Abstract
Sesame (Sesamum indicum L.) is an erect herbaceous annual plant with flat seeds. It is one of the oldest cultivated oilseed plants in the world, especially popular in Africa and Asia.The present research objective was to describe a sesame protein isolate, i.e., its amino acid profile, functional and physicochemical properties, zeta potential, and hydrodynamic diameter. The surface charge and hydrodynamic diameter in aqueous solutions were obtained for standard sesame seeds, defatted sesame seeds, and the sesame protein isolate.
Defatted sesame seeds yielded the following optimal parameters: salt concentration – 0.6 M, pH – 7, iso-electric point (pI) – 4. The sesame protein isolate was rich in methionine content, which is rare in other plant proteins, but its lysine content was lower than in other isolates. The sesame protein isolate displayed almost identical zeta potential profiles with its pH. The decreasing pH increased the zeta values gradually from the lowest negative value to the highest positive value. The zeta potentials of standard and defatted sesame seeds at pH 7 were –23.53 and –17.30, respectively. The hydrodynamic diameter of the sesame protein isolate (0.33 μm) was smaller than that of sesame seeds (2.64 μm) and defatted sesame seeds (3.02 μm). The sesame protein isolate had a water holding capacity of 1.26 g/g and an oil holding capacity of 3.40 g/g. Its emulsifying properties looked as follows: emulsion capacity – 51.32%, emulsion stability – 49.50%, emulsion activity index – 12.86 m2/g, and emulsion stability index – 44.96 min, respectively. These values are suitable for the sesame protein isolate and are consistent with the literature.
The sesame protein isolate was a good source of protein (88.98%). Using sesame proteins as functional components can be an important basis for better knowledge of the relationship between electrical charge interactions in food matrices and the structure, stability, shelf life, texture, structural and functional properties of food. Research prospects include the effects of sesame protein isolates on various food systems.
Keywords
Sesame protein isolate, amino acid profile, zeta potential, functional properties, solubilityINTRODUCTION
Sesame (Sesamum indicum L.) is an erect herbaceous annual plant. Its seeds are considered one of the oldest oilseed plants in human history. For instance, peoples of Africa and Asia have cultivated them for many centuries. Nowadays, sesame seeds and oil are used in food production and different industries. Global sesame production was approximately 2.5 million tons in 1997 and 6.5 million tons in 2019 [1].
Sesame seeds are remarkably rich in oil and protein. They contain 45.46–59.28% oil, 21.43–25.77% protein, 2.70–5.10% moisture, 2.89–5.44% ash, 3.20–7.31% fiber, and 4.33–19.33% carbohydrates [2, 3]. The chemical composition of sesame seeds varies according to many factors, e.g., genetics, climate, environment, growth, maturation stage, harvest time, analytical methods, etc. [4].
Sesame proteins have a high potential to be used as an ingredient in the food industry because they increase the nutritional value of foods. One of the main properties of sesame proteins is that they contain such essential amino acids as tryptophan and methionine. Sesame flour and protein isolates are rich in sulfurcontaining amino acids (3.8–5.5%) with methionine prevailing (2.5–4.0%) [5–7]. Most plant proteins, e.g., soy protein, wheat flour, barley grain, ground rice, cornmeal, etc., contain low sulfur amino acids. Therefore, sesame proteins are unique because they contain high amounts of sulfur amino acids [4]. As a result, sesame proteins can serve as a complementary food to legumes and cereals. The polymeric structure of sesame proteins also improves the textural and rheological properties of the food they complement by increasing the acceptability of the final product [8].
Emulsifying capacity and emulsion stability are essential parameters in selecting a protein for particular industrial processes. Proteins prevent coalescence and reduce tension of the water-oil interface. The stabilizing effect of a protein in an emulsion comes from the membrane matrix that surrounds the oil droplet and prevents it from coalescing [5]. Proteins also have a good oil and water holding capacity, foaming stability, and solubility. The importance of these characteristics depends on the type of food product where the protein isolate is used. For example, high foaming and emulsifying protein isolates are preferred in salad dressings and soups.
In contrast, protein isolates with high water and fat holding capacity are preferred in products that contain meat fat [4]. Onsaard et al. reported that the emulsifying properties and solubility of sesame protein isolates can be used to replace soy protein isolates [9]. Achouri et al. found that sesame protein isolates had lower water retention and oil holding capacities than soy protein isolates [3]. They also proved that the foaming and emulsifying properties of sesame protein isolates are pH-dependent.
Sesame protein isolates or concentrates are easy to obtain by the isoelectric precipitation method. Due to its different functional properties and easy extraction procedures, sesame protein has become a popular commercial protein with a wide range of applications. Sesame protein isolates have a good heat stability and high molecular weight, which makes them suitable for film-forming utilization [10]. However, very few publications feature the zeta potential of sesame protein isolates and the average particle size (hydrodynamic diameter) in an aqueous solution. Most of them focus on the zeta potential and hydrodynamic size measurement of emulsions produced from sesame protein isolates [11, 12].
The industrial role of sesame seeds as a potential source of protein is growing. As a result, composition, physicochemical profile, and functional properties of sesame-isolated proteins are attracting more and more scientific attention. For proper industrial use, isolated proteins need to have a proper effect on specific processed foods. Therefore, food science needs more data on utilization of a specific protein [6]. For instance, very little information is available about how extraction conditions, e.g., salt concentration and pH, affect the extractability of proteins from sesame seeds.
Therefore, the main objectives of this study were as follows: (I) to investigate the extractability of proteins from sesame seeds in water and at different NaCl concentrations (0, 0.3, 0.6, 0.9, and 1.2 M) as a function of pH; (II) to describe the sesame protein isolate and to examine some of its functional properties; (III) to investigate the zeta potential and particle size of the sesame protein isolate in aqueous solution, (IV) to examine the amino acid profile of the sesame protein isolate.
STUDY OBJECTS AND METHODS
Materials and chemicals. The sesame (Sesamum indicum L.) seeds were supplied by Pro Gıda, Olam Group Co (Samsun, Turkey). The corn oil was purchased from a local market (Samsun, Turkey). The diethyl ether and sodium hydroxide (NaOH) were obtained from Isolab (Wertheim, Germany). All other chemicals and standards were obtained from Merck (Darmstadt, Germany) and Sigma-Aldrich (USA). A Milli-Q water purification system (Millipore, Massachusetts, USA) was used to obtain deionized water.
Optimal pH, NaCl concentration, and iso-electric point for protein extractability. Sesame seeds were milled in a kitchen blender (AR1133, Arzum, Turkey) and defatted by constant stirring with diethyl ether (1:4 w/v, sesame/solvent) at room temperature for 3 h. The resulting mix was defatted four more times using the same sesame/solvent proportion. After that, the defatted sesame was filtered using filter paper and dried at 40°C overnight [9]. After drying, the defatted sesame seeds were used for protein extraction.
In order to find the optimal conditions for protein isolation, we determined the optimal salt concentration and pH by modifying the method described by Achouri et al. in [3]. Dispersions of defatted sesame seeds (10% w/v) were prepared using different concentrations of sodium chloride (0, 0.3, 0.6, 0.9, and 1.2 M) and shaken at 300 pm at 35°C for 2 h. The dispersions were divided into test tubes by approximately 10 mL. There, their pH was adjusted from 2 to 11 (in 1 unit increments) using 1N HCl or 1N NaOH. The dispersions were continuously stirred at room temperature for 2 h, and their pH was kept at the desired level (2–11). The samples were sonicated for 30 min and centrifuged at 2060×g for 30 min (NF 1200R, Nuve, Ankara, Turkey).
Then, 0.1 mL was taken from the supernatant and added to 3 mL of Bradford’s reagent. After 5 min, the absorbance of the mix was registered using a T80+ spectrophotometer (PG Instruments Limited, Leicestershire, UK) at 595 nm wavelength. The pH and salt concentration of the samples with the highest protein content were chosen as optimal conditions to maximize protein isolation.
The iso-electric point (pI) precipitation method was used for protein isolation. The most appropriate pI was determined by the method described by Yuzer et al. in [13]. First, a 10% sample dispersion was prepared with the optimal concentration of NaCl solution and adjusted to the optimal pH value for extraction. The dispersion was shaken at 300 rpm for 2 h. After shaking, 10 mL of the liquid part from each sample was poured into six centrifuge tubes. Their pH values were adjusted between 3.5–6.0 with a 0.5-unit change and vortexed for 1 min in a WN-2800 Vortex Mixer (Weightlab Instruments, Istanbul, Turkey). The samples were centrifuged at 3000×g 4°C for 20 min. The protein amount in the supernatants was determined using the Bradford method. The pH value with the lowest protein amount was determined as the iso-electric point of the sample.
Preparation of the sesame protein isolate. The optimal salt concentration and pH values were determined as 0.6 and 7 M, respectively (Fig. 1), to produce a 1:10 (w/v) sample suspension. The suspension was stirred at 35°C at 300 rpm for 2 h. The supernatant portion containing the soluble proteins was obtained by filtration. In order to increase the protein amount and yield, we mixed the remaining solid using the same salt solution and pH and removed the supernatant by filtration.
Afterward, the pH values of the samples were adjusted to the isoelectric point (pH 4, Fig. 1). The proteins were allowed to precipitate and kept at +4°C for 18 h. At the end of the period, the supernatant was removed by centrifugation at 3000×g at 4°C for 30 min to recover the precipitated proteins. The resulting precipitates were washed with distilled water. The pH was then neutralized to 7. The protein isolates were dried with a lyophilizer at –105°C and 100 mbar (ScanVac Coolsafe 110-4 Pro, Labogene, Lynge, Denmark). The resulting sesame protein isolate was stored at –20°C. The protein yield, %, was calculated as follows [3]:
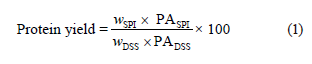
where SPI is the sesame protein isolate; DSS is the defatted sesame seeds; w is the weight, g; PA is the protein amount, %.
Proximate composition and pH analysis. Standard sesame seeds, defatted sesame seeds, and sesame protein isolates were tested for moisture, crude protein (N×6.25), ash, and crude fat according to the AOAC methods [14]. The pH values were determined by a Starter 2100 pH-meter (OHAUS, USA) as follows: 10% (w/v) dispersions of each sample were tested in triplicates.
Zeta potential and hydrodynamic size. The hydrodynamic size (μm) of the samples was determined using a Malvern Zetasizer Nano Zetasizer (Malvern Instruments, Malvern, UK). The device employed the dynamic light scattering principle with refractive indices 1.330 and 1.476 for water and sesame, respectively. The zeta potential (mV) was measured with the same equipment based on the electrophoretic action of protein solutions. We prepared 0.05% (w/v) protein solutions of the sesame protein isolate with distilled water. Their pH was adjusted from 2 to 11 with 1N HCl or 1N NaOH. For standard sesame seeds and defatted sesame seeds, the sample dispersions contained 0.1% (w/v) deionized water. Their pH was adjusted to 7 with 1N HCl or 1N NaOH. Each sample was homogenized and measured in triplicates.
Amino acid analysis. The amino acid analysis of the sesame protein isolate relied on a liquid chromatography tandem mass spectrometry (LC-MS/MS). The Agilent Infinity 1260 HPLC system consisted of a binary pump, a degasser, and an autosampler coupled with an Agilent 6460 Triple Quad Mass Spectrometer (Agilent Technologies, CA, USA) using the method described by Bilgin et al. in [15]. We used a Jasem LC-MS/MS amino acid analysis kit (Sem Laboratories Devices Pazarlama San. ve Tic. Inc., Istanbul, Turkey) with a modified sample preparation procedure to measure amino acid concentrations.
The samples were hydrolyzed as follows. We placed 0.1 g of sample in a screw-cap glass tube, added 4 mL of acidic hydrolysis reagent, and hydrolyzed it at 110°C for 24 h. When the hydrolysate was cooled to room temperature, it was centrifuged at 4000 rpm for 5 min. Then, 100 μL of supernatant was poured into a vial with 900 μL of distilled water. This dilution process was repeated once more to obtain a 4000-fold diluted hydrolysate of the sample. Following the hydrolysis process, the kit sample preparation was applied as follows. We poured 50 μL of diluted hydrolysate into a vial and added 50 μL of the stable isotope-labeled internal standard mix and 700 μL of reagent 1. The mix was vortexed for 5 s. After all the samples underwent the same procedure, they were injected into the LCMS/ MS system. The calibration curve required for quantifying amino acids was obtained by preparing the five-point calibration set in accordance with the kit sample preparation without hydrolysis and reading in the LC-MS/MS system.
We injected 3 μL of the prepared sample into the Jasem amino acid analytical column set at 30°C in HPLC. The chromatographic separation analysis took 7.5 min with mobile phase A and B gradient programmed for 0.7 mL/min. The tandem electrospray ionization mass spectrometer performed mass spectrometric detection in the positive ionization mode. The mass detector parameters were as follows: gas temperature – 150°C, gas flow – 10 L/min, capillary voltage – +2000 V, and nebulizer pressure – 40 psi.
Scanning electron microscopy (SEM). The surface morphology of the sesame protein was determined by a scanning electron microscope (SEM, JSM-700 LF JEOL, Japan) at a voltage acceleration of 10 kV. In order to increase the electrical conductivity of the samples and obtain a clearer image, the surface of the samples was coated with a 10-nm gold-palladium alloy (Quorum SC7620, UK) before measurement. The scanning electron microscopy images had three different magnifications: 5000×, 10 000×, and 30 000×.
X-ray diffraction (XRD). The X-ray diffraction analysis (Rigaku Smartlab, Germany) was performed to investigate the crystallinity of the samples. The analysis conditions were as follows: room temperature, 2 θ from 5 to 40°, scanning rate – 1°/min, step size – 0.01°, power – 40 kV, Cu Kα radiation – 30 mA [11].
Fourier transform infrared spectroscopy (FTIR). Fourier transformed infrared spectroscopy determined the functional groups of the samples and their interactions. The secondary structure of sesame protein isolates was determined using a Platinum ATR equipped with a FTIR Tensor 27 spectrophotometer (Bruker Optics Inc., USA). The measurements were made at the wavelength range between 400 and 4000 cm–1.
Differential scanning calorimetry (DSC). The thermal properties of the samples were studied by differential scanning calorimetry (DSCQ2000, TA Instruments, USA). Approximately 10 mg of each sample was hermetically sealed in aluminum containers. The samples were then analyzed under the following conditions: nitrogen atmosphere, flow rate – 30 mL/min, heating rate – 10°C/min between 25 and 400°C [16]. An empty aluminum pan served as a reference. The initial temperature (T0), peak temperature (Tр), and reaction enthalpy (ΔH) of the samples were determined using Universal Analysis 2000, version 4.5A software (TA Instruments, USA).
Thermogravimetric analysis (TGA). The thermal stability of the samples was evaluated using thermogravimetry (Q600 SDT, TA Instruments, USA). Approximately 10 mg of each sample in platinum cups was studied under the following conditions: nitrogen atmosphere, heating rate – 10°C/min between 30 and 600°C, flow rate – 50 mL/min. An empty platinum capsule served as a reference [17]. The results were evaluated using Universal Analysis 2000 software 4.5A (TA Instruments, USA).
Solubility. Solubility percentage was determined as a function of pH (2–11). We prepared 0.5% (w/v) protein dispersions of the sesame protein isolate with deionized water. The pH of the dispersions was adjusted 2 to 11 using 1N HCl or 1N NaOH. The suspensions were stirred in a magnetic stirrer for 1 h at ambient temperature. Then, the suspensions were centrifuged at 3000×g at room temperature for 30 min [18]. After centrifugation, we performed the protein analysis in the supernatant using the Bradford method. The bovine serum albumin standard curve was used to calculate the amount of protein. The solubility, %, was found according to Eq. (2):
![]()
Water and oil holding capacities. Approximately 50 mg of the protein isolate was dispersed in a 2-mL centrifuge tube by adding 1.5 mL of distilled water or commercial corn oil and mixed with a vortex at room temperature for 20 s. After mixing, the tubes were capped and held at room temperature for 30 min. The tubes were centrifuged at 14 000 rpm for 20 min. After the supernatant was carefully decanted at a 45° angle, we weighed the centrifuge tube with the sediment. The water or oil retained content was determined by weighing the tubes with the sediment. The water (WHC) and oil (OHC) holding capacities were expressed as 1 g of water or oil absorbed per 1 g of the sesame protein isolate, respectively. They were found according to Eq. (3):
![]()
where M0 is the weight of the sample; M1 is the weight of the centrifuge tube; M2 is the weight of the centrifuge tube after the oil or water has been removed [18].
Emulsifying properties. We diluted 0.25 g of the protein isolate with 5 mL of distilled water. The pH value was adjusted to 7.0 using 1N HCl or 1N NaOH. After that, we added 5 mL of corn oil to the protein solution and mixed using a vortex for 1 min. Afterward, the emulsion was centrifuged at 1100×g for 5 min, and the emulsion capacity (EC, %) was determined as in Eq. 4. To define the emulsion stability, the samples were heated in a water bath at 80°C for 30 min and cooled rapidly under running water and ice. The samples were again centrifuged at 2291×g for 5 min, and emulsion stability (ES, %) was calculated as in Eq. (5) [19]:

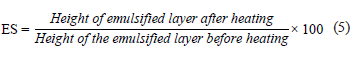
The emulsion activity index and emulsion stability index were determined according to the method developed by You et al. [20]. We mixed 300 mg of the protein isolate sample with 30 mL of purified water (1% protein dispersion) and then added 10 mL of corn oil. The pH of the mix was adjusted to 7 with 1N HCl or 1N NaOH. The mix went through a homogenizer (Ultra-Turrax T25 digital, IKA, Staufen, Germany) at 20 000 rpm for 1 min. Immediately after the emulsion was formed, 50 μL of emulsion sample from its lower part (liquid phase) was put into a tube and diluted by adding 5 mL of a 0.1% (w/v) sodium dodecyl sulfate (SDS) solution. The absorbance of the resulting mix at 500 nm was measured in the spectrophotometer. The emulsion activity index (EAI, m2/g) was calculated as in Eq. (6):
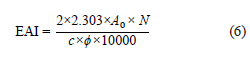
where, A0 is the absorbance at 0 min; N is the dilution factor (100); c is the concentration of the protein dispersion (0.01 g/mL); ϕ is the volumetric fraction of the oil (10/40 = 0.25).
After 10 min, 50 μL of the emulsion was taken from its lower part (liquid phase) and diluted with 5 mL of a 0.1% (w/v) sodium dodecyl sulfate solution. The absorbance registered at 500 nm was used in Eq. (7) to calculate the emulsion stability index (ESI, min):
![]()
where, A10 is the absorbance at 10 min after the homogenization process; t is the holding time of the emulsion (10 min).
Foaming properties. We diluted 3% (w/v) dispersions of the sample with distilled water and adjusted to pH 7 using 1 N HCl or 1 N NaOH. Then the mix was homogenized at 11 000 rpm for 2 min using an Ultra- Turrax homogenizer. The mix was immediately transferred to a 100-mL graduated cylinder. We recorded the total volumes, liquid volumes, and volumes of the remaining foams after 10 and 30 min of storage at room temperature. The foaming capacity (FC, %) and foaming stability (FS, %) were calculated using Eqs. (8 ) and (9) [18]:
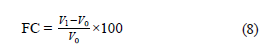
![]()
where V1 is the volume of foam instantly after homogenization; V0 is the volume before homogenization; V2 is the volume of foam remaining after 10 and 30 min at room temperature.
Statistical analysis. We used SPSS Statistics 26.0 (IBM, New York, USA) to evaluate the data and Duncan’s multiple comparison test to evaluate significant (p < 0.05) differences.
RESULTS AND DISCUSSION
Proximate composition and pH analysis. Table 1 illustrates the proximate composition of standard sesame (Sesamum indicum L.) seeds, defatted sesame seeds, sesame protein isolate, and protein extraction yield. Defatted sesame seeds had a greater content of ash and protein. The protein content rose from 25.55% in the standard sesame seeds to 57.37% in the defatted sesame seeds. Probably, the ash content increased as a result of the proportional decrease in the amount of crude fat. The crude fat content of the sesame seeds decreased because it was affected, first, by the solvent during the defatting process and, second, by the further separation of oil during centrifugation. As a result, the crude fat content of the standard sesame seeds, defatted sesame seeds, and sesame protein isolate were 56.83, 6.64, and 0.82%, respectively. The moisture content of the samples varied between 1.26 and 2.68%. These results confirmed those reported in [2, 3, 21]. The protein content of the sesame protein isolate was 88.98%. It was slightly lower than 90.50% achieved by Sharma et al., but higher than 86.33% obtained by Fathi et al. and 81.66% obtained by Saatchi et al. [6, 10, 12]. The protein extraction yield was determined as 43.76% according to the extraction conditions used in this study.
The pH values of the standard sesame seeds, defatted sesame seeds, and sesame protein isolates were 6.41, 6.23, and 6.98, respectively (Table 1). An insignificant change in pH occurred between standard and defatted sesame seeds (p > 0.05), followed by an increase in the sesame protein isolates as a result of the neutralization in the last stages of isolate production. Unfortunately, very few publications are available on the pH of sesame and its products.
Zeta potential and hydrodynamic size. Food systems include electrically charged particles that interact with the environment and with each other. Electric charge interactions significantly affect food structure, stability, rheological behavior, precipitation, texture, color, shelf life, and flavor. The zeta potential (ζ) is one of the most practical characteristics in investigating electrical relations in food matrices. Functional characteristics of proteins are affected by their foaming and emulsifying capacity, solubility, gelation, surface activity, conformational stability, and interaction with polysaccharides. The zeta potential is important for determining the aggregation and interactions of compounds [22].
Figure 2 shows the zeta potential of the sesame protein isolates at pH 2–11. The aqueous solution of the sesame protein isolate had a neutral charge at pH 4–5. This range included the iso-electric point pH 4 determined for precipitation during the isolation process. The sesame protein isolate displayed almost identical zeta potential profiles with its pH. As the pH increased from 2 to 11, the zeta values gradually changed from the highest (positive) value to the lowest (negative) value. This result was consistent with the fact that the surface charge can change gradually from negative to positive due to the gradual protonation of carboxyl groups and deprotonation of amino groups of proteins [23]. The zeta potential of standard and defatted sesame seeds at pH 7 were –23.53 and –17.30 mV, respectively (Table 1). We found no previous publications on the surface charge in aqueous solutions of standard and defatted sesame seeds, which makes this research relevant. The obtained data revealed how electrical charge interactions in sesame seeds and sesame-containing food components may affect the structure, stability, shelf life, and texture of the finished product.
Table 1 illustrates the hydrodynamic dimension measurements for the standard sesame seeds, defatted sesame seeds, and sesame protein isolates. The functionality and emulsifying properties of proteins depend on their molecular size [24]. The hydrodynamic diameter of the sesame protein isolates (0.33 μm) was smaller than that of standard sesame seeds (2.64 μm) and defatted sesame seeds (3.02 μm). Ordinary and defatted sesame seeds may have formed larger aggregates through strong hydrophobic interactions in water. These values were similar to those reported in previous scientific publications. For instance, Rahmati et al. determined the hydrodynamic dimensions between 100–200 nm at pH 7 in bean protein isolates [24]. Mozafarpour et al. reported the hydrodynamic radius of soy protein isolates as 0.32– 0.68 μm [25].
Amino acid composition. Table 2 shows the amino acid composition of the sesame protein isolates. Glutamic acid, arginine, and aspartic acid were 17.78, 11.86, and 6.92 g/100 g, respectively. They proved to be the predominant amino acids in the isolate, which confirmed other sesame protein studies [11, 26–29]. Leucine (5.68 g/100 g) appeared to be the most abundant essential amino acid, which approximated the results reported by Yang et al. and Lawal et al. [27, 28]. The sesame protein isolates also contained sulfurous amino acids, primarily methionine, which are rarely found in other plant proteins. However, methionine was more abundant than cysteine, which was also observed by Saatchi et al. and Fasuan et al. [11, 26].
The sesame protein isolates owed their acidic quality to their high acidic/basic amino acid ratio. The fact that the iso-electric point of the protein is in the acidic pH region can be attributed to the high amount of acidic amino acids, e.g., glutamic and aspartic acids, in its content (Figs. 2 and 5).
The sesame protein isolate was richer in such essential amino acids as phenylalanine, methionine, threonine, valine, and histidine than hemp, soy, and pea proteins reported by Gorissen et al. [30]. They also had more leucine and isoleucine than hemp and soy proteins but less than pea protein. However, the sesame protein isolates were insufficient in lysine compared to soy and pea proteins.
Scanning electron microscopy. The structural morphology of the sesame protein isolate was examined with the aid of scanning electron microscopy at three different magnifications (Fig. 3). The particles of the sesame protein isolate powder were irregular in shape and not uniform. The surface was wrinkled, and the particles were agglomerated. Saini et al. also reported that the alkali extraction and iso-electric precipitation technique applied to sesame protein isolates transformed the microstructure of the protein into a compact structure with a wrinkled surfacee [7].
X-ray diffraction (XRD). Figure 4 shows the crystal structure of the sesame protein isolate obtained by X-ray diffraction. The image revealed a broad and diffuse background at 19.5°, which means that the major structure of the isolate was amorphous. Other sharp peaks ( 2θ = 2 7.3° and 31.6°) could be attributed to minerals or other compounds remaining after protein isolation. These results were consistent with those reported in previous publications [7, 11, 31, 32].
Fourier transform infrared spectroscopy (FTIR). Figure 5 shows the spectra demonstrated by the Fourier transform infrared spectroscopy. The obtained spectra made it possible to determine the functional groups. The sesame protein isolate showed some typical features of the protein spectrum, namely Amide I (1642 cm–1), Amid II (1516 cm–1), and Amid III (1238 and 1396 cm–1) that result from the stretching and bending vibrations, typical of the protein backbone [12, 27, 33]. The characteristic peaks in Amid I and Amid II were predominantly due to N-H stretching vibrations. In Amid III, they were attributed to C-N stretching and N-H bending vibrations [12, 27]. In particular, the density of the Amid I group was stronger than that of Amid II and Amid III. Saatchi et al. observed the Amid I, Amid II, and Amid III bands of sesame protein concentrate at 1635, 1518, and 1234 cm–1, respectively [12]. These results are very similar to the Fourier transform infrared spectroscopy results obtained for the sesame protein isolate in the current study. Our results were also compatible with other studies [7, 27].
Differential scanning calorimetry (DSC). Differential scanning calorimetry is a convenient method to evaluate protein thermal stability and conformational changes. Figure 6 shows differential scanning calorimetry results for the sesame protein isolate. In our study, the thermograms exhibited endothermic peaks, which are usually attributed to protein denaturation [18].
For the sesame protein isolate, T0 started at 88.12°C, and peak temperature Tp was at 122.56°C, while the ΔH value was 28.47 J/g. The amorphous fractions of the protein formed a glass transition (Tg), and the Tg value was 85.56°C. Available publications on the thermal stability of sesame proteins give a wide range of results. Sharma and Singh determined the Tg value of the sesame protein isolate as 37°C, T0 as 98.25°C, and Tp as 207°C [32]. Saini et al. reported T0 as 176.07°C, Tp as 210.78°C, and ΔH as 63.72 J/g [7]. They attributed the high denaturation temperature to higher non-polar residues in the proteins. However, Saatchi et al. reported the Tg temperature of the sesame protein concentrates as 100.54°C and the ΔH value as 51.10 J/g [11]. Yang et al. determined T0 as 55.55–61.26°C, Tp as 112.39–113.69°C, and ΔH as 203.4–220.8 J/g [27].
Thermogravimetric analysis (TGA). Thermogravimetry presents the change in mass with progression in temperature and, therefore, can determine the physical and chemical structural changes during the thermal conversion of biomass to food products [27, 34]. Table 3 describes the thermogravimetric analysis while Fig. 7 illustrates the thermogram of the sesame protein isolate. The samples exhibited weight loss in two stages. A slight weight loss was observed in the samples at temperatures below 100°C, probably, due to water removal. The second and significant primary decrease in the mass of the samples was observed in the range of 250–450°C.
For the sesame protein isolate, the first degradation started at 31.71°C with a weight loss of 3.80%, while the second degradation started at 203.81°C with a weight loss of 62.56%. The weight loss below 100°C could be attributed to the water loss and the decomposition of the quaternary structure of proteins. Temperatures above 100°C are known to denature protein subunits and promote protein aggregate formation through electrostatic, hydrophobic, and disulfide exchange binding mechanisms [35]. At 203°C, the weight loss resulted mainly from the cleavage of the covalent bond between the peptide bonds of amino acids. Further heating caused dissociation of α-globulins (2S), β-globulins (11S), and the secondary structure opening in the subunit.
The sesame protein isolate was rich in sulfurcontaining amino acids methionine and cysteine, which decomposed between 220 and 250°C. The weight loss continued at around 300°C, probably, due to the splitting of S–S, O–N, and O–O junctions [7]. Our thermogravimetry results were consistent with those obtained by Saini et al., Yang et al., and Sharma and Singh [7, 27, 32]. Yang et al. reported a two-stage weight loss [27]. They attributed the first weight loss to water evaporation at 59.02°C, while the second weight loss, which occurred at 182.58°C, was probably caused by protein weight loss.
Solubility. Figure 8 shows the protein solubility profile for the sesame protein isolate. The pH-solubility profile was similar for all proteins, and the solubility in water had a typical U-shaped curve. The sesame protein isolate showed minimal solubility in the isoelectric point region (pH 4–5, Fig. 2). The low protein solubility in the region close to the iso-electric point (pI) values could be attributed to the low net charge in this region. The solubility increased as the pH moved away from the isoelectric point. At pH = 2 and 3, the solubility for the sesame protein isolate was at its highest: 41.11 and 36.99%, respectively. The lowest solubility value of 6.87% was registered at pH 4. These results were consistent with those reported by Achouri et al. [3]. The solubility remained low at pH 5–10 because high salt concentration (0.6 M NaCl) during protein isolation might contribute to the adsorption of chloride ions by proteins, thus reducing repulsive interactions between protein molecules [3].
Water and oil holding capacities. The water holding capacity of the sesame protein isolate was 1.26 g/g, and the oil holding capacity was 3.40 g/g. The oil holding capacity exceeded the water holding capacity probably because the solubility of the sesame protein isolate was low at neutral pH (10.28%, Fig. 8). Similarly, Gundogan and Can Karaca reported that the water and oil holding capacities of bean protein isolates were between 1.8–2.1 and 4.0–5.4 g/g, respectively [18]. They also reported that protein isolates with a high oil holding capacity could serve as food components to improve such properties as content, consistency, and viscosity in many products.
Emulsifying properties. Table 4 shows such emulsifying properties of sesame protein as emulsion capacity, emulsion stability, emulsion activity index, and emulsion stability index. The emulsion capacity and stability were 51.32 and 49.50%, respectively. Cano- Medina et al. reported the highest emulsion capacity values of sesame protein concentrates as 38.0% and the lowest as 19.2% [5]. In the same study, they reported the highest emulsion stability as 50.6% and the lowest as 35.6%. Sharma et al. determined the emulsifying activity of sesame protein isolates as 8.70% at pH 9 and 28.96% at pH 12 [6]. Apparently, the differences in the extraction methods and conditions of sesame proteins affect the emulsifying properties.
The emulsion activity index measures the ability of the protein to provide a rapid and adequate coating of the interface area to prevent immediate association and aid in the dispersion of the fat phase [9]. In our study, the emulsion activity index of the sesame protein isolates was 12.86 m2/g. Onsaard et al. reported a similar emulsion activity index (14.95 m2/g) for sesame protein concentrates extracted at pH 9 [9]. However, they had a higher emulsion activity index for the samples extracted at pH 11 and in salt conditions: 96.66 and 49.70 m2/g, respectively. Still, our results were consistent with those reported in other studies [3, 26].
Emulsion stability is a time-related property. Interactions of proteins in the oil and aqueous phase affect the stability of the protein film formed at the emulsion interface [9]. Emulsion stability index was 44.96 min, which coincided with the results reported in [9, 26].
However, sesame protein had lower emulsion activity and stability indices compared to other protein isolates, e.g., beans and soy [9, 18]. This result can be explained by the relatively low solubility of sesame protein, compared to that of proteins from other sources.
Foaming properties. Table 4 shows the foaming capacity and stability of the sesame protein isolates at pH 7. The foaming capacity and stability after 10 min were 36.21 and 22.79%, respectively. The foaming stability after 30 min was 20.26%. Good foamability can be associated with flexible protein molecules that reduce surface tension. In contrast, low foaming may be associated with highly regular globular proteins that resist surface denaturation [9]. Sesame flour has the following protein content: 67.3% globulin, 8.6% albumin, 6.9% glutelin, and 1.4% prolamine. The alkaline protein isolate extracted in water at pH 10 and precipitated at pH 4.0 was reported to contain 41.3% albumin, 41.0% glutelin, 14.8% globulin, and 0.8% prolamin [4]. However, an excess of globular proteins can be cited among the reasons for low foaming capacity and stability of the sesame protein isolates. Previous studies also reported relatively low foaming capacity and stability values of sesame protein isolates and concentrates [6, 9, 26].
CONCLUSION
This study featured sesame (Sesamum indicum L.) protein isolates produced from defatted sesame seeds, as well as various properties of standard sesame seeds, defatted sesame seeds, and sesame protein isolates. The extractability of sesame proteins proved sensitive to pH more than to pH 4 and correlated with the increasing NaCl content. The sesame protein isolate appeared to be a good source of protein (88.98%). The sesame protein isolates contained sulfurous amino acids, e.g., methionine (2.78 g/100 g), which is rarely found in plant proteins.
The study is novel in that it reports the zeta potential and hydrodynamic diameter of the sesame protein isolate. The solubility was found to correlate with the zeta potential. Thus, sesame proteins can serve as prospective functional components. They can cast more light on the relationship between the electrical charge interactions in food matrices and the structure, stability, shelf life, texture, and functions of food.
More data on how these proteins interact with each other and behave in foods can help develop appropriate methods that could detect the effects of sesame protein isolates in foods. In this regard, the behavior of sesame protein isolates in various food systems seems a promising research direction. Our next step will be to study the sesame protein isolate we produced and described in this article in various food systems. We also plan to concentrate on the interactions between sesame paste and sesame isolates, salt concentration, and pH conditions.
Contribution
The authors were equally involved in writing the manuscript and are equally responsible for plagiarism. All authors of this study have read the manuscript and agreed to publish it.CONFLICTS OF INTEREST
The authors declare no conflict of interests regarding the publication of this article.ACKNOWLEDGEMENTS
The authors are also thankful to Pro Gıda, Olam Group Co. and Mr. Tufan Osman Yayla for supporting this study.FUNDING
The present research was part of Mustafa O. Yuzer’s Ph.D. thesis supported by Ondokuz Mayis University (OMÜ) Research Foundation (Project No. PYO.MUH.1904.20.012), Turkey.REFERENCES
- FAOSTAT [Internet]. [cited 2021 Sep 07]. Available from: https://www.fao.org/faostat/en/#home
- Fasuan TO, Omobuwajo TO, Gbadamosi SO. Optimization of simultaneous recovery of oil and protein from sesame (Sesamum indicum) seed. Journal of Food Processing and Preservation. 2018;42(1). https://doi.org/10.1111/jfpp.13341
- Achouri A, Nail V, Boye JI. Sesame protein isolate: Fractionation, secondary structure and functional properties. Food Research International. 2012;46(1):360–369. https://doi.org/10.1016/j.foodres.2012.01.001
- Onsaard E. Sesame proteins. International Food Research Journal. 2012;19(4):1287–1295.
- Cano-Medina A, Jiménez-Islas H, Dendooven L, Herrera RP, González-Alatorre G, Escamilla-Silva EM. Emulsifying and foaming capacity and emulsion and foam stability of sesame protein concentrates. Food Research International. 2011;44(3):684–692. https://doi.org/10.1016/j.foodres.2010.12.015
- Sharma L, Singh C, Sharma HK. Assessment of functionality of sesame meal and sesame protein isolate from Indian cultivar. Journal of Food Measurement and Characterization. 2016;10(3):520–526. https://doi.org/10.1007/s11694-016-9330-3
- Saini CS, Sharma HK, Sharma L. Thermal, structural and rheological characterization of protein isolate from sesame meal. Journal of Food Measurement and Characterization. 2018;12(1):426–432. https://doi.org/10.1007/s11694-017-9655-6
- Gómez-Arellano A, Jiménez-Islas H, Castrejón-González EO, Medina-Torres L, Dendooven L, Escamilla-Silva EM. Rheological behaviour of sesame (Sesamum indicum L.) protein dispersions. Food and Bioproducts Processing. 2017;106:201–208. https://doi.org/10.1016/j.fbp.2017.09.010
- Onsaard E, Pomsamud P, Audtum P. Functional properties of sesame protein concentrates from sesame meal. Asian Journal of Food and Agro-Industry. 2010;3(4):420–431.
- Fathi N, Almasi H, Pirouzifard MK. Effect of ultraviolet radiation on morphological and physicochemical properties of sesame protein isolate based edible films. Food Hydrocolloids. 2018;85:136–143. https://doi.org/10.1016/j.foodhyd.2018.07.018
- Saatchi A, Kiani H, Labbafi M. Structural characteristics and functional properties of sesame protein concentrate – maltodextrin conjugates. Journal of Food Measurement and Characterization. 2021;15(1):457–465. https://doi.org/10.1007/s11694-020-00655-2
- Saatchi A, Kiani H, Labbafi M. A new functional protein-polysaccharide conjugate based on protein concentrate from sesame processing by-products: Functional and physico-chemical properties. International Journal of Biological Macromolecules. 2019;122:659–666. https://doi.org/10.1016/j.ijbiomac.2018.10.122
- Yuzer MO. Obtaining isolate from sesame proteins and production of nanofibers by electrospinning method: Its effects on oil separation in sesame paste. Ondokuz Mayıs University; 2021.
- Official methods of analysisTM, 21st edition. Arlington: Association of Official Analytical Chemists; 2019.
- Bilgin Ö, Çarlı U, Erdogan S, Maviş ME, Göksu Gürsu G, Yılmaz M. Determination of amino acids composition in different tissues of whiting, Merlangus merlangus euxinus (Nordmann, 1840) from the Black Sea, Turkey. Alinteri Journal of Agriculture Sciences. 2019;34(2):142–147. https://doi.org/10.28955/alinterizbd.665228
- Wang H, Hao L, Wang P, Chen M, Jiang S, Jiang S. Release kinetics and antibacterial activity of curcumin loaded zein fibers. Food Hydrocolloids. 2017;63:437–446. https://doi.org/10.1016/j.foodhyd.2016.09.028
- El Halal SLM, Fonseca LM, do Evangelho JA, Bruni GP, dos Santos Hackbart HC, da Rosa Zavareze E, et al. Electrospun ultrafine fibers from black bean protein concentrates and polyvinyl alcohol. Food Biophysics. 2019;14(4):446–455. https://doi.org/10.1007/s11483-019-09594-y
- Gundogan R, Can Karaca A. Physicochemical and functional properties of proteins isolated from local beans of Turkey. LWT. 2020;130. https://doi.org/10.1016/j.lwt.2020.109609
- Ngui SP, Nyobe CE, Bakwo Bassogog CB, Nchuaji Tang E, Minka SR, Mune Mune MA. Influence of pH and temperature on the physicochemical and functional properties of Bambara bean protein isolate. Heliyon. 2021;7(8). https://doi.org/10.1016/j.heliyon.2021.e07824
- You Y, Yang L, Chen H, Xiong L, Yang F. Effects of (−)-epigallocatechin-3-gallate on the functional and structural properties of soybean protein isolate. Journal of Agricultural and Food Chemistry. 2021;69(7):2306–2315. https://doi.org/10.1021/acs.jafc.0c07337
- Gharby S, Harhar H, Bouzoubaa Z, Asdadi A, El Yadini A, Charrouf Z. Chemical characterization and oxidative stability of seeds and oil of sesame grown in Morocco. Journal of the Saudi Society of Agricultural Sciences. 2017;16(2):105–111. https://doi.org/10.1016/j.jssas.2015.03.004
- Cano-Sarmiento C, Téllez-Medina DI, Viveros-Contreras R, Cornejo-Mazón M, Figueroa-Hernández CY, García-Armenta E, et al. Zeta potential of food matrices. Food Engineering Reviews. 2018;10(3):113–138. https://doi.org/10.1007/s12393-018-9176-z
- Tang C-H, Sun X. A comparative study of physicochemical and conformational properties in three vicilins from Phaseolus legumes: Implications for the structure – function relationship. Food Hydrocolloids. 2011;25(3):315–324. https://doi.org/10.1016/j.foodhyd.2010.06.009
- Rahmati NF, Koocheki A, Varidi M, Kadkhodaee R. Introducing Speckled sugar bean (Phaseolus vulgaris) protein isolates as a new source of emulsifying agent. Food Hydrocolloids. 2018;79:498–508. https://doi.org/10.1016/j.foodhyd.2018.01.022
- Mozafarpour R, Koocheki A, Milani E, Varidi M. Extruded soy protein as a novel emulsifier: Structure, interfacial activity and emulsifying property. Food Hydrocolloids. 2019;93:361–373. https://doi.org/10.1016/j.foodhyd.2019.02.036
- Fasuan TO, Gbadamosi SO, Omobuwajo TO. Characterization of protein isolate from Sesamum indicum seed: In vitro protein digestibility, amino acid profile, and some functional properties. Food Science and Nutrition. 2018;6(6):1715–1723. https://doi.org/10.1002/fsn3.743
- Yang K, Xu T-R, Fu Y-H, Cai M, Xia Q-L, Guan R-F, et al. Effects of ultrasonic pre-treatment on physicochemical properties of proteins extracted from cold-pressed sesame cake. Food Research International. 2021;139. https://doi.org/10.1016/j.foodres.2020.109907
- Lawal SO, Idowu AO, Malomo SA, Badejo AA, Fagbemi TN. Effect of toasting on the chemical composition, functional and antioxidative properties of full fat and defatted sesame (sesamum indicum L) seed flours. Journal of Culinary Science and Technology. 2021;19(1):18–34. https://doi.org/10.1080/15428052.2019.1681333
- Lu X, Sun Q, Zhang L, Wang R, Gao J, Jia C, et al. Dual-enzyme hydrolysis for preparation of ACE-inhibitory peptides from sesame seed protein: Optimization, separation, and identification. Journal of Food Biochemistry. 2021;45(4). https://doi.org/10.1111/jfbc.13638
- Gorissen SHM, Crombag JJR, Senden JMG, Waterval WAH, Bierau J, Verdijk LB, et al. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids. 2018;50(12):1685–1695. https://doi.org/10.1007/s00726-018-2640-5
- Fathi N, Almasi H, Pirouzifard MK. Sesame protein isolate based bionanocomposite films incorporated with TiO2 nanoparticles: Study on morphological, physical and photocatalytic properties. Polymer Testing. 2019;77. https://doi.org/10.1016/j.polymertesting.2019.105919
- Sharma L, Singh C. Sesame protein based edible films: Development and characterization. Food Hydrocolloids. 2016;61:139–147. https://doi.org/10.1016/j.foodhyd.2016.05.007
- López-Monterrubio DI, Lobato-Calleros C, Alvarez-Ramirez J, Vernon-Carter EJ. Huauzontle (Chenopodium nuttalliae Saff.) protein: Composition, structure, physicochemical and functional properties. Food Hydrocolloids. 2020;108. https://doi.org/10.1016/j.foodhyd.2020.106043
- Müsellim E, Tahir MH, Ahmad MS, Ceylan S. Thermokinetic and TG/DSC-FTIR study of pea waste biomass pyrolysis. Applied Thermal Engineering. 2018;137:54–61. https://doi.org/10.1016/j.applthermaleng.2018.03.05
- Swain SN, Rao KK, Nayak PL. Biodegradable polymers. III. Spectral, thermal, mechanical, and morphological properties of cross-linked furfural – soy protein concentrate. Journal of Applied Polymer Science. 2004;93(6):2590–2596. https://doi.org/10.1002/app.20729

