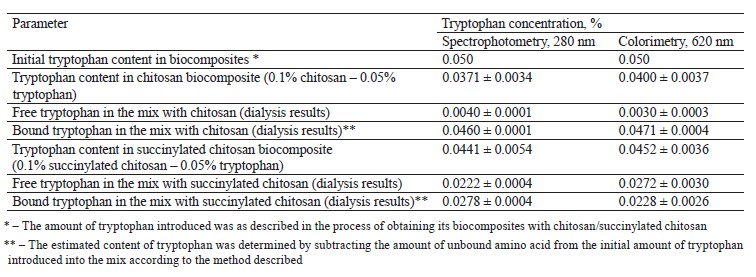
The content of unbound amino acid in the solution did not change after dialysis. To determine the proportion of tryptophan in the complex, we subtracted the amount of dialyzed amino acid from its initial content (0.05%) (Table 2).
Both spectrophotometric and colorimetric methods yielded similar results. The mixes of chitosan/succinylated chitosan with amino acids had a low signal level in the ultraviolet and visible spectra, compared to the control 0.05% tryptophan solution. The resulting effect depended on the binding of the amino acid to the complexing agent and the change in the access to the amino acid radical.
Spectrophotometry and colorimetry also showed that the 0.1% chitosan solution bound 0.046 and 0.047% tryptophan, respectively, which means that 94% of the introduced amino acid entered the complex. The 0.1% succinylated chitosan solution bound 0.0278 and 0.0228% amino acid, respectively, which indicated a complex formation of 56% tryptophan. Thus, tryptophan bound with chitosan 1.7 times as effectively as with succinylated chitosan.
When the molar ratio of tryptophan:glucosamine was 1:0.25, a complete complexation of the amino acid occurred at 0.05% tryptophan and 0.2% succinylated chitosan. The optimal mass ratio of tryptophan:succinylated chitosan was 1:4 (Table 2).
As for the mix of amino acids and chitosan, the initial molar ratio of tryptophan:glucosamine was 1:2. When the solution contained 0.1% chitosan and 0.05% tryptophan (mass ratio of 2:1), almost all the amino acid entered the complex, probably, as a result of the twofold excess of protonated amino groups. We expected an equimolar binding at a mass ratio of 1:1. The formation of biocomposites of chitosan/succinylated chitosan with tryptophan was confirmed by spectrophotometry and colorimetry.
The optimal mass ratio of tryptophan and chitosan/ succinylated chitosan was 1:1 and 1:4, respectively. The binding of succinylated chitosan with tryptophan was quite weak because succinylation blocked amino groups. According to Deka & Bhattacharyya, it is the NH2- groups of the polysaccharide that have the most vigorous interaction with amino acids [19].
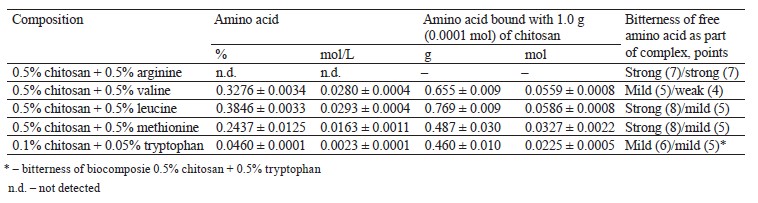
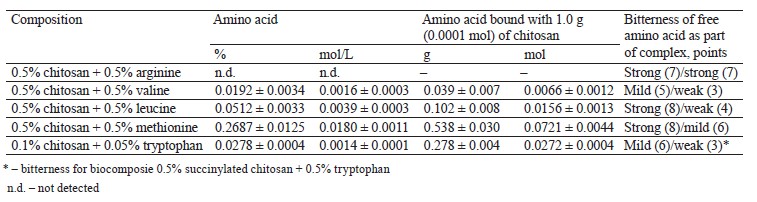
The experimental biocomposites of chitosan/suc- cinylated chitosan with arginine/valine/leucine/methio- nine were filtered with a 10-kDa cut-off. The concentration of total nitrogen in the initial biocomposites and the proportion of amino acids in the biocomposites and filtrates were determined by calculation. Tables 3 and 4 illustrate the experimental data on the content of chitosan-bound amino acids.
Based on the preparation procedure and calculations, the biocomposites with arginine, valine, leucine, or methionine included 0.5% chitosan or succinylated chitosan, i.e., 5×10–5 mol/L of chitosan and 2.5×10–5 mol/L of succinylated derivative (0.0272 and 0.0032 mol/L of glucosamine monomer relative to chitosan and succinylated chitosan). Valine and leucine demonstrated the most effective interaction with chitosan, as evidenced by the equimolar saturation of glucosamine residues (NH2- groups) with amino acids (Tables 3 and 4). In case of tryptophan, almost all the introduced amino acid bound with chitosan (Table 2–4). The complexing properties of succinylated chitosan with amino acids decreased as follows: methionine – tryptophan – leucine – valine.
The amount of the amino acids in the complexes with succinylated chitosan was low because succinylation blocked amino groups (75.1%). Arginine did not bind to polysaccharides (Tables 3 and 4).
In general, the properties of amino acid radicals are responsible for polysaccharide complexing. Thus, polycationic chitosan binds with protonated amino groups if amino acids are in their anionic form (valine, leucine, methionine, and tryptophan). Arginine, on the contrary, remains in its protonated state in the solution, which prevents it from interacting with chitosan and its derivatives.
The sensory assessment of chitosan with tryptophan for bitterness was based on standard tryptophan samples (Table 1). A 0.5% chitosan solution is usually slightly bitter and astringent, which is also typical of succinylated chitosan (Table 1). Chitosan and succinylated chitosan improve the sensory properties of tryptophan: its bitterness decreases by 1–3 points compared to the pure amino acid sample (Tables 3 and 4). Bitterness was entirely absent when the concentration of polysaccharides and tryptophan was as low as 0.01– 0.10%.
The bitterness of complexes of chitosan/succinylated chitosan with valine, leucine, and methionine lost 2–4 points, compared with the pure amino acid samples (Tables 3 and 4). Arginine biocomposites demonstrated no significant change in the level of bitterness. The resulting sensory profiles corresponded with the efficiency of complexation for valine, leucine, methionine, and tryptophan and the inability of arginine to bind with chitosan/succinylated chitosan.
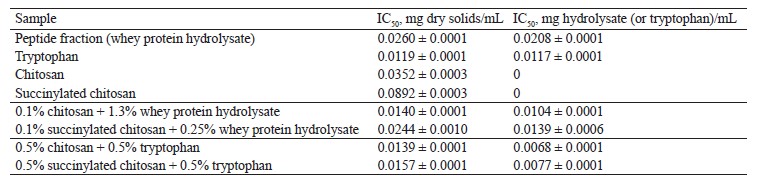
Effect of complexation with chitosan and succinylated chitosan on the antioxidant properties of hydrolysate and tryptophan. This research also featured the antioxidant properties of tryptophan and whey protein hydrolysate in their complexes with chitosan and succinylated chitosan. We studied the antioxidant potential of tryptophan, hydrolysate, chitosan, succinylated chitosan, and their biocomposites by restoring the fluorescein fluorescence at different antioxidant concentrations (0.0005–0.5 mg dry solids/mL). All the compounds were able to restore the fluorescein fluorescence (81–97%). The capacity to inhibit 50% hydroxyl radicals (IC50) served as the main indicator of antioxidant activity.
The antioxidant effect of peptides is known to depend on the reducing effect of amino acid radicals, mainly methionine, cysteine, tyrosine, and tryptophan [32–34]. Natural polysaccharides, including chitin and chitosan, are potential antioxidants [35, 36]. The antioxidant activity index (IC50) took into account the concentration of dry solids and the content of tryptophan (or protein) in the complexes (Table 5).
The restored fluorophore fluorescence reached 95 and 90% when chitosan and succinylated chitosan were introduced into the experimental system, respectively. Thus, the antioxidant activity index IC50 for chitosan reached 0.0352 mg of dry solids/mL and 0.0892 mg of dry solids/mL for succinylated chitosan. The chitosan samples had a better antioxidant profile probably because their amino groups were blocked by succinylation.
The peptide fraction had a relatively high antioxidant effect: its IC50 was as high as 0.0260 mg dry solids/mL and 0.0208 mg protein/mL.
Tryptophan had a much higher antioxidant effect than peptides: it increased by 2.2 and 1.8 times, respectively, in terms of nitrogen and solids.
The antioxidant effect of the peptide fraction increased by 2.0 and 1.5 times as a result of interaction with chitosan and succinylated chitosan, respectively. The antioxidant activity of tryptophan increased by 1.7 times after binding with chitosan and by 1.5 times after binding with succinylated chitosan. The chitosan biocomposites of hydrolysate and tryptophan demonstrated a better antioxidant effect. The interaction of peptides and amino acids with succinylated chitosan proved less efficient. Probably, peptides and tryptophan had a better solubility during their biocomposing with chitosan and its derivative, which explains the antioxidant effect.
CONCLUSION
These experiments featured biocomposites of chitosan and succinylated chitosan with whey protein hydrolysate. The spectrophotometry and pH-metric analysis revealed that 1.0 g of chitosan interacted with 13.0 g of hydrolysate and 2.5 g of succinylated chitosan. The complexation of chitosan with whey peptides proved effective. The complexes had better sensory profiles than the original substances. Their bitterness score lost 1–2 points relative to the pure hydrolysate samples.
We also described the complexes of chitosan and succinylated chitosan with such amino acids as arginine, valine, leucine, methionine, and tryptophan. Valine, leucine, and tryptophan interacted with glucosamine residues in chitosan in an equimolar ratio. Succinylated chitosan had a low complexing potential because it contained few free amino groups, which were affected by succinic acid residues introduced into the structure. Arginine demonstrated no complexing with the polysaccharides. Valine, leucine, methionine, and tryptophan were anionic, which allowed them to bind with polycationic chitosan, whereas arginine was cationic. The sensory evaluation revealed that the complexes of chitosan/succinylated chitosan with valine, leucine, methionine, and trypphan had a lower bitterness (minus 1–4 points). The biocomplexes of chitosan/succinylated chitosan with arginine demonstrated no changes in bitterness.
Tryptophan and peptides of whey proteins demonstrated a good antioxidant potential. The antioxidant activity index IC50 was 0.0117 mg tryptophan/mL for the amino acid and 0.0208 mg protein/mL for peptides. The experiments confirmed the antioxidant effect of chitosan (0.0352 mg chitosan/mL) and succinylated chitosan (0.0892 mg chitosan/mL). The antioxidant effect of tryptophan and peptides increased by 1.7 and 2.0 times, respectively, in the chitosan biocomposites and by 1.5 times in the succinylated chitosan biocomposites. Peptides and tryptophan were more effective in their binding with chitosan because they interacted with the amino groups of the polysaccharide, which improved their antioxidant properties.
The biocomposites of chitosan with whey peptides and amino acids had a high antioxidant activity and an improved sensory profile. As a result, they can be used in hypoallergenic functional foods.
Contribution
T. N. Halavach supervised the project, designed the research, collected and interpreted the data, and wrote the manuscript. V. P. Kurchenko, A. D. Lodygin, and I. A. Evdokimov created the research concept and proofread the manuscript. E. I. Tarun, R. V. Romanovich, N. V. Mushkevich, and A. D. Kazimirov were responsible for the experimental part of the study and the methodology.CONFLICTS OF INTEREST
The authors declared no conflict of interest regarding the publication of this article.
FUNDING
This research was supported by the Ministry of Education of the Republic of Belarus as part of the research in Mechanisms of Amino Acids and Peptides Interaction with Chitosan and Its Derivatives, grant no. 20211584.REFERENCES
- Shivanna SK, Nataraj BH. Revisiting therapeutic and toxicological fingerprints of milk-derived bioactive peptides: An overview. Food Bioscience. 2020;38. https://doi.org/10.1016/j.fbio.2020.100771
- Zhao C, Ashaolu TJ. Bioactivity and safety of whey peptides. LWT. 2020;134. https://doi.org/10.1016/j.lwt.2020.109935
- Ye H, Tao X, Zhang W, Chen Y, Yu Q, Xie J. Food-derived bioactive peptides: production, biological activities, opportunities and challenges. Journal of Future Foods. 2022;2(4):294–306. https://doi.org/10.1016/j.jfutfo.2022.08.002
- Nutten S, Schuh S, Dutter T, Heine RG, Kuslys M. Design, quality, safety and efficacy of extensively hydrolyzed formula for management of cow’s milk protein allergy: What are the challenges? Advances in Food and Nutrition Research. 2020;93:147–204. https://doi.org/10.1016/bs.afnr.2020.04.004
- Liceaga AM, Hall F. Nutritional, functional and bioactive protein hydrolysates. In: Melton L, Shahidi F, Varelis P, editors. Encyclopedia of food chemistry. Reference work. Vol. 3. Elsevier; 2019. pp. 456–464. https://doi.org/10.1016/B978-0-08-100596-5.21776-9
- Iwaniak A, Minkiewicz P, Darewicz M, Hrynkiewicz M. Food protein-originating peptides as tastants – Physiological, technological, sensory, and bioinformatic approaches. Food Research International. 2016;89:27–38. https://doi.org/10.1016/j.foodres.2016.08.010
- Murray NM, Jacquie JC, O’Sullivan M, Hallihan A, Murphy E, Feeney EL, et al. Using rejection thresholds to determine acceptability of novel bioactive compounds added to milk-based beverages. Food Quality and Preference. 2019;73:276–283. https://doi.org/10.1016/j.foodqual.2018.10.014
- Gonzalez Pereira A, Carpena M, García Oliveira P, Mejuto JC, Prieto MA, Simal Gandara J. Main applications of cyclodextrins in the food industry as the compounds of choice to form host–guest complexes. International Journal of Molecular Sciences. 2021;22(3). https://doi.org/10.3390/ijms22031339
- Rudolph S, Riedel E, Henle T. Studies on the interaction of the aromatic amino acids tryptophan, tyrosine and phenylalanine as well as tryptophan-containing dipeptides with cyclodextrins. European Food Research and Technology. 2018;244(9):1511–1519. https://doi.org/10.1007/s00217-018-3065-9
- Li J, Geng S, Liu B, Wang H, Liang G. Self-assembled mechanism of hydrophobic amino acids and β-cyclodextrin based on experimental and computational methods. Food Research International. 2018;112:136–142. https://doi.org/10.1016/j.foodres.2018.06.017
- Halavach TM, Savchuk ES, Bobovich AS, Dudchik NV, Tsygankow VG, Tarun EI, et al. Antimutagenic and antibacterial activity of β-cyclodextrin clathrates with extensive hydrolysates of colostrum and whey. Biointerface Research in Applied Chemistry. 2021;11(2):8626–8638. https://doi.org/10.33263/BRIAC112.86268638
- Halavach TM, Kurchenko VP, Tsygankow VG, Bondaruk AM, Tarun EI, Asafov VA. β-Cyclodextrin nanocomplexes with biologically active peptides from hydrolysed bovine whey and colostrum. Biointerface Research in Applied Chemistry. 2022;12(6):8502–8514. https://doi.org/10.33263/BRIAC126.85028514
- Irastorza A, Zarandona I, Andonegi M, Guerrero P, de la Caba K. The versatility of collagen and chitosan: From food to biomedical applications. Food Hydrocolloids. 2021;116. https://doi.org/10.1016/j.foodhyd.2021.106633
- Silva AO, Cunha RS, Hotza D, Francisco Machado RA. Chitosan as a matrix of nanocomposites: A review on nanostructures, processes, properties, and applications. Carbohydrate Polymers. 2021;272. https://doi.org/10.1016/j.carbpol.2021.118472
- Ahmad SI, Ahmad R, Khan MS, Kant R, Shahid S, Gautam L, et al. Chitin and its derivatives: Structural properties and biomedical applications. International Journal of Biological Macromolecules. 2020;164:526–539. https://doi.org/10.1016/j.ijbiomac.2020.07.098
- Wani TU, Pandith AH, Sheikh FA. Polyelectrolytic nature of chitosan: Influence on physicochemical properties and synthesis of nanoparticles. Journal of Drug Delivery Science and Technology. 2021;65. https://doi.org/10.1016/j.jddst.2021.102730
- Benchamas G, Huang G, Huang S, Huang H. Preparation and biological activities of chitosan oligosaccharides. Trends in Food Science and Technology. 2021;107:38–44. https://doi.org/10.1016/j.tifs.2020.11.027
- Lee JS, Nah H, Moon H-J, Lee SJ, Heo DN, Kwon IK. Controllable delivery system: A temperature and pH-responsive injectable hydrogel from succinylated chitosan. Applied Surface Science. 2020;528. https://doi.org/10.1016/j.apsusc.2020.146812
- Deka BC, Bhattacharyya PK. DFT study on host-guest interaction in chitosan–amino acid complexes. Computational and Theoretical Chemistry. 2017;1110:40–49. https://doi.org/10.1016/j.comptc.2017.03.036
- Medeiros Borsagli FGL, Carvalho IC, Mansur HS. Amino acid-grafted and N-acylated chitosan thiomers: Construction of 3D bio-scaffolds for potential cartilage repair applications. International Journal of Biological Macromolecules. 2018;114:270–282. https://doi.org/10.1016/j.ijbiomac.2018.03.133
- Wang S, Shi L, Zhang S, Wang H, Cheng B, Zhuang X, et al. Proton-conducting amino acid-modified chitosan nanofibers for nanocomposite proton exchange membranes. European Polymer Journal. 2019;119:327–334. https://doi.org/10.1016/j.eurpolymj.2019.07.041
- Rafiee F, Rezaie Karder F. Bio-crosslinking of chitosan with oxidized starch, its functionalization with amino acid and magnetization: As a green magnetic support for silver immobilization and its catalytic activity investigation. International Journal of Biological Macromolecules. 2020;146:1124–1132. https://doi.org/10.1016/j.ijbiomac.2019.09.238
- Taketa TB, Mahl CRA, Calais GB, Beppu MM. Amino acid-functionalized chitosan beads for in vitro copper ions uptake in the presence of histidine. International Journal of Biological Macromolecules. 2021;188:421–431. https://doi.org/10.1016/j.ijbiomac.2021.08.017
- Fernandes J, Ghate MV, Basu Mallik S, Lewis SA. Amino acid conjugated chitosan nanoparticles for the brain targeting of a model dipeptidyl peptidase-4 inhibitor. International Journal of Pharmaceutics. 2018;547(1–2):563–571. https://doi.org/10.1016/j.ijpharm.2018.06.031
- Hefni HHH, Nagy M, Azab MM, Hussein MHM. O-Acylation of chitosan by L-arginine to remove the heavy metals and total organic carbon (TOC) from. Egyptian Journal of Petroleum. 2020;29(1):31–38. https://doi.org/10.1016/j.ejpe.2019.10.001
- Wang Y, Han Q, Wang Y, Qin D, Luo Q, Zhang H. Self-assembly, rheological properties and antioxidant activities of chitosan grafted with tryptophan and phenylalanine. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2020;597. https://doi.org/10.1016/j.colsurfa.2020.124763
- Pereira MBB, França DB, Araújo RC, Silva-Filho EC, Rigaud B, Fonseca MG, et al. Amino hydroxyapatite/chitosan hybrids reticulated with glutaraldehyde at different pH values and their use for diclofenac removal. Carbohydrate Polymers. 2020;236. https://doi.org/10.1016/j.carbpol.2020.116036
- Torkaman S, Rahmani H, Ashori A, Najafi SHM. Modification of chitosan using amino acids for wound healing purposes: A review. Carbohydrate Polymers. 2021;258. https://doi.org/10.1016/j.carbpol.2021.117675
- Zhong Y, Shahidi F. Methods for the assessment of antioxidant activity in foods. In: Shahidi F, editor. Handbook of antioxidants for food preservation. A volume in Woodhead Publishing Series in Food Science, Technology and Nutrition. Woodhead Publishing; 2015. pp. 287–333. https://doi.org/10.1016/b978-1-78242-089-7.00012-9
- Tarun EI, Zaitseva MV, Kravtsova OI, Kurchenko VP, Golovach TN. Effect of whey protein peptides on recovery of fluorescence level in system with activated form of oxygen. Proceedings of the Belarusian State University. Series of Physiological, Biochemical and Molecular Biology Sciences. 2016;11(1):231–236. (In Russ.). https://www.elibrary.ru/ymgpfb
- Hu Z, Qin YQ, Guang J, Cai Y. Preparation and characterization of chitosan amino acid salts. IOP Conference Series: Materials Science and Engineering. 2019;504. https://doi.org/10.1088/1757-899X/504/1/012023
- Daroit DJ, Brandelli A. In vivo bioactivities of food protein-derived peptides – a current review. Current Opinion in Food Science. 2021;39:120–129. https://doi.org/10.1016/j.cofs.2021.01.002
- Bielecka M, Cichosz G, Czeczot H. Antioxidant, antimicrobial and anticarcinogenic activities of bovine milk proteins and their hydrolysates – A review. International Dairy Journal. 2022;127. https://doi.org/10.1016/j.idairyj.2021.105208
- Galland F, de Espindola JS, Lopes DS, Taccola MF, Pacheco MTB. Food-derived bioactive peptides: Mechanisms of action underlying inflammation and oxidative stress in the central nervous system. Food Chemistry Advances. 2022;1. https://doi.org/10.1016/j.focha.2022.100087
- Anraku M, Gebicki JM, Iohara D, Tomida H, Uekama K, Maruyama T, et al. Antioxidant activities of chitosans and its derivatives in in vitro and in vivo studies. Carbohydrate Polymers. 2018;199:141–149. https://doi.org/10.1016/j.carbpol.2018.07.016
- Abd El-Hack ME, El-Saadony MT, Shafi ME, Zabermawi NM, Arif M, Batiha GE, et al. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. International Journal of Biological Macromolecules. 2020;164:2726–2744. https://doi.org/10.1016/j.ijbiomac.2020.08.153